Abstract
The capacity of proteins to adapt their structure in response to various perturbations including covalent modifications, and interactions with ligands and other proteins plays a key role in biological processes. Here, we explore the ability of molecular dynamics (MD), replica exchange molecular dynamics (REMD) and a library of structures of crystal-ligand complexes, to sample the protein conformational landscape and especially the accessible ligand binding site geometry. The extent of conformational space sampled is measured by the diversity of the shapes of the ligand binding sites. Since our focus here is the effect of this plasticity on the ability to identify active compounds through virtual screening, we use the structures generated by these techniques to generate a small ensemble for further docking studies, using binding site shape hierarchical clustering to determine four structures for each ensemble These are then assessed for their capacity to optimize enrichment and diversity in docking. We test these protocols on three different receptors: androgen receptor (AR), HIV protease, and CDK2. We show that REMD enhances structural sampling slightly as compared both to MD, and the distortions induced by ligand binding as reflected in the crystal structures. The improved sampling of the simulation methods doesn’t translate directly into improved docking performance however. The ensemble approach did improve enrichment and diversity, and, the ensemble derived from the crystal structures performed somewhat better than those derived from the simulations.
Keywords: Molecular dynamics, REMD, clustering, enrichment, diversity, binding site
Introduction
In a previous paper we investigated the use of a small ensemble of protein structures based on the diversity of binding site shapes to account for protein flexibility in docking. We found that the use of four diverse structures indeed improves consistency in results, database enrichment rates, and diversity of the hits obtained from docking. The studies were performed on crystal structures of HIV-1 protease and CDK2 and also on a structural ensemble generated from molecular dynamics (MD) simulations of androgen receptor (AR). We found that the MD ensemble of structures produced improved docking enrichment over a single crystal structure, consistent with the findings from the crystal structure ensembles. However, using multiple MD structures for AR did not improve results as significantly as using multiple crystal structures did for the HIV-1 protease and CDK2 systems and, perhaps surprisingly, the diversity of shapes was smaller than from the different crystal structures.
In this paper we apply MD to the same three systems (HIV-1 protease, CDK2, and AR) and study more rigorous sampling through the use of replica exchange molecular dynamics (REMD)1. The diversity of ligand binding site (LBS) shapes and the enrichment achieved from docking into the ensemble of structures obtained in these studies is compared to those obtained from the previous crystal studies. In addition we generated an ensemble of ligand binding shapes from the universe of AR-ligand crystal structures and carried out the same analysis of docking results using these.
Our objective is twofold. First, we explore whether the use of MD to improve enrichment through generating diverse structures is a generally applicable approach - or if the AR results were in some way unique to that system. Secondly, we investigate whether the apparently limited sampling of ligand binding site structure by the MD was due to the use of a limited portion of a single trajectory or is also a general phenomenon. REMD was performed to improve the sampling over standard MD with the hopes that the additional sampling from the single initial crystal structure would improve the virtual screening results. In addition, we performed an LBS SiteMap clustering of the REMD “trajectories” as well as on 44 AR crystal structures to have a complete set of the three systems. The results of docking into the small ensemble of diverse LBS structures resulting from this clustering was then analyzed to ascertain if the enrichment and diversity achieved with REMD is comparable or perhaps superior to that achieved from crystal structures or MD.
Methods
Crystal Structures and Initial Preparation
We searched the PDB for AR ligand binding domain (LBD) structures and selected 44 for clustering. The protein structures were prepared using the Schrodinger Protein Preparation Wizard (PrepWizard)2. This preparation protocol added hydrogens, built side chains and loops with missing atoms, determined the optimal protonation states for ionizable residues, optimized the hydrogen-bonding network, and performed a restrained minimization to obtain the final structure for running docking or simulations.
Choice of Systems for MD Studies
As noted in the introduction, we studied the three systems (HIV-1 protease, CDK2, and AR) used previously to assess database enrichments and diversity of retrieved ligands in ensemble docking. For HIV-1 protease, we used the 1EBZ structure from the PDB for the MD since 1EBZ was the representative structure of the cluster with the largest number of structures from our previous clustering of 135 HIV-1 protease structures3. Two CDK2 structures were chosen, one with cyclin bound and another without cyclin bound. These two forms have significant structural changes due to re-folding of the sections of the CDK2 structure involved in cyclin binding. Our previous clustering of 92 structures3 separated these two forms into different clusters, and we chose the non-cyclin bound structure as the cluster representative from the largest cluster of non-cyclin bound structures (1W0X) and the cluster representative of the single cyclin bound cluster (1OI9) for the MD simulations. For AR we used the 1T63 AR-DHT complex we investigated previously4.
Preparation of Protein Systems for MD and REMD
The PDB structures were prepared with the Schrodinger PrepWizard utility as described above. The prepared systems were solvated using the System Builder program of the Desmond suite with a dodecahedral solvent box and a solvent buffer extending 10 Å beyond the protein in all directions. The systems were neutralized with counterions, which entailed adding different numbers of Na+ or Cl− ions to each system. 4 Cl− ions were added for HIV-1 protease, 5 and 2 Cl− ions were added for apo and cyclin bound CDK2 respectively. For the AR structure, we used the existing prepared starting structure for the solvated MD studies as in our previously published AR work4.
Molecular Dynamics
MD simulations were carried out using the Desmond suite5 with long-range electrostatic interactions computed using a smooth Particle-Mesh Ewald (PME) approximation6 with a cutoff radius of 9.0 Å for the transition between the particle-particle and particle-grid calculations. Van der Waals (vdW) interactions were truncated at 9.0 Å. Dodecahedral periodic boundary conditions were used for all simulations. MD steps were integrated using a two time-step algorithm, with 2 fs steps for bonded and short-range interactions within the 9.0 Å cutoff and 10 fs for long-range non-bonded interactions. The system was relaxed using a protocol consisting of an initial minimization, MD for 20 ps at 0.1K, MD for 20 ps at 310K both with restraints of 250 kcal/mol/Å2 on all heavy atoms using the Berendsen7 thermostat followed by 100 ps with restraints of 2.5 kcal/mol/Å2 performed using 1 fs short range and 5 fs long range timesteps to reduce numerical issues with large initial forces. This was followed by 100 ps using the NPT ensemble and Berendsen thermostat and barostat and 100 ps Nose-Hoover thermostat and Martyna-Tuckerman-Klein barostat8. This was extended to 1ns with no restraints. The production MD simulations were carried out at 310 K using the NVT ensemble with a Nose-Hoover thermostat9,10. Trajectories were run for 5 ns, with the equilibration being carried out over the first nanosecond and analysis carried out on the final four nanoseconds.
Replica Exchange Molecular Dynamics
REMD is a procedure for improving sampling by performing multiple simulations of the same system at different temperatures in parallel1,11–13. At fixed time intervals a so-called “exchange” is attempted in which both the coordinates and velocities from a pair of adjacent temperature simulations are swapped. A Metropolis criterion14 is used to determine if the exchange should be accepted or not (eqn 1).
| (1) |
This procedure guarantees that in the long run for each simulation in the REMD set, averages over the trajectory are thermodynamically valid. The ability for configurations from higher temperatures to swap to lower temperatures improves the sampling of configurations at that temperature compared to performing a standard molecular dynamics trajectory.
The rate at which exchanges are accepted should be similar for each trajectory and allow a reasonable residence time for an exchanged structure at that temperature (around 10 ns). The temperature differences needed to achieve this can be estimated using an algorithm published by Patriksson15. This algorithm was used to determine the temperature distribution in this work. Given our initial choice of a 100-degree temperature range, from 298–398 K, the total number of replicas was determined as a function of the system size (i.e. number of atoms). The 100-degree temperature range was intended to improve sampling but not to introduce unfolding at the higher temperatures. Forty-five replicas were required for HIV-1 protease, while 55 and 53 replicas were used for the two CDK2 systems and AR required 44 replicas. REMD simulations at each temperature were performed for 3 ns using the Desmond program suite5 with the same methods as described above for the MD, resulting in approximately 150 ns of total simulation time for each system.
Ligand Binding Site Shape Clustering
As in our previous study3, the program SiteMap16,17 was used to compute the binding site shape for each receptor structure. Structures were superimposed using Cα atoms of common residues within 8 Å of the ligands. The volume overlap of the sites for each pair of structures was computed to create the volume overlap matrix. Hierarchical clustering was applied to this matrix using average linkage to create a clustering order. A cluster partitioning of four was chosen as a balance between time and completeness of sampling. For each partition the cluster representative was determined to realize a set of diverse binding site shapes.
Ligand Datasets
The DUD dataset18 was chosen as the test dataset to provide active and decoy ligands. This included 52 unique active ligands and 1884 decoys for HIV-1 protease and 49 unique active ligands and 1778 decoys for CDK2. For androgen receptor (AR), the set included 73 active ligands and 2627 decoys.
Docking
As in our previous work3, the Glide SP algorithm was used for docking19,20 with the final scoring using the GlideScore. The GlideScore has empirical terms to account for desolvation effects and special reward terms to account for interactions that are difficult to accurately compute with force fields, such as pi-pi and pi-cation interactions.
Database Enrichment and Diversity Assessment
The enrichment factor (EF) was computed using eqn 2:
| (2) |
where Fa is the fraction of actives found and Fd is the fraction of the database sampled. In this study, as previously, we have calculated enrichment factors for the actives found in the top scoring 1% and 4% of the total compounds docked (denoted EF (1%) and EF (4%), respectively).
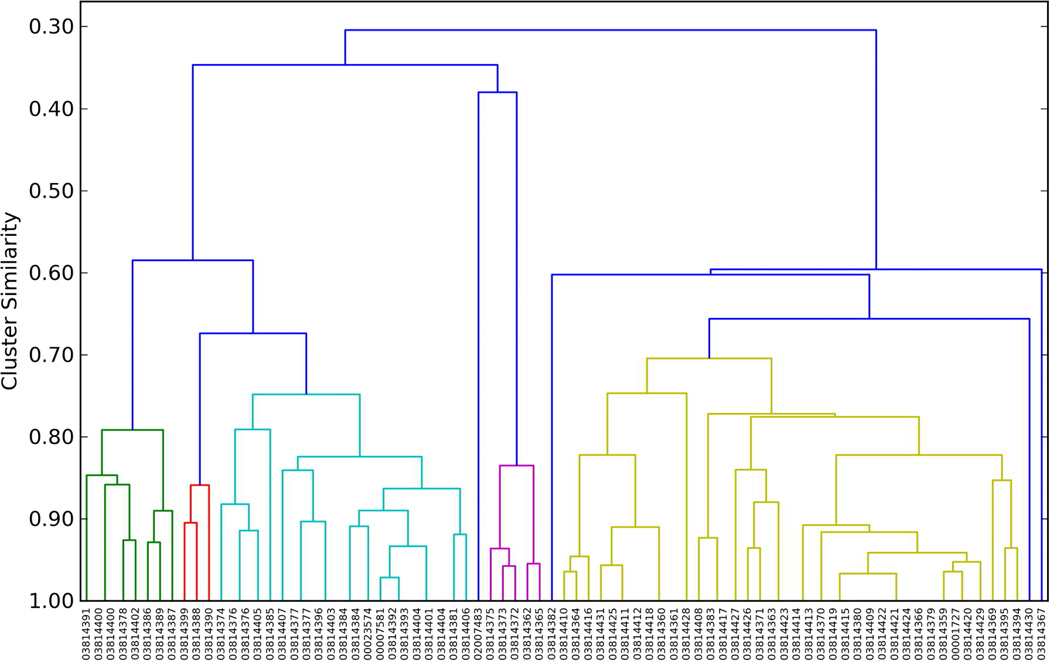
The diversity of ligands selected was computed as follows: The MACCs fingerprints21 of the active ligands were determined and hierarchical clustering was performed on the matrix of pair similarities determined using the Tanimoto metric using the program Canvas22. From inspection of the resulting clustering of all actives in each DUD ligand dataset (figure 1 shows the clustering of AR with those of HIV and CDK2 in supplementary material figures S4 and S5), a clustering level of 0.7 similarity was chosen as separating the active ligands into different chemotypes. For each cluster representative, after docking the actives in the 1% ligands were clustered and the number of clusters at the 0.7 level was determined. This number of clusters gives an estimate of the diversity of the hits found from docking.
Figure 1.
Dendrogram of clustering the 74 active ligands of the AR DUD dataset by their MACCs fingerprints, defining clusters at the 0.7 level and using the same color for the members of each cluster and blue for singleton clusters. The x-axis is the ZINC ID numbers.
Results
Molecular dynamics
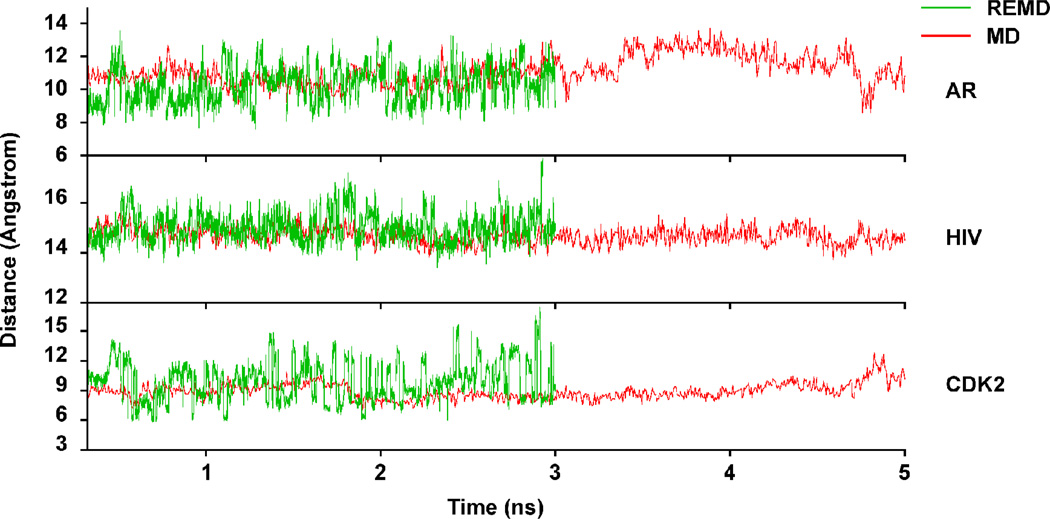
In order to assess the ability of molecular dynamics (MD) to sample the accessible configuration space of the ligand binding site, we carried out standard MD and replica exchange MD (REMD) simulations of AR, CDK2, and HIV-1 protease. The MD and REMD trajectories of the distance between residues roughly characterizing the LBS width are given in Figure 2 for the three proteins. As can be seen from the figure the distances visited during the trajectories for all three systems span a greater range for the REMD, as might be expected from it’s superior sampling ability1,11–13 even though the REMD trajectories are slightly shorter. This is especially true for the HIV-1 protease and CDK2 systems, while in the case of AR the MD eventually visits almost the whole span of distances, albeit briefly in some cases.
Figure 2.
Trajectory of residue-residue distances spanning the binding sites for the AR, HIV-1 protease and CDK2 MD (red) and REMD (green) simulations. The AR trajectory is characterized by the distance between the Gln 711 side chain amide nitrogen and the Met 895 sulfur. The HIV-1 protease trajectory corresponds to the Val 82 – Val 182 side chain distance, the CDK2 trajectory by the Tyr 15 – Asn 132 side chain distance.
Exploration of LBS Shapes By MD Versus Crystal Structures
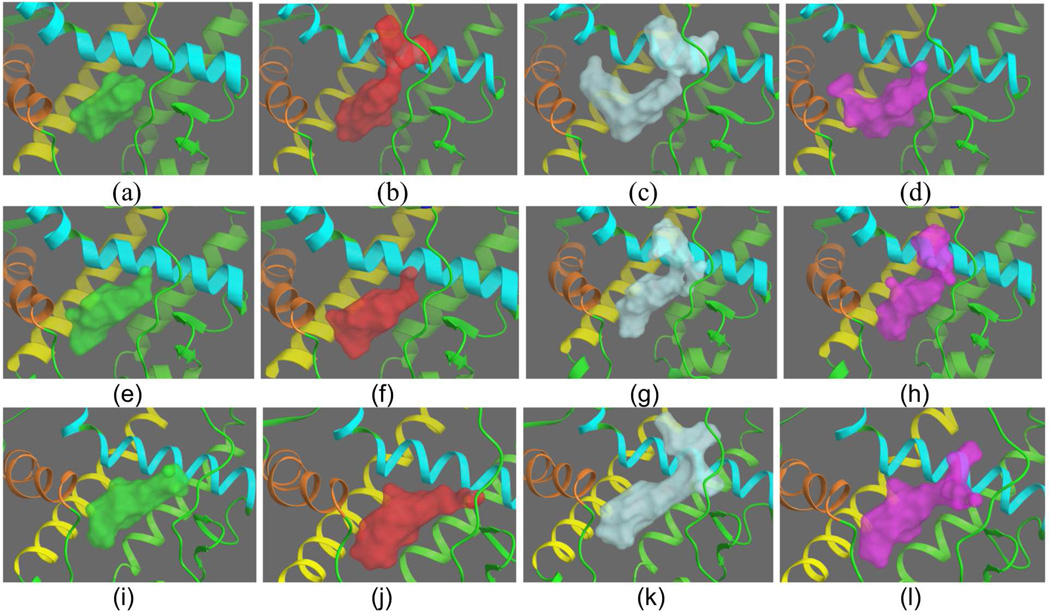
The structures obtained from the three sources (crystal, MD, and REMD) were then used to generate structural ensembles for virtual screening through hierarchical clustering. The resulting representative structures from MD and REMD were then compared with those obtained from the crystal structures3. A sense of the ligand binding site plasticity can be obtained by examining the LBS volumes of the cluster representatives sampled by the different methods. These are given for AR in Figure 3 and for CDK2 and HIV-1 protease in supplementary material. As can be seen in Figure 3, the crystal structures appear to show a greater diversity of volumetric shape than the MD structures, with four distinctly different shapes. The MD structures show essentially two different shapes as opposed to the greater variation in the crystal structures. This is consistent with the results in our previous study, where the volume overlap matrices indicated the crystal complexes of HIV-1 protease and CDK2 sampled the accessible LBS volumetric space more effectively than the (limited) MD did for AR3 (and was one of the motivating factors for this study).The REMD cluster representatives are distinctly different from the crystal and MD volumes but show some similarities in overall shape, with Figure 3 (k) (cyan shaded) showing similar features to (b) from the crystal and MD (h), although as seen from the overlap matrix given below, similarity of features doesn’t necessarily equate to a global volume similarity.
Figure 3.
Active site shapes for the androgen receptor from crystal structures, MD and REMD. The four crystal structure cluster representatives of AR are given in panels a–d: The four cluster representatives from MD are given in structures e–h, while the four cluster representatives from REMD are given in i–l. Helix 3 of AR, which runs roughly vertically in front of the LBS, has been removed from view for clarity.
Overlap volumes of representative AR structures
The greater diversity of the crystal shapes is further demonstrated by the normalized overlap volume matrix for the crystal and MD cluster representatives of AR (Table 1). The overlap matrix of the representative structures from MD confirms that the two pairs of structures are more similar to each other than to the other pair (e–f, g–h, shaded yellow and light blue) with the smallest similarity between the two pairs being 0.55.
Table 1.
Normalized overlap volume matrix of the four crystal cluster representatives for AR, the four MD cluster representatives for AR and the four REMD cluster representatives for AR 310 K. Cross normalized overlap volumes between the crystal, MD and REMD shapes shaded green
| Crystal | ||||||||||||
| 1T74 | 2AMA | 2AXA | 2HVC | |||||||||
| 1T74 | 1.00 | 0.69 | 0.45 | 0.66 | ||||||||
| 2AMA | 0.69 | 1.00 | 0.56 | 0.57 | ||||||||
| 2AXA | 0.45 | 0.56 | 1.00 | 0.51 | ||||||||
| 2HVC | 0.66 | 0.57 | 0.51 | 1.00 | ||||||||
| MD | e | f | g | h | ||||||||
| e | 0.70 | 0.57 | 0.41 | 0.57 | 1.00 | 0.71 | 0.59 | 0.66 | ||||
| f | 0.71 | 0.59 | 0.46 | 0.60 | 0.71 | 1.00 | 0.55 | 0.61 | ||||
| g | 0.57 | 0.57 | 0.48 | 0.46 | 0.59 | 0.55 | 1.00 | 0.69 | ||||
| h | 0.56 | 0.55 | 0.49 | 0.46 | 0.66 | 0.61 | 0.69 | 1.00 | ||||
| REMD | i | j | k | l | ||||||||
| i | 0.63 | 0.57 | 0.38 | 0.50 | 0.64 | 0.64 | 0.57 | 0.54 | 1.00 | 0.70 | 0.60 | 0.64 |
| j | 0.70 | 0.59 | 0.38 | 0.53 | 0.69 | 0.70 | 0.54 | 0.53 | 0.70 | 1.00 | 0.58 | 0.55 |
| k | 0.56 | 0.53 | 0.45 | 0.44 | 0.57 | 0.57 | 0.59 | 0.56 | 0.60 | 0.58 | 1.00 | 0.50 |
| l | 0.51 | 0.42 | 0.29 | 0.41 | 0.53 | 0.51 | 0.50 | 0.41 | 0.64 | 0.55 | 0.50 | 1.00 |
By comparison the least similar structures from the crystals have an overlap volume of 0.45. The average normalized overlap volume for all pairs is 0.64 for the MD cluster representatives versus 0.57 for the crystal, showing the crystal structure volumes sample a larger binding site structural space, on average, than the MD volumes in this system. The average of the normalized overlap matrix of the four cluster representatives from the REMD sampling of AR is 0.60 which is somewhat lower than the MD average (0.64) indicating that REMD has increased the diversity of volumes slightly, but still does not achieve the sampling of LBS volume induced by different ligands (average crystal overlap volume 0.57).
LBS volumetric sampling of HIV-1 protease and CDK2 yield different trends then AR
To assess the generality of this result we compare the sampling achieved by the three methods for the HIV-1 protease and CDK2 systems as well. Figures of the representative structures arising from the clustering of the crystal and dynamic trajectories are given in the supplementary material (Figures S1 and S2). Figure S1 shows the HIV-1 protease REMD shapes are more varied than the MD, with more open space in most of the representatives suggesting that the REMD has searched more of conformational space. This is confirmed by the overlap matrix for the HIV-1 protease representative structures, as seen in Table 2. In this system, unlike the behavior for AR, we see that a greater sampling efficiency is indeed achieved by the REMD method, than by either the crystal structures or the MD, consistent with the trend seen in the distance trajectory in Fig 1.Thus the average of the normalized overlap volume matrices is 0.41 and 0.43, and 0.32 for the crystal, MD, and REMD sampling, respectively, reflecting a significantly more extensive sampling by the REMD method.
Table 2.
Normalized overlap volume matrix of the four crystal cluster representatives for HIV, the four MD cluster representative s for HIV-1 protease and the four REMD cluster representatives for HIV-1 protease 310 K. Cross normalized overlap volumes between the crystal, MD and REMD shapes shaded green.
| Crystal | ||||||||||||
| 1EBZ | 1HVS | 1XL2 | 3AID | |||||||||
| 1EBZ | 1.00 | 0.56 | 0.35 | 0.39 | ||||||||
| 1HVS | 0.56 | 1.00 | 0.38 | 0.40 | ||||||||
| 1XL2 | 0.35 | 0.38 | 1.00 | 0.37 | ||||||||
| 3AID | 0.39 | 0.40 | 0.37 | 1.00 | ||||||||
| MD | e | f | g | h | ||||||||
| e | 0.29 | 0.29 | 0.25 | 0.30 | 1.00 | 0.38 | 0.43 | 0.41 | ||||
| f | 0.38 | 0.33 | 0.32 | 0.30 | 0.38 | 1.00 | 0.42 | 0.46 | ||||
| g | 0.26 | 0.27 | 0.23 | 0.28 | 0.43 | 0.42 | 1.00 | 0.50 | ||||
| h | 0.29 | 0.29 | 0.25 | 0.27 | 0.41 | 0.46 | 0.50 | 1.00 | ||||
| REMD | i | j | k | l | ||||||||
| i | 0.36 | 0.37 | 0.30 | 0.32 | 0.35 | 0.36 | 0.35 | 0.36 | 1.00 | 0.24 | 0.33 | 0.26 |
| j | 0.24 | 0.22 | 0.24 | 0.26 | 0.34 | 0.37 | 0.38 | 0.34 | 0.24 | 1.00 | 0.37 | 0.35 |
| k | 0.37 | 0.38 | 0.28 | 0.39 | 0.34 | 0.28 | 0.33 | 0.29 | 0.33 | 0.37 | 1.00 | 0.37 |
| l | 0.15 | 0.19 | 0.24 | 0.24 | 0.27 | 0.34 | 0.29 | 0.30 | 0.26 | 0.35 | 0.37 | 1.00 |
Comparison of LBS volumes obtained from different methods
As might be expected, at least for the dynamic methods, which trace the trajectory of a single structure, the “cross” overlap matrices between volumes obtained from different methods, tend to show a greater variation in volumes than those from any of the individual techniques. Thus, for example, the overlap between the first representative structure from the crystal with the fourth REMD structure is only 0.15, lower than any of the overlaps within a set. The average overlaps for representative structures from the HIV-1 protease crystal clustering with those from MD is 0.29 and for the crystal – REMD, and MD – REMD 0.28 and 0.33 respectively. This trend is also observed in the AR structures.
CDK2 – Cyclin vs. non-cyclin bound
Finally, in Table 3, we give the overlap matrices for the CDK2 system. The CDK2 structures separate globally into two basic classes; the cyclin and non-cyclin bound structures. In the crystal the former assemble into a single cluster while the non-cyclin structures are spread among the remaining three clusters with the exception of two non-cyclin structures, which appear in the cyclin bound cluster (Fig S3). Similar results are found in the distribution of structures from the MD. Namely, all cyclin bound structures are found in a single cluster, which interestingly contains two of the 800 non-cyclin structures that are visited along the MD trajectory. The REMD results are similar in that again all cyclin bound structures along the REMD “trajectory” group together in a single cluster. However in this case 103 of the 800 non-cyclin structures sampled in the REMD simulation group with the cyclin bound cluster. The excursion of the non-cyclin structures into the cyclin bound configuration space is consistent with the enhanced ability of REMD to sample the energy landscape.
Table 3.
Normalized overlap volume matrix of the four crystal cluster representatives for CDK2, the four MD cluster representatives for CDK2 and the four REMD cluster representatives for CDK2 310 K. Cross normalized overlap volumes between the crystal, MD and REMD shapes shaded green.
| Crystal | ||||||||||||
| 1OI9 | 2BHE | 1GZ8 | 1W0X | |||||||||
| 1OI9 | 1.00 | 0.46 | 0.40 | 0.49 | ||||||||
| 2BHE | 0.46 | 1.00 | 0.38 | 0.52 | ||||||||
| 1GZ8 | 0.40 | 0.38 | 1.00 | 0.50 | ||||||||
| 1W0X | 0.49 | 0.52 | 0.50 | 1.00 | ||||||||
| MD | e | f | g | h | ||||||||
| e | 0.36 | 0.32 | 0.45 | 0.44 | 1.00 | 0.37 | 0.31 | 0.29 | ||||
| f | 0.32 | 0.26 | 0.38 | 0.35 | 0.37 | 1.00 | 0.41 | 0.39 | ||||
| g | 0.28 | 0.27 | 0.30 | 0.31 | 0.31 | 0.41 | 1.00 | 0.43 | ||||
| h | 0.52 | 0.41 | 0.37 | 0.45 | 0.29 | 0.39 | 0.43 | 1.00 | ||||
| REMD | i | j | k | l | ||||||||
| i | 0.51 | 0.43 | 0.33 | 0.42 | 0.30 | 0.35 | 0.37 | 0.50 | 1.00 | 0.49 | 0.41 | 0.38 |
| j | 0.47 | 0.39 | 0.40 | 0.42 | 0.31 | 0.37 | 0.28 | 0.51 | 0.49 | 1.00 | 0.37 | 0.40 |
| k | 0.44 | 0.43 | 0.44 | 0.55 | 0.49 | 0.35 | 0.31 | 0.43 | 0.41 | 0.37 | 1.00 | 0.35 |
| l | 0.37 | 0.28 | 0.46 | 0.37 | 0.46 | 0.47 | 0.40 | 0.38 | 0.38 | 0.40 | 0.35 | 1.00 |
Simulations sample greater volume diversity than crystal structures in CDK2
As in the case of the AR and HIV-1 protease systems, another measure of the plasticity of the protein sampled by the different techniques is the similarity of the LBS volumes of the representative structures. Here the results differ slightly from what might be expected from the nature of the clustering and from the results of the AR and HIV-1 protease systems. In this case, as can be seen from the normalized overlap volumes in Table 3, both the MD and REMD simulations visit more diverse volumes than induced by the ligands in the crystal structures but in this case the MD simulations also span slightly more “volume space” than the REMD. This is reflected in the average overlap volumes of 0.46, 0.37 and 0.40 for the crystal, MD, and REMD respectively.
Does the ensemble of structures obtained from simulations improve enrichment over docking into a single structure?
The purpose of these studies is to develop methods for improving enrichment in virtual screening campaigns. Thus, the bottom line is whether docking into small ensembles obtained from simulations of dynamics improve enrichment over docking into a single structure, and secondly how this enrichment compares with that achieved with ensembles derived from crystal structures.
In Table 4 we show the results of a docking into the individual four cluster representatives obtained from crystal structures, MD, and REMD for the HIV-1 protease system. The enrichment achieved by exploiting the ensemble is better or equal (in the case of MD) to the average enrichment, which would be achieved by taking the same number of hits from the single structures. For example, 13 actives are found in the combined hits from the top 1% of hits from each representative structure of the crystal, while if we just took the top 4% of the hits from individual structures we would only recover ~10 actives on average. As noted previously3, there are individual structures, for example representative structure 2 in the case of HIV-1 protease, which would yield a greater enrichment than the ensemble. Unfortunately there is no clear way to a priori choose the best structure for docking.
Table 4.
HIV-1 protease Active Ligands and Decoy Ligands Docking to 4 Representative Structures from Crystal, MD and REMD. Active ligand counts, enrichment factors and Diversity for each structure at the 1% and 4% level
| Count of Active Ligands | Enrichment Factor | Diversity of Actives | ||||||
|---|---|---|---|---|---|---|---|---|
| Cluster Representative |
Top 19 (1%)† | Top 76 (4%) | Top 19 (1%) | Top 76 (4%) | Top 19 (1%) | Top 76 (4%) | ||
| Crystal | 1 | 6 | (6) | 10 | 12 | 4.8 | 3 | 5 |
| 2 | 10 | (5) | 16 | 19 | 7.7 | 5 | 6 | |
| 3 | 3 | (0) | 6 | 6 | 2.9 | 2 | 4 | |
| 4 | 7 | (2) | 9 | 13 | 4.3 | 3 | 5 | |
| Ensemble | 13 | 10.3†† | 6.3 | 4.9 | 6 | 5.0 | ||
| MD | 1 | 8 | (2) | 13 | 15 | 6.3 | 4 | 6 |
| 2 | 2 | (0) | 8 | 3.8 | 3.8 | 1 | 3 | |
| 3 | 6 | (1) | 12 | 12 | 5.8 | 1 | 4 | |
| 4 | 7 | (7) | 8 | 13 | 3.8 | 3 | 3 | |
| Ensemble | 10 | 10.3 | 4.8 | 4.9 | 2.3 | 4.0 | ||
| REMD | 1 | 6 | (6) | 11 | 12 | 5.3 | 1 | 3 |
| 2 | 8 | (6) | 17 | 15 | 8.2 | 5 | 6 | |
| 3 | 2 | (0) | 6 | 3.9 | 2.9 | 2 | 2 | |
| 4 | 4 | (1) | 8 | 7.7 | 3.8 | 2 | 3 | |
| Ensemble | 13 | 10.5 | 6.3 | 5.0 | 5 | 3.5 | ||
Additional unique ligand count shown in brackets – these are the ligands of the current structure that are different from any active ligand of structures before this structure
This is average over the active ligands recovered in the top 4% of hits
Diversity of hits
As noted above, not only are we interested in optimizing the recovery of actives in a virtual screening campaign, but equally important, we would also like to find a diverse set of hits on which to base further drug design efforts. The diversity of ligands is measured by number of clusters (~chemotypes) found at the 0.7 similarity level from a hierarchical clustering of the ligands, as described in methods. As with enrichment of HIV-1 protease hits, we see from Table 4 that the ensemble produces greater diversity than is achieved by docking into single structures, with the exception of the MD simulation. Again, one could achieve similar diversity from a single structure if one were fortunate enough to pick the best structure in which to dock.
Summary of enrichment and diversity comparison for the three systems
The results for all three systems studied here are summarized in Table 5, and the detailed tables for AR and CDK2 are given in the supplementary material (Tables S1 and S2). As we see from Table 5, in all cases the ensemble produces a better enrichment and greater diversity of hits than from the same number of compounds taken from docking into a single structure for the crystal structures. For example, the ensemble docking yields enrichment factors of 13.0, 6.3, and 9.1 for the crystal systems of AR, HIV, and CDK2, respectively while the average of the comparable enrichment factors for docking into single structures for the same systems is 9.7, 4.9, and 6.3 respectively. The diversity, as measured by the number of clusters/“chemotypes” recovered from the ensemble, is also superior (4, 6, and 11) as compared to the average crystal values (3.5, 5.0, and 7.0), respectively. Similar results are found from the structures derived from REMD, with the exception of AR. The MD results don’t follow this trend with both the AR and HIV-1 protease systems showing greater or equal enrichment from docking into a single structure and the average diversity achieved is also greater in the top 4% of the hits from single structures, as opposed to the combined top 1% of each of the individual representative structures. In CDK2, docking into the ensemble again produces better enrichment and diversity than the single structures.
Table 5.
Comparison of ensemble 1% results with average of 4% results for Crystal, MD and REMD studies of AR, HIV-1 protease and CDK2
| Count of Active Ligands | Enrichment Factor | Diversity of Actives | |||||
|---|---|---|---|---|---|---|---|
| System | Ensemble 1%a | Average 4%b | Ensemble 1%c | Average 4%d | Ensemble 1%e | Average 4%f | |
| AR | Crystal | 39 | 29 | 13 | 9.7 | 4 | 3.5 |
| MD | 25 | 27 | 8.5 | 9.0 | 3 | 2.3 | |
| REMD | 20 | 29 | 6.8 | 9.7 | 2 | 2.3 | |
| HIV | Crystal | 13 | 10.3 | 6.3 | 4.9 | 6 | 5.0 |
| MD | 10 | 10.3 | 4.8 | 4.9 | 2 | 4.0 | |
| REMD | 13 | 10.5 | 6.3 | 5.0 | 5 | 3.5 | |
| CDK2 | Crystal | 18 | 12 | 9.1 | 6.3 | 11 | 7.0 |
| MD | 12 | 7.8 | 6.1 | 3.9 | 8 | 5.3 | |
| REMD | 12 | 7.5 | 6.1 | 3.8 | 8 | 5.5 | |
Count of unique active ligands from all 4 Structures at the 1% level
Average of active ligand count for the 4 Structures at the 4% level
EF computed using the count of unique active ligands from all 4 structures at the 1% level
Average of the EF computed at the 4% level for each structure
Diversity computed using the unique active ligands from all 4 structures at the 1% level
Average of the Diversity computed at the 4% level for each structure
Discussion and Conclusions
We have shown that MD and REMD simulation techniques can be used to generate diverse structures that may be exploited to improve database enrichment and diversity in virtual screening campaigns as compared with the use of a single receptor structure. Furthermore, a procedure was described to select an ensemble of structures based on diversity of the binding site shapes. The ensemble selection is an unbiased procedure based on hierarchical clustering to select diverse binding site shapes.
The results presented here show that structures generated from MD and REMD simulations can be used in docking studies and provide significant enrichment and diversity of hits. In all three systems, docking into structures taken from MD and REMD yield good enrichment of actives, ranging from 3.8-fold improvement over random in the case of MD structures of CDK2 to 9.7-fold over random in AR, when analyzing the top 4% of the virtual screening hits. However, when many crystal structures are available, docking into an ensemble of binding site shape diverse crystal structures yields better enrichment and diversity of active ligands than docking into structures derived from simulations. Thus, one should use crystal structures if enough structures are available to achieve binding site diversity sufficient to accommodate the diversity of actives sought. The results from AR also confirm the conclusion drawn previously that docking into a small ensemble of representative crystal structures will yield better enrichment and diversity than docking into a single structure.
The value of using REMD versus MD for the generation of receptor structures is not clear from the limited studies performed here. In HIV-1 protease, greater enrichment is achieved by the REMD ensemble docking whereas in CDK2 there is no difference in enrichment between MD and REMD. In AR, equal or better enrichment is achieved by docking into any of the single representative structures from the simulation techniques than exploiting the top 1% of each structure in the ensemble. The reason for this is the subject of further study.
It was hypothesized that the relatively greater effectiveness of the crystal structures in the CDK2 and HIV-1 protease to yield enrichment in docking over the MD structures of AR was due to the limited sampling in the 1 ns portion of the MD trajectory exploited. Here, we employed REMD simulations to test this hypothesis. As seen from the resulting docking studies carried out in all three systems, the improved sampling afforded by the REMD did not translate into improved enrichment or diversity. The reason for this remains to be elucidated. The ligand binding site volume overlap matrices of the REMD with the crystal structures indicate that the simulations sample regions of LBS volumes which are not sampled by the crystal-ligand systems. This raises the question as to whether the REMD energy surface may not be adequately true to the actual energy surface of the protein ligand system, leading to slight artifactual excursions in the configuration space of the protein. That these deviations are not too extreme is demonstrated by the success of the structures in the trajectory to yield significant enrichment in docking, albeit not quite as competently as the crystal structures.
Perhaps a more plausible explanation is that binding site diversity alone is not a sufficient descriptor to determine the best structures to use for virtual screening. An extreme scenario can be constructed where a very diverse structure results from the binding site completely collapsing, yielding a “diverse” structure that would not accommodate any active compounds. While such an extreme situation was not encountered in the work presented here, more subtle situations may exist with side chains moving into positions that create diverse shapes that cannot accommodate active ligands. One possible solution would be to generate a diverse ensemble of structures under the constraint that certain other characteristics of the binding site are maintained, such as total volume, degree of enclosure, etc. Such a study is beyond the scope of the work presented here and will be the focus of future studies.
Supplementary Material
Acknowledgements
ATH would like to acknowledge Prof Harold Scheraga, a mentor and friend for many years.
This work was supported by the National Institutes of Health Grant R43 CA132538, and the National Science Foundation Grant CNS 0551500.
Footnotes
Supporting Information Description: This lists the PDB IDs for the AR crystal clustering, figures of the active site shapes for HIV and CDK2 from crystal, MD and REMD structures clustering, a dendrogram of the CDK2 crystal clustering, tables of detailed results for active ligand counts, enrichment factors and diversity estimates for docking to each individual crystal, MD and REMD cluster representative for AR, CDK2 and HIV and a dendrogram of HIV and CDK2 active ligand clustering using MACCs fingerprints. The supporting information includes additional figures and tables referenced in the paper. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Sugita Y, Okamoto Y. Chem. Phys. Lett. 1999;314:141–151. [Google Scholar]
- 2.Schrodinger Software Suite. 2010 [Google Scholar]
- 3.Osguthorpe DJ, Hagler AT, Sherman W. Chem Biol Drug Des. doi: 10.1111/j.1747-0285.2012.01396.x. in press. [DOI] [PubMed] [Google Scholar]
- 4.Osguthorpe DJ, Hagler AT. Biochemistry. 2011;50:4105–4113. doi: 10.1021/bi102059z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desmond Molecular Dynamics System. 2009 [Google Scholar]
- 6.Darden T, York D, Pedersen L. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 7.Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
- 8.Martyna GJ, Tuckerman ME, Tobias DJ, Klein ML. Mol. Phys. 1996;87:1117–1157. [Google Scholar]
- 9.Nose S. J. Chem. Phys. 1984;81:511–519. [Google Scholar]
- 10.Hoover WG. Physical Review A. 1985;31:1695–1697. doi: 10.1103/physreva.31.1695. [DOI] [PubMed] [Google Scholar]
- 11.Hukushima K, Nemoto K. J. Phys. Soc. Jpn. 1996;65:1604–1608. [Google Scholar]
- 12.Garcia AE, Sanbonmatsu KY. Proteins. 2001;42:345–354. doi: 10.1002/1097-0134(20010215)42:3<345::aid-prot50>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Garcia AE, Onuchic JN. Proc Natl Acad Sci U S A. 2003;100:13898–13903. doi: 10.1073/pnas.2335541100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metropolis N, Ulam S. J Am Stat Assoc. 1949;44:335–341. doi: 10.1080/01621459.1949.10483310. [DOI] [PubMed] [Google Scholar]
- 15.Patriksson A, van der Spoel D. PCCP. 2008;10:2073–2077. doi: 10.1039/b716554d. [DOI] [PubMed] [Google Scholar]
- 16.Tom H. Chem Biol Drug Des. 2007;69:146–148. doi: 10.1111/j.1747-0285.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 17.Halgren TA. J. Chem. Inf. Model. 2009;49:377–389. doi: 10.1021/ci800324m. [DOI] [PubMed] [Google Scholar]
- 18.Huang N, Shoichet BK, Irwin JJ. J Med Chem. 2006;49:6789–6801. doi: 10.1021/jm0608356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 20.Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL. J Med Chem. 2004;47:1750–1759. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- 21.MDL Information Systems “MACCS keys.”
- 22.Sastry M, Lowrie JF, Dixon SL, Sherman W. J. Chem. Inf. Model. 2010;50:771–784. doi: 10.1021/ci100062n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.