Abstract
Cone photoreceptors are assembled by unknown mechanisms into geometrically regular mosaics in many vertebrate species. The formation and maintenance of photoreceptor mosaics are speculated to require differential cell-cell adhesion. However, the molecular basis for this theory has yet to be identified. The retina and many other tissues express Crumbs (Crb) polarity proteins. The functions of the extracellular domains of Crb proteins remain to be understood. Here we report cell-type specific expression of the crb2a and crb2b genes at the cell membranes of photoreceptor inner segments and Müller cell apical processes in the zebrafish retina. We demonstrate that the extracellular domains of Crb2a and Crb2b mediate a cell-cell adhesion function, which plays an essential role in maintaining the integrity of photoreceptor layer and cone mosaics. Because Crb proteins are expressed in many types of epithelia, the Crb-based cell-cell adhesion may underlie cellular patterning in other epithelium-derived tissues as well.
Keywords: retina, Crumbs, cell-cell adhesion, photoreceptor mosaics, pattern formation, apicobasal polarity, zebrafish
Introduction
In many vertebrate species, cone photoreceptors assemble by unknown mechanisms into exquisite two-dimensional arrays, namely cone mosaics (Raymond et al., 1995; Ahnelt and Kolb, 2000; Raymond and Barthel, 2004). In the zebrafish retina, cones are organized orthogonally in rows and columns (Figures 1A–1D; Raymond and Barthel, 2004). In each row (Figure 1D, large arrowheads), cones are aligned in tandem in the sequence of ultraviolet (UV), green, red, blue, red and green cones. Neighboring rows are shifted relative to each other by a distance of three cells, resulting in one column of alternating UV and blue cones (Figure 1D, arrow1) for every two columns of alternating green and red cones (Figure 1D, arrow2, arrow3). Previous studies have recognized the structural coupling between a green cone and a red cone in certain vertebrate species; these coupled cone pairs are named “double cones” (Rodieck, 1973). The structural coupling between the members of double cones occurs at a junctional plane that is parallel to the axes of cone columns (Figure 1D, small arrowheads). The geometric mosaic organization of cones are believed to be important for high spatial resolution of color vision (Solomon and Lennie, 2007; Dacey and Packer, 2003). Computer modeling suggests that differential cell-cell adhesion can lead to the formation of cone mosaics (Mochizuki 2002). However, the molecular basis for this theory has yet to be identified.
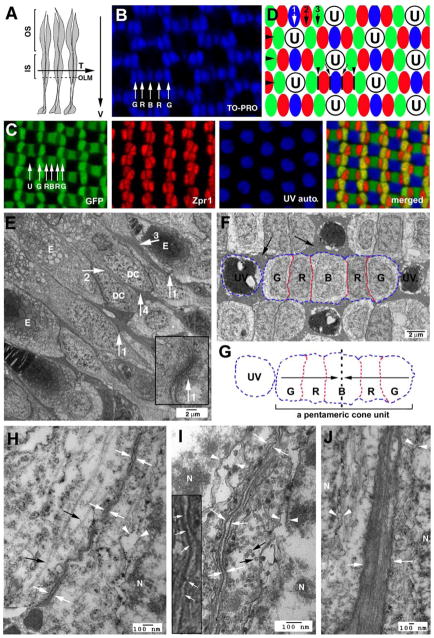
Figure 1.
Cones are organized in regular mosaics in the zebrafish retina, and their inner segment cell membranes are closely juxtaposed. A. A schematic illustrates the transverse/tangential (arrow T) and vertical (arrow V) section planes of photoreceptors (grey). The OLM, the outer limiting membrane (dashed line); IS, inner segments; OS, outer segments. B. TO-PRO nuclear staining illustrates the geometric alignments of the cell nuclei of green (G), red (R) and blue (B) cones. C. Confocal images of four types of cones in wildtype Tg(RH2-1:GFP) pt112 adult zebrafish (Zou et al., 2010), which express GFP strongly in green (G) and blue (B) cones and weakly in red (R) cones. Double cones (red) were visualized with zpr1 antibodies. UV cones (blue) were visualized by auto-fluorescence signals. D. A schematic illustrates the orthogonal planar arrangements of rows (large arrowheads) and columns of cones (arrows 1, 2, and 3). The junctional interfaces (small arrowheads) between the members of double cones are parallel to the axes of cone columns. The junctional interfaces between red and blue cones are indicated with black lines and small arrows. The letter U stands for UV cones; green, red and blue cones are color-coded in green, red, and blue, respectively. E. Transmission electron microscopic (TEM) imaging of the inner segment vicinity of a vertically-sectioned adult retina revealed the neatly aligned OLM (arrow1s), direct juxtaposition between double cone (DC) members (arrow2), and the bundles of long (arrow3) and short (arrow4) apical Müller cell processes. The ellipsoids are indicated with the letter E. The inset shows an OLM junction at a higher magnification. F. TEM imaging of a tangentially-sectioned wildtype adult retina revealed the flat junctional interfaces (red-dashed lines) between members of a pentameric cone unit, which contains a blue cone (B) and two pairs of double cones (R, for red cones; and G for green cones). UV cones, surrounded by clusters of Müller cell apical processes and the inner segments of rods (arrows), are round and do not establish any flat junctional interfaces with other cone types at the level of inner segments. The blue contour lines indicate the convex cell membrane regions of the cones. G. The contours of a UV cone and a pentameric cone unit in panel F are duplicated here to better illustrate the overall shape of cones at cross sections. The dashed line and arrows illustrate the mirror-symmetrical alignment of the cone members within a pentameric cone unit. H, I. TEM imaging of the junctions between the members of double cones (opposing white arrows), either sectioned vertically (H) or transversly (I). The inset at a higher magnification shows the fine filaments (arrows) that connect the neighboring cell membranes. J. Müller cell apical processes are tightly bundled together (opposing white arrows). The letter N, the cell nuclei; the black arrows, the microtubules (H, I, J).
The planar organization of cone mosaics suggests differential cell-cell adhesion at the lateral surfaces of these photoreceptors. A well-known mechanism for lateral adhesion is the outer limiting membrane (OLM), which is composed of a narrow band of a special type of cell-cell junctions between photoreceptors and Müller cells in the photoreceptor layer (Figure 1E, arrow1s; Dowling 1970; Rodieck, 1973; Raviola 1977; Paffenholz et al., 1999). While the OLM is required for the overall integrity of the photoreceptor layer, there is no evidence that the OLM directly correlates with the formation of photoreceptor mosaics. Besides the OLM, evidence suggests that the cell membranes of both photoreceptor inner segments (Figure 1E, arrow2 and 1H) and Müller cell apical processes (Figures 1E, arrow3 and 1J) participate in the stabilization of the photoreceptor layer (Wei et al., 2006a; Gosens, et al., 2008). These cell membranes (Figure 1E, arrow2, arrow3) are associated with many apicobasal polarity proteins (Gosens, et al. 2008). Many of these polarity proteins regulate the formation of the adherens junctions and tight junctions in various epithelia (Knust and Bossinger, 2002). It is thus tempting to determine if the cell membranes of the inner segments and Müller cell apical processes directly mediate cell-cell adhesion and, if so, whether or not the adhesion varies between subtypes of photoreceptors and consequently contributes to the formation or maintenance of photoreceptor mosaics.
Among the apicobasal polarity proteins, the Crumbs (Crb) proteins have been speculated to directly mediate photoreceptor adhesion through their extracellular domains (Wei et al., 2006a). The prototype fly Crb has a large extracellular domain that contains multiple EGF-like and laminin G-like motifs (Tepass et al., 1990). In conjunction with its cytoplasmic partners, Crb maintains the apicobasal polarity of epithelia and photoreceptor morphogenesis in the fruit fly (Pellikka et al., 2002; Izaddoost et al., 2002; Laprise et al., 2006; Hong et al., 2001; Bachmann et al., 2001). Many vertebrate species have multiple crb genes; for example, zebrafish have crb1, crb2a, crb2b, crb3a, and crb3b (Omori and Malicki, 2006). The vertebrate Crb homologs can be divided into two groups: the Crb1/Crb2 group contains the typical large extracellular domain, whereas the Crb3 group does not (Omori and Malicki, 2006; Makarova et al., 2003). The expression patterns of Crb proteins in vertebrate retina are complex and controversial. In mouse retina, immuno-EM suggested CRB1 localizes to the apical processes of the Müller cells, whereas CRB2 and CRB3 are expressed in both Müller cells and photoreceptors (van Rossum et al., 2006). Interestingly, CRB1 was also found on the plasma membranes of cone outer segments in mice (Pellikka et al., 2002). However, in situ hybridization analyses suggested that CRB1 is expressed in the photoreceptor layer as well as in the bipolar cells (den Hollander et al., 2002). In addition, the crb2 transcript was found in all retinal cell layers (van den Hurk et al., 2005). In zebrafish retina, only Crb1, Crb2a and Crb2b are expressed (Crb3a and Crb3b are expressed in the otic vesicle and digestive system); however, it is unclear how they differ in terms of functions and expression patterns at the cellular and subcellular levels (Omori and Malicki. 2006).
The functions of the extracellular domains of Crb proteins are poorly understood. On the one hand, some studies suggest that the domains are functionally dispensable. The expression of a Crb deletion construct that lacks the extracellular domain fully rescued the epithelial polarity defects in a fly crb mutant (Wodarz et al., 1995). Similarly, the expression of Crb3 by mRNA injection rescued retinal neuroepithelial polarity defects in crb2a mutant zebrafish (Omori and Malicki, 2006). On the other hand, accumulating evidence suggests that the extracellular domains of Crb have significant biological functions in the retina. Certain mutations in the extracellular domain of humans CRB1 cause retinitis pigmentosa and Leber Congenital Amaurosis (den Hollander et al. 1999; Lotery et al., 2001; den Hollander et al., 2004; Gosens, et al., 2008). In addition, the extracellular domain of Crb plays roles in the development of the stalk membrane and rhabdomere morphogenesis in flies (Pellikka et al., 2002; Richard et al., 2009). However, the exact functions of the extracellular domains of Crb in photoreceptor morphogenesis remain to be elucidated.
Here, we analyzed the expression patterns and functions of Crb2a and Crb2b during retinal development in zebrafish. We report that Crb2a and Crb2b localize in a cell-type specific manner to the cell membranes of photoreceptor inner segments and/or Müller cell apical processes. We demonstrate that the extracellular domains of the Crb2 proteins carry a previously unrecognized cell-cell adhesion function, which plays an essential role in maintaining cone mosaics.
Results
Close juxtaposition of the cell membranes of photoreceptor inner segments and Müller cell apical processes
To investigate the cell-cell adhesion function of photoreceptor inner segments and Müller cell apical processes, we first compared their ultrastructural features with that of the OLM under transmission electron microscopy. Like other typical cell-cell junctions such as adherens junctions, intracellular plaques of high electron density are associated with the OLM (Figure 1E, arrow 1s and inset). Although the inner segments and Müller cell apical processes do not display typical electron-dense plaque-like structures, the cell membranes of green, red, and blue cones are tightly juxtaposed at the inner segments, showing an average distance of 12.06 nanometers (N=5; SEM=1.8 nm; Figures 1E, arrow2; 1H and 1I; opposing white arrows). This distance is only about 1.5 nm larger than that of the OLM (N=4; SEM=0.9 nm). Furthermore, numerous fine filaments span the narrow clefts between the neighboring membranes and appear to connect them together (Figure 1I, inset, arrows). At transverse sections, the junctional interfaces at the inner segments between the members of double cones and between red and blue cones are strikingly straight (Figures 1F and 1G, red contour lines,); by contrast, the cell surfaces outside of the junctional regions are convex (Figure 1F and 1G, blue contour lines). The flat inner segment junctional interface suggests the presence of regional cell-cell adhesion that is strong enough to overcome the surface tension of individual cells. The adhesion between two pairs of double cones and a blue cone suggest that they exist as a structural unit, designated a pentameric cone unit hereafter (Figure 1G). Pentameric cone units are mirror-symmetric: the blue cones are positioned at the midline axes; the green cones are positioned distal to the axes; and the red cones are positioned proximal to the axes (Figure 1G). Unlike pentameric cones, the contours of the cross sections of UV cones are quite round (Figures 1F and 1G). As for the apical processes of the Müller cells, they can be categorized in two groups according to how far they protrude apically: the long processes, clustering around the UV cones, extend to the level of the outer segments of the UV cones (Figures 1E, arrow3; 1F, black arrows; 1J, opposing arrows; 2A; 7G), whereas the short apical processes extend only to the bases of the inner segment interfaces between members of pentameric cones (Figures 1E, arrow4; 2A; 7G). Thus, the close juxtaposition and fiber connections among the cell membranes of photoreceptor inner segments and Müller cell apical processes support the hypothesis that these regions mediate lateral cell-cell adhesion within the photoreceptor layer.
Distinct expression patterns of Crb2a and Crb2b
The Crb proteins have been speculated to directly mediate cell-cell adhesion through their large extracellular domains (Wei et al., 2006a). Because zebrafish Crb3a and Crb3b do not bear typical large extracellular domains and are not expressed in the retina (Omori and Malicki, 2006), we focused our attention on Crb1, Crb2a, and Crb2b. We performed RT-PCR and RACE analyses and confirmed the retinal expression patterns of crb1, crb2a and crb2b (Omori and Malicki, 2006). Furthermore, we found that the crb2b gene is also transcribed at a second start site located in its seventh intron, producing a shorter transcript, designated crb2b-shorter form (crb2b-sf) hereafter. We thus designated the previously reported crb2b (Omori and Malicki, 2006) crb2b-longer form (crb2b-lf). Crb2b-sf does not contain the first eleven EGF-like motifs of Crb2b-lf (Figure S1A). Consequently, they differ in their first exon and signal peptides (Figure S1A). However, the downstream cDNA sequences of crb2b-sf (52–3,033 nt) and crb2b-lf (1,396–4,377 nt) are identical. The two Crb2b isoforms are expressed at a similar level in the retina, as suggested by twelve distinct RT-PCR reactions (Figures S1A and S1B; data not shown).
Due to the lack of specific antibodies against different Crb homologs, it was unknown how Crb1, Crb2a and Crb2b differ in their retinal expression patterns (Omori and Malicki, 2006). To address this issue, we generated antibodies specific for Crb1, Crb2a amino acid 97–457, or Crb2b-lf amino acid 466–773/Crb2b-sf amino acids 18–325 (Figure S1C). With these antibodies, we revealed that Crb2a and Crb2b but not Crb1 are expressed at the photoreceptor inner segments and/or Müller cell apical processes. The expression patterns of Crb2a and Crb2b also differ significantly: Firstly, Crb2a is expressed throughout retinal development, whereas Crb2b expression coincides with photoreceptor genesis (Figure S1E). Secondly, in adult retina, Crb2a appears to be expressed in the inner segments of all types of photoreceptors and the Müller cell apical processes (Figures 2A and 2C), whereas Crb2b proteins are predominantly expressed at the inner segment junctional interfaces between the members of pentameric cones (Figures 2B and 2D). The membrane localization of Crb2a and Crb2b makes it difficult to tell unequivocally which cell types express these two proteins by conventional immunohistology. We thus took advantage of the random distribution of photoreceptors and the cellular internalization of Crb proteins in the nagie oko (nok) mutant retinas (Wei et al., 2006a) to address this issue. Nok, a zebrafish homolog of fly Stardust (Wei and Malicki, 2002), is required to target Crb proteins to the cell membranes in the fish retina (Wei et al., 2006a). Using this technique as well as the clustering distribution of rod inner segments in wildtype retina, we revealed that all types of photoreceptors and Müller cells express Crb2a, whereas Crb2b is predominantly expressed in green, red, and blue cones (Figures 2E, 2F and S2). These results raised an interesting possibility: If Crb2a and Crb2b mediate lateral cell-cell adhesion, the differences in their spatial and temporal expression patterns would suggest that they play different roles in the planar organization of photoreceptors.
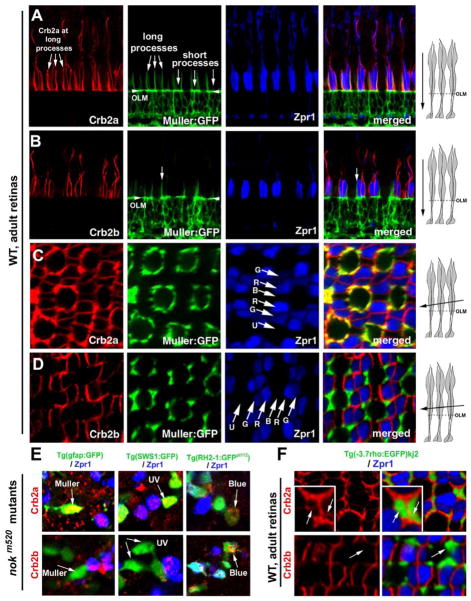
Figure 2.
Crb2a is expressed in all types of photoreceptors and Müller cells, whereas Crb2b is predominantly expressed in green, red and blue cones. A, B. Vertical imaging of the photoreceptor layer showed that Crb2a localizes to inner segment interfaces between photoreceptors and to the apical processes of Müller cells, which were highlighted by GFP expression in the Tg(gfap:GFP) mi2001 background (Bernardos and Raymond, 2006). By contrast, Crb2b localizes predominately at the junctional interfaces between the green, red and blue cone inner segments but not to the Müller cell apical processes. The long and short Müller cell apical processes are indicated with arrows. The arrowheads indicate the OLM. C, D. Slightly tilted tangential imaging of the photoreceptor layer revealed broader Crb2a expression and more restricted Crb2b localization to the junctional regions between green, red, and blue cones. Lettered arrows in panels C and D indicate UV (U), green (G), red (R), and blue (B) cones. The drawings on the right illustrate the planes of imaging (solid arrows) relative to the orientations of photoreceptors. E. Unlike Crb2a, Crb2b was not detected in Müller cells (left panels, arrows) or UV cones (middle panels, arrows) in 5-dpf nokm520 mutant retinas. However, both Crb2a and Crb2b were detected in blue cones (right panels, arrow, GFP positive and Zpr1 negative) and double cones (Zpr1 positive cells). The backgrounds of various transgenic fish used here are indicated above the panels. F. In wildtype adult rod:GFP fish, Crb2a but not Crb2b was detected at the inner segments of rods (green). Also see Figures S1 and S2.
The extracellular domains of Crb2a and Crb2b mediate physical adhesion
To determine if the extracellular domains of Crb2a, Crb2b-lf and Crb2b-sf mediate physical adhesion, we co-express GST- or His-fusions of these domains in a pairwise fashion in the SF9 cells, and then performed GST-pulldown analyses. We found that GST-fusions could pulldown His-fusions in all combinations examined (Figure 3A). In addition, taking the advantage that Crb2b-sf is much shorter than Crb2a and Crb2b-lf, we also performed competition analyses and found that Crb2b-sf preferred binding homophilically to itself over heterophilically to Crb2b-lf or Crb2a because the signal ratios of Crb2b-sfex-His : Crb2aex-His and Crb2b-sfex-His : Crb2b-lfex-His were higher in the pulldown fractions than in the input fractions (Figure 3B). The binding among the extracellular domains was specific as suggested by the negative results of GST pulldown and α-tubulin contamination controls (Figure 3). Furthermore, the binding was strong and withstood 1M NaCl high ionic washes (Figures 3A and 3B). Thus, we concluded that the extracellular domains of Crb2a, Crb2b-lf, and Crb2b-sf could mediate both homophilic and heterophilic physical adhesion.
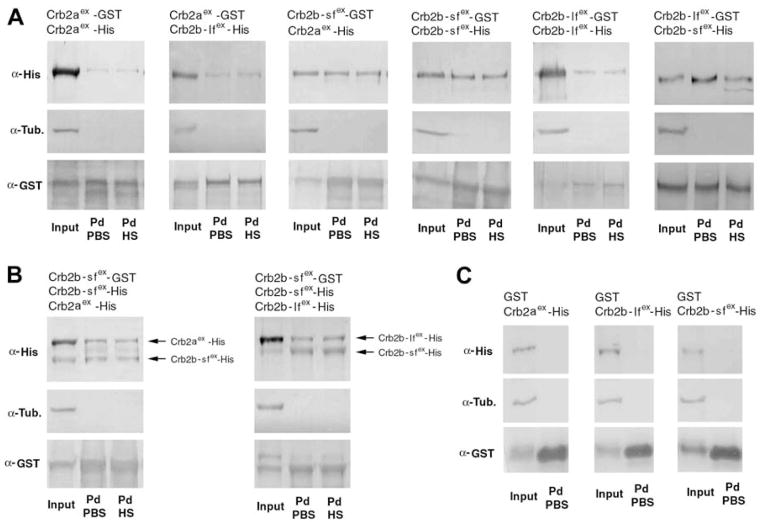
Figure 3.
The extracellular domains of Crb2a, Crb2b-lf, and Crb2b-sf mediate homophilic and heterophilic physical adhesion. Various combinations (indicated above the blots) of GST and His fusions of the extracellular domains of Crb2a, Crb2b-lf, and Crb2b-sf were co-expressed in sf9 cells. The cell lysates were analyzed by GST pulldown and Western blotting assays. Anti-GST blots confirmed the efficient pulldown of GST fusion proteins. Anti-α-tubulin blotting indicated the absence of cytoplasmic protein contamination in the pulldown fractions (Pd). The pulldown fractions were washed either with PBS (PBS) only or with PBS as well as 1M NaCl high salt solution (HS). A. Pulldown analyses of pair-wisely co-expressed GST- and His-fusions indicated that the extracellular domain of Crb2a, Crb2b-lf, and Crb2b-sf physically adhered to each other both homophilically and heterophilically. In addition, the adhesion was strong enough to withstand high ionic washes. B. Competition pulldown analyses revealed that Crb2b-sfex-GST bound to Crb2b-sfex-His more efficiently than it bound to Crb2b-lfex-His or Crb2aex-His, as suggested by the higher ratios between Crb2b-sfex-His and its Crb2b-lfex-His or Crb2aex-His competitors in the pulldown fractions than in the input fractions. C. As specificity controls, when co-expressed with Crb2aex-His, Crb2b-lfex-His, or Crb2b-sfex-His, GST itself did not pull-down any Crb-His fusions, indicating that these His-fusions did not bind to GST or glutathione resin non-specifically.
Expression of Crb2a or Crb2b in HEK293 cells directly prompted cell aggregation through their extracellular domains
To determine if Crb2a and Crb2b can mediate cell-cell adhesion, we generated stable monoclonal HEK293 (HEK) cell lines that expressed full-length Crb2a or Crb2b-lf. Untransfected HEK cells normally prefer to grow solitarily until they reach contact confluence, even though small clusters are frequently found (Figures 4A and 4E). By contrast, the Crb2a- and Crb2b-lf-expressing HEK cells tended to grow in large aggregates (Figures 4A and 4E). Immunochemistry confirmed that Crb2a and Crb2b were expressed on the cell membranes (Figure 4D). Similarly, transient expression of Crb2b-sf in HEK cells also prompted cell-cell adhesion (Figure S3A). The cell aggregation was blocked upon suppression of Crb2 with corresponding anti-crb2 siRNAs, and the treated cells resumed their flat and process-bearing wildtype morphologies (Figures S3B–D). To verify whether the extracellular domains of Crb2a and Crb2b is directly required for cell aggregation, we then generated monoclonal HEK cell lines that express truncated Crb2a-Δex and Crb2b-Δex or Crb3a that do not bear the typical large extracellular domains (Figures 4B and S3E–F). As expected, these cells did not display a stronger tendency towards cell aggregation than wildtype HEK cells (Figure 4B and 4E). These findings support the role of the extracellular domain of Crb2 in cell-cell adhesion.
Figure 4.
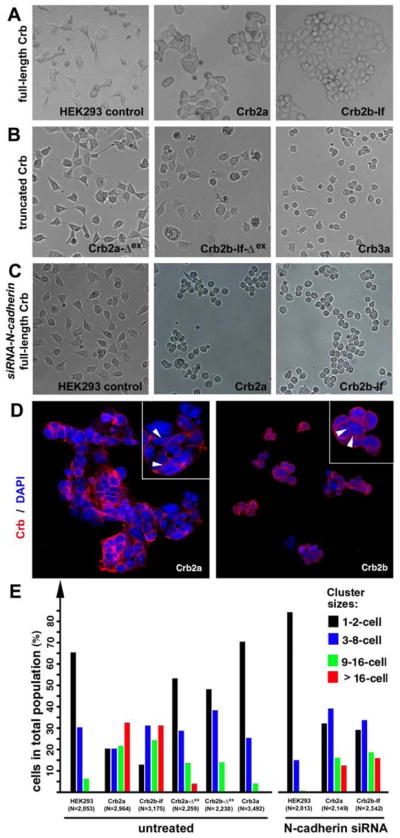
The extracellular domains of Crb2a- or Crb2b-lf mediated cell aggregation in HEK cells. A. Unlike wildtype HEK cells, it is more common for monoclonal HEK cells that express full length Crb2a or Crb2b-lf to grow in large aggregates. B. Expression of Crb3a, Crb2a-Δex, and Crb2b-Δex, which lack typical Crb extracellular domains, did not prompt HEK cell aggregation. C. Suppression of N-Cadherin by N-cadherin siRNA treatment further reduced the tendency for wildtype HEK cells to form aggregates, suggesting that the low level cell-cell adhesion in wildtype HEK cells was dependent on N-cadherin. However, loss of N-cadherin did not prevent Crb2a- or Crb2b-lf-expressing HEK cells from aggregating, suggesting that the Crb2-mediated cell-cell adhesion was independent of N-Cadherin. D. Crb2a and Crb2b-lf localized to the cell membrane in HEK cells with various degrees of enrichment at the cell-cell junctions (arrowheads, insets). The cell nuclei were stained with DAPI (blue). E. To quantitatively evaluate the adhesion property of the extracellular domain of Crb proteins, various Crb-expressing HEK cells were counted and categorized according to their cluster sizes. The total numbers of cells are indicated below the histograms. The symbol Δex indicates that the extracellular domain of the corresponding Crb protein was deleted (Figure 6A). Also see Figures S3.
Previous studies had shown that Crb plays a critical role in maintaining the tight junctions and adherens junctions in various epithelia (Tepass et al., 1990; Tepass et al., 2001; Knust and Bossinger, 2002). This raised the question of whether Crb2-based HEK cell adhesion was directly mediated by their extracellular domains or indirectly through other transmembrane cell-cell adhesion molecules. Although no adherens junctions or tight junctions are present in wildtype HEK cells, nor do the cells express E-cadherin of the adherens junctions (Hogan, et al., 2004) or claudins and occludins of the tight junctions (Piontek et al., 2008; Inai, 2011), HEK cells do in fact express N-cadherin (Hogan, et al., 2004), which localizes to small and fragmented foci at the regions where HEK cells make small process contacts with their neighbor cells. To test whether or not Crb2-mediated cell-cell adhesion is actually dependent on the adhesive function of N-cadherin, we treated Crb2a- and Crb2b-lf expressing HEK cells as well as wildtype HEK cells with anti-N-cadherin siRNAs. As expected, suppression of N-cadherin almost completely abolished the weak adhesive property of wildtype HEK cells (Figures 4C, 4E, S3G, and S3H), suggesting that the weak cell-cell adhesion in wildtype HEK cells is based on N-cadherin. By contrast, the treatment of Crb2a- and Crb2b-lf-expressing HEK cells with N-cadherin siRNAs did not abolish cell aggregation (Figures 4C, 4E, S3G, and S3H), even though the percentages of Crb2a- or Crb2b-lf-expressing cells in larger cluster categories reduced slightly compared to untreated cells (Figure 4E). The tendency for Crb2-expressing HEK cells to form large aggregates was greater than the summed tendency of wildtype HEK cells and the Crb2-expressing cells treated with N-cadherin siRNA (Figure 4E). Thus, these results strongly suggest that the expression of Crb2a or Crb2b on the cell membrane can sufficiently and directly mediate cell-cell adhesion through their extracellular domains. Nevertheless, N-cadherin and Crb2 can synergistically enhance cell aggregation in HEK cells.
Crb2a is required for photoreceptor adhesion in vivo
Although mutations of many apicobasal polarity genes lead to the disruption of retinal lamination, their effects on the distribution of photoreceptors vary. For example, in the N-cadherinm117 mutants, photoreceptors form rosettes (Wei et al., 2006a). In a three-dimensional space, photoreceptor rosettes are actually spherical aggregations of photoreceptors with their apical ends pointing interiorly. When cross-sectioned, the aggregates display a rosetted organization of cells, hence the name photoreceptor rosettes. The formation of photoreceptor rosettes reflects the presence of a coherent adhesion among polarized photoreceptors; thus, it can serve as an assay to analyze the adhesion property of photoreceptors in various genetic backgrounds. Unlike in the N-cadherinm117 mutants, photoreceptors in regular crb2am289 mutants do not form rosettes; rather, they scatter all over the retina (Fig. 5B; Malicki and Driever, 1999; Pujic and Malicki, 2001; Wei et al., 2006a). The lack of photoreceptor aggregation in crb2am289 mutants suggests that either Crb2a is directly required for photoreceptor adhesion, or that the loss of certain Crb2a functions prior to photoreceptor differentiation indirectly compromises photoreceptor adhesion later on. To distinguish between these two possibilities, we analyzed the 5-dpf (days postfertilization) crb2am289 mutant embryos that were injected with a construct to express full-length wildtype Crb2a using the RH2-1 green opsin promoter (Figure 5A). In these embryos, because the opsin promoter-driven transgenes were activated after photoreceptor differentiation (Tsujimura et al., 2007), the earlier role of Crb2a in regulating the polarity of undifferentiated retinal neuroepithelium was not rescued, allowing us to analyze the cell-cell adhesion function of Crb2a in photoreceptors. Thus, if transgenic expression of wildtype Crb2a in the photoreceptors of the crb2am289 mutants results in photoreceptor rosettes, it would indicate that Crb2a confers adhesion property to these photoreceptors.
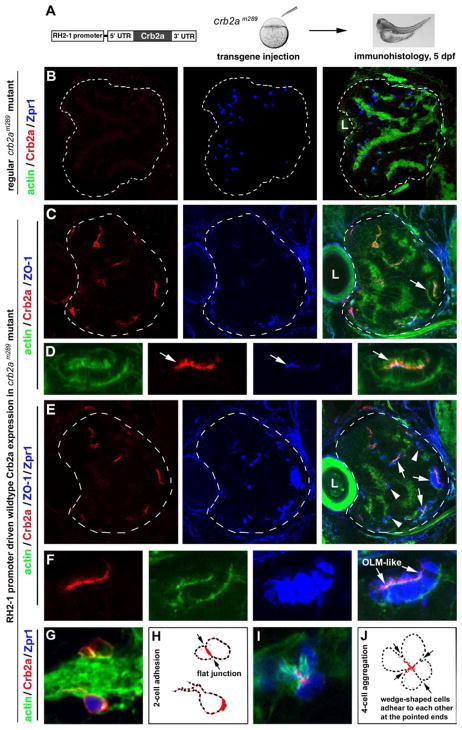
Figure 5.
Transgenic expression of wildtype Crb2a in crb2am289 mutants led to photoreceptor aggregation. A. A schematic illustrates the structure of the transgene construct that was injected into embryos at 1–4-cell stages to express wildtype Crb2a with the RH2-1 green opsin promoter in photoreceptors (Tsujimura et al., 2007). It should be noted that the number of transgenic photoreceptors varies from embryo to embryo due to varying efficiencies of transgenesis in the injected fish. B. In regular crb2am289 mutant retinas, Zpr1 positive cones (blue) scatter everywhere in the retina; note the lack of Crb2a signals (red). C, D. Retinal cells that transgenically expressed wildtype Crb2a (red) tended to form aggregates. The OLM-like structures, visualized by the OLM marker ZO-1 (arrows), were present in the aggregates. Actin staining (green) illustrated the overall structure of the retina as well as the OLM-like structures (D, arrows). Panels D show a cell aggregate in panel C (arrow) at a higher magnification. E, F. The identical tissue section showed in C and D was subsequently immunostained with zpr1 antibodies; this confirmed that the aggregating cells were mostly double cones (F, higher magnification). G, H. Two Crb2a-expressing transgenic cells established a flat junctional interface which was highly enriched with Crb2a (arrows). Panel H shows the contours of transgenic cells in panel G; I, J. Four wedge-shaped transgenic double cones adhered to each other to form a mini rosette and Crb2a localized to the central region of the rosette (arrows). Panel J shows the contours of transgenic cells in panel I. Dashed lines in panels B, C, and E mark the boundary of the retinas and the letters L indicate the lens. Also see Figure S4.
As expected, over 70% of Crb2a-expressing transgenic cells did form aggregates in the crb2am289 mutants (Figures 5C–J and 6A). These cells were confirmed to be photoreceptors because they were positive for double cone marker Zpr1(Figures 5E and 5F). Moreover, in these aggregates, photoreceptors were elongated and polarized coherently, forming apically localized OLM-like structures that were enriched with OLM marker ZO-1 (Figures 5D and 5F). The formation and patterns of photoreceptor aggregation varied according to the density of transgenic cells. At low density, in one example, two transgenic cells formed a flat junctional interface (Figures 5G, 5H, and S4A), whereas in another example, a few cells took on a wedge shape and formed a mini rosette, in which Crb2a was enriched at the junctional interface at the center (Figures 5I, 5J, and S4B). These results are consistent with the hypothesis that Crb2a directly mediates photoreceptor adhesion in vivo through its extracellular domain.
Figure 6.
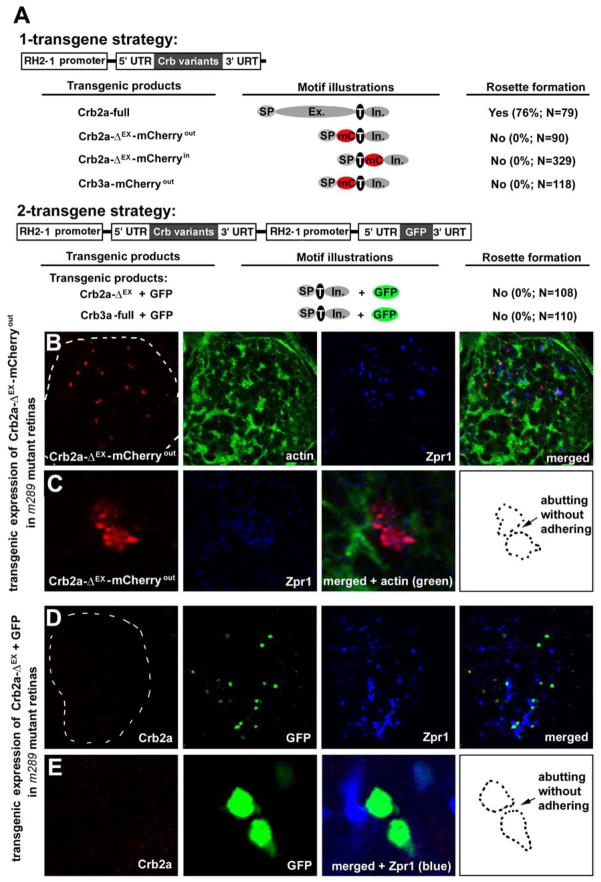
The extracellular domain of Crb2a is required for Crb2a to mediate photoreceptor adhesion. A. The schematics illustrate the two strategies to transgenically express Crb2a, Crb2a-ΔEX-mCherryout, Crb2a-ΔEX-mCherryin, Crb2a-ΔEX, Crb3a-mCherryout, or Crb3a in zebrafish retina. Individual protein domains are labeled as follows: SP, the signal peptide; Ex., the extracellular domain; T, the transmembrane domain; In., the intracellular domain; mC, mCherry; GFP, green florescent protein; -ΔEX, absence of the extracellular domain. The percentages of transgenic photoreceptors that formed rosettes in 5-dpf crb2am289 mutant retinas were quantified and summarized in the table. B, C. Crb2a-ΔEX-mCherryout-expressing photoreceptors (red) scattered randomly in the crb2am289 mutant retinas. Panels C are magnified images of two transgenic cells that abutted each other but did not appear to adhere (the contours illustrates the lack of flat adhering junctional interface, arrow). D, E. Transgenic photoreceptors that co-expressed Crb2a-ΔEX and GFP also scattered randomly in the crb2am289 mutant retinas. Panels E show magnified images of three transgenic cells, two of which abutted but did not adhere to each other to establish a flat junctional interface (contours, arrow). The lack of Crb2a staining confirmed that the embryo was a crb2am289 mutant. See Figure S5 for effects of transgenic expressing Crb2a-ΔEX-mCherryin, Crb3a-mCherryout, and Crb3a in either wildtype or crb2am289 mutant embryos. Also see Figure S5.
However, the above notion seems to contradict previous findings of the dispensability of the extracellular domain of the Crb proteins for maintaining epithelial polarity and integrity: In the fly, the expression of truncated fly Crb lacking the extracellular domain rescued the epithelial polarity and integrity defects in the crb mutant fly embryos (Wodarz et al., 1995), and zebrafish Crb3a, which lacks the typical extracellular domain, rescued retinal polarity defects in crb2a mutant retina (Omori and Malicki, 2006). These studies suggest that the intracellular domain of Crb is essential and sufficient for interacting with adherens junctions or tight junctions to maintain epithelial polarity and integrity indirectly. The seeming contradiction may however suggest that the mechanisms by which polarity proteins regulate tissue polarity and integrity actually vary dramatically in different biological contexts. This kind of variation is consistent with a significant change in the composition of the Crb complex during retinal development. For example, Lin7c, which binds Crb’s cytoplasmic partner Nok in undifferentiated neuroepithelium, is almost completely downregulated in the developed photoreceptor layer (Yang et al., 2009; Wei et al., 2006b). Therefore, it is possible that the extracellular domain of Crb2 may play unique roles in photoreceptors but not necessarily in undifferentiated retinal neuroepithelium.
To investigate whether or not the extracellular domain of Crb2a directly mediates photoreceptor adhesion, we next transgenically expressed in crb2am289 mutant retinas, three Crb2a variants that lacked the extracellular domain (Figure 6A) and then analyzed their efficacy on photoreceptor aggregation. We found that these Crb2a deletion variants localized diffusively in the cell membranes of wildtype photoreceptors, suggesting that the extracellular domain plays a role in targeting Crb2a to the inner segment cell membranes, possibly through an adhesive anchoring interaction with the Crb molecules on the neighboring photoreceptors (Figures S5A–E). This notion is consistent with the findings that in the fly, Crb needs to be present in both neighboring cells for it to be enriched at the cell-cell contacts in larval eye disc epithelium as well as the requirement of the extracellular domain for Crb to be targeted to the stalk membranes, which are the subdomain of fly photoreceptors equivalent to the inner segment membranes in vertebrate photoreceptors (Pellikka et al., 2002). Furthermore, photoreceptors expressing these Crb2a variants were incapable of adhering to each other (Figures 6A–E and S5F). Photoreceptors can be positioned next to each other by chance simply because of the limited retinal space. However, in no case did we find two abutting photoreceptors forming a flat junctional interface, let alone multi-cell rosetted aggregates (Figures 6C, 6E, and S5F). These results contrasted sharply with those photoreceptors expressing wildtype full-length Crb2a (Figures 5 and S4). Similarly, expression of Crb3a in photoreceptors did not prompt the aggregation of photoreceptors (Figures S5G and S5H). Considering that the intracellular domains of four of these Crb2a and Crb3a variants were intact or not directly linked to the mCherry tag, their capability of interacting with cytoplasmic OLM components was likely unaffected. Thus, these results suggest that the intracellular domain of Crb variants are not sufficient to establish or maintain the OLM of developed photoreceptors, contrasting their role in establishing and maintaining the adherens junctions in undifferentiated neuroepithelia (Wodarz et al., 1995; Omori and Malicki, 2006). More importantly, the results suggest a direct role for the extracellular domain of Crb2a in mediating photoreceptor adhesion independent of the OLM. This notion is consistent with two other observations: First, photoreceptor rosettes still form in the absence of N-cadherin, which is the critical transmembrane adhesion molecule of the OLM (Wei et al., 2006a). Second, many Crb2a proteins in the inner segments do not colocalize with the OLM (Figures 2A and 2B). The Crb molecules likely mediate photoreceptor adhesion independent of the OLM.
An essential role for Crb2b in maintaining photoreceptor mosaics
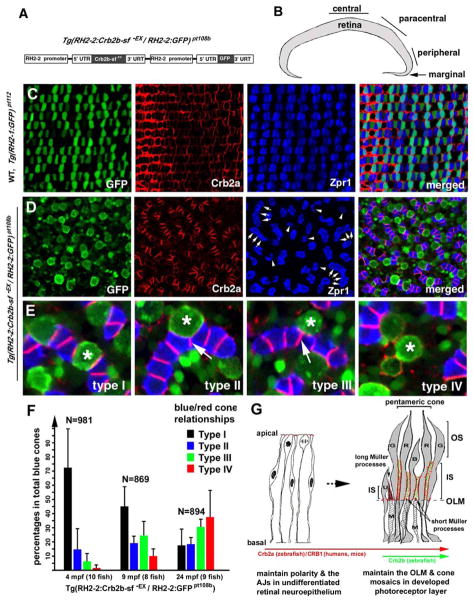
The predominant expression of Crb2b at the inner segment junctional interfaces between members of pentameric cones suggests that Crb2b proteins may play a direct role in the assembly of pentameric cones. To test this hypothesis, we generated a transgenic zebrafish line, designated Tg(RH2-2:Crb2b-sf−EX/RH2-2:GFP) pt108b, that expresses a secreted form of the extracellular domain of Crb2b-sf (Crb2b-sf −EX) as well as an intracellular GFP reporter (Figure 7A). In this fish line, the transgenes are expressed mainly in the blue cones at the peripheral, paracentral, and central retina (Figure S6B) and also in some rods and green cones at the marginal zone (Figure S6A). The strong Crb2b-sf−EX signals were observed throughout the entire surface of the photoreceptors, with a majority accumulated in the inner segment regions (Figure S6A, arrowheads). The enrichment of Crb2b-sf−EX in the inner segment region is consistent with the prediction that the secreted molecules would directly bind to the extracellular domains of endogenous Crb2 molecules and compromise their adhesion function. Thus, this fish line serves as a tool to analyze the function of the extracellular domains of Crb2 in developed retina.
Figure 7.
Transgenic expression of a secreted form of the extracellular domain of Crb2b-sf (Crb2b-sf−EX) disrupts cone mosaics. A. A schematic illustrates the tandem arrangement of the Crb2b-sf−EX and GFP transgenes driven by the RH2-2 green opsin promoter (Tsujimura et al., 2007). B. The central, paracentral, peripheral, and marginal regions of the retina are indicated. C. Photoreceptors are organized in regular mosaic in the paracentral retina of wildtype Tg(RH2-1:GFP) pt112 fish (Zou et al., 2010) at 24 months postfertilization (mpf). D. Transgenic expression of Crb2b-sf−EX in blue cones disrupted photoreceptor mosaics in the paracentral retina in Tg(RH2-2:Crb2b-sf−EX/RH2-2:GFP) pt108b at 24 mpf. Some double cones were dissociated into solitary Zpr1 positive cells (arrowheads); other double cones abnormally aligned into chains (arrows). E. In Tg(RH2-2:Crb2b-sf−EX/RH2-2:GFP) pt108b, blue cones (asterisks) were found in four different spatial relationships with red cones: Type I, sandwiched between red cones; Type II, partially expelled from the red cone sandwich; Type III, completely expelled from the red cone sandwich, but still attached to the side of the red cones; Type IV, solitary without adhering to any Zpr1 positive cones. The arrows indicate the de novo junctions formed between two presumable red cones. F. Blue cone-secreted Crb2b-sf−EX gradually compromised the association between blue cones and red cones, resulting in an increase in the percentages of dissociated blue cones over time. Error bars = Standard deviations. G. A diagram summarizes the temporal and spatial expression patterns and functions of Crb proteins during the vertebrate retinal development. Zebrafish Crb2a and its mammalian equivalent CRB1 are broadly expressed and regulate the polarity and integrity of both the undifferentiated neuroepithelium and the developed photoreceptor layer. Crb2b homologs are likely more restrictively expressed in certain types of photoreceptors in a subgroup of vertebrates such as fish. The restricted expression of Crb2b at the inner segments between green (G), red (R), and blue (B) cones may underlie their structural assembly into pentameric cones in zebrafish; consequently, Crb2b plays a critical role in maintaining cone mosaics. The long apical processes of Müller cells (M) cluster around the UV cones (U), likely preventing them from adhering to pentameric cones. The apical processes of Müller cells between the members of pentameric cones are very short and are thus no impediment to their inner segment associations. Also see Figure S6.
Histological analyses of Tg(RH2-2:Crb2b-sf−EX/RH2-2:GFP) pt108b retinas revealed gross defects in the patterning of cones (Figures 7D–F). Contrasting with wildtype retinas (Figures 1C and 7C), blue cones in these fish were observed in four different types of spatial relationships with red cones: Type I blue cones were completely sandwiched between red cones as seen in wildtype; Type II blue cones were partially sandwiched between red cones, with de novo junctions partially formed between two presumable red cones; Type III blue cones were completely expelled from the red cone sandwich, but still attached to the sides of red cones; And type IV were solitary blue cones that showed no specific association with any Zpr1 positive cones. The percentages of Type II, Type III and IV increased over time (Figure 7F), suggesting that the blue cone-secreted Crb2b-sf-EX most strongly compromised their adhesion to the neighboring red cones, thus sorting out blue cones from the normal pentameric cone organization. Such defects in blue-red association may explain the formation of chains of Zpr1 positive cones (Figure 7D, arrow clusters). In these Zpr1 cone chains, the planes of the junctional interfaces that join green and red cones into double cones were perpendicular to the long axes of the chains. Such Zpr1 cone chains do not exist in regular cone mosaics because all double cones are separated by blue cones in the cone rows (Figures 1D and 7C). The axes of the Zpr1 cone chains are often curved and oriented randomly, further disrupting the global orthogonal arrangements of cone rows and columns (Figures 1C, 1D, 7C, and 7D). In addition, many Zpr1 positive cones existed individually, suggesting the dissociation of some double cones as well (Figure 7D, arrowheads). Despite the disruption of photoreceptor mosaics in Tg(RH2-2:Crb2b-sf−EX/RH2-2:GFP) pt108b, no photoreceptors mislocalized to the ganglion or inner nuclear layers, and the OLM appeared largely intact (Figures S6C and S6D). Taken together, these results suggest that Crb2b plays an important role in stabilizing the planar organization of cone mosaics.
Discussion
In this study, we analyzed the expression patterns of Crb2a and Crb2b in zebrafish retina and showed that Crb2-based cell-cell adhesion plays an important role in maintaining photoreceptor mosaics. Because Crb proteins are also expressed in other tissues, such as the brain, kidney, skin, and intestine (Knust and Bossinger, 2002), Crb-based cell-cell adhesion may play a general role in cellular patterning during embryogenesis.
Crb proteins as a distinct class of cell-cell adhesion molecules
The structures of Crb2a and Crb2b are different from the established families of transmembrane adhesion proteins, namely cadherins, integrins, selectins, and cell adhesion molecules of the immunoglobulin super family (Chothia and Jones, 1997). Although the EGF-like motifs and the laminin G-like motifs are shared among these protein families, they show major differences in motif composition and organization. Thus, Crb2a and Crb2b constitute a distinct family of transmembrane cell-cell adhesion molecules.
Roles of Crb2a in the organization of photoreceptors in the retina
The organization of photoreceptor mosaics is a complex process which may be simplified into three steps: the generation of various types of photoreceptors in proper ratios, the alignment of photoreceptors into specific spatial patterns, and the stabilization of photoreceptor patterns. Many studies have addressed photoreceptor differentiation (Cepko et al., 1996; Stenkamp et al., 1997; Hu and Easter, 1999; Adler, 2000; Fadool, 2003; Raymond and Barthel, 2004; Yang, 2004; Deeb, 2006; Kitambi and Malicki, 2008), but little is known about how photoreceptors in the vertebrate retinas adjust and stabilize their spatial relationships with their neighbors.
The differences in the expression patterns between Crb2a and Crb2b suggest that they play different roles in maintaining photoreceptor mosaics. Because the Crb2a is universally expressed in all types of photoreceptors and Müller glial cells, Crb2a-based homophilic adhesion may utilize two mechanisms to mediate the overall integrity of the photoreceptor layer but rather specific cell-cell adhesion between distinct cone types (Figure 7G).
First, for Crb2a proteins that colocalize with the OLM, they may indirectly stabilize the photoreceptor layer through maintaining the OLM. This mechanism is supported by three observations: 1) Loss of the Crb2a function disrupts the retinal neuroepithelial polarity and the adherens junctions, the precursor of the OLM (Malicki and Driver, 1999; Wei et al., 2006a); 2) Expression of wildtype Crb2a in crb2a mutant photoreceptors introduced the formation of the OLM-like structures in the photoreceptor aggregates (Figure 5); 3) Despite the lack of proper planar organization of cones in Tg(RH2-2:Crb2b-sf−EX / RH2-2:GFP)pt108b, the integrity of the OLM is largely maintained (Figures S6C and S6D). The mechanism by which Crb2a regulates the OLM integrity might be similar to that by which the fly Crb regulates the zonula adherens in fly photoreceptors (Pellikka et al., 2002), and might not require its extracellular domain because both a truncated fly Crb that lacked the extracellular domain or zebrafish Crb3a was able to rescue the epithelial integrity and the OLM precursor adherens junctions (Wodarz et al., 1995; Omori and Malicki, 2006).
Second, for Crb2a proteins that do not colocalize with the OLM, they may directly mediate lateral adhesion among photoreceptors and Müller cells through its adhesive extracellular domain. This mechanism might not be conserved in fly ommatidia because the Crb-expressing stalk membranes of developed photoreceptors are separated far apart from each other. However, the role for Crb in regulating stalk membrane formation in the fly (Pellikka et al., 2002) might be conserved in fish because we observed on average a 1.79 fold increase in the cross sections of the inner segments of cones that overexpressed the full-length Crb2a (Figures S5I). A similar increase was observed in the diameter of inner segments in rods that overexpressed Crb2a (Hsu and Jensen, 2010).
The above two mechanisms are not mutually exclusive. Rather, they should synergistically maintain the integrity of the photoreceptor layer in the vertebrate retinas. This notion is consistent with the observed synergy between N-cadherin and Crb2 in mediating HEK cells to form large aggregates (Figure 4E).
Roles of Crb2b in the organization of photoreceptors in the retina
Unlike Crb2a, the Crb2b proteins are predominantly expressed at the inner segments between the members of pentameric cones and play an essential role in mediating specific geometric patterns of these cones through regional adhesion. This of course does not exclude the possibility that Crb2a participates in mosaic formation by heterophilically interacting with Crb2b because Crb2a also localizes to the inner segment regions where Crb2b is enriched (Figure 2), and because in vitro data suggest that they can interact with each other (Figure 3). In the Tg(RH2-2:Crb2b-sf−EX / RH2-2:GFP) pt108b line, in which the Crb2b-sf−EX is primarily secreted by blue cones, it was the inner segment adhesion between blue cones and neighboring red cones that was most compromised. The causal relationship between a reduction in the adhesion strength of blue cones and the selective sorting out of blue cones from the pentameric cone unites strongly suggests that Crb2 proteins maintain a delicately-balanced lateral adhesion among cones and are therefore essential for cone mosaics. This retinal phenomenon attests to the “differential adhesion hypothesis”, which posits that cell surface adhesive properties underlie the sorting out of heterotypic cell mixtures to form a particular anatomical configuration during embryogenesis (Steinberg, 1996).
Apical processes of the Müller cells
It is not yet clear whether or not the apical processes of the Müller cell plays an active role in the mosaic organization of cones in zebrafish. The physical inner segment conjunction between the members of pentameric cones may be facilitated by the shortening of Müller cell apical processes in the pentameric cone regions so as not to impede the inner segment junction (Figure 7G). As for the long Müller cell apical processes around the UV cones, it is unclear whether or not they directly prevent the physical association between UV cones and other cones at the inner segments.
Future perspectives
Our study raised several interesting questions for future research. First, what are the molecular mechanisms that target Crb proteins to distinct subcellular regions? For example, is Ponli responsible for targeting Crb2b to the inner segments since Ponli, a homolog of Nok, displays identical expression patterns as Crb2b in cones (Zou et al., 2010)? Second, do Crb2b homologs exist and play conserved roles in conjunctions of cones such as double cones in certain species of reptiles and birds (Dowling, 1970; Rodieck, 1973; Mariani, 1986)? Third, are there other types of lateral cell adhesions that are responsible for the mirror-symmetric alignment of the members of pentameric cones in zebrafish? Finally, are the roles of Crb in cell adhesion, Notch signaling (Ohata et al., 2011), and Hippo signaling (Martin-Belmonte and Perez-Morenoto, 2011) coordinated to regulate photoreceptor survival? Answers to this question may shed light on the etiology of CRB1-related Retinitis Pigmentosa and Leber’s Congenital Amaurosis in humans (den Hollander, 2004).
Experimental Procedures
5′ RACE and RT-PCR analyses
Total RNA was isolated from adult AB wildtype zebrafish eyes and reversely transcribed to make cDNAs. 5′ RACE analyses identified crb2b-sf (GenBank #: JQ740885) and confirmed crb2b-lf (GenBank #: DQ314737).
Rabbit polyclonal anti-Crb2a and anti-Crb2b antibodies
The polypeptides of Crb2a amino acids 97–457 and Crb2b-lf amino acids 466–773/Crb2b-sf amino acids 18–325 were expressed in E. coli and purified as antigens for antibody production.
Baculoviral expression and GST-pulldown analysis
Baculovirus expression vectors pAcGP67a and pAcSecG2T (BD Biosciences) were used to generate recombinant Baculovirus strains to transfect and express GST- and His-tagged Crb fusions in Sf9 cells. Four days after transfection, cell lysates were extracted and used for GST-pulldown assays.
Expression of Crb variants in HEK cells
Crb2a, Crb2b-sf, Crb2b-lf, Crb3a, Crb2a-Δex, and Crb2b-Δex were cloned in the pcDNA3.1 vector (Invitrogen) and used to transfect HEK cells. For Crb2a-Δex and Crb2b-Δex, a CMV-driven mCherry reporter gene was cloned downstream of the Crb genes (Figure S3E). Monoclonal Crb-expressing HEK cell lines were generated by transfecting cells with linearized constructs, followed by a selection with G-418.
siRNA knock-down
Suppression of Crb2a, Crb2b-lf, or N-cadherin in HEK cells were achieved with siRNA transfection with Lipofectamine™ RNAiMAX (Invitrogen).
Generation of transgenic zebrafish
The RH2-1 or RH2-2 green opsin promoters (Tsujimura et al., 2007) were used to express transgenes in photoreceptors. Various Crb transgene cassettes were integrated into fish genome with either the Tol2-based (Kawakami, 2007) or I-SceI meganuclease-based systems (Thermes et al., 2002). Experimental animals were maintained in accordance with University of Pittsburgh guidelines.
Supplementary Material
Highlights.
In zebrafish, all types of photoreceptors and Müller cells express Crb2a
Only green, red, and blue cones express Crb2b
The extracellular domains of Crb2a and Crb2b mediate specific cell-cell adhesion
Crb2-based cell adhesion is required to maintain pentameric cones and cone mosaics
Acknowledgments
This work was supported by an NIH Core Grant (P30EY008098), Eye and Ear Foundation of Pittsburgh, an unrestricted grant from Research to Prevent Blindness, and a NIH R01 grant (EY016099) and a RPB Wasserman Merit Award to XW. The authors thank Ming Sun, Fengli Guo, and Donna Stolz for assistance in preparing TEM samples; Shoji Kawamura for providing the RH2-1 and RH2-2 promoters as well as Tg(SWS1:GFP) and Tg(-3.7rho:EGFP)kj2 fish; Pamela Raymond for Tg(gfap:GFP) mi2001 fish; David Hyde and Thomas Vihtelic for anti-blue opsin antibodies; Michael Tsang for the pIsce-I vector; Nathan Bahary for the pCS2-GFP plasmid; Cen Zhang for participating in the initial characterization of the Tg(RH2-2:Crb2b-sf−EX/RH2-2:GFP) pt108b fish line; Gary Gorbsky for critical reading of the manuscript; Sue-Hwa Lin for suggestions on cell aggregation analyses; and Lynne Sunderman and Sarah Bonaffini for proofreading the manuscript.
Abbreviations
- nok
nagie oko
- hpf
hours postfertilization
- dpf
days postfertilization
- mpf
months postfertilization
- ypf
years postfertilization
- RPE
retinal pigment epithelium
- Crb
Crumbs
- OLM
outer limiting membrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler R. A model of retinal cell differentiation in the chick embryo. Prog Retin Eye Res. 2000;19:529–557. doi: 10.1016/s1350-9462(00)00008-2. [DOI] [PubMed] [Google Scholar]
- Ahnelt PK, Kolb H. The mammalian photoreceptor mosaic-adaptive design. Prog Retin Eye Res. 2000;19:711–777. doi: 10.1016/s1350-9462(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Bachmann A, Schneider M, Theilenberg E, Grawe F, Knust E. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 2001;414:638–643. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C, Jones EY. The molecular structure of cell adhesion molecules. Annu Rev Biochem. 1997;66:823–862. doi: 10.1146/annurev.biochem.66.1.823. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Packer OS. Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Curr Opin Neurobiol. 2003;13:421–427. doi: 10.1016/s0959-4388(03)00103-x. [DOI] [PubMed] [Google Scholar]
- Deeb SS. Genetics of variation in human color vision and the retinal cone mosaic. Curr Opin Genet Dev. 2006;16:301–307. doi: 10.1016/j.gde.2006.04.002. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, ten Brink JB, de Kok YJ, van Soest S, van den Born LI, van Driel MA, van de Pol DJ, Payne AM, Bhattacharya SS, Kellner U, et al. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12) Nature Genetics. 1999;23:217–221. doi: 10.1038/13848. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Ghiani M, de Kok YJ, Wijnholds J, Ballabio A, Cremers FP, Broccoli V. Isolation of Crb1, a mouse homologue of Drosophila crumbs, and analysis of its expression pattern in eye and brain. Mechanisms of Development. 2002;110:203–207. doi: 10.1016/s0925-4773(01)00568-8. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Davis J, van der Velde-Visser SD, Zonneveld MN, Pierrottet CO, Koenekoop RK, Kellner U, van den Born LI, Heckenlively JR, Hoyng CB, et al. CRB1 mutation spectrum in inherited retinal dystrophies. Hum Mutat. 2004;24:355–369. doi: 10.1002/humu.20093. [DOI] [PubMed] [Google Scholar]
- Dowling JE. Organization of vertebrate retinas. Invest Ophthalmol. 1970;9:655–680. [PubMed] [Google Scholar]
- Fadool JM. Rod genesis in the teleost retina as a model of neural stem cells. Exp Neurol. 2003;184:14–19. doi: 10.1016/s0014-4886(03)00309-1. [DOI] [PubMed] [Google Scholar]
- Gosens I, den Hollander AI, Cremers FP, Roepman R. Composition and function of the Crumbs protein complex in the mammalian retina. Exp Eye Res. 2008;86:713–726. doi: 10.1016/j.exer.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Hamaoka T, Takechi M, Chinen A, Nishiwaki Y, Kawamura S. Visualization of rod photoreceptor development using GFP-transgenic zebrafish. Genesis. 2002;34:215–220. doi: 10.1002/gene.10155. [DOI] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414:634–638. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Jensen AM. Multiple domains in the Crumbs Homolog 2a (Crb2a) protein are required for regulating rod photoreceptor size. BMC Cell Biol. 2010;11:60. doi: 10.1186/1471-2121-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Easter SS. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Developmental Biology. 1999;207:309–321. doi: 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- Inai T. Vol Methods in Molecular Biology. Springer Science+Business Media, LLC; 2011. The Coculture Method to Examine Interactions Between Claudin Isoforms in Tight Junction-Free HEK293 Cells and Tight Junction-Bearing MDCK II Cells. [DOI] [PubMed] [Google Scholar]
- Izaddoost S, Nam SC, Bhat MA, Bellen HJ, Choi KW. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature. 2002;416:178–183. doi: 10.1038/nature720. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Tol2: a versatile gene transfer vector in vertebrates. Genome Biol. 2007;8(Suppl 1):S7. doi: 10.1186/gb-2007-8-s1-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitambi SS, Malicki JJ. Spatiotemporal features of neurogenesis in the retina of medaka, Oryzias latipes. Dev Dyn. 2008;237:3870–3881. doi: 10.1002/dvdy.21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- Laprise P, Beronja S, Silva-Gagliardi NF, Pellikka M, Jensen AM, McGlade CJ, Tepass U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev Cell. 2006;11:363–374. doi: 10.1016/j.devcel.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery AJ, Jacobson SG, Fishman GA, Weleber RG, Fulton AB, Namperumalsamy P, Heon E, Levin AV, Grover S, Rosenow JR, et al. Mutations in the CRB1 gene cause Leber congenital amaurosis. Arch Ophthalmol. 2001;119:415–420. doi: 10.1001/archopht.119.3.415. [DOI] [PubMed] [Google Scholar]
- Makarova O, Roh MH, Liu CJ, Laurinec S, Margolis B. Mammalian Crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1) Gene. 2003;302:21–29. doi: 10.1016/s0378111902010843. [DOI] [PubMed] [Google Scholar]
- Malicki J, Driever W. oko meduzy mutations affect neuronal patterning in the zebrafish retina and reveal cell-cell interactions of the retinal neuroepithelial sheet. Development. 1999;126:1235–1246. doi: 10.1242/dev.126.6.1235. [DOI] [PubMed] [Google Scholar]
- Mariani AP. Photoreceptors of the larval tiger salamander retina. Proc R Soc Lond B Biol Sci. 1986;227:483–492. doi: 10.1098/rspb.1986.0035. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2011;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- Mochizuki A. Pattern formation of the cone mosaic in the zebrafish retina: a cell rearrangement model. J Theor Biol. 2002;215:345–361. doi: 10.1006/jtbi.2001.2508. [DOI] [PubMed] [Google Scholar]
- Ohata S, Aoki R, Kinoshita S, Yamaguchi M, Tsuruoka-Kinoshita S, Tanaka H, Wada H, Watabe S, Tsuboi T, Masai I, Okamoto H. Dual roles of Notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron. 2011;69:215–230. doi: 10.1016/j.neuron.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Omori Y, Malicki J. oko meduzy and related crumbs genes are determinants of apical cell features in the vertebrate embryo. Curr Biol. 2006;16:945–957. doi: 10.1016/j.cub.2006.03.058. [DOI] [PubMed] [Google Scholar]
- Paffenholz R, Kuhn C, Grund C, Stehr S, Franke WW. The arm-repeat protein NPRAP (neurojungin) is a constituent of the plaques of the outer limiting zone in the retina, defining a novel type of adhering junction. Exp Cell Res. 1999;250:452–464. doi: 10.1006/excr.1999.4534. [DOI] [PubMed] [Google Scholar]
- Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. Faseb J. 2008;22:146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- Pujic Z, Malicki J. Mutation of the zebrafish glass onion locus causes early cell- nonautonomous loss of neuroepithelial integrity followed by severe neuronal patterning defects in the retina. Developmental Biology. 2001;234:454–469. doi: 10.1006/dbio.2001.0251. [DOI] [PubMed] [Google Scholar]
- Raviola G. The structural basis of the blood-ocular barriers. Exp Eye Res. 1977;25(Suppl):27–63. doi: 10.1016/s0014-4835(77)80009-2. [DOI] [PubMed] [Google Scholar]
- Raymond P, Barthel L, Curran G. Developmental patterning of rod and cone photoreceptors in embryonic zebrafish. Journal of Comparative Neurology. 1995;359:537–550. doi: 10.1002/cne.903590403. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK. A moving wave patterns the cone photoreceptor mosaic array in the zebrafish retina. Int J Dev Biol. 2004;48:935–945. doi: 10.1387/ijdb.041873pr. [DOI] [PubMed] [Google Scholar]
- Richard M, Muschalik N, Grawe F, Ozuyaman S, Knust E. A role for the extracellular domain of Crumbs in morphogenesis of Drosophila photoreceptor cells. Eur J Cell Biol. 2009;88:765–777. doi: 10.1016/j.ejcb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. Principles of Structure and Function. San Francisco, California: W. H. Freeman & Co; 1973. The Vertebrate Retina. [Google Scholar]
- Solomon SG, Lennie P. The machinery of colour vision. Nat Rev Neurosci. 2007;8:276–286. doi: 10.1038/nrn2094. [DOI] [PubMed] [Google Scholar]
- Steinberg MS. Adhesion in development: an historical overview. Dev Biol. 1996;180:377–388. doi: 10.1006/dbio.1996.0312. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Barthel LK, Raymond PA. Spatiotemporal coordination of rod and cone photoreceptor differentiation in goldfish retina. J Comp Neurol. 1997;382:272–284. doi: 10.1002/(sici)1096-9861(19970602)382:2<272::aid-cne10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Takechi M, Hamaoka T, Kawamura S. Fluorescence visualization of ultraviolet-sensitive cone photoreceptor development in living zebrafish. FEBS Lett. 2003;553:90–94. doi: 10.1016/s0014-5793(03)00977-3. [DOI] [PubMed] [Google Scholar]
- Takechi M, Kawamura S. Temporal and spatial changes in the expression pattern of multiple red and green subtype opsin genes during zebrafish development. J Exp Biol. 2005;208:1337–1345. doi: 10.1242/jeb.01532. [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev. 2002;118:91–98. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Tsujimura T, Chinen A, Kawamura S. Identification of a locus control region for quadruplicated green-sensitive opsin genes in zebrafish. Proc Natl Acad Sci U S A. 2007;104:12813–12818. doi: 10.1073/pnas.0704061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk JA, Rashbass P, Roepman R, Davis J, Voesenek KE, Arends ML, Zonneveld MN, van Roekel MH, Cameron K, Rohrschneider K, et al. Characterization of the Crumbs homolog 2 (CRB2) gene and analysis of its role in retinitis pigmentosa and Leber congenital amaurosis. Mol Vis. 2005;11:263–273. [PubMed] [Google Scholar]
- van Rossum AGAW, Meuleman J, Klooster J, Malysheva A, Versteeg I, Arsanto JP, Le Bivic A, Wijnholds J. Pals1/Mpp5 is required for correct localization of Crb1 at the subapical region in polarized Muller glia cells. Hum Mol Genet. 2006;15:2659–2672. doi: 10.1093/hmg/ddl194. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Doro CJ, Hyde DR. Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis Neurosci. 1999;16:571–585. doi: 10.1017/s0952523899163168. [DOI] [PubMed] [Google Scholar]
- Wei X, Malicki J. nagie oko, encoding a MAGUK-family protein, is essential for cellular patterning of the retina. Nature Genetics. 2002;31:150–157. doi: 10.1038/ng883. [DOI] [PubMed] [Google Scholar]
- Wei X, Zou J, Takechi M, Kawamura S, Li L. Nok plays an essential role in maintaining the integrity of the outer nuclear layer in the zebrafish retina. Exp Eye Res. 2006a;83:31–44. doi: 10.1016/j.exer.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Luo Y, Hyde DR. Molecular cloning of three zebrafish lin7 genes and their expression patterns in the retina. Exp Eye Res. 2006b;82:122–131. doi: 10.1016/j.exer.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zou J, Hyde DR, Davidson LA, Wei X. Stepwise maturation of apicobasal polarity of the neuroepithelium is essential for vertebrate neurulation. J Neurosci. 2009;29:11426–11440. doi: 10.1523/JNEUROSCI.1880-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Yang X, Wei X. Restricted localization of ponli, a novel zebrafish MAGUK-family protein, to the inner segment interface areas between green, red, and blue cones. Invest Ophthalmol Vis Sci. 2010;51:1738–1746. doi: 10.1167/iovs.09-4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.