Abstract
We studied the outcome of allogeneic transplantation after lower-intensity conditioning regimens (reduced-intensity [RIC] and non-myeloablative [NST]) in non-Hodgkin lymphoma (NHL) relapsing after autologous transplantation. Non-relapse mortality (NRM), lymphoma progression/relapse, progression-free survival (PFS) and overall survival (OS) were analyzed in 263 NHL patients. All had relapsed after a prior autologous transplant and then received allogeneic transplantation from related (n = 26) or unrelated donors (n= 237) after RIC (n = 128) or NST (n = 135), and were reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) between 1996 and 2006. Median follow-up of survivors was 68 months (range, 3–111). Three-year NRM was 44% (95% CI, 37%–50%). Lymphoma progression/relapse at three years was 35% (95% CI, 29%–41%). Three-year probabilities of PFS and OS were 21% (95% CI, 16%–27%) and 32% (95% CI, 27%–38%) respectively. Superior performance score, longer interval between transplants, total-body irradiation-based conditioning regimen and lymphoma remission at transplantation correlated with improved PFS. Allogeneic transplantation after lower-intensity conditioning is associated with significant NRM, but can result in long-term PFS. We describe a quantitative risk model based on pretransplant risk factors in order to identify those likely to benefit from this approach.
Keywords: Non-Hodgkin Lymphoma, Allogeneic, Relapse
INTRODUCTION
Autologous hematopoietic progenitor cell transplantation (autotransplant) is widely used to treat recurrent or refractory non-Hodgkin lymphoma (NHL).1,2 Unfortunately, relapse is common after autologous transplantation and the prognosis for these patients is poor.(3) Conventional chemotherapy is non-curative after autotransplant failure, and a second autotransplant mostly benefits a small group of patients relapsing after a long lymphoma-free interval.4,5 The results of conventional myeloablative allogeneic transplantation (allotransplant) performed in this setting are also poor (5% progression-free survival [PFS] at five years), as previously reported.6 Also, many patients are not candidates for myeloablative conditioning because of age or co-morbidities.
Reduced-intensity conditioning (RIC) and non-myeloablative conditioning (NST) regimens are increasingly used in patients with NHL. These lower-intensity conditioning regimens are reported to have lower non-relapse mortality (NRM) and can be used in older persons with co-morbidities.7 Lower-intensity regimens for allotransplant use lower doses of conditioning chemotherapy and radiation, and rely on an immune-mediated graft-versus-lymphoma (GVL) effect for disease control. The magnitude of this effect in NHL is unclear.8,9
Prior studies reporting on RIC or NST allotransplant for NHL relapsing after autotransplant have limited numbers of patients, variable histologies and variable follow-up limiting comparisons.10–14 In order to analyze the wider applicability and effectiveness of this modality, we analyzed long-term outcomes of lower-intensity (RIC/NST) allotransplant for relapsed B-cell NHL (B-NHL) after a prior autotransplant using data from the Center for International Blood and Marrow Transplant Research (CIBMTR). To date, this represents the largest study of patients with NHL treated with lower-intensity conditioning allotransplant after autotransplant failure.
SUBJECTS AND METHODS
Data Sources
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR) and the National Marrow Donor Program (NMDP) established in 2004, which comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplants to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for discrepancies, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority, and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Boards of the National Marrow Donor Program and the Medical College of Wisconsin since 1985.
Subjects
Outcomes of 263 adult patients (> 21 years) with B NHL relapsing after autotransplantation who then received lower-intensity conditioning regimens followed by allotransplantation between 1996 and 2006 were analyzed. Follicular, diffuse large B-cell (DLBCL) and mantle-cell lymphoma histologies were included. Recipients of planned tandem auto-allotransplants and those in first complete remission at the time of their allotransplantation were excluded. Donors were HLA-matched siblings for 26 recipients and HLA-matched unrelated (URD) for 237 recipients.
Only a limited number of those relapsing after autotransplantation subsequently receive an allotransplant. In the period between 1990 and 2006, a total of 6395 patients with post-autotransplantation relapse of B-NHL registered with the CIBMTR, 5.8% (373) received a subsequent allogeneic transplant after RIC/NST conditioning regimens. The cohort studied in this report is a subset of those patients for whom comprehensive data were available, with high-level reporting, with complete case report forms. We confirmed that the global cohort and the study subset had similar outcomes.
Definitions
Lower-intensity conditioning regimens and HLA matching
Lower-intensity conditioning regimens were categorized as RIC or NST using established consensus criteria.15 Previously established validated criteria for categorizing degree of HLA matching were used.16 Well-matched cases had either no identified HLA mismatch and informative data at four loci, or allele matching at HLA-A, B & DRB1 (6/6).
Endpoints
Primary outcomes were NRM, relapse/progression, PFS and survival. NRM was defined as death from any cause in the first 28 days or death without evidence of lymphoma progression/relapse. Progression was defined as an increase of ≥ 25% in the sites of lymphoma or development of new sites of lymphoma. Relapse was defined as recurrence of lymphoma after a complete response (CR). For PFS patients were considered treatment failures at the time of relapse/progression or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow up and the PFS event was summarized by a survival curve. The OS interval variable was defined as the time from date of transplant to date of death or last contact and summarized by a survival curve. Other outcomes analyzed included acute and chronic graft-versus-host disease (AGVHD and CGVHD) and cause of death (COD). AGVHD was defined and graded based on the pattern and severity of organ involvement using established criteria.17 CGVHD was defined as the development of any chronic GVHD based on clinical criteria. Both these events were summarized by the corresponding cumulative incidence estimate with death without development of GVHD as the competing risk.
Statistical Analyses
Probabilities of PFS and survival were calculated using the Kaplan-Meier product limit estimate. Probabilities of NRM, lymphoma progression/relapse and acute and chronic GVHD were calculated using cumulative incidence curves to accommodate competing risks.18,19 Associations between subject-, disease-, and transplant-related factors and outcomes of interest were assessed using multivariate Cox proportional hazards regression. A stepwise forward selection multivariate model was built to identify covariates that influenced outcomes. Covariates with a p-value <0.05 were considered significant. The proportionality assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome.20 All variables met the proportional hazards assumption. Results were expressed as relative risks (RR) or the relative rate of occurrence of the event.
The following variables were considered in multivariate analyses: age at allotransplant, sex, Karnofsky Performance Score (KPS) at allotransplant, time from diagnosis to autotransplant, time from autotransplant to allotransplant, NHL histology, disease status and sensitivity to chemotherapy at allotransplant, conditioning regimen intensity (RIC vs. NST), donor type (HLA identical related vs. HLA well-matched URD vs. HLA partially-matched URD), donor-recipient gender match (female donor and male recipient versus all other combinations), donor-recipient cytomegalovirus (CMV) state (donor and recipient CMV-seronegative vs. all other combinations), graft source (bone marrow vs. blood), year of allotransplant (1996–2003 versus 2004–2006) and type of GVHD prophylaxis. The interval from autotransplant to relapse was not available in all patients. Therefore, the interval from the autotransplant to allotransplant was used as a surrogate variable, combining the intervals from autotransplant to relapse and the interval from such relapse to allotransplant.
RESULTS
Subject- and Transplant-Related Variables
Subject-, disease-, and transplant-related characteristics are listed in Table 1. Two hundred sixty-three patients from 69 centers received an allotransplant for NHL with lower-intensity conditioning after relapse following a prior autotransplant. Median age at allotransplantation was 52 years (range, 23–70 years). Eighty-nine (34%) had KPS < 90 at time of allotransplant.
Table 1.
Patient-, disease- and transplant-related characteristics
| Variable | N (%) |
|---|---|
| Number of patients | 263 |
| Age at allotransplant, median (range), years | 52 (23–70) |
| Age at allotransplant, years | |
| 21–30 | 14 (5) |
| 31–40 | 34 (13) |
| 41–50 | 71 (27) |
| 51–60 | 107 (41) |
| ≥61 | 37 (14) |
| Male sex | 168 (64) |
| Karnofsky score at allotransplant | |
| < 90 | 89 (34) |
| Histology at allotransplant | |
| Follicular large/DLBCL | 147 (56) |
| Follicular | 44 (17) |
| Mantle cell | 72 (27) |
| Histologic transformation after diagnosis | 57 (22) |
| Time from diagnosis to first autotransplant, median (range), months | 19 (2–278) |
| Time from auto- to allotransplant, median (range), months | 25 (4–159) |
| Time from auto- to allotransplant, months | |
| <12 | 52 (20) |
| 12–24 | 80 (30) |
| >24 | 131 (50) |
| Disease status at allotransplant | |
| CR2+ | 67 (27) |
| PIF (never in CR) | 22 (9) |
| REL-sensitive | 90 (36) |
| REL-resistant | 58 (23) |
| REL-unknown/untreated | 14 (6) |
| Chemosensitivity disease at allotransplant | |
| Sensitive | 159 (63) |
| Others | 104 (37) |
| Donor type | |
| Related | 26 (10) |
| Unrelated | 237 (90) |
| Donor/Recipient gender match | |
| M-M | 112 (43) |
| M-F | 54 (21) |
| F-M | 56 (21) |
| F-F | 41 (16) |
| Donor/Recipient CMV status | |
| +/+ | 50 (19) |
| +/− | 23 (9) |
| −/+ | 90 (34) |
| −/− | 87 (33) |
| Not tested/inconclusive | 11 ( 4) |
| Conditioning regimen allotransplant | |
| Low dose TBI based (< 500 cGy) | 9 (3) |
| Melphalan dose ≤ 150 mg/m2 | 65 (25) |
| Busulfan dose ≤ 9 mg/kg | 54 (21) |
| TBI dose=200 cGy | 66 (25) |
| Fludarabine + Cyclophosphamide | 62 (24) |
| Fludarabine only | 7 (3) |
| Conditioning regimen at 2nd transplant | |
| Reduced-intensity (RIC) | 128 (49) |
| Non-myeloablative (NST) | 135 (51) |
| Rituximab pre-allotransplant | 195 (74) |
| Type of donor | |
| Well-matched | 150 (57) |
| Partially matched | 69 (26) |
| Mismatched | 12 (5) |
| Unrelated, matching unknown | 6 (2) |
| Related | 26 (10) |
| Graft source | |
| Bone marrow | 56 (21) |
| Peripheral blood | 207 (79) |
| Year of allotransplant | |
| 1996–1997 | 2 (1) |
| 1998–1999 | 8 (3) |
| 2000–2001 | 41 (16) |
| 2002–2003 | 71 (27) |
| 2004–2006 | 141 (54) |
| GVHD prophylaxis at allotransplant | |
| MTX + CsA ± other | 35 (13) |
| CsA ± other | 96 (37) |
| MTX + FK506 ± other | 72 (27) |
| FK506 ± other | 51 (19) |
| T-cell depletion ± other | 4 (2) |
| Other/unspecified | 5 (2) |
| Donor lymphocyte infusion after allotransplanta | 17 (6) |
| Median follow-up of survivors, months | 68 (3–111) |
Abbreviations: DLCL=diffuse large cell lymphoma; CR=complete remission; PIF=primary induction failure; REL=relapse; CMV=cytomegalovirus; GVHD=graft-versus-host-disease; MTX=methotrexate; CsA =cyclosporine; FK506=tacrolimus; EVAL=evaluable
5 (29%) are alive and 12 (71%) are dead. Sixteen patients (95%) relapse/progressed after 2nd transplant. Completeness index follow up=90%.
One hundred forty-seven patients (56%) had DLBCL or follicular large-cell NHL, 72 (27%) had mantle cell lymphoma, and 44 (17%) had follicular lymphoma. Fifty-seven of the DLBCL patients were reported to be the consequence of histologic transformation from a lower grade lymphoma. Median interval from diagnosis to autotransplant was 19 months (range, 2–278 months). Eighty-five patients (33%) had their autotransplant < 1 year after diagnosis. Median interval between auto- and allotransplant was 25 months (range, 4–159 months). Fifty-two (20%) patients received their allotransplant < 1 year after their autotransplant, 80 (30%) patients received them between 1–2 years, and 131 received their allotransplant (50%) > 2 years after their autotransplant. Only 67 patients (27%) were in complete remission (≥ 2nd CR) at the time of allotransplant. One hundred fifty-nine (63%) patients were considered to have chemotherapy-sensitive disease at allotransplant.
Conditioning regimens were classified as RIC in 128 (49%) patients and NST in 135 (51%). Sixty-six (25%) patients received total body radiation (TBI) of 2 Gy, 65 patients (25%) received lower dose melphalan < 150 mg/m2, and 62 (24%) received fludarabine and cyclophosphamide regimens. Three-fourths of patients received rituximab as treatment at some point before allotransplant. A bone marrow graft source was used in 21%. One hundred forty-one (54%) patients received their allotransplant between 2004 and 2006. Seventeen (6%) received donor lymphocyte infusions (DLI) for relapse or failure to achieve CR after their allotransplant. Median follow-up of survivors was 68 months (range, 3–111 months).
Outcomes
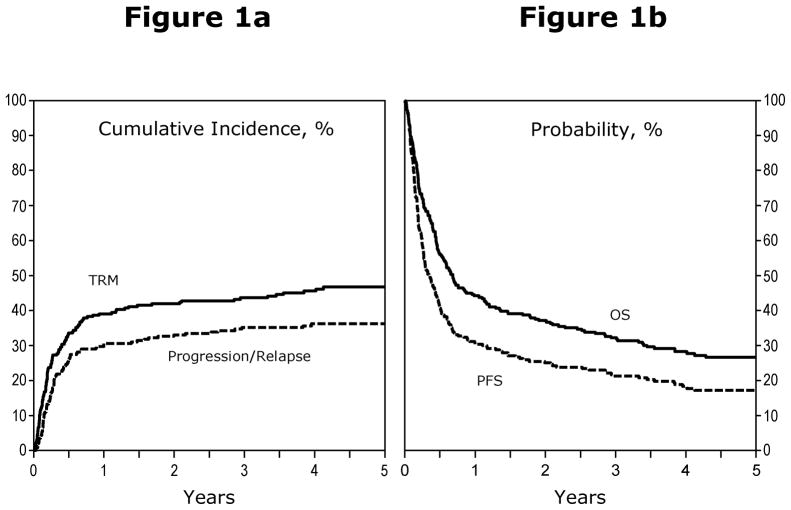
Outcomes are summarized in Table 2. One hundred ninety-four patients died (74%). Twenty-three (9%) were alive with lymphoma and 46 (18%) were alive lymphoma-free without relapse at last follow-up. The 100 day mortality rate was 30% (95% confidence interval [95% CI], 25%–36%). NRM rates were 39% (95% CI, 33%–45%), 44% (95%CI, 37%–50%) and 47% (95% CI, 40%–53%) at 1, 3 and 5 years after allotransplantation. Incidences of lymphoma progression/relapse were 31% (95% CI, 25%–36%), 35% (95% CI, 29%–41%) and 36% (95% CI, 30%–42%) at 1, 3 and 5 years after allotransplant. Figure 1a illustrates cumulative incidences of NRM and lymphoma progression/relapse.
Table 2.
Univariate outcome probabilities
| Outcome event | Prob. (95% CI)a |
|---|---|
| 30 day mortality | 10 (7–15) |
| 100 day mortality | 30 (25–36) |
| Absolute neutrophil count>0.5 × 109/L | |
| @ 28 days | 91 (87–95) |
| @ 100 days | 95 (92–97) |
| Acute GVHD @ 100 days, grades (2–4) | 39 (34–45) |
| Chronic GVHD | |
| @ 1 year | 37 (31–43) |
| @ 3 years | 40 (34–46) |
| @ 5 years | 40 (34–46) |
| NRM | |
| @ 1 year | 39 (33–45) |
| @ 3 years | 44 (37–50) |
| @ 5 years | 47 (40–53) |
| Progression/relapse | |
| @ 1 year | 31 (25–36) |
| @ 3 years | 35 (29–41) |
| @ 5 years | 36 (30–42) |
| PFS | |
| @ 1 year | 30 (25–36) |
| @ 3 years | 21 (16–27) |
| @ 5 years | 17 (13–22) |
| Overall survival | |
| @ 1 year | 44 (38–50) |
| @ 3 years | 32 (27–38) |
| @ 5 years | 27 (21–32) |
Probabilities of neutrophil, acute and chronic GVHD, NRM, and progression/relapse were calculated using the cumulative incidence estimate. 100-day mortality, PFS and overall survival were calculated using the Kaplan-Meier product limit estimate.
Figure 1.
a) Cumulative incidence of NRM and disease progression after RIC/NST in patients who experience relapse after auto-HSCT for NHL. b) Probabilities of PFS and OS after RIC/NST in patients who experienced relapse after auto-HSCT for NHL.
Figure 1b illustrates actuarial probabilities of PFS and survival. PFS rates were 30% (95% CI, 25%–36%), 21% (95% CI, 16%–27%) and 17% (95% CI, 13%–22%) at 1, 3 and 5 years after allotransplant. Survival rates were 44% (95% CI, 38%–50%), 32% (95% CI, 27%–38%) and 27% (95% CI, 21%–32%) at 1, 3 and 5 years after allotransplant.
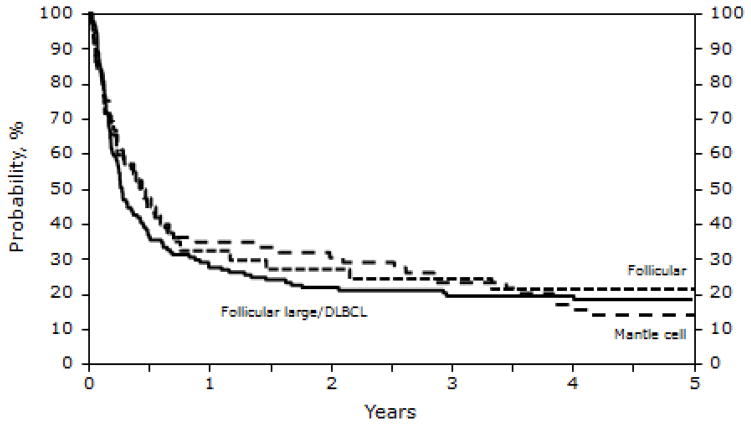
The incidence of ≥ grade 2 acute GVHD within 100 days of transplantation was 39% (95% CI, 34%–45%). The incidences of chronic GVHD were 37% (95% CI, 31%–43%) and 40% (95% CI, 34%–46%) at 1 and 5 years after allotransplant. PFS did not correlate with histologic type of NHL (Figure 2), except for lower PFS (but not survival) in patients with transformed large-cell lymphoma.
Figure 2.
Probability of progression-free survival after RIC/NST in patients who experienced relapse after auto-HSCT for NHL, according to histology at the time of RIC/NST.
Seventeen patients received DLI post allotransplant for lymphoma progression/relapse. Survival after DLI was short –12% (95% CI, 2%–31%), 6% (95% CI, 0–24%) and 6% (95% CI, 0–24%) at 1, 3 and 5 years, respectively.
Causes of death were lymphoma-relapse/progression in 50 (26%), infection in 33 (17%), organ failure in 32 (16%) and acute and chronic GvHD in 23 (12%) patients. Table 5 illustrates the causes of death.
Table 5.
Causes of death
| Causes of death | N eval | N (%) |
|---|---|---|
| Number of patients | 194 | |
| Primary disease | 50 (26) | |
| GVHD | 23 (12) | |
| Pulmonary syndrome | 11 ( 6) | |
| Infection | 33 (17) | |
| Organ failure | 32 (16) | |
| Hemorrhage | 5 ( 3) | |
| New malignancy | 2 ( 1) | |
| Vascular | 2 ( 1) | |
| Unknown | 36 (19) |
Multivariate analyses
NRM
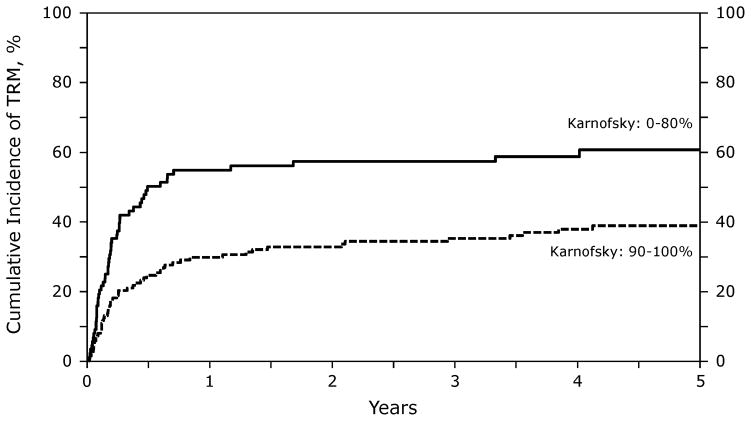
KPS significantly correlated with NRM. Those patients with a KPS < 90 had higher risk of NRM (RR 2.57 [95% CI, 1.57–3.25]; p<0.001). Figure 3 illustrates the probability of NRM by KPS.
Figure 3.
Probability of NRM after RIC/NST according to KPS in patients who experienced relapse after auto-HSCT for NHL.
Lymphoma Progression/Relapse
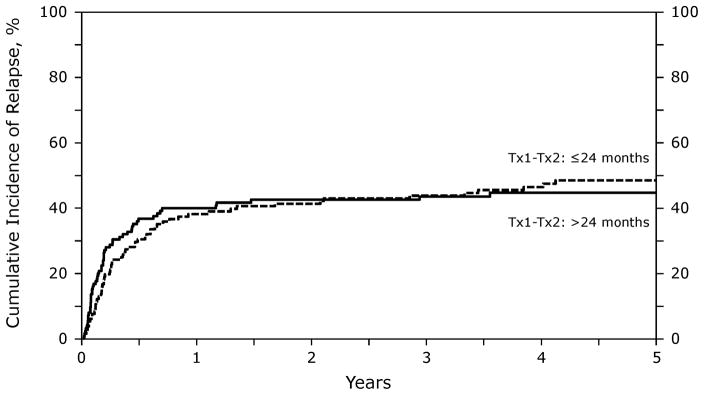
Interval from autologous to allogeneic transplantation significantly correlated with the risk of lymphoma progression/relapse. Recipients of an allotransplant < 2 years after autotransplant were at higher risk of progression/relapse (RR 2.09 [95% CI, 1.37–3.18]; p=0.001) (Figure 4).
Figure 4.
Probability of relapse after RIC/NST in patients who experience relapse after auto-HSCT for NHL, according to time interval between transplants
PFS and Treatment Failure
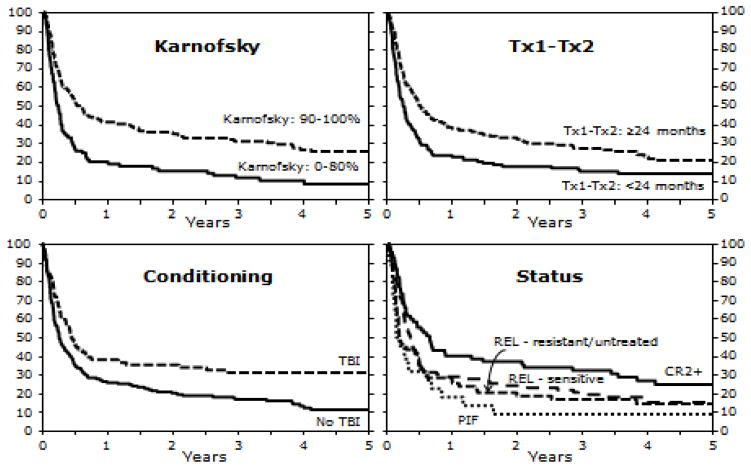
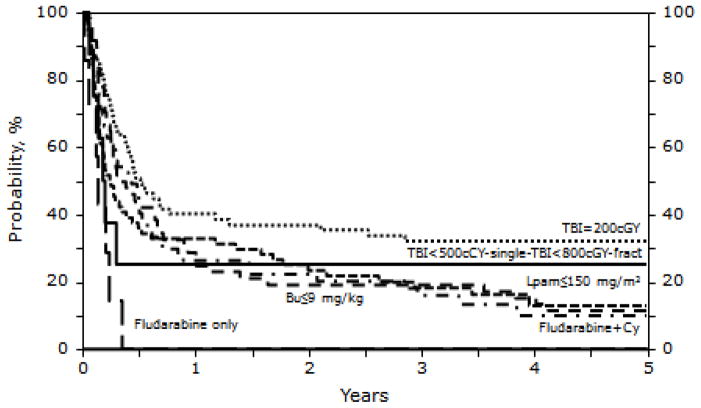
Table 3 shows the multivariate analysis of PFS. Patients with a KPS < 90 had nearly a two-fold increased risk of treatment failure, and lower PFS, compared to patients with a higher KPS (RR 1.78 [95% CI,1.33–2.40]; p < 0.001). Those receiving an allotransplant within two years after a prior autotransplant had a lower PFS and higher risk of treatment failure (RR 1.49 [95% CI, 1.13–1.96]; p=0.004). Recipients of conditioning regimens without TBI had lower PFS (RR of treatment failure =1.66 [95% CI, 1.20–2.29]; p=0.002). Supplemental Table 1 compares the clinical characteristics of patients who received TBI versus patients receiving non-TBI based regimens. Patients who had never achieved a CR (primary induction failure [PIF]) had lower PFS (RR of treatment failure = 1.89 [95% CI, 1.12–3.18], p= 0.017). Figure 5 shows probabilities of PFS according to risk factors. Figure 6 illustrates PFS after allotransplantation by individual conditioning regimens. The type of conditioning regimen, RIC vs. NST, did not impact PFS.
Table 3.
Multivariate analysis for progression-free survival
| Variables: | N | Relative Risk of relapse/progression or death (95% CI) | P-value |
|---|---|---|---|
| Karnofsky score | |||
| ≥90 | 138 | 1.00 | |
| <90 | 119 | 1.78 (1.33 – 2.40) | <0.001 |
| Time from auto to allotransplant | |||
| >24 months | 128 | 1.00 | |
| ≤24 months | 129 | 1.49 (1.13 – 1.96) | 0.004 |
| Conditioning regimen allotransplant | |||
| TBI-based | 73 | 1.00 | |
| Non-TBI | 184 | 1.66 (1.20 – 2.29) | 0.002 |
| Disease status at allotransplant* | Poverall=0.043 | ||
| (1) CR2+ | 67 | 1.00 | |
| (2) Relapse | 156 | 1.26 (0.90 – 1.75) | 0.177 |
| (3) PIF | 22 | 1.89 (1.12 – 3.18) | 0.017 |
| (4) Unknown | 12 | 0.75 (0.37 – 1.51) | 0.418 |
Abbreviations: CI = confidence interval.
Figure 5.
Probability of PFS after RIC/NST in patients who experienced relapse after auto-HSCT for NHL according to KPS, interval between auto-HSCT and RIC/NST, use of TBI as part of conditioning regimen, and disease status at the time of RIC/NST.
Figure 6.
Probability of PFS after RIC/NST in patients who experienced relapse after auto-HSCT for NHL according to conditioning regimen.
GVHD
Patients with KPS < 90, those receiving TBI-based regimens, and those receiving grafts from female donors had higher risk of developing ≥ grade 2 acute GVHD. The only variable correlated with chronic GVHD was the graft source: those receiving blood cell grafts had an increased risk compared to bone marrow (RR 2.45 [95% CI, 1.33–4.48]; p=0.004). Patients with ≥ grade 2 acute GVHD were less likely to develop lymphoma progression/relapse (RR = 0.55 [95% CI, 0.34–0.90]; p = 0.0166) in univariate analysis, but this was not statistically significant in the multivariate model. Chronic GVHD had no impact on probability of lymphoma relapse/progression (RR = 0.71, [(95% CI, 0.37–1.34]; p = 0.2869).
Survival
Survival was significantly correlated with KPS. Patients with a KPS of < 90 had a higher risk of death (RR 1.92 [95% CI 1.43–2.56]; p<0.001).
Risk Model
Based on the significant pretransplant variables identified in the multivariate model we developed a risk scoring system outlined in Table 4. Those with all four adverse risk factors (KPS <90, never in CR, non-TBI-based conditioning, and interval between autotransplant and allotransplant ≤24 months) had an 8.32 times higher risk of death or relapse than patients with no risk factors. Similarly, those with three risk factors (KPS <90, never in CR and non-TBI-based conditioning) had a 5.58 times higher risk of death or relapse. Those with two risk factors (KPS <90 and never in CR) had a 3.36 times higher risk of death or relapse.
Table 4.
Risk Factor model for progression-free survival
| Combination of Variables: | Relative Risk of relapse/progression or death (95% CI) |
|---|---|
| KPS <90 + PIF at allotransplant+ Time between transplants ≤24 months + Non-TBI based conditioning | 8.32 (4.00–17.33) |
| KPS <90 + PIF at allotransplant + Non-TBI based conditioning | 5.58 (2.82–11.04) |
| KPS <90 + PIF at allotransplant | 3.36 (1.84–6.13) |
| Time between transplants ≤24 months + Non-TBI-based conditioning | 2.47 (1.61–3.81) |
DISCUSSION
Our aims were to define outcomes after allogeneic transplantation using lower-intensity conditioning regimens in patients with B-cell NHL relapsing after an autotransplant, and to identify subject-, disease- and treatment-related variables correlated with outcomes. This study represents a large cohort of patients, from multiple centers with long follow-up, thereby providing a perspective on the feasibility and effectiveness of this treatment strategy.
Despite the lower intensity of the conditioning regimens, three-year NRM was high at 44% (95% CI, 38%–46%). In multivariate analysis, KPS was the sole predictor of NRM: those with a KPS < 90 had two-fold higher NRM than patients with a KPS ≥ 90. NRM in our study was higher than previously reported. In the study by Branson et al using HLA-identical sibling donors, 14-month mortality was 20%.21 Martino et al reported 24% NRM (95% CI, 15%–41%) at one year with HLA-identical sibling donors.7 Escalon et al reported 5% NRM in patients with chemo-sensitive lymphoma receiving transplants from HLA-identical related donors.22 Baron et al reported 28% NRM at three years after allotransplant from unrelated donors.23 A recently published study by the European Group for Blood and Marrow Transplantation (EBMT) reported a three-year non-relapse mortality of 28.2%.24 It is likely that differences in NRM between studies reflect subject selection, proportion of unrelated donors and wide confidence intervals. About 40% of the patients in our study had a KPS < 90. Also, 90% of patients in our study received unrelated donor transplants. Furthermore, the proportion of unrelated donor transplants that were well matched was only about 60%, compared to a higher proportion of well-matched unrelated donors in other studies.22,23 Another significant difference is that our study cohort was almost a decade older than patients in most prior studies.
The risk for lymphoma progression/relapse was 31% (95% CI, 25%–36%) at one year, increasing to 36% (95% CI, 30%–42%) at five years. These data are similar to other studies.23,25 The major risk factor correlated with risk of lymphoma progression/relapse was a shorter time interval between autologous and allogeneic transplantation, likely a surrogate for a short time to relapse after autotransplant. In multivariate analyses, superior KPS, longer interval between autologous and allogeneic transplantation, the use of TBI and more favorable disease status at the time of transplantation correlated with superior PFS. As in previous studies, disease status at the time of allotransplant correlated with PFS. Patients with PIF (who had never achieved a prior CR) had the highest risk of treatment failure.7,23,26 In prior studies, these patients were excluded or had worse outcomes.22,27 Interestingly, the use of TBI for conditioning substantially improved PFS, which is consistent with our prior study of myeloablative allotransplants in this setting.6 Use of TBI was also found to decrease the rate of recurrence in a prior CIBMTR study of follicular lymphomas.8 The quantitative risk model we describe is predictive of progression free survival and helps define the risks and benefits of allogeneic transplantation in this setting in practice.
Most previous studies had limited statistical power to detect differences in outcomes between lymphoma subtypes. The survival of patients with DLBCL, follicular and mantle cell lymphoma was similar in our study. Although we found shorter PFS in patients with histological transformation of follicular lymphoma, it did not translate into shorter overall survival.
The use of lower-intensity allotransplants is predicated on a GVL effect. However, it has been difficult to consistently detect a GVL effect in this setting.8,9 In our study, persons with ≥ grade 2 acute GVHD were less likely to develop lymphoma progression/relapse, but this effect was not significant in multivariate analysis. Mohty et al, in a small study, reported a correlation between the acute GVHD and lymphoma relapse.12 Others reported a correlation between chronic GVHD lymphoma progression/relapse, while the EBMT study did not demonstrate a beneficial effect of either acute or chronic GvHD.23–25 In aggregate, these data do not support the presence of a strong, consistent GVL effect in this population of patients with advanced relapsed NHL.
Our study has several limitations. The time interval between autotransplant and relapse, and the time to allotransplant following relapse, are relevant disease-related variables that were not available to us. Instead, we used the time interval between autotransplant and allotransplant as a surrogate incorporating both time intervals. Furthermore, our study population does not include all patients who relapsed after an autotransplant and were eligible for RIC/NST allotransplant. In fact, only a minority of patients relapsing after autotransplant undergoes allotransplantation. The reasons are beyond the scope of our analysis, but may relate to the failure of salvage therapies for NHL relapse, early mortality after relapse, ineligibility for allotransplant, or patient/physician choices. Our results are only applicable to NHL patients who receive an allogeneic transplant.
The survival of patients with NHL who relapse after autotransplant is poor.28,29 Our previous study reported only a 5% PFS five years after myeloablative allotransplant for patients failing an autotransplant.6 Myeloablative conditioning in this setting has been largely abandoned in favor of lower-intensity conditioning regimens, as illustrated by this study and the recent EBMT report.24 Relapse or progression of NHL in this cohort of advanced, high-risk patients who underwent lower intensity allogeneic transplantation was 36% at five years, with the vast majority of relapses happening within the first year after transplantation. However, NRM was also high, contributing to the five-year PFS of 17% and overall survival of 27%. More effective and less toxic conditioning regimens as well as post transplant anti-lymphoma therapy need to be developed to improve these outcomes since the most common causes of failure were disease progression and NRM. Despite these sobering results, our risk model based on pretransplant characteristics defines a subset of patients that can benefit from lower intensity allogeneic transplantation after autologous transplant failures. Patients with late relapses, superior KPS and controlled disease are especially likely to benefit from this approach and they should be considered for this modality.
Supplementary Material
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; Swedish Orphan Biovitrum; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
We thank Osman Ilhan, MD, James L. Gajewski, MD, Andrew L. Pecora, MD, David Rizzieri, MD, Edmund K. Waller, MD, PhD, Gustavo Milone, MD, Mitchell S. Cairo, MD, Brian J. Bolwell, MD, Leona A. Holmberg, MD, Nalini Janakiraman, MD, Ginna Laport, MD for their helpful comments and insights.
Footnotes
Presented in part at the 50th American Society of Hematology Annual Scientific Meeting, San Francisco, CA, December 2008
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vose JM, Zhang MJ, Rowlings PA, et al. Autologous transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2001;19:406–413. doi: 10.1200/JCO.2001.19.2.406. [DOI] [PubMed] [Google Scholar]
- 2.Lazarus HM, Loberiza FR, Jr, Zhang MJ, et al. Autotransplants for Hodgkin’s disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR) Bone Marrow Transplant. 2001;27:387–396. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 3.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, van Besien K, Carreras J, et al. Second autologous stem cell transplantation for relapsed lymphoma after a prior autologous transplant. Biol Blood Marrow Transplant. 2008;14:904–912. doi: 10.1016/j.bbmt.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenberghe E, Pearce R, Taghipour G, Fouillard L, Goldstone AH. Role of a second transplant in the management of poor-prognosis lymphomas: a report from the European Blood and Bone Marrow Registry. J Clin Oncol. 1997;15:1595–1600. doi: 10.1200/JCO.1997.15.4.1595. [DOI] [PubMed] [Google Scholar]
- 6.Freytes CO, Loberiza FR, Rizzo JD, et al. Myeloablative allogeneic hematopoietic stem cell transplantation in patients who experience relapse after autologous stem cell transplantation for lymphoma: a report of the International Bone Marrow Transplant Registry. Blood. 2004;104:3797–3803. doi: 10.1182/blood-2004-01-0231. [DOI] [PubMed] [Google Scholar]
- 7.Martino R, Caballero MD, de la Serna J, et al. Low transplant-related mortality after second allogeneic peripheral blood stem cell transplant with reduced-intensity conditioning in adult patients who have failed a prior autologous transplant. Bone Marrow Transplant. 2002;30:63–68. doi: 10.1038/sj.bmt.1703606. [DOI] [PubMed] [Google Scholar]
- 8.van Besien K, Loberiza FR, Jr, Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 9.Bierman PJ, Sweetenham JW, Loberiza FR, Jr, et al. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin’s lymphoma: a comparison with allogeneic and autologous transplantation--The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2003;21:3744–3753. doi: 10.1200/JCO.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 10.Nagler A, Or R, Naparstek E, Varadi, Slavin S. Second allogeneic stem cell transplantation using nonmyeloablative conditioning for patients who relapsed or developed secondary malignancies following autologous transplantation. Exp Hematol. 2000;28:1096–1104. doi: 10.1016/s0301-472x(00)00511-7. [DOI] [PubMed] [Google Scholar]
- 11.Dey BR, McAfee S, Sackstein R, et al. Succesful allogeneic stem cell transplantation with nonmyeloablative conditioning in patients with relapsed hematologic malignancy following autologous transplantation. Biol Blood Marrow Transplant. 2001;7:604–612. doi: 10.1053/bbmt.2001.v7.pm11760148. [DOI] [PubMed] [Google Scholar]
- 12.Mohty M, Fegueux N, Exbrayat C, et al. Reduced intensity conditioning: enhanced graft-versus-tumor effect following dose-reduced conditioning and allogeneic transplantation for refractory lymphoid malignancies after high-dose therapy. Bone Marrow Transplant. 2001;28:335–339. doi: 10.1038/sj.bmt.1703134. [DOI] [PubMed] [Google Scholar]
- 13.Porter DL, Luger SM, Duffy KM, et al. Allogeneic cell therapy for patients who relapse after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2001:7230–238. doi: 10.1053/bbmt.2001.v7.pm11349810. [DOI] [PubMed] [Google Scholar]
- 14.Fung HC Cohen S, Rodriguez R, et al. Reduced -intensity allogeneic stem cell transplantation for patients whose prior autologous stem cell transplantation for hematologic malignancy failed. Biol Blood Marrow Transplant. 2003;9:649–656. doi: 10.1016/s1083-8791(03)00241-6. [DOI] [PubMed] [Google Scholar]
- 15.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 18.Klein J, Moeschberger M. Survival Analysis: Techniques of censored and truncated data. 2. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 19.Kaplan E. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 21.Branson K, Chopra R, Kottaridis PD, et al. Role of nonmyeloablative allogeneic stem-cell transplantation after failure of autologous transplantation in patients with lymphoproliferative malignacies. J Clin Oncol. 2002;20:4022–4031. doi: 10.1200/JCO.2002.11.088. [DOI] [PubMed] [Google Scholar]
- 22.Escalon MP, Champlin RE, Saliba RM, et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin’s lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol. 2004;22:2419–2423. doi: 10.1200/JCO.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 23.Baron F, Storb R, Storer BE, et al. Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopoietic cell transplantation. J Clin Oncol. 2006;24:4150–4157. doi: 10.1200/JCO.2006.06.9914. [DOI] [PubMed] [Google Scholar]
- 24.van Kampen RJW, Canals C, Schouten HC, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell Non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. 2011;29:1342–1348. doi: 10.1200/JCO.2010.30.2596. [DOI] [PubMed] [Google Scholar]
- 25.Rezvani AR, Norasetthada L, Gooley T, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol. 2008;143:395–403. doi: 10.1111/j.1365-2141.2008.07365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devine SM, Sanborn R, Jessop E, et al. Fludarabine and melphalan-based conditioning for patients with advanced hematological malignancies relapsing after a previous hematopoietic stem cell transplant. Bone Marrow Transplant. 2001;28:557–562. doi: 10.1038/sj.bmt.1703198. [DOI] [PubMed] [Google Scholar]
- 27.Freytes CO, Lazarus HM. Second hematopoietic SCT for lymphoma in patients who relapse after autotransplantation: another autograft or switch to allograft? Bone Marrow Transplant. 2009;44:559–569. doi: 10.1038/bmt.2009.214. [DOI] [PubMed] [Google Scholar]
- 28.Vose JM, Bierman PJ, Anderson JR, et al. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood. 1992;80:2142–2148. [PubMed] [Google Scholar]
- 29.Kewalramani T, Nimer SD, Zelenetz AD, et al. Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin’s disease or aggressive non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2003;32:673–679. doi: 10.1038/sj.bmt.1704214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.