Abstract
Ethanol vapor chambers have been utilized widely in alcohol research since their introduction in 1971, and implementations of these systems are now available commercially. Here, we present a modification of the chamber that can be built at lower cost and greater simplicity of operation. The six-chamber system for rats has multiple air pumps. Ethanol vapor levels are adjusted with the air flow rate, ethanol drip rate, and dilution with room air, without a heater or fans. Ethanol vapor concentrations are measured with a breathalyzer, using room air to dilute the vapor chamber output into the range of the breathalyzer. Multiple pumps provide backup to ensure animal survival in the case of failure of the primary air pump. Tests in animals demonstrated comfortable and stable elevation of blood ethanol, with tight control of the ethanol vapor concentrations and the ability to select from a broad range of levels. The ethanol vapor measurement was rapid and efficient. The parts cost was a few thousand U.S. dollars. This vapor chamber system features low cost, ease of use, and convenient and inexpensive measurement of ethanol vapor concentrations. The lack of a heater and electrical components that could come into contact with ethanol in our case facilitated institutional approval.
Keywords: Ethanol Vapor Chamber, Ethanol Administration, Rats, Alcohol
1. Introduction
In the 1970's it was shown that high blood ethanol concentrations can be achieved in pyrazole-treated mice for 3 days using inhalation of alcohol vapor in a chamber as the route of administration (Goldstein, 1975, 1972; Goldstein and Pal, 1971). Those results demonstrated that blood ethanol concentrations (BEC) could be predicted from the alcohol vapor levels (Gilpin et al., 2009; Goldstein, 1972). Ferko and Bobyock (Ferko and Bobyock, 1977) then modified the vapor chamber and provided a reliable method for the induction of physical dependence on ethanol without the use of pyrazole. Later, an ethanol inhalation device was developed with a detailed description of the construction and operation (Karanian et al., 1986). The ethanol vapor chamber system has provided an additional method of ethanol administration now finds widespread use in alcohol research (Gilpin et al., 2008).
Here we describe a modified vapor chamber design with a detailed characterization of the chamber construction and operation, with a demonstration of application in rats.
2. Material and methods
2.1 Materials
The primary parts used in the vapor chamber are the following:
Rat cages: Model GR900SU (Tecniplast, USA, Inc., Philadelphia, PA, USA).
Fluid Metering QG6 Lab Pump 110V: (Fmipump, Syosset, NY, USA);
3L 5-Neck spherical glass flask (Glassblower, Department of Chemistry, Yale University, New Haven, CT, USA);
Flow meters with ranges of 4-50 liters per minute, 0-3.0 standard cubic feet per hour (Cole-Parmer, Vernon Hills, IL, USA);
Three PondMaster AP-100 Air Pumps: (Antonline, Roswell, GA, USA);
Alcotest 6510 breathalyzer (Dräger, Telford, PA, USA).
Tubing, valves, and connectors (Cole-Parmer, Vernon Hills, IL, USA).
190- and 200-proof ethanol, and silica gel desiccant (SilicaGelPackets, Harrisburg, NC, USA).
2.2 Construction of the Vapor Chamber System
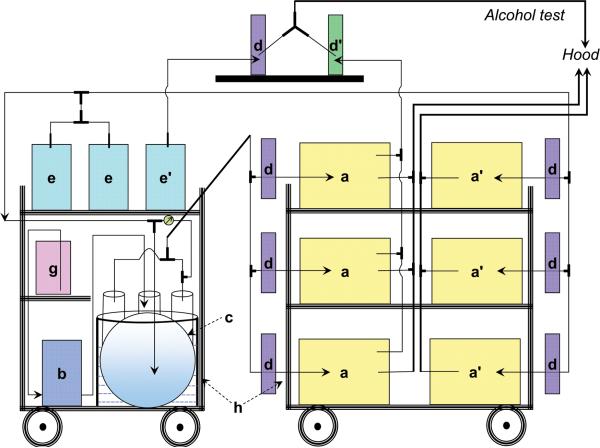
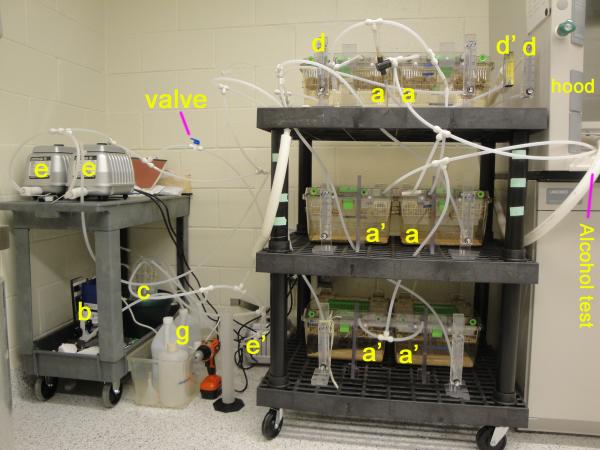
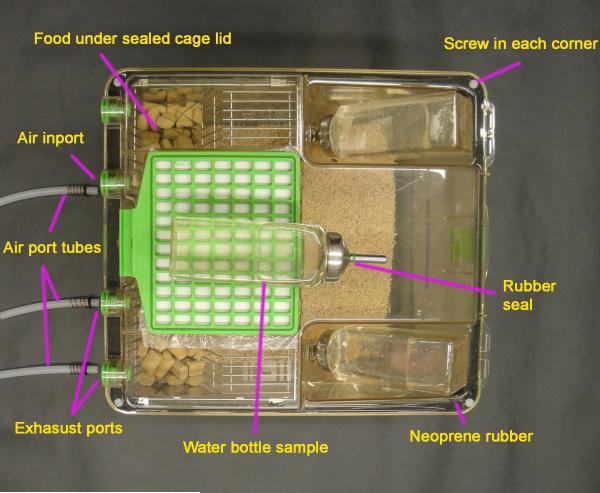
The ethanol vaporization and control system was constructed as shown in Fig. 1. Fig. 1A and B show three cages (denoted by lower case a) used for the ethanol vapor exposure group, and three other cages (a’) for use as control environments with room air. All the cages were sealed with neoprene rubber (McMaster-Carr, Princeton, NJ, USA) beneath the lid and the seal was enhanced with the addition of screws (Fig. 1C). The cages have four access ports, one of which is used for inflow of vapor and two for exhaust and monitoring of vapor concentrations. The water bottles that the manufacturer supplies have a gasket around the nozzle that maintains a sufficient seal. Ethanol is vaporized by pumping room air (e) into a spherical, 3-liter flask with 5 necks (c) while liquid ethanol is pumped (b) from a four-quart Nalgene jug (g) into the flask to create a pool of ethanol at the bottom of the flask. The ethanol in the flask evaporates and is carried to the chambers (a). Many vapor systems use a heater to increase the rate of evaporation of ethanol, but institutional concerns about the safe heating of ethanol excluded the use of a heater in our case. To increase the rate of evaporation without heating, the flask has a centered inlet for air and a side inlet for ethanol liquid. With sufficient air speed, the air flow becomes turbulent and violently disturbs the surface of the liquid ethanol, increasing the surface area and the rate of evaporation. Meanwhile, the liquid ethanol drips along the side and spreads over an area of the inner surface of the flask, which further increases the surface area and the rate of evaporation of the ethanol. In continuous operation, the flows of air and liquid ethanol maintain a steady level of ethanol liquid and a constant rate of evaporation that yield a stable concentration of ethanol vapor in the chambers. The four side necks of the flask are each connected with an adaptor through polyethylene tubing (1/4″ × 3/8″, Cole-Parmer, Vernon Hills, IL, USA) to a larger air hose (Masterflex C-FLEX Tubing, 3/4″ × 1″, Cole-Parmer, Vernon Hills, IL, USA) to combine the ethanol vapor into a single source. The reason to combine the outflows of the four tubes is that the ethanol vapor concentration varies up to three-fold from one neck to another, so the combination provides a reliable, uniform vapor supply to all of the cages. The mixed ethanol/air vapor is introduced into the sealed Polysulfone cages (Model GR900SU, Tecniplast USA, Inc.) via a flowmeter (d) whose rate is adjusted to 11 L/min for each cage. The outflows of all cages are combined and vented to an exhaust hood.
Fig. 1.
Schematic representation (A) and photographs (B and C) of ethanol metering and vaporization apparatus for inhalation chambers. In the configuration shown, three of the cages are connected to the alcohol vapor source, and three of them to room air. a and a’: Cages; b: Fluid Pump; c: five-mouth glass flask with connectors to tubing; d: Air flow meter with ranges of 4-50 liters per minute; d’: Air flow meter with 0-2 standard cubic feet per hour; e and e’: Air Pump; g: Ethanol Container; h: Cart. ⊥: T connectors; Y: Y connectors; →: Air flow direction;  : valve to change dilution of ethanol vapor.
: valve to change dilution of ethanol vapor.
2.3 Adjustment of the Ethanol Vapor Concentration
In this system, the depth of the ethanol was an influential parameter for the adjustment of the ethanol vapor concentration, a feature already incorporated in other vapor chambers in use today. If the depth of the ethanol is increased in the spherical flask, its surface area grows, and so does the rate of evaporation. In this case, the system was designed to achieve a vapor concentration that was greater than the target value, so the output of an additional room air pump was used to dilute the vapor with room air to reach the desired ethanol vapor concentration (Fig. 1). Although the air flow rate through the flask also can be used to adjust the ethanol vapor concentration, we chose to maintain total air flow into each chamber constant, for more uniform environments for the rats, regardless of ethanol vapor level.
Various aspects of the system design were tested for their impact on the vapor concentration in the chambers, and some of these are likely to be relevant for other vapor chamber systems that are now in use. For those evaluations, no rats were in the chambers. The following parameters were assessed with regard to their impact on the ethanol vapor concentration in the chambers:
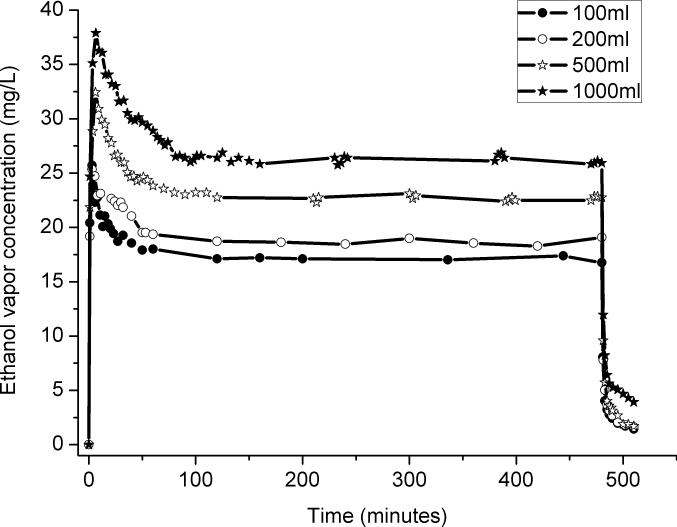
The ethanol depth in the flask. Different depths were achieved by putting different volumes of ethanol in the flask (100ml, 200ml, 500ml, and 1000ml). The volume was maintained by adjusting the ethanol drip rate to lower or higher values.
The temperature of the liquid ethanol. The ethanol was started at three different temperatures (22 °C, 14 °C, and 4 °C), and then during the test the ethanol was allowed to change its steady-state temperature.
The presence of water contamination in the liquid ethanol. Water content was varied by using 190- and 200-proof ethanol and by allowing ambient humidity in the room air to dissolve in the ethanol. The elimination of water with silica gel was tested as a remedy for water contamination.
The dilution of ethanol vapor with room air. The range of possible values and the speed of change of vapor concentration were assessed by changing the valve position for the mixture with room air.
The influence of the food and bedding in the chambers. The time courses of vapor concentrations were tested under four different conditions with 1000ml ethanol in the flask, starting at room temperature: 1) with food and bedding, 2) only with food, 3) only with bedding and 4) without food and bedding. At the end of each session, the chamber flow was switched to room air, and the vapor level decreased rapidly in all the tests.
During all tests, except for the food/bedding test (test #5), every ethanol vapor chamber was equipped with bedding, 400 grams standard rat chow, and a full water bottle.
2.4 Measurement of the Ethanol Concentration
There exist various methods to measure the ethanol vapor concentrations in the chambers. An early approach (Goldstein and Pal, 1971) adopted an enzymatic assay (Lundquist, 1959) that used 0.5-ml air samples injected through serum stoppers into the head space of tubes that contained 3.0 ml of the alcohol assay mixture (including the enzyme). This method has been used by other investigators (Becker and Hale, 1993; Becker and Lopez, 2004; Ferko and Bobyock, 1977). Several other groups (Ehlers and Chaplin, 1991; Ehlers and Slawecki, 2000; Rogers et al., 1979) measured the ethanol concentration periodically with gas chromatography. Another approach was the use of a temperature-controlled thermal conductivity detector oven to calibrate the vapor concentrations (Karanian et al., 1986), with a gas chromatographic system to calibrate the measurements. These approaches are effective but incur significant costs and labor. Thus, it was desirable to develop a fast and simple method to test the vapor concentration. A simple but effective approach is to use a breathalyzer. However, the measurement range of most breathalyzers is 0-0.500% breath alcohol concentration, which is equivalent to 0-2.4mg/L, much lower than the chamber's typical concentrations of 20-27 mg/L. A practical solution is to use a large syringe to sample a small amount of ethanol vapor from a cage and then to dilute the sample by drawing in a larger volume of room air, with the resulting mixture expelled into a breathalyzer to obtain the measurement (Personal Communication, Professor Howard Becker, Medical University of South Carolina). Inexpensive plastic syringes of a size sufficient to provide the volume needed for a breathalyzer were difficult to find, so we adapted Dr. Becker's concept of dilution to work directly with the outflow from the chamber system, using a breathalyzer (Alcotest 6510, Dräger Instruments, Telford, PA, U.S.A.). The outflow was diluted into the range of the breathalyzer with an additional air pump (Fig. 1: e’) to push room air through a flow meter (Fig. 1: d’) adjusted to achieve a mixture of 16:1 (room air: chamber output). Here the connection had to be the one Y connector in the design (Fig. 1 connection between d and d’), instead of a T-junction, to prevent the more powerful flow of room air from forcing its way into the chamber The diluted outflow was directed through the breathalyzer using the standard breathalyzer mouthpiece. The breathalyzer was set to read in units of mg/L and then calibrated by the manufacturer at our request. This procedure provided simple and rapid measurements of the concentration of ethanol vapor.
2.5 Application to Rats
The animal experiments were carried out in accordance with protocols approved by the Yale Animal Care and Use Committee. For the first test, three weeks of exposure were tested, measuring BEC once per week. Three male Sprague-Dawley rats (199.1±3.6g) were maintained on a 12h/12h light-dark cycle, with food and water available ad libitum. To accustom the rats to human interaction and minimize stress on the day of the experiment, they were handled and weighed once a day for three weeks before exposure was begun. The rats were exposed to ethanol for eight hours during the day, the light cycle. Then, for the remaining 4 hours of the light cycle and the 12 hours of the dark cycle, the chamber flow was changed to room air. For the rats’ first exposure, the ethanol vapor was set to ~15.5 mg/L, which was lower than the final target range of 23-27 mg/L, to allow the rats to adapt to the ethanol vapor. If they were immediately exposed to the target vapor concentration, they would be constantly intoxicated and sleeping, unable to eat or drink water. The ethanol vapor concentration was gradually increased over two weeks, reaching a final value ~25 mg/L. To monitor the BEC, 200 μL of blood was drawn from the saphenous vein once a week after 7 hours of that day's exposure. The blood samples were treated with centrifugation to obtain plasma, and the ethanol concentration measured with an Analox GM7 (Analox Instruments, Inc., Lunenburg, MA, USA).
The next experiment was designed to measure daily stability of the BEC. Three different rats (252.7±2.8g) were exposed just as the first group was, only this time, the alcohol vapor was increased to the target value over three days, and BEC were measured daily, after 7 hours of exposure.
3. Results
3.1 Influences of Liquid Volume and Temperature on Ethanol Vapor Concentration
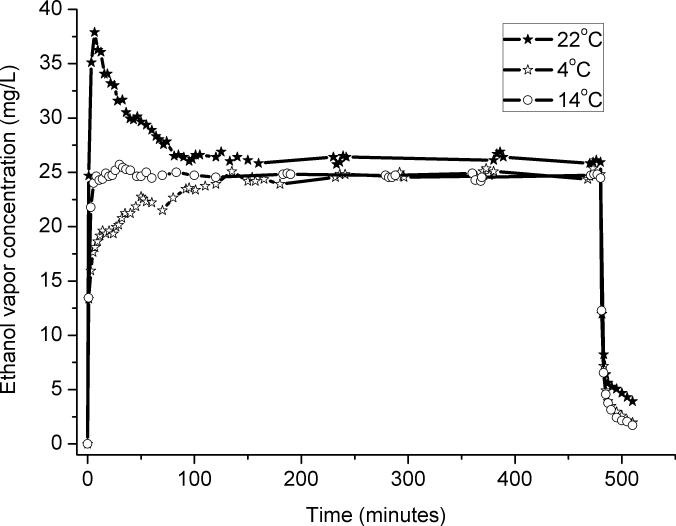
The two primary factors that control the ethanol vapor concentration are the surface area and temperature of the ethanol. The surface area is largely determined by the volume of ethanol in the spherical flask, so to test the influence of these two parameters, a range of volumes and temperatures of ethanol were tested. To keep the volume of the ethanol in the flask constant for each of the different volumes, different pump rates were used in each volume test. Fig. 2 shows the results of the test of the influence of ethanol depth in the flask. The greater the volume, the greater the surface area, and therefore, the greater the ethanol vapor concentration. Also of note was that that the shape of the profiles was similar for all of the volumes of ethanol that were tested: the ethanol concentration always increased rapidly at the system startup, decreased over the following 90 minutes, and then maintained a consistent level over the rest of the test. That decrease from the initial peak value led us to evaluate the impact of the temperature of the liquid ethanol on the concentration of ethanol vapor.
Fig. 2.
The effect of the volume of liquid ethanol in the glass flask on the ethanol vapor concentration in the chambers. Larger volumes were associated with greater surface area and so yielded higher concentrations of ethanol vapor.
When the different volumes were tested, the final temperature of the ethanol in the sphere was always ~14°C, although the room temperature and the initial temperature of the ethanol were 22 °C. We hypothesized that the decrease from the initial peak in ethanol vapor concentration was due to the evaporative cooling to 14 °C from the initial 22 °C temperature of the ethanol liquid. To verify this time-dependent temperature hypothesis, the ethanol in the flask was tested at three different temperatures: 4 °C, 14 °C , and 22 °C. Those temperatures were chosen for practical reasons. 14 °C was the steady-state temperature of the ethanol during the standard operation of the system. 22 °C was the room temperature in the facility where the system is used. 4 °C was a typical temperature if the ethanol is stored in the refrigerator. To measure the temperature, the ethanol sphere was placed in a bowl of water initially matched to the temperature of the ethanol, monitored with a thermometer, and then as the hours passed, the water was used as a surrogate marker for the temperature of the ethanol in the flask. Fig. 3 shows that the temperature of the 4°C group gradually increased to the constant value (14°C) as the flask was warmed by the room, while the ethanol that was initially 14 °C maintained a nearly constant level of vapor. The 22 °C test looked like the standard vapor chamber behavior shown in Fig. 2. Fig. 3 demonstrates that the temperature is a key to the control of the vapor concentration, which is why a heater is used in many designs. The reader may note that at the end of the experiment, the test that began with 22 °C yielded a slightly higher steady-state vapor level compared to the 4 °C and 14 °C tests. This is due to greater condensation of humidity from room air by colder ethanol. Humidity is addressed with the next test.
Fig. 3.
The effect of the initial temperature of liquid ethanol in the glass flask on the ethanol vapor concentration in the chambers. Although all three temperatures yielded a similar steady-state concentration of ethanol vapor, the lower temperature caused a slow rise in vapor concentration until the fluid warmed up, and the higher temperature caused an overshoot that decreased as evaporation cooled the liquid. The higher temperature retained a slightly higher concentration of ethanol throughout, because its warmer temperature condensed less humidity from the air and so was less diluted by water.
3.2 Influence of the water content in the liquid ethanol
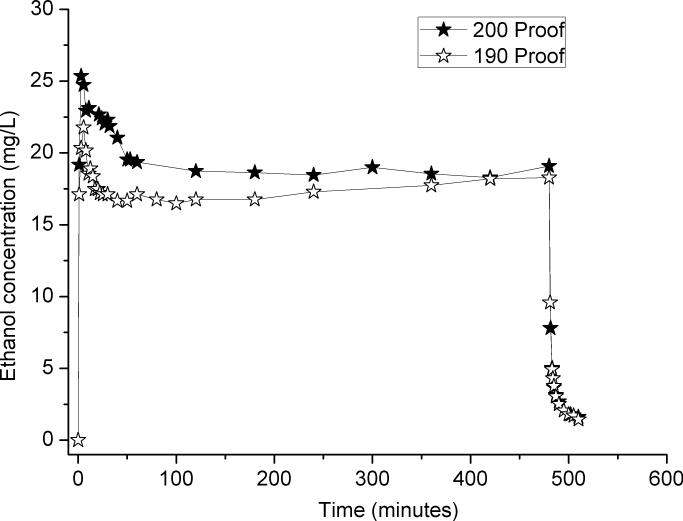
During the system development and testing, we observed that several days of use without discarding the ethanol in the flask resulted in much lower concentrations of ethanol vapor for the same ethanol pump rate and air flow. A possible explanation was that humidity from the room air was diluting the ethanol in the flask. To test this theory, the effects of starting the system with 190-proof ethanol relative to 200 proof were assessed over 8 hours. At the end of the study, the volume of liquid ethanol in the flask was measured. Fig. 4 shows that it took several hours for the vapor levels with 190- and 200-proof to converge. However, the final volumes were of liquid in the flasks differed greatly (455 mL and 255 mL for 190-proof and 200-proof ethanol, respectively), which indicates different rates of evaporation with different proofs of ethanol. Furthermore, with the same volume, the 190-proof ethanol generated lower ethanol concentrations at the beginning of the experiment. Furthermore, with four days’ exposure, the ethanol concentration of the 200-proof ethanol was unable to maintain the initial concentration. The source of the decreasing vapor concentrations after the ethanol has been exposed to room air for extended periods of time appears to come from dilution of the ethanol by condensation of humidity from the air, diluting the ethanol.
Fig. 4.
The differences of the time courses of ethanol vapor concentration for 190-proof and 200-proof ethanol in the flask.
To further test this theory, silica gel (SilicaGelPackets, Harrisburg, NC, USA) was added to the ethanol flask, and the ethanol vapor levels were constant over a week (as will be shown with the stability testing in animals).
3.3 Adjustment of the mixture of room air and ethanol vapor to regulate the ethanol vapor concentration
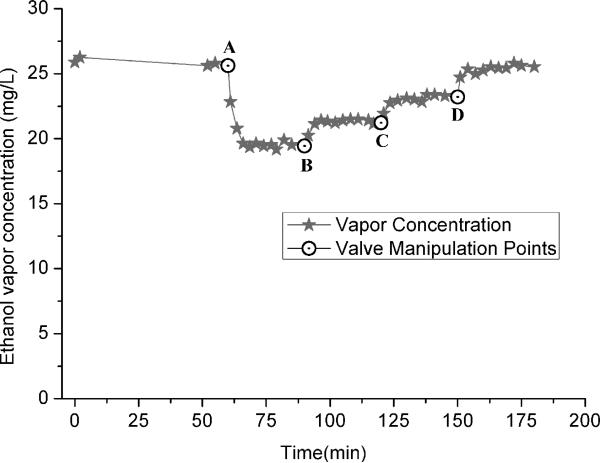
The valve that controls the dilution with room air was tested to assess its range of influence on the ethanol vapor concentration in the chambers (Fig. 1A, labeled  ). With the valve fully closed, the system was allowed to run to a steady-state level of 26 mg/L. Then the valve was opened fully for 30 minutes and afterward was gradually closed to reduce the dilution in steps over the next few hours (Fig. 5). The ethanol concentration during this time ranged between 18 and 26 mg/L, corresponding to valve positions of fully open and fully closed, respectively. This result shows that if the ethanol vapor level begins to drop because of water contamination or some other effect, the valve can be used to compensate. For lower or higher ranges, a different ethanol liquid pump rate and flask size can be used.
). With the valve fully closed, the system was allowed to run to a steady-state level of 26 mg/L. Then the valve was opened fully for 30 minutes and afterward was gradually closed to reduce the dilution in steps over the next few hours (Fig. 5). The ethanol concentration during this time ranged between 18 and 26 mg/L, corresponding to valve positions of fully open and fully closed, respectively. This result shows that if the ethanol vapor level begins to drop because of water contamination or some other effect, the valve can be used to compensate. For lower or higher ranges, a different ethanol liquid pump rate and flask size can be used.
Fig. 5.
The ethanol vapor concentration for different settings of the valve that regulates dilution with room air. The procedure was begun with the value fully closed (no dilution) to establish a steady state concentration, and then the valve was opened fully (A) to dilute the vapor and lower the concentration to 18 mg/L. Over the next two hours, the valve das closed in incremental steps (B, C, D), and the concentration of ethanol vapor increased step-wise to 26 mg/L (fully closed, D).[d1]
3.4 Influence of the food and bedding
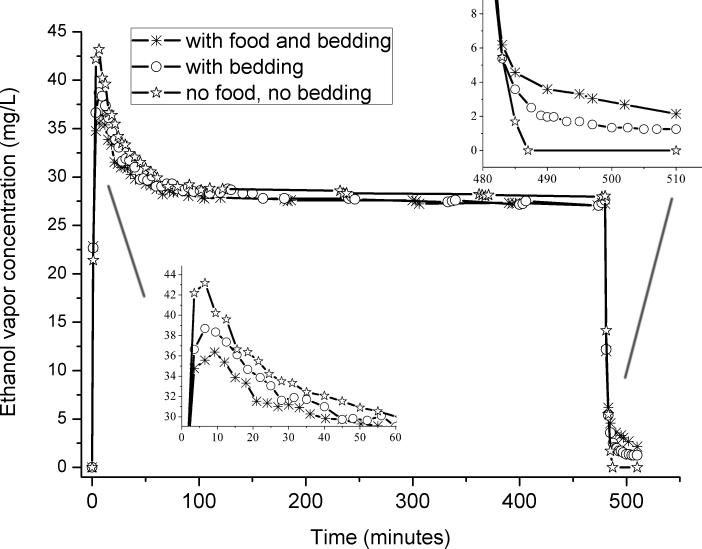
The presence of food and bedding transiently lowers the early peak ethanol vapor level (Fig. 6), probably by absorption. Later, when ethanol vapor is discontinued, the food and bedding delay slightly the elimination of vapor from the cage. After 8 hours without food and bedding, when the vapor was switched to room air, the ethanol vapor vanished after 7 minutes, but in the presence of food, bedding, or both, some ethanol vapor remained for more than half an hour. Therefore, the food and bedding cause some delay in the initial rise and the later discontinuation of ethanol vapor. However, on the time scale of most studies, the influence is probably negligible, because only about 1.1 mg/L ethanol remained after half an hour, and the ethanol was undetectable after overnight flow of room air through the chambers.
Fig. 6.
The effects of food and bedding on the ethanol vapor concentration. The bedding blunted the peak vapor concentration upon system startup, and it delayed the washout of the ethanol when the system was turned off.
3.5 Application to Rats
Rats require proper temperature, humidity, and fresh air. During the tests with rats, the temperature and humidity in the chambers differed by less than 1.5 °C and 2%, respectively, compared to the room air in the animal care facility (22.5°C and 45%). The flow rate provided ~36 air changes/hour, which is sufficient to maintain proper air quality for the animals (Krohn et al., 2003). In the presence of vapor, the rats showed no obvious signs of discomfort. When the vapor flow was first turned on, some rats continued sleeping, while others ran for several seconds, then walked around, sniffing the sides of the cage for 30-60 seconds, and then their behavior returned to pre-vapor conditions.
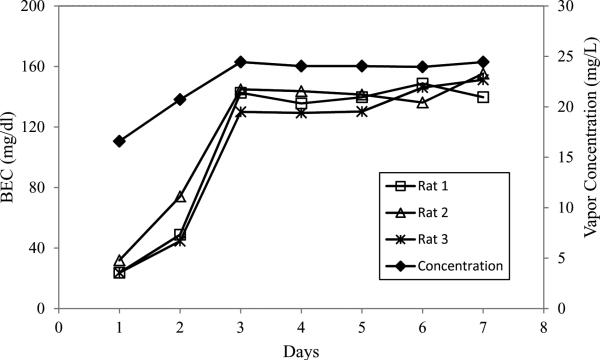
For the rats exposed over three weeks, with BEC measured once per week, the BEC was 81.4± 19.4, 149± 24.6, and 168.8 ± 22.3 mg/dL, after each week (mean ± SD), corresponding to respective ethanol vapor concentrations of 18.9mg/L, 22.1mg/L, and 25.8mg/L. For the rats whose BEC was assessed daily for one week, their BEC levels rose as the level was increased over the first three days and then remained constant, as did the ethanol vapor concentration (Fig. 7). The final, target level was in the range that has been used for studies of ethanol dependence (Funk et al., 2007; Rimondini et al., 2002; Schulteis et al., 1995), although the purpose of this study was only to demonstrate the feasibility of maintaining that level and did not assess dependence. It should be noted that previous work shows that at least the first several hours of each day's exposure can be expected to yield a linear rise of BEC (Gilpin et al., 2009) .
Fig. 7.
The relationship between daily blood ethanol concentration (BEC) and the daily vapor concentration during one week's exposure. The initial vapor levels were purposely set low to allow the rats time to adapt to the vapor, and then a steady level was reached.
4. Discussion
We have designed and built a vapor chamber to administer ethanol to small animals. Institutional concerns precluded the use of heaters, fans, and other electrical components that might come into contact with the vapor. Simplicity of ethanol vapor measurement was desirable, as was an economical design for limited grant budgets. After construction, we tested various parameters for their influence on the concentrations and stability of ethanol vapor, and we verified stability in rats.
The chamber testing revealed that temperature, volume, and water content of the liquid ethanol are important influences on the ethanol vapor concentration. Temperature in particular had a powerful influence. In our system the ethanol temperature stabilized at ~14 °C, whether the ethanol liquid started at higher or lower temperatures. If a user wants a more immediate arrival at the steady-state concentration of ethanol vapor, then the ethanol flask can be cooled to 14 °C before turning on the system. If some delay can be tolerated, then an easier alternative is to let the system run for 90 minutes to allow the temperature and the vapor level to reach their steady state. Regarding volume effects, more liquid in the flask had a greater surface area and so led to more evaporation and a higher ethanol vapor level. If the liquid ethanol contains water, such as occurs with 190-proof ethanol or with condensation of humidity from the air, then silica gel can be added to the liquid ethanol in the flask to absorb the water and maintain a stable ethanol vapor concentration. The presence of food and bedding did not affect the steady-state ethanol concentration, but they did blunt the initial overshoot in the ethanol vapor and delayed changes in ethanol vapor level.
The current design of the ethanol vapor chamber has several positive features: (1) The parts cost less than $5000, including the breathalyzer and associated tubing for the vapor measurement. (2) No heating or other electrical components are needed in the system except for the air pumps, which do not come into contact with ethanol vapor. (3) The measurement of the ethanol vapor concentration is fast, simple, and inexpensive. (4) The ethanol vapor concentration can be changed quickly and easily between high and low values. (5) There are three air pumps in this system, so if one pump fails, the animals still have enough air to breathe. (6) All cages receive the same ethanol vapor concentration. (7) The initial vapor concentration of the ethanol can be altered by cooling to achieve a gradual increase of vapor level or an immediate arrival at the steady-state vapor concentration. The equipment has some limitations: (1) The noise of the air pumps has the potential to influence animal behavior. That effect could be reduced with physical separation and sound-proofing between the pumps and the cages. (2) A minor inconvenience is the need to make a physical change of air tube connections to switch between ethanol vapor or room air, but a valve system could be implemented simply. (3) There is no exhaust fan or outflow pump, so the air pressure in the cages will be a little bit higher than the atmospheric pressure, which was caused by the resistance of the tubes. However, the animals showed no symptoms of discomfort in this environment, and their behavior did not differ from their behavior in standard cages. Any increase in air pressure did not interfere with the dispensing of water from the water bottles, although the bottles sit outside the cages with their nozzles penetrating the top of the cage through a seal. (4) The current design forces all cages to have the same ethanol vapor concentration. If there is a need to provide different vapor concentrations to individual cages, it would be necessary to modify the design with additional valves to create different dilutions with room air.
It is important to recognize that the particular range of ethanol vapor levels (18-26 mg/L) and schedule of exposure (8 h/day) in the tests on the animals were appropriate for our own future applications. Investigators who plan to use the system for other species or other ranges of vapor will require their own times of exposure and chamber settings, such as air and ethanol flow rates and size of the ethanol flask. The detailed parts list and advice for implementation are available upon request.
Highlights.
▶ A six-chamber system was designed for control of vapor levels for small animals.
▶ Different ethanol vapor levels were achieved without use of a heater or fan.
▶ The vapor concentrations were measured with a breathalyzer.
▶ The system is low cost, and simple to use and to measure vapor levels.
Acknowledgements
The authors thank Andrew Cameron of Oregon Health and Science University who provided information on the flow pumps and valuable discussion on practicalities of the use and design. We also thank Pietro Cottone and Valentina Sabina at Boston University for demonstrations of their vapor chamber system, Don Pouliot at Draeger Safety Diagnostics for the calibration of the breathalyzer, and Peter Brown and Scott McIntyre at Yale University for help and advice during the construction of the system. The research was supported by NIH grants R21 AA018210 (GM) and R21 AA019803 (GM) and Natural National Science Foundation of China Y2Z1141001 (JW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “Kindling”. Alcohol Clin Exp Res. 1993;17:94–8. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Ehlers C, Chaplin R. EEG and ERP response to chronic ethanol exposure in rats. Psychopharmacol. 1991;104:67–74. doi: 10.1007/BF02244556. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slawecki CJ. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–9. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Ferko AP, Bobyock E. Induction of physical dependence in rats by ethanol inhalation without the use of pyrazole. Toxicol Appl Pharmacol. 1977;40:269–76. doi: 10.1016/0041-008x(77)90097-7. [DOI] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee M-J, Rice KC, Koob GF. Corticotropin-Releasing Factor 1 Antagonists Selectively Reduce Ethanol Self-Administration in Ethanol-Dependent Rats. Biol Psychiatr. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008 doi: 10.1002/0471142301.ns0929s44. Chapter 9: Unit 9.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant Behavior and Alcohol Levels in Blood and Brain of Alcohol-Dependent Rats. Alcohol Clin Exp Res. 2009;33:2113–23. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB. Physical-Dependence on Alcohol in Mice. Fed Proc. 1975;34:1953–61. [PubMed] [Google Scholar]
- Goldstein DB. Relationship of Alcohol Dose to Intensity of Withdrawal Signs in Mice. J Pharmacol Exp Ther. 1972;180:203–15. [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: Grading the withdrawal reaction. Science. 1971;172:288–90. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Karanian J, Yergey J, Lister R, Souza N, Linnoila M, Salem N. Characterization of an Automated Apparatus for Precise Control of Inhalation Chamber Ethanol Vapor and Blood Ethanol Concentrations. Alcohol Clin Exp Res. 1986;10:443–7. doi: 10.1111/j.1530-0277.1986.tb05121.x. [DOI] [PubMed] [Google Scholar]
- Krohn TC, Hansen AK, Dragsted N. The impact of cage ventilation on rats housed in IVC systems. Lab Anim. 2003;37:85–93. doi: 10.1258/00236770360563714. [DOI] [PubMed] [Google Scholar]
- Lundquist F. The determination of ethyl alcohol in blood and tissues. Methods Biochem Anal. 1959;7:217–51. [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Markus H. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. The FASEB Journal. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–86. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Decreased brain reward produced by ethanol withdrawal. 1995;92:5880–4. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]