Abstract
Objective
Previous Vagus Nerve Stimulation (VNS) studies have demonstrated anti-nociceptive effects, and recent non-invasive approaches; termed transcutaneous-VNS, or t-VNS, have utilized stimulation of the auricular branch of the vagus nerve in the ear. The dorsal medullary vagal system operates in tune with respiration, and we propose that supplying vagal afferent stimulation gated to the exhalation phase of respiration can optimize t-VNS.
Design
counterbalanced, crossover study.
Patients
patients with chronic pelvic pain (CPP) due to endometriosis in a specialty pain clinic.
Interventions/Outcomes
We evaluated evoked pain analgesia for Respiratory-gated Auricular Vagal Afferent Nerve Stimulation (RAVANS) compared with Non-Vagal Auricular Stimulation (NVAS). RAVANS and NVAS were evaluated in separate sessions spaced at least one week apart. Outcome measures included deep tissue pain intensity, temporal summation of pain, and anxiety ratings, which were assessed at baseline, during active stimulation, immediately following stimulation, and 15 minutes after stimulus cessation.
Results
RAVANS demonstrated a trend for reduced evoked pain intensity and temporal summation of mechanical pain, and significantly reduced anxiety in N=15 CPP patients, compared to NVAS, with moderate to large effect sizes (eta2>0.2).
Conclusion
Chronic pain disorders such as CPP are in great need of effective, non-pharmacological options for treatment. RAVANS produced promising anti-nociceptive effects for QST outcomes reflective of the noted hyperalgesia and central sensitization in this patient population. Future studies should evaluate longer-term application of RAVANS to examine its effects on both QST outcomes and clinical pain.
Introduction
Previous Vagus Nerve Stimulation (VNS) studies have demonstrated anti-nociceptive effects [1], particularly in patients with depression [2]. However, moderate morbidity has been associated with the surgical procedure and maintenance of VNS [3]. Furthermore, it is still unclear whether VNS is an analgesic treatment in general or for a specific chronic pain syndrome. In this study, we propose a novel, non-invasive procedure based on the neurobiology of VNS treatment - Respiratory-gated Auricular Vagal Afferent Nerve Stimulation (RAVANS), which synchronizes stimulation to the respiratory cycle. The auricular branch of the vagus nerve extends to the pinna of the ear and can be electrically depolarized with minimal invasiveness, a procedure referred to as transcutaneous-VNS, or t-VNS [4, 5]. Respiration is known to cyclically modulate activity in both input and output vagal brainstem regions. Hence, the brainstem vagal input-output system operates in tune with respiration and t-VNS can be synchronized with respiratory events to better optimize stimulation, which may improve the analgesic benefits of VNS.
Multiple studies have suggested that VNS can produce anti-nociceptive effects. Studies in a rat model have linked stimulation of vagal afferents with antinociception [6, 7]. Both animal studies [8] and a recent study in humans [9], suggest that during active VNS, pro-nociception can occur when stimulus intensity is low, but anti-nociceptive effects predominate when stimulus intensity is high (non-noxious, detectable stimulation, in mA range). Moreover, Kirchner et al. have found in humans that chronic VNS (at mean 0.7 to 1.4mA) raises pain thresholds for both tonic pinch and heat pain, as well mitigating pain wind-up phenomenon for mechanical stimuli [10, 11]. These results demonstrate promising analgesic effects of VNS, although it is unclear whether findings involving implanted vagal stimulators in patients with intractable seizure disorders will generalize to trials of t-VNS in patients with chronic pain.
Classical VNS involves surgery, with the stimulator lead implanted within the carotid sheath, wrapped around the vagus nerve in the left neck [12]. This can induce morbidity stemming either from co-activation of efferent vagal fibers (e.g. bradycardia, asystole [13], larynx/pharynx disorders [14], dysphagia [15]), or from infection or hardware failure [15]. Ultimately, as the mechanisms for VNS likely involve afferent, and not efferent vagal fibers [16], isolation of afferent fibers in vagal stimulation would eliminate potential negative effects due to efferent stimulation, while accessing these fibers without surgical intervention would eliminate infection-associated morbidity. In sum, there are many advantages to a minimally invasive and less costly vagal nerve stimulation device, which would serve to benefit a larger number of chronic pain patients.
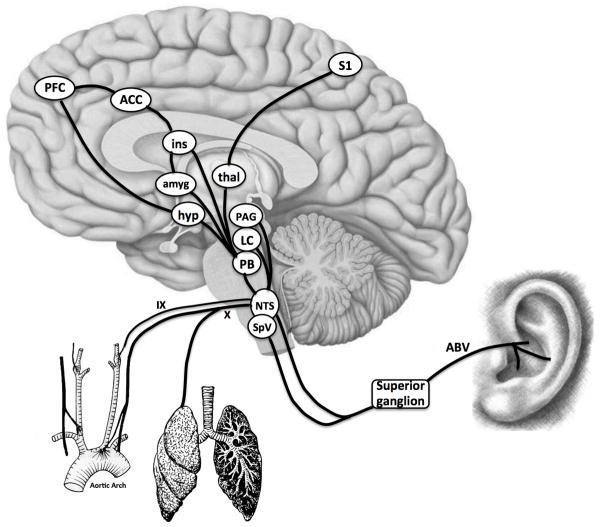
The analgesic mechanisms of VNS have not been fully elucidated, but are likely mediated by afferent (not efferent) input to supraspinal brain regions [16]. Vagal afference is relayed to the nucleus tractus solitarious (NTS) in the medullary brainstem. Importantly, the NTS also receives somatosensory afference via the auricular branch of the vagus (ABV) nerve from specific portions of the auricle [17]. ABV afference is transmitted to both the NTS [17] and the spinal trigeminal nucleus (SpV) [18], by neurons located in the superior (jugular) ganglion of the vagus nerve. Respiration can modulate NTS activity directly (the lungs are innervated by the vagus nerve) and indirectly. In regard to the latter, inspiration increases venous return to the thorax, which increases arterial pressure, and hence vagal (and glossopharyngeal n.) afference to the NTS via aortic and carotid baroreceptors, respectively [19]. The NTS then inhibits efferent vagal outflow to the heart [20, 21], leading to a transient inspiratory tachycardia with every breath. This feedback loop is termed “respiratory sinus arrhythmia” [22]. Hence, the dorsal medullary vagal system operates in tune with respiration, and we propose that supplying vagal afferent stimulation gated to the exhalation phase of respiration (i.e. when thoracic baroreceptor afference does not enter the NTS), will optimize t-VNS therapy (see Figure 1 for schematic). Furthermore, such intermittent, irregular stimulation (i.e., varying with respiration) will also mitigate classical neuronal adaptation/accomodation, which can occur with continuous stimulation of NTS neurons [23].
Figure 1. Schematic of Integrative Innervation of the NTS.
The nucleus tractus solitarius (NTS) in the medulla integrates afferent inputs from the cervical vagus (X, e.g. aortic arch baroreceptors, lungs), glossopharyngeal nerve (IX, e.g. carotid baroreceptors), and auricular branch of the vagus (ABV). NTS input to higher brain regions processing different aspects of pain is thought to underlie the anti-nociceptive effects of vagus nerve stimulation (VNS). N.b. SpV = trigeminal nucleus, PB = parabrachial nucleus, LC = locus ceruleus, PAG = periaqueductal gray, hyp = hypothalamus, amyg = amygdala, thal = thalamus, ins = insula, ACC = anterior cingulate cortex, PFC = prefrontal cortex, S1 = primary somatosensory cortex.
While VNS is a general analgesic mechanism at the level the brain, perhaps enhancing the activity of descending inhibitory systems, the vagus nerve has widespread projections throughout the abdominal and pelvic viscera. Thus, a likely target of VNS in initial clinical use could be abdominal and/or pelvic pain. Chronic pelvic pain (CPP) is a syndrome in urgent need of innovative and effective therapies [24]. CPP encompasses a number of common and debilitating syndromes including interstitial cystitis, endometriosis-mediated pain, and cancer pain [25]. Evidence from quantitative sensory testing (QST) studies has indicated that hyperalgesic mechanisms and central sensitization play a role in the chronicity and severity of this pain syndrome [26–28], supporting the use of QST measures as primary outcomes in evaluating potential therapeutic interventions for pelvic pain. In this study, we evaluated the effects of RAVANS on evoked, experimental pain ratings in patients with CPP due to endometriosis, using a counterbalanced crossover design. Patients completed two sessions utilizing QST evaluations before and after either RAVANS or an active control procedure, Non-Vagal Auricular Stimulation (NVAS). This was identical to RAVANS, except for the auricular location of stimulation. We hypothesized that RAVANS would produce greater evoked pain analgesia compared to the NVAS control.
Methods
Our randomized, crossover, pilot study was conducted at the Pain Management Center in the Department of Anesthesiology at Brigham and Women’s Hospital in Boston, MA. All patients completed informed consent procedures according to the protocol approved by the Partners Human Research Committee (PHRC).
Subjects
In an effort to select a more homogenous pelvic pain condition, patients with CPP due to endometriosis were recruited from the Pain Management Center of Brigham and Women’s Hospital. However, we recognize that endometriosis-linked CPP is difficult to classify and characterize as well, and that the etiology of pain is often unclear. For this initial study of RAVANS treatment, we targeted a sample size of approximately 15 patients. Crossover studies in patient groups which use QST as outcome measures often employ sample sizes of 20 or lower (e.g., n=10 in Staahl et al., 2007).
Inclusion criteria consisted of the following: a) female volunteers between 21 and 64 years of age with chronic pelvic pain for more than six months thought to be due to endometriosis by self report (six months of chronic pain is the criteria most often used in CPP research [24]); b) confirmed by determination of a gynecologist or pain physician specializing in pelvic pain (AV); c) average pain intensity of ≥4 on a scale from 0 to 10; and d) at least an 8th grade English-reading level. In addition, exclusion criteria consisted of the following: a) any interventional procedure for CPP two weeks prior to the study or during the two-week study period, such as lumbar epidural steroids, nerve root blocks, etc.; b) any etiology for CPP due to a known other local somatic lesion for the pain (e.g. fibroids) documented by the patient’s gynecologist, surgery and/or imaging; c) opioid usage, either oral or intrathecal; d) surgical therapy in the previous 12 weeks, the intent to undergo surgery during the study period, or any clinically unstable systemic illness that is judged to interfere with the trial; e) non-ambulatory status; f) history of severe cardiac or nervous system disease; g) cancer or other malignant disease; and i) pregnancy.
We did not evaluate study subjects during a specific phase of menstrual cycle. While the effects of menstrual cycle phase on pain sensitivity have been controversial [29], we chose not to control for this factor as (1) we anticipated that multiple subjects would be menopausal due to either post-hysterectomy, or other endometriosis treatment, (2) our study outcomes focused on within-session change scores, and (3) multiple subjects would be on oral contraceptives which are known to blunt any potential cycle related variability in pain sensitivity [30, 31].
Session Protocol
Subjects completed two experimental sessions, spaced at least one week apart, though given the duration of the treatment, we did not expect any carryover effects. The two sessions included either RAVANS (patent pending by Massachusetts General Hospital, not by the authors) or non-vagal auricular stimulation (NVAS), occurring in a counter-balanced order.
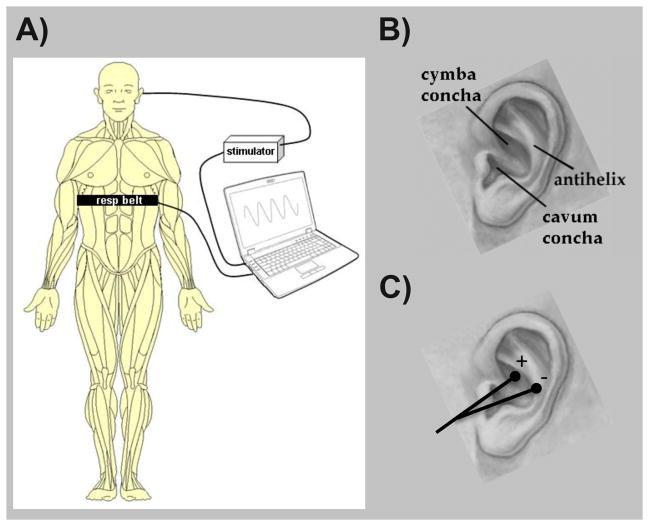
Subjects were seated in a reclined position for both sessions. During the RAVANS stimulation session, two 0.20 x 1.5mm modified press-tack electrodes (DBC, Korea and Vinco, China) were inserted in the left ear. Auricular locations were (1) the cymba concha and (2) the slope between the antihelix and cavum concha (Figure 2). These locations were chosen based on previous knowledge of vagus innervation of the human auricle. While variability exists, anatomic dissection in 7 cadavers (14 ears) found that the cymba concha, anti-helix, and cavum concha were innervated by the afferent branch of the vagus nerve in 100%, 75%, and 45% of ears, respectively [32]. During the control, NVAS, procedure, two electrodes were inserted into the ear lobe of the left auricle. Peuker et al. found that the ear lobe was innervated by the great auricular nerve in 100% of ears studied [32]. The stimulus duration, intensity, pulse frequency, and all other stimulation parameters were the same between RAVANS and NVAS. As all aspects of the protocol including transcutaneous electrical stimulation parameters, but not site of stimulation, were matched in the 2 treatment conditions, NVAS should be considered an active control.
Figure 2. Schematic of the RAVANS procedure.
(A) Subjects were outfitted with a thoracic belt to measure respiratory excursions. This signal was transduced and fed into a laptop controller, allowing for left t-VNS stimulation to occur only during the expiratory phase of respiration. (B) Auricular anatomy includes important regions including the cymba and cavum conchae, as well as the antihelix. (C) Auricular electrodes were placed within the cymba concha and antihelix, the two regions found to be most consistently innervated by the ABV nerve [32].
Electrical stimulation was provided by a Cefar Acus II (Cefar Medical, Lund, Sweden). Stimuli consisted of rectangular pulses with 450 μS pulse width, delivered at 30Hz. Stimulus duration was 0.5 seconds, and was gated to the exhalation phase of respiration (see below). Current intensity was set to achieve moderate to strong (but not painful) sensation, and pulse frequency/duration was set following pilot testing to achieve a subjectively comfortable stimulus sensation.
Respiratory gating for stimulation required real-time evaluation of the respiratory cycle. A pneumatic belt was placed around the subject’s lower thorax. Low-compliance tubing connected this belt to a pressure transducer (PX138-0.3D5V, Omegadyne, Inc., Sunbury, Ohio), thereby producing voltage data that corresponded to changes in respiratory volume [33]. The voltage signal from the transducer was acquired by a laptop-controlled device (National Instruments USB DAQCard 6009, 14bit i/o, with Labview 7.0 data acquisition software). Computer code detected end-inspiration and end-expiration in real-time and a TTL signal was output to a miniature high-frequency relay (G6Z-1P-DC5, Omron Electronics Components, Schaumburg, IL). The TTL pulse was output to the relay 0.5 second after end-inspiration (i.e. during expiration), which allowed stimuli to pass to the ear electrodes for 0.5 seconds. Real-time evaluation of respiratory cycle is non-trivial, and an adaptive threshold detection method was employed. Correct expiratory-cycle stimulation was confirmed in real-time by the experimenter via running chart of the respiration signal and stimulus pulse. Post-hoc review of these tracings was also performed and demonstrated accurate expiratory stimulation.
Physiological Monitoring
In addition to QST and clinical outcomes, we also collected physiological monitoring data, as our RAVANS intervention was mediated by the afferent vagus nerve, and efferent vagal feedback may have also been affected at medullary and higher brain levels. We collected both respiratory and electrocardiography (ECG) data, at 400Hz. Respiration was monitored with a pneumatic belt as part of the RAVANS procedure.
Respiration and ECG data were used to calculate respiratory rate, heart rate (HR), and heart rate variability (HRV). HRV analysis has been applied to indirectly estimate sympathetic and parasympathetic modulation to the heart [34–37]. While some controversy in interpretation remains, the spectral peak in a low frequency band (LF, 0.01–0.15Hz) is thought to be influenced by both parasympathetic and sympathetic activity, while the peak in a high frequency band (HF, 0.15–0.50Hz) is influenced solely by parasympathetic (cardiovagal) activity [36]. The LF/HF ratio has been used to approximate the balance between sympathetic and parasympathetic modulation to the heart. All physiological metrics were evaluated for 5-minute windows at baseline, and at the end of stimulation (window ending at termination of stimulus). ECG data were processed with the WFDB (WaveForm DataBase) Software Package [38] and MATLAB 7.4.0 (The Mathworks, Inc. Natick, MA). Data were automatically annotated with careful manual correction for QRS peak detection in order to form an R-R interval time series. Respiration rate and HRV were evaluated using spectral methods over the window of interest. We used a conventional FFT-based analysis using the Yule-Walker algorithm, a parametric spectral estimation method.
Within each window of interest, modulation of physiological metrics (HR, respiratory rate, LF-HRV, HF-HRV, LF/HF) was evaluated with a 2 x 2 ANOVA (PASW Statistics 18, SPSS Inc., Chicago, IL) was performed with factors STIM (RAVANS and NVAS) and TIME (baseline and end-stim) as independent variables. Post-hoc testing was performed with Student’s t-tests, significant at alpha = 0.05.
Quantitative Sensory Testing (QST)
Our primary outcome measures included psychophysical responses to several forms of noxious mechanical stimulation. QST measures serve as markers of sensitization and hyperalgesia, and have been studied as predictors of pain treatment outcomes. Prior research in a variety of patient samples has indicated that QST measures predict responses to opioid medications in both patients [39] and controls [40]. Other treatment studies have revealed that changes in responses to standardized noxious stimuli are associated with changes in clinical pain [41–44].
Since numerous studies have demonstrated that CPP is associated with generalized hyperalgesia at various body sites [26, 45, 46], we elected to study RAVANS’ impact on indices of hyperalgesia and central sensitization. Hence, we evaluated repeated mechanical stimuli that produce windup (a phenomenon related to central sensitization) and tonic, deep-tissue mechanical pain.
During the session, subjects were seated comfortably in a reclining chair. Tonic, deep tissue mechanical pain was assessed using an inflatable cuff. Cuff pressure algometry (CPA) is a recently-characterized method that is now included in many quantitative sensory testing studies. In brief, tonic, deep-tissue, mechanical stimulation is applied using a pneumatic tourniquet cuff, which is inflated to and maintained at a particular pressure [47]. One advantage to the application of cuff algometry is that unlike more superficial methods of evaluating mechanical sensitivity, cuff pain responses are unaffected by sensitization or desensitization of the skin, indicating that this procedure primarily assesses sensitivity in muscle and other deep tissues [48, 49]. The present protocol utilized a Hokanson rapid cuff inflator, as in some of our previous cuff studies [50, 51]. A standard blood pressure cuff was wrapped comfortably around the lower leg, over the gastrocnemius muscle. A computer-controlled air compressor maintained the pressure at a level that was individually tailored, for each subject, to produce a pain intensity rating of 40/100. Cuff inflation was maintained for 2 minutes, and subjects rated pain intensity and unpleasantness at 30-second intervals. Cuff pain intensity and unpleasantness were averaged across the 2-minute cuff stimulation period.
Mechanical probes were used to assess windup. First, as in previous work [52], participants underwent an assessment of mechanical temporal summation using a set of seven custom-made weighted pinprick stimulators developed by the German research Network on Neuropathic Pain [53, 54]. These punctuate mechanical probes have a flat contact area of 0.2 mm in diameter, and exert forces between 8 and 512 mN. Punctate stimuli were delivered to the skin on the dorsum of the middle finger of the right hand. In each session, we determined the lowest force stimulator that produced a sensation of mild to moderate pain (128 or 256 mN for most subjects), and then applied a train of 10 stimuli at the rate of 1 per second. Participants rated the painfulness of the first, fifth, and tenth stimulus. All ratings were on a 0–100 verbal pain intensity scale used in previous studies [55, 56]. We used these ratings to evaluate temporal summation of mechanical pain (i.e., the human analog to “wind-up”), a frequently used index of central pain facilitation. The assessment of temporal summation involves rapidly applying a series of identical noxious stimuli and determining the increase in pain across trials. Animal studies have suggested that temporal summation occurs centrally in second-order neurons in the spinal cord as a consequence of sustained C-fiber afferent input [57].
We also evaluated the two measures described above, concurrently, in order to study the modulatory effects of one stimulus on the other. Recent psychophysical pain research has recognized the role of endogenous inhibitory systems in shaping an individual’s perception of pain. In particular, diffuse noxious inhibitory controls (DNIC), refers to one noxious stimulus inhibiting the pain produced by a second noxious stimulus [58]. DNIC depends on opioid-mediated supraspinal mechanisms [59], is a sensitive measure of deficits in pain modulation in fibromyalgia and related disorders [60] and predicts the development of long-term clinical pain [61]. In this study, we assessed the effects of RAVANS and NVAS stimulation on the magnitude of DNIC by assessing changes in the painfulness of punctuate mechanical stimulation during cuff algometry. That is, at the conclusion of the 2-minute cuff stimulus, the sequence of 10 punctate mechanical probe stimuli was repeated while maintaining cuff inflation around the gastrocnemius.
Each set of pain responses (temporal summation, cuff algometry, DNIC) was assessed at baseline, at the midpoint (15 minutes) of a half-hour-long period of RAVANS (or NVAS), immediately post-RAVANS (or post-NVAS), and 15 minutes after the conclusion of RAVANS (or NVAS). A 2 X 3 repeated measures ANOVA was performed on change scores from baseline. The factor with 2 levels was STIM (RAVANS vs. NVAS), and the factor with 3 levels was TIME (change from baseline at the 3 time points: during stimulation, immediately after stimulation, and 15 min following the end of stimulation). Post-hoc testing was performed with Student’s t-tests, significant at alpha = 0.05.
Exploratory Outcomes
Exploratory, or secondary, outcome measures included clinical pain ratings (on a 0–10 scale), which were obtained at numerous time points during the psychophysical testing session. Sensations evoked by RAVANS and NVAS stimulation were assessed using a psychophysical instrument, the MASS scale, developed for acupuncture and acupuncture-like interventions [62]. The MASS scale can be summarized by the MASS Index, which aggregates the breadth and depth of different sensations evoked by needle penetration and stimulation [62]. In addition, as in prior QST studies (Edwards, Smith et al. 2006; Kuzminskyte, Kupers et al. 2010), current verbal ratings of anxiety (on a 0–100 scale, with “no anxiety” and “severe anxiety” as the respective anchors) were also obtained during the testing session. Finally, we performed exploratory correlation analyses to evaluate if changes in perceived anxiety were correlated to changes in pain report for both cuff pain ratings and windup scores.
Results
A total of eighteen (18) women were enrolled in the study. Fifteen (15) women completed the study. Their mean age was 36.3 years old (SD = 10.6, range = 20–58 years), and the mean pelvic pain duration was 12.3 years (SD = 9.2, range = 1–39 years). Subjects completed two experimental sessions, spaced at least one week apart (Mean: approximately 2 weeks, Range: 1 week to 6 weeks). 3 more subjects completed a single session but did not return for the second session. No subjects dropped out due to stimulus discomfort.
All of the subjects tolerated the RAVANS and NVAS procedures. The average electrical current intensity used for stimulation did not differ (p=0.31) between RAVANS (0.43 ± 0.25 mA, μ±σ), and NVAS (0.34 ± 0.20 mA). Similarly, the intensity of sensations evoked by the stimulation did not differ, as MASS Index (assessed in only 9 of the 15 patients due to a paperwork error) did not differ (p=0.18) across the testing sessions (RAVANS: 3.3 ± 2.3, μ±σ; NVAS: 2.5 ± 1.4).
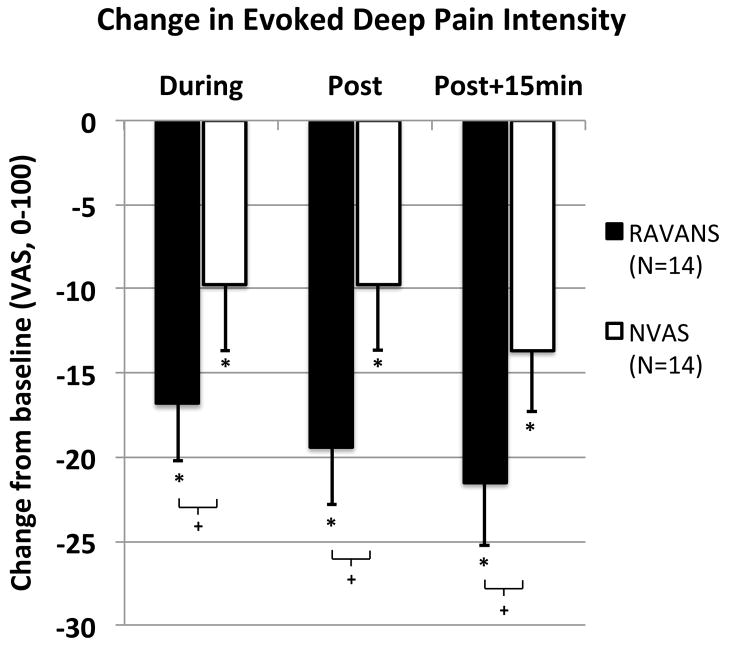
Subjects rated the pain intensity and unpleasantness evoked by cuff pressure. One subject’s cuff algometry data was dropped due to inadvertent within-session alterations in the cuff pressure. Hence, 14 participants are included in this analysis. Average cuff inflation pressure to reach a 40/100 pain rating did not differ (p=0.34) between RAVANS (133.8 ± 43.0 mmHg, μ±σ) and NVAS (144.6 ± 45.4 mmHg) visits. In addition, baseline cuff pain intensity and unpleasantness ratings did not differ across study visits (p’s > 0.5). A 2 x 3 repeated measures ANOVA with factors STIM and TIME demonstrated that for cuff pain intensity, a significant main effect of STIM was observed [F(1,13)= 4.7, p=0.049, eta2 = 0.27], with no significant main effect of TIME [F(2, 12)= 1.8, p=0.21] or interaction [F(2,12)= 0.5, p=0.65]. Follow-up t-tests (see Figure 3) revealed that cuff pain intensity ratings were reduced from baseline at each time point in both sessions (p’s< 0.05), but that the reduction tended to be larger in the RAVANS session for each time period: during the stimulation [t(13)=1.9, p= 0.08], immediately after the stimulation [t(13)= 2.0, p= 0.07], and 15 minutes after the end of stimulation [t(13)= 1.9, p= 0.08]. For cuff pain unpleasantness, neither main effect nor the interaction was significant (p’s> .1).
Figure 3. Response of deep pain intensity to RAVANS vs. NVAS.
Evoked deep pain intensity was reduced (p<0.05) during, immediately after, and 15 minutes following cessation of both RAVANS and NVAS, with a trend (p=0.07–0.08) for greater pain reduction following RAVANS stimulation. N.b. * = p<0.05, + = 0.05 < p < 0.1; error bars represent SEM.
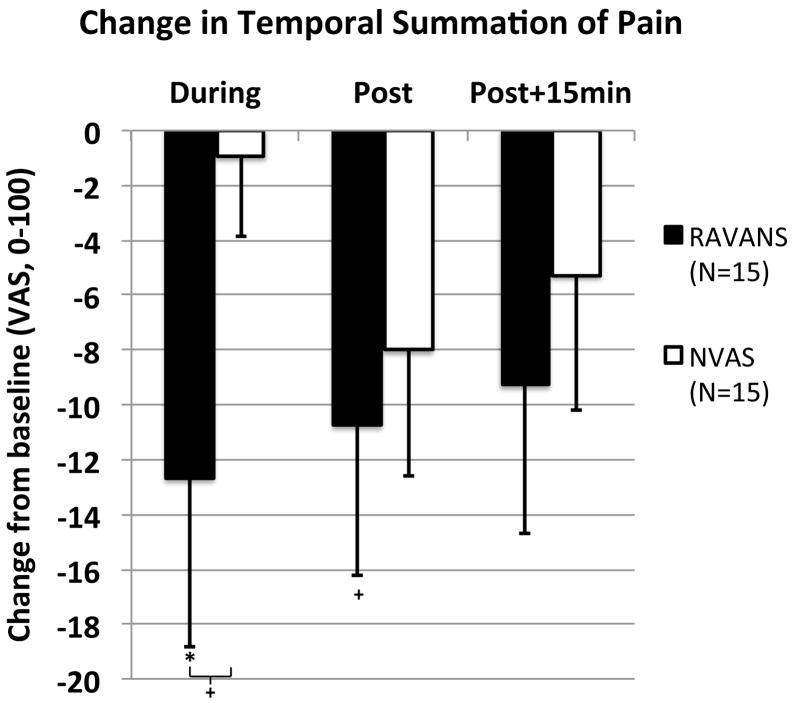
Temporal summation of mechanical pain was calculated by subtracting the pain rating of the first stimulus from the maximum pain rating during the sequence of 10 punctate stimuli. The amount of temporal summation at baseline did not differ significantly (p=0.16) between the RAVANS (30.7 ± 20.8, μ±σ) and NVAS (20.7 ± 18.9) sessions. As with the cuff algometry data, a 2 (STIM) X 3 (TIME) repeated measures ANOVA was performed on temporal summation change scores from baseline. While no significant main effects of STIM [F(1,14)= 1.1, p=0.33] or TIME [F(2, 13)= 0.8, p=0.45] were observed, the interaction was significant [F(2,13)= 3.6, p=0.04, eta2 = 0.20]. Follow-up t-tests (Figure 4) revealed that the only significant change from baseline was observed during stimulation in the RAVANS session (p=0.05), and there was a similar trend for windup to be reduced immediately post-stimulation in the RAVANS session (p=0.07). At 15 minutes after RAVANS stimulation, and at all 3 time points in the NVAS session, there was no significant change from baseline in windup (p’s > 0.1). Comparing change scores in the RAVANS and NVAS sessions, there was a trend for reductions in windup to be greater during stimulation in the RAVANS relative to the NVAS session [t(15)= 1.8, p=0.09), but no trend for any session differences at the immediate post-stimulation and 15-minute post-stimulation time points (p’s>0.4).
Figure 4. Response of temporal summation of pain to RAVANS vs. NVAS.
Temporal summation of pain was reduced (p=0.05) during RAVANS stimulation, while a trend (p=0.07) was found for reduction immediately following RAVANS stimulation, and comparing RAVANS and NVAS during stimulation. N.b. * = p<0.05, + = 0.05 < p < 0.1; error bars represent SEM.
DNIC was explored by evaluating temporal summation on the fingers during cuff algometry on the leg, at both RAVANS and NVAS sessions. At baseline, temporal summation of mechanical pain was unchanged during cuff algometry (p’s> 0.1 for both RAVANS and NVAS), suggesting an absence of DNIC effects in these patients. A 2 X 3 repeated measures ANOVA on change scores from baseline revealed no significant main effects of TIME or STIM and no interaction (p’s> 0.1).
Clinical Pain was also explored by having patients rate (0–100) the intensity of their pelvic pain prior to QST and at each of the study time points. Pain ratings at baseline differed significantly (p=0.02) between the RAVANS (32.8 ± 28.7, μ±σ) and NVAS (mean= 44.0 ± 27.0) sessions. However, a 2 (STIM) X 3 (TIME) repeated measures ANOVA on change scores from baseline revealed no significant main effects of Time or Session and no interaction (p’s> 0.3).
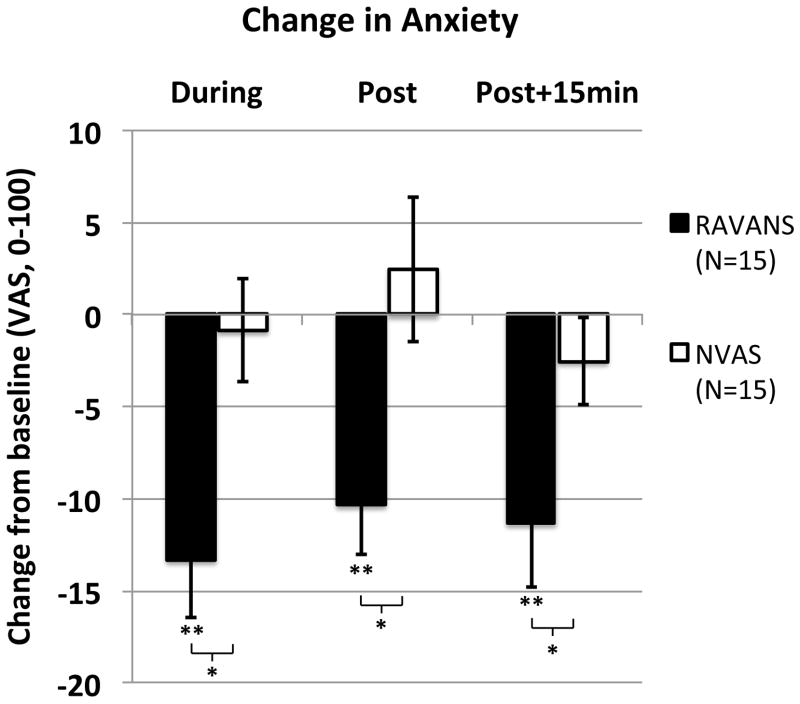
We also specifically assessed anxiety prior to QST at each of the study time points (Figure 5). Anxiety ratings at baseline did not differ significantly (p=0.12) between the RAVANS (17.0 ± 16.9, μ±σ) and NVAS (10.5 ± 17.2) sessions. A 2x3 ANOVA on change scores revealed a significant main effect of STIM [F(1,14)= 9.1, p< 0.01, eta2 = 0.40], but no main effect of TIME or interaction (p’s> 0.2). Follow-up t-tests revealed that anxiety scores were lower (compared to baseline) at each of the subsequent time points in the RAVANS session (p’s< 0.01), but there was no change from baseline at any time points in the NVAS session (p’s> 0.3). Direct comparison of change scores at each time point indicated that reductions in anxiety were significantly larger in the RAVANS than the NVAS session at each time point (p’s< 0.05).
Figure 5. Response of anxiety ratings to RAVANS vs. NVAS.
Anxiety was reduced (p’s < 0.01, compared to baseline) during, immediately after, and 15 minutes after cessation of RAVANS. There was no change from baseline (p’s> 0.3) at any time points in the NVAS session. Reductions in anxiety were significantly larger in the RAVANS than the NVAS session at each time point (p’s< .05). N.b. * = p<0.05, ** = p<0.01; error bars represent SEM.
We examined associations between changes in anxiety and changes in pain responses using correlation coefficients. Because correlations can be strongly affected by outlying values, we evaluated the distributions of change scores. Visual inspection of these distributions did not reveal any obvious outliers, and Grubb’s test indicated that no individual values were significant outliers (p’s> 0.05). After Bonferroni correction for multiple comparisons, none of the correlations were significant (p’s> 0.05), suggesting that treatment-associated changes in anxiety and treatment-associated changes in pain responses were largely independent.
While RAVANS stimulation specifically targeted afferent, and not efferent, vagal stimulation, physiological outflow variables (HR, respiratory rate, and HRV metrics) were evaluated to investigate potential feedback modulation of ANS outflow. These metrics were evaluated at baseline, before QST, and at the very end of RAVANS and NVAS stimulation. Due to excessive noise in the ECG signal (stemming from concurrent stimulation and line noise), the ECG data for some subjects were excluded from HR and HRV analyses. Due to variable cross-interference between electrical stimulation and ECG signal acquisition, we were only able to successfully annotate ECG data for 10 RAVANS and 12 NVAS sessions. A 2x2 ANOVA demonstrated no significant effect of STIM, TIME, or interaction (p’s>0.7) on HR. A similar result was also found for HRV indices HF-HRV, LF-HRV, and LF/HF ratio (p’s>0.7), with 1 subject’s data dropped because their respiratory rate (at both baseline and end-stimulation) was below the HF frequency band cutoff). Respiratory rate was assessed in 11 RAVANS sessions and 14 NVAS sessions. A 2x2 ANOVA demonstrated no significant effect of STIM, TIME, or interaction (p’s>0.8) on respiratory rate (RAVANS: baseline = 14.6 ± 1.3 breaths per minute, μ±σ; end-stim = 14.6 ± 1.2 bpm; NVAS: baseline = 15.1 ± 1.0 bpm; end-stim = 16.1 ± 1.0 bpm).
Discussion
Our pilot counterbalanced, crossover study found that RAVANS demonstrated a trending reduction of both evoked deep pain intensity and temporal summation of mechanical pain (windup) in patients with chronic pelvic pain due to endometriosis. RAVANS was also found to reduce anxiety levels. These reductions in pain responses and anxiety showed moderate to large effect sizes (eta2>0.2 [63]) and tended to be greater than those produced by control stimulation at auricular sites not innervated by the ABV nerve. Furthermore, analgesic responses were independent of reductions in anxiety, suggesting independent mechanisms. These results are promising and further longitudinal studies are warranted, utilizing QST and clinical outcomes as primary endpoints.
Analgesic effects of the auricular, non-invasive variant of VNS, t-VNS, have been evaluated by several studies in the previous decade. In healthy adults, Johnson et al. found that electrical stimulation of auricular locations, including the cavum concha (a noted site of ABV receptors), increased experimental pain threshold by 30% to 50% in a subset of subjects [64]. While we also did not find modulation of autonomic variables such as HR or HRV, we did find significant evoked pain analgesia in our group of CPP patients. Notable differences between our study and that of Johnson et al. included the group of subjects evaluated (i.e. CPP patients versus healthy adults), and the duration of stimulation, which was only 15 minutes in the study by Johnson et al, and was 30 minutes in our study. Interestingly, several previous longitudinal trials of electrical stimulation on three points on the auricle (one of which was the anti-helix, noted to be innervated by vagal afferents [32]) have demonstrated analgesia for chronic low back pain [65], cervical pain [66], and for acute pain during in-vitro fertilization [67]. Similarly, future studies should also evaluate potential analgesia produced by RAVANS in a longitudinal trial in CPP and other chronic pain populations.
While RAVANS produced more significant analgesia compared to NVAS, some mild analgesia was also noted following this active control stimulation. Auricular vagal stimulation accesses higher brain regions through both the NTS and SpV [17]. As our control stimulation provided input to great auricular nerve receptors localized on the ear lobe, the SpV nucleus would also be processing NVAS stimulation. Thus, the mild evoked pain analgesia imparted by NVAS stimulation, suggests that input to the SpV might also contribute to anti-nociception, though less significant compared to vagal input relayed by both NTS and SpV. In addition to the scientific rationale for being an active control, the lack of differences in stimulation parameters (e.g. current amplitude) or in subject ratings of the stimulation sensations between treatment conditions support the credibility of NVAS as an active control.
Our lack of response in different cardiac autonomic variables (reflecting the safety of RAVANS) may simply reflect the innervation of the auricle, which is innervated by afferent, and not efferent, fibers of the vagus nerve [32], the latter of which innervate the chronotropic sinoatrial node of the heart. In fact, this specificity of innervation is one of the advantages of t-VNS stimulation over that of classical VNS stimulation, which affects both afferent and efferent branches of the main vagus nerve, with multiple side-effects resulting from the stimulation of the latter. However, side-effects for t-VNS via afferent-efferent vagal reflexes may also exist and include Arnold’s cough reflex (incidence 2.3–4.2%) [18, 68], ear-gag reflex (~1.8%), ear-lacrimation reflex (~2%), and ear-syncope reflex (~0.6%). Thus, feedback loops, similar to the more extensively studied autonomic baroreflex [69], also exist for ABV signaling, but are rare and we did not encounter any side effects consistent with such reflexes in our study. Interestingly, somatosensory afference tuned to the respiratory rhythm has been found in previous studies to modulate autonomic outflow. For instance, when stimulation was applied to the arm, gated to respiration, heart rate was found to decrease more substantially than for continuous stimulation at the same location [70]. Thus, future studies should continue to evaluate cardiac and other autonomic measures in response to RAVANS, as subtle modulations noted in this study may demonstrate significance with larger sample sizes, and may ultimately relate to clinically-relevant outcomes.
There is a dearth of studies exploring t-VNS mechanisms of action. The afferent vagus nerve, including the ABV, synapses bilaterally on the nucleus tractus solitarius (NTS) in the dorsal medulla of the brainstem. The NTS sends information to efferent (premotor) parasympathetic nuclei, including the dorsal motor nucleus of the vagus (DMNX) and the nucleus ambiguus (NAmb), as well as higher brain regions known to modulate pain, such as the rostral ventromedial medulla, periaqueductal gray, and anterior cingulate cortex [71–74]. Thus the NTS connects with a diffuse system of brain regions modulating pain. This supraspinal network of brain regions has been hypothesized to be the mechanistic substrate of VNS therapeutic effects [16]. In humans, Fallgetter et al. report evoked brainstem potentials following t-VNS [75]. Additionally, fMRI has demonstrated that t-VNS modulates limbic brain regions and induces positive effects on mood [4]. The latter finding is supported by our data, which showed reduced anxiety following RAVANS, and not NVAS. Reduced anxiety was not correlated with reductions in pain outcomes, suggesting an independent mechanism specific to ABV stimulation. More study is needed on the neural mechanisms of t-VNS and on the optimum location for stimulation, as neither of these neuroimaging studies stimulated the cymba or cavum concha, instead focusing on the tragus, which was found by Peuker et al. to be innervated by the ABV in only 45% of ears studied [32].
Future studies will need to more thoroughly optimize various stimulus parameters for longitudinal application of RAVANS. In clinical application, classical VNS uses stimulus parameters that vary depending on patient tolerance. However, typical usage includes a 30–90 second, 20–50hz (0.5mS pulse width) burst of stimulation with current amplitude 1–3mA, which is applied every 5–10 minutes throughout the day [12]. Furthermore, the specific contribution of respiratory gating should be addressed by adding control intervention groups with ABV stimulation only during inspiration, intermittently irrespective of respiratory cycle, and/or continuously throughout the stimulus period. Important design parameters would have to be addressed, including whether stimulation in this control group is continuous at the same frequency (perhaps leading to greater energy input, but also more chance for habituation or sensitization, compared to respiratory-gated stimulation). Another option would be to have pulsed stimuli gated to exhalation (similar to RAVANS), but instead of a fixed delay, these control stimuli could occur after a random delay, i.e. during exhalation or inhalation for the next breath.
Several limitations should be noted. We did not find any reduction of clinical pain by either RAVANS or the control NVAS stimulation. This is not surprising given that chronic pain was assessed after a single treatment. Future studies may need to include longer-duration RAVANS stimulation over the course of multiple treatment sessions. Another issue is the effect size of the analgesia observed. Clinically significant analgesia for clinical pain outcomes is at least a 30% improvement [76]. For evoked pain outcomes (i.e., QST), no consensus has emerged to define the magnitude of clinically meaningful analgesia, and effects vary as a function of numerous factors such as the modality of the noxious stimulus, the location of its application, etc. [77]. However, recent studies of oral opioids have revealed that oxycodone reduces deep-tissue mechanical pain by approximately 15–25% in healthy volunteers [78, 79] and by 40–50% in a study of chronic pain patients [80]. We report RAVANS-associated reductions of evoked pain ratings of approximately 30–50% in models of deep tissue mechanical pain and mechanical temporal summation. This suggests that RAVANS stimulation may have effects on deep-tissue evoked pain that are comparable in magnitude to those of potent opioids such as oxycodone, though direct comparison studies would be necessary to confirm this. An additional limitation stems from the possibility that CPP patients may have disrupted central pain modulation circuitry [26–28]. While we did not find any significant DNIC effects during RAVANS stimulation, healthy subjects, who would have intact DNIC circuitry, should also be evaluated in future studies, as a comparison group. While we have included our rationale for not controlling for phase of menstrual cycle in our patient cohort, this lack of control should nevertheless be noted as another limitation. Finally, due to technical difficulties we were not able to use the ECG signal in all subjects. Thus, the negative findings of RAVANS effects on autonomic outflow to the heart, while consistent with similar investigations in healthy adults [64], should be confirmed in future studies.
In conclusion, RAVANS demonstrated a trend for reduced evoked pain intensity and temporal summation of mechanical pain, and significantly reduced anxiety in CPP patients. Chronic pain disorders such as CPP are in great need of effective, non-pharmacological options for treatment. RAVANS produced promising anti-nociceptive effects for QST outcomes reflective of the noted hyperalgesia and central sensitization in this patient population. Future studies should evaluate RAVANS for longitudinal reduction of both QST outcomes and clinical pain.
Acknowledgments
We would like to thank NIH for funding support (VN: R01-AT004714, P01-AT002048; RRE: R01 AG034982, R21 AR057920; KP: F05-AT003770; ADW: K23-DA020681), Dr. Park was also supported by the Institute of Information Technology Advancement, Korea IITA-2008-(C1090-0801-0002). The content is solely the responsibility of the authors and does not necessarily represent the official views of our sponsors.
References
- 1.Multon S, Schoenen J. Pain control by vagus nerve stimulation: from animal to man...and back. Acta Neurol Belg. 2005;105(2):62–7. [PubMed] [Google Scholar]
- 2.Borckardt JJ, Kozel FA, Anderson B, Walker A, George MS. Vagus nerve stimulation affects pain perception in depressed adults. Pain Res Manag. 2005;10(1):9–14. doi: 10.1155/2005/256472. [DOI] [PubMed] [Google Scholar]
- 3.Fahy BG. Intraoperative and perioperative complications with a vagus nerve stimulation device. Journal of clinical anesthesia. 2010;22(3):213–22. doi: 10.1016/j.jclinane.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Kraus T, Hosl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm. 2007;114(11):1485–93. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 5.Ventureyra EC. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv Syst. 2000;16(2):101–2. doi: 10.1007/s003810050021. [DOI] [PubMed] [Google Scholar]
- 6.Ren K, Randich A, Gebhart GF. Vagal afferent modulation of spinal nociceptive transmission in the rat. J Neurophysiol. 1989;62(2):401–15. doi: 10.1152/jn.1989.62.2.401. [DOI] [PubMed] [Google Scholar]
- 7.Ren K, Zhuo M, Randich A, Gebhart GF. Vagal afferent stimulation-produced effects on nociception in capsaicin-treated rats. J Neurophysiol. 1993;69(5):1530–40. doi: 10.1152/jn.1993.69.5.1530. [DOI] [PubMed] [Google Scholar]
- 8.Randich A, Gebhart GF. Vagal afferent modulation of nociception. Brain Res Brain Res Rev. 1992;17(2):77–99. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- 9.Ness TJ, Fillingim RB, Randich A, Backensto EM, Faught E. Low intensity vagal nerve stimulation lowers human thermal pain thresholds. Pain. 2000;86(1–2):81–5. doi: 10.1016/s0304-3959(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 10.Kirchner A, Birklein F, Stefan H, Handwerker HO. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology. 2000;55(8):1167–71. doi: 10.1212/wnl.55.8.1167. [DOI] [PubMed] [Google Scholar]
- 11.Kirchner A, Stefan H, Bastian K, Birklein F. Vagus nerve stimulation suppresses pain but has limited effects on neurogenic inflammation in humans. Eur J Pain. 2006;10(5):449–55. doi: 10.1016/j.ejpain.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Hatton KW, McLarney JT, Pittman T, Fahy BG. Vagal nerve stimulation: overview and implications for anesthesiologists. Anesth Analg. 2006;103(5):1241–9. doi: 10.1213/01.ane.0000244532.71743.c6. [DOI] [PubMed] [Google Scholar]
- 13.Asconape JJ, Moore DD, Zipes DP, Hartman LM, Duffell WH., Jr Bradycardia and asystole with the use of vagus nerve stimulation for the treatment of epilepsy: a rare complication of intraoperative device testing. Epilepsia. 1999;40(10):1452–4. doi: 10.1111/j.1528-1157.1999.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 14.Vassilyadi M, Strawsburg RH. Delayed onset of vocal cord paralysis after explantation of a vagus nerve stimulator in a child. Childs Nerv Syst. 2003;19(4):261–3. doi: 10.1007/s00381-003-0722-4. [DOI] [PubMed] [Google Scholar]
- 15.Smyth MD, Tubbs RS, Bebin EM, Grabb PA, Blount JP. Complications of chronic vagus nerve stimulation for epilepsy in children. J Neurosurg. 2003;99(3):500–3. doi: 10.3171/jns.2003.99.3.0500. [DOI] [PubMed] [Google Scholar]
- 16.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(6 Suppl 4):S3–14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 17.Nomura S, Mizuno N. Central distribution of primary afferent fibers in the Arnold’s nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain Res. 1984;292(2):199–205. doi: 10.1016/0006-8993(84)90756-x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta D, Verma S, Vishwakarma SK. Anatomic basis of Arnold’s ear-cough reflex. Surg Radiol Anat. 1986;8(4):217–20. doi: 10.1007/BF02425070. [DOI] [PubMed] [Google Scholar]
- 19.Piepoli M, Sleight P, Leuzzi S, Valle F, Spadacini G, Passino C, Johnston J, Bernardi L. Origin of respiratory sinus arrhythmia in conscious humans. An important role for arterial carotid baroreceptors. Circulation. 1997;95(7):1813–21. doi: 10.1161/01.cir.95.7.1813. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Irnaten M, Mendelowitz D. Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res. 2001;889(1–2):78–83. doi: 10.1016/s0006-8993(00)03112-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, Mettenleiter TC, Mendelowitz D. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann N Y Acad Sci. 2001;940:237–46. doi: 10.1111/j.1749-6632.2001.tb03680.x. [DOI] [PubMed] [Google Scholar]
- 22.Eckberg DL. The human respiratory gate. J Physiol. 2003;548(Pt 2):339–52. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z, Champagnat J, Poon CS. Phasic and long-term depression in brainstem nucleus tractus solitarius neurons: differing roles of AMPA receptor desensitization. The Journal of neuroscience :the official journal of the Society for Neuroscience. 1997;17(14):5349–56. doi: 10.1523/JNEUROSCI.17-14-05349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams RE, Hartmann KE, Steege JF. Documenting the current definitions of chronic pelvic pain: implications for research. Obstet Gynecol. 2004;103(4):686–91. doi: 10.1097/01.AOG.0000115513.92318.b7. [DOI] [PubMed] [Google Scholar]
- 25.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Human reproduction update. 2011;17(3):327–46. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain. 2003;4(7):372–80. doi: 10.1016/s1526-5900(03)00720-x. [DOI] [PubMed] [Google Scholar]
- 27.Neziri AY, Haesler S, Petersen-Felix S, Muller M, Arendt-Nielsen L, Manresa JB, Andersen OK, Curatolo M. Generalized expansion of nociceptive reflex receptive fields in chronic pain patients. Pain. 2010;151(3):798–805. doi: 10.1016/j.pain.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Pukall CF, Baron M, Amsel R, Khalife S, Binik YM. Tender point examination in women with vulvar vestibulitis syndrome. Clin J Pain. 2006;22(7):601–9. doi: 10.1097/01.ajp.0000210903.67849.af. [DOI] [PubMed] [Google Scholar]
- 29.de Tommaso M. Pain Perception during Menstrual Cycle. Current pain and headache reports. 2011 doi: 10.1007/s11916-011-0207-1. [DOI] [PubMed] [Google Scholar]
- 30.Hooper AE, Bryan AD, Eaton M. Menstrual cycle effects on perceived exertion and pain during exercise among sedentary women. Journal of women’s health. 2011;20(3):439–46. doi: 10.1089/jwh.2010.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. The journal of pain : official journal of the American Pain Society. 2006;7(3):151–60. doi: 10.1016/j.jpain.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15(1):35–7. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 33.Binks AP, Banzett RB, Duvivier C. An inexpensive, MRI compatible device to measure tidal volume from chest-wall circumference. Physiol Meas. 2007;28(2):149–59. doi: 10.1088/0967-3334/28/2/004. [DOI] [PubMed] [Google Scholar]
- 34.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–2. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 35.Luczak H, Laurig W. An analysis of heart rate variability. Ergonomics. 1973;16(1):85–97. doi: 10.1080/00140137308924484. [DOI] [PubMed] [Google Scholar]
- 36.Malik M, Bigger JT, Jr, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–81. [PubMed] [Google Scholar]
- 37.Sayers BM. Analysis of heart rate variability. Ergonomics. 1973;16(1):17–32. doi: 10.1080/00140137308924479. [DOI] [PubMed] [Google Scholar]
- 38.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–20. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 39.Edwards RR, Haythornthwaite JA, Tella P, Max MB, Raja S. Basal heat pain thresholds predict opioid analgesia in patients with postherpetic neuralgia. Anesthesiology. 2006;104(6):1243–8. doi: 10.1097/00000542-200606000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Eisenberg E, Midbari A, Haddad M, Pud D. Predicting the analgesic effect to oxycodone by ‘static’ and ‘dynamic’ quantitative sensory testing in healthy subjects. Pain. 2010;151(1):104–9. doi: 10.1016/j.pain.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 41.Bialosky JE, Bishop MD, Robinson ME, Price DD, George SZ. Heightened pain sensitivity in individuals with signs and symptoms of carpal tunnel syndrome and the relationship to clinical outcomes following a manual therapy intervention. Manual therapy. 2011 doi: 10.1016/j.math.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poitras P, Riberdy Poitras M, Plourde V, Boivin M, Verrier P. Evolution of visceral sensitivity in patients with irritable bowel syndrome. Digestive diseases and sciences. 2002;47(4):914–20. doi: 10.1023/a:1014729125428. [DOI] [PubMed] [Google Scholar]
- 43.Kleinbohl D, Gortelmeyer R, Bender HJ, Holzl R. Amantadine sulfate reduces experimental sensitization and pain in chronic back pain patients. Anesthesia and analgesia. 2006;102(3):840–7. doi: 10.1213/01.ane.0000196691.82989.67. [DOI] [PubMed] [Google Scholar]
- 44.Fenton BW, Palmieri PA, Durner C, Fanning J. Quantification of abdominal wall pain using pain pressure threshold algometry in patients with chronic pelvic pain. The Clinical journal of pain. 2009;25(6):500–5. doi: 10.1097/AJP.0b013e31819a3cf9. [DOI] [PubMed] [Google Scholar]
- 45.Granot M, Friedman M, Yarnitsky D, Zimmer EZ. Enhancement of the perception of systemic pain in women with vulvar vestibulitis. BJOG. 2002;109(8):863–866. doi: 10.1111/j.1471-0528.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 46.Pukall CF, Binik YM, Khalife S, Amsel R, Abbott FV. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain. 2002;96(1–2):163–175. doi: 10.1016/s0304-3959(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 47.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010 doi: 10.1038/nrrheum.2010.107. [DOI] [PubMed] [Google Scholar]
- 48.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Modality-specific facilitation and adaptation to painful tonic stimulation in humans. Eur J Pain. 2002;6(6):475–84. doi: 10.1016/s1090-3801(02)00058-7. [DOI] [PubMed] [Google Scholar]
- 49.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Pressure-pain function in desensitized and hypersensitized muscle and skin assessed by cuff algometry. J Pain. 2002;3(1):28–37. doi: 10.1054/jpai.2002.27140. [DOI] [PubMed] [Google Scholar]
- 50.Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. J Gerontol A Biol Sci Med Sci. 2001;56(3):M180–M185. doi: 10.1093/gerona/56.3.m180. [DOI] [PubMed] [Google Scholar]
- 51.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratory-induced pain. Pain. 2004;111(3):335–341. doi: 10.1016/j.pain.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 52.Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN. Elevated Pain Sensitivity in Chronic Pain Patients at Risk for Opioid Misuse. The journal of pain : official journal of the American Pain Society. 2011 doi: 10.1016/j.jpain.2011.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 54.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10(1):77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. 2009 doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edwards RR, Wasan AD, Bingham CO, III, Bathon J, Haythornthwaite JA, Smith MT, Page GG. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther. 2009;11(3):R61. doi: 10.1186/ar2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. Peripheral mechanisms of pain and analgesia. Brain research reviews. 2009;60(1):90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain. 2009;144(1–2):16–19. doi: 10.1016/j.pain.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 59.Sprenger C, Bingel U, Buchel C. Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain. 2011;152(2):428–439. doi: 10.1016/j.pain.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 60.van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11(5):408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138(1):22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 62.Kong J, Gollub R, Huang T, Polich G, Napadow V, Hui K, Vangel M, Rosen B, Kaptchuk TJ. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med. 2007;13(10):1059–70. doi: 10.1089/acm.2007.0524. [DOI] [PubMed] [Google Scholar]
- 63.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 64.Johnson MI, V, Hajela K, Ashton CH, Thompson JW. The effects of auricular transcutaneous electrical nerve stimulation (TENS) on experimental pain threshold and autonomic function in healthy subjects. Pain. 1991;46(3):337–42. doi: 10.1016/0304-3959(91)90116-F. [DOI] [PubMed] [Google Scholar]
- 65.Sator-Katzenschlager SM, Scharbert G, Kozek-Langenecker SA, Szeles JC, Finster G, Schiesser AW, Heinze G, Kress HG. The short-and long-term benefit in chronic low back pain through adjuvant electrical versus manual auricular acupuncture. Anesth Analg. 2004;98(5):1359–64. doi: 10.1213/01.ane.0000107941.16173.f7. table of contents. [DOI] [PubMed] [Google Scholar]
- 66.Sator-Katzenschlager SM, Szeles JC, Scharbert G, Michalek-Sauberer A, Kober A, Heinze G, Kozek-Langenecker SA. Electrical stimulation of auricular acupuncture points is more effective than conventional manual auricular acupuncture in chronic cervical pain: a pilot study. Anesth Analg. 2003;97(5):1469–73. doi: 10.1213/01.ANE.0000082246.67897.0B. [DOI] [PubMed] [Google Scholar]
- 67.Sator-Katzenschlager SM, Wolfler MM, Kozek-Langenecker SA, Sator K, Sator PG, Li B, Heinze G, Sator MO. Auricular electro-acupuncture as an additional perioperative analgesic method during oocyte aspiration in IVF treatment. Hum Reprod. 2006;21(8):2114–20. doi: 10.1093/humrep/del110. [DOI] [PubMed] [Google Scholar]
- 68.Tekdemir I, Aslan A, Elhan A. A clinico-anatomic study of the auricular branch of the vagus nerve and Arnold’s ear-cough reflex. Surg Radiol Anat. 1998;20(4):253–7. [PubMed] [Google Scholar]
- 69.Karemaker JM, Wesseling KH. Variability in cardiovascular control: the baroreflex reconsidered. Cardiovasc Eng. 2008;8(1):23–9. doi: 10.1007/s10558-007-9046-4. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka TH, Leisman G, Nishijo K. The physiological responses induced by superficial acupuncture: a comparative study of acupuncture stimulation during exhalation phase and continuous stimulation. Int J Neurosci. 1997;90(1–2):45–58. doi: 10.3109/00207459709000625. [DOI] [PubMed] [Google Scholar]
- 71.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68(10):988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 72.Janig W. The Integrative Action of the Autonomic Nervous System. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 73.Loewy A, Spyer K. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. p. 390. [Google Scholar]
- 74.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 75.Fallgatter AJ, Neuhauser B, Herrmann MJ, Ehlis AC, Wagener A, Scheuerpflug P, Reiners K, Riederer P. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm. 2003;110(12):1437–43. doi: 10.1007/s00702-003-0087-6. [DOI] [PubMed] [Google Scholar]
- 76.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 77.Staahl C, Olesen AE, Andresen T, Arendt-Nielsen L, Drewes AM. Assessing efficacy of non-opioid analgesics in experimental pain models in healthy volunteers: an updated review. British journal of clinical pharmacology. 2009;68(3):322–41. doi: 10.1111/j.1365-2125.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olesen AE, Upton R, Foster DJ, Staahl C, Christrup LL, Arendt-Nielsen L, Drewes AM. A pharmacokinetic and pharmacodynamic study of oral oxycodone in a human experimental pain model of hyperalgesia. Clinical pharmacokinetics. 2010;49(12):817–27. doi: 10.2165/11536610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 79.Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM. A comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain model. Pain. 2006;123(1–2):28–36. doi: 10.1016/j.pain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Staahl C, Dimcevski G, Andersen SD, Thorsgaard N, Christrup LL, Arendt-Nielsen L, Drewes AM. Differential effect of opioids in patients with chronic pancreatitis: an experimental pain study. Scandinavian journal of gastroenterology. 2007;42(3):383–90. doi: 10.1080/00365520601014414. [DOI] [PubMed] [Google Scholar]