Abstract
Previous reports have shown ambiguous findings regarding the possible associations between ischaemic stroke (IS) and single nucleotide polymorphisms (SNPs) in the phosphodiesterase 4D (PDE4D) gene region. The SNP rs12188950 (or SNP45) has often been studied in this context. We performed a multi-centre study involving a large sample of 2599 IS patients and 2093 control subjects from the south and west regions of Sweden to replicate previous studies regarding IS risk and rs12188950. Subjects from Lund Stroke Register (LSR), Malmö Diet and Cancer Study (MDC) and Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS) were enroled. Subgroups of participants with hypertension and participants <55 years of age, as well as the TOAST subgroups large vessel disease, small vessel disease and cardioembolism, were also assessed. Univariate odds ratios (ORs) and ORs controlling for hypertension, diabetes and current smoking were calculated. We additionally performed a meta-analysis including 10 500 patients and 10 102 control subjects from 17 publications (including the present study). When assessing pooled data from LSR, MDC and SAHLSIS we obtained no association between IS and rs12188950 for all participants (OR=0.93; 95% confidence interval (CI): 0.83–1.05). Significant associations were not found for hypertensive participants or participants with age <55, or when separately evaluating patients from the three different TOAST subgroups. The meta-analysis showed no significant overall estimate (OR=0.96; 95% CI: 0.89–1.04) with significant heterogeneity for random effect (P=0.042). No effect from rs12188950 on IS was found from either our pooled multi-centre data or the performed meta-analysis. We did not find any association between the examined subgroups and rs12188950 either.
Keywords: stroke, polymorphism, PDE4D, rs12188950, meta-analysis
Introduction
Stroke is a major cause of death and functional disability in industrialised countries. The clinical presentation of stroke includes intracranial bleedings as well as cerebral diseases with a cardiovascular aetiology. A genetic contribution to stroke risk, also for common ischaemic stroke (IS) subtypes, is considered likely, but results have been equivocal.
Two previous reports, one from an Icelandic study in 2003 and one reported by us in 2008, suggested that the minor allele in the single nucleotide polymorphism (SNP) in rs12188950 (also denoted SNP45), located at the 5′ end of the phosphodiesterase 4D (PDE4D) gene in chromosome 5, might have an inhibiting effect on IS risk.1, 2 Our previous study also showed a significant inhibiting effect of rs12188950 minor (T) allele on IS among subjects with hypertension.2 However, a meta-analysis in our previous publication in 2008 showed ambiguous results concerning the possible association between IS risk and variants in rs12188950.3, 4, 5, 6, 7, 8, 9, 10, 11, 12
We were aware of three replicating studies, that showed significant allelic association with IS by detecting and examining haplotype blocks located at the 5′ end region of the PDE4D gene.4, 13, 14 Moreover, a meta-analysis including four studies indicated significantly increased IS risk when considering an at-risk haplotype, including rs12188950.15 These findings suggest that rs12188950, as a polymorphism located in the centre of the 5′ end region of PDE4D, might be particularly interesting for further study to confirm or refute a relation to IS.
In the present multi-centre study involving three case–control groups from the west and south of Sweden, we intended to test for replication of previous findings regarding rs12188950 by assessing a large number of individuals. We also updated our previous meta-analysis by including new publications and our new results regarding the subject of the possible association between rs12188950 and IS. We focused on possible discrepancies between included populations and subpopulations because of sex, nationality or ethnicity, rather than discrepancies between the publications themselves in this updated meta-analysis.
Material and methods
Study population and sample characteristics
Phenotypic and genotypic data for subjects from Lund Stroke Register (LSR), the Malmö Diet and Cancer study (MDC) and the Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS) were included in our analyses. Originally, the total number of individuals consisted of 2682 IS patients and 2139 control subjects. After exclusion of 129 subjects owing to genotyping failure for rs12188950, the remaining 2599 patients and 2093 control subjects were included in our multi-centre study.
Complete characteristics of the data used are described elsewhere.2, 16, 17, 18, 19, 20, 21 Briefly, LSR is a prospective, consecutive epidemiological study from which we have included 920 patients with first-ever stroke from the local catchment area of Skåne University Hospital in Lund, and 566 control subjects from the same geographical area, selected randomly after stratification by age and sex to match the patient group (when including intracranial bleedings as well as cerebral infarctions).2, 17 The LSR data was collected between March 2001 and August 2007. In a previous study by us from 2008 we assessed 932 IS patients and 396 control subjects from LSR.2 In order to perform a replication being independent of assessments made previously, we did not include any of these participants in the current study. However, the results from 2008 were included as a separate study/population in our meta-analysis.
MDC is a prospective population-based study that was initiated in the 1990s as a cohort study at Skåne University Hospital in Malmö, Sweden.16, 21 MDC initially included a total of 28 499 randomly selected men, born between 1923 and 1945, and women, born between 1923 and 1950. Cases with a first stroke after the baseline examination (n=874) were matched for age, sex and month of the baseline examination with control subjects (n=881) free from stroke at the time of the stroke event of the corresponding patient.
SAHLSIS is a consecutive study comprising patients from four stroke units situated in the west of Sweden. SAHLSIS involves patients presenting with first-ever or recurrent acute IS before reaching the age of 70 years.18, 19, 20 A total of 805 IS patients and 646 controls, recruited between 1998 and 2008 within the SAHLSIS project, were included in this study.
All three above mentioned studies are approved by the ethics committee of the University of Gothenburg (SAHLSIS data) and by the ethics committee of Lund University (LSR and MDC data). All participants or, when necessary, their next of kin provided informed consent before enrolment.
Definition of IS
The diagnosis of IS was determined in accordance with World Health Organization criteria.22 All patients in the study had computer tomography or magnetic resonance scan, or autopsy findings, compatible with IS. In addition, a classification of IS according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) nomenclature was made for the LSR and SAHLSIS populations. Three main TOAST subgroups of IS were defined: (i) Large vessel disease/large artery atherosclerosis (LVD); (ii) small vessel disease/small artery occlusion (SVD) and iii) cardioembolism or significant aortic plaques (CE).23 Three patients diagnosed with aortic plaques were included in the LVD group in SAHLSIS.
Definition of intermediate phenotypes
Three risk factors were ascertained for all participants in the study. (1) Hypertension was defined as medical treatment for hypertension, or blood pressure 160/90 mm Hg or higher at the time of discharge or after at least 1 week of hospitalisation. (2) Diabetes mellitus was defined as dietary or medical treatment for diabetes mellitus or measurements at discharge or at least 1 week of hospitalisation as follows: blood glucose 6.1 mmol/l or higher at two occasions, plasma glucose 7.0 mmol/l or higher at two occasions or alternatively more than 11 mmol/l at one occasion with concurrent symptoms suggestive of diabetes mellitus (measurements of hypertension and diabetes mellitus among SAHLSIS cases were performed at 3 months follow-up).18 (3) Current smoking was defined through self-report (current smokers versus previous smokers and never smokers).
For LSR and SAHLSIS, age was defined at first-time stroke onset for patients and at inclusion for control subjects. A nested case–control design was used in MDC. Age at the time of the stroke event was used both for MDC stroke cases and their corresponding control subjects (age was omitted for 63 control subjects of the MDC owing to the absence of matching patients).
Genotyping
Genotyping of data from the three included populations was performed by matrix-assisted, laser desorption ionisation time-of-flight mass spectrometry on a MASSarray platform with iPLEX genotyping technology (Sequenom, San Diego, CA, USA).20 All laboratory analyses were performed by the SWEGENE Resource Center for Profiling Polygenic Disease (Skåne University Hospital, Malmö, Sweden), with no access to case/control status or personal identities of the samples. Data were stored at the Bioinformatics Unit at the same facility.
Data analysis and statistical methods
Allelic association tests were used to compare patients and control subjects regarding possible allelic association between variants in rs12188950 and IS. Odds ratio (OR) estimates of the minor (alternative) T allele over the major (reference) C allele against cases and controls are presented with 95% confidence intervals (CI). OR estimates for each subpopulation (LSR, MDC and SAHLSIS) were calculated as well as an overall pooled OR estimate for the total of these subpopulations were joined together. We correspondingly assessed a specific subgroup of 1727 patients and 1056 control subjects with hypertension. Similarly, we defined a subgroup of 382 patients and 321 control subjects below 55 years of age for a separate assessment. We also evaluated a subsample of patients with either SVD, CE or LVD according to TOAST classification criteria compared with control subjects for LSR and SAHLSIS data joined together.23
Furthermore, we carried out unweighted and weighted simple logistic regression (LR) as well as weighted multiple LR for individuals genotyped using an additive model counting 0, 1 or 2 minor alleles per individual. Regression weights were created by sex and age (categorized into three age groups: participants below 55 years; participants between 55 and 65 years; and participants 65 years or more). Patients and control subjects of LSR, MDC and SAHLSIS (ie six strata referring to cases/controls in three geographical domains of study) were thus adjusted within each stratum to a weight variable referring to sex and age distribution in uniformity with the age and sex distribution of the entire sample of data.
We performed a goodness-of-fit test (a χ2 test using one degree of freedom) for possible departure from Hardy–Weinberg equilibrium in control subject data.
Statistical computations were performed by using PASW (SPSS) Statistics (version 18) statistical software (PASW/SPSS software, IBM Corporation, Armonk, NY, USA). Power tests were carried out by using the PS Power and Sample Size Calculations software (version 3.0.14; Department of Biostatistics, Vanderbilt University, Nashville, TN, USA).
Meta-analysis
We searched the electronic PubMed database (using http://www.ncbi.nlm.nih.gov/pubmed/) for studies including the search keywords ‘STRK1', ‘PDE4D' or ‘Phosphodiesterase 4D' in combination with keywords ‘Stroke' or ‘Cerebrovascular'. All keywords were searched in titles or abstracts of publications issued between January 2003 and August 2011. A total of 58 publications were found and thereafter manually reviewed. We excluded 42 publications owing to the following reasons: 17 publications were studies comprising reviews or meta-analyses; 3 publications were editorials or correspondence articles; 5 publications were association studies excluding rs12188950 because the SNP was found to be monomorphic after being genotyped; 8 were association studies that did not consider rs12188950 at all; whereas 9 publications were not allelic association studies assessing genotypes versus IS risk.
After this process, 16 publications, comprising 19 distinguishable populations suitable for the meta-analysis, remained.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 24, 25, 26 The meta-analysis thus involved a total of 20 populations from 17 publications when our current study was included.
The 42 publications that remained after the above selection for the meta-analyses were either studies comprising reviews or meta-analyses (11 publications); or editorials or correspondence articles (3 publications); or association studies where rs12188950 was excluded because of non-polymorphic traits (5 publications), or studies that were not including rs12188950 or in other respects were irrelevant for our meta-analysis (23 publications).
We performed a random effects DerSimonian–Laird meta-analysis, and our intention was to examine the effect of rs12188950 on IS in a large population providing genetic information, as well as comparing different population subgroups regarding the possible association between rs12188950 allelic variation and IS risk. R version 2.12.0 (The R Foundation for Statistical Computing c/o Institute for Statistics and Mathematics, Vienna, Austria) was used for the meta-analysis calculations.
Results
The 2599 IS patients were older than the 2030 control subjects with non-missing values of age (see Table 1). The LSR subpopulation showed a higher rate of men in control subjects than in patients, whereas the SAHLSIS subpopulation conversely showed a higher rate of women. As expected, there were higher prevalences of the vascular risk factors hypertension, diabetes mellitus and current smokers among patients compared with control subjects.
Table 1. Phenotype characteristics of patients and control subjects included in Lund Stroke Register (LSR), Malmö Diet and Cancer study (MDC) and the Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS).
| Patients | Control subjects | P-valuea | |
|---|---|---|---|
| LSR (N=1486) | N=920 | N=566 | |
| Age: mean (±SD) | 74.8 (±12.3) | 73.9 (±12.4) | 0.163b |
| Age: median, min, max | 76.7, 18.2, 101.7 | 76.3, 18.0, 96.4 | 0.207c |
| Age: non-missing N | 920 | 566 | |
| Men: frequency/non-missing N (%) | 463/920 (50.3) | 324/566 (57.2) | 0.010 |
| Hypertension: frequency/non-missing N (%) | 618/896 (69.0) | 319/566 (56.4) | <0.001 |
| Diabetes mellitus: frequency/non-missing N (%) | 256/875 (29.3) | 45/562 (8.0) | <0.001 |
| Current smokers: frequency/non-missing N (%) | 171/907 (18.9) | 60/565 (10.6) | <0.001 |
| MDC (N=1755) | N=874 | N=881 | |
| Age: mean (±SD) | 69.3 (±7.0) | 69.4 (±7.0) | 0.680b |
| Age: median, min, max | 69.9, 48.4, 82.7 | 70.1, 48.4, 82.7 | 0.630c |
| Age: non-missing N | 874 | 818d | |
| Men: frequency/non-missing N (%) | 481/874 (55.0) | 476/881 (54.0) | 0.701 |
| Hypertension: frequency/non-missing N (%) | 645/873 (73.9) | 513/880 (58.3) | <0.001 |
| Diabetes mellitus: frequency/non-missing N (%) | 86/874 (9.8) | 25/881 (2.8) | <0.001 |
| Current smokers: frequency/non-missing N (%) | 283/854 (33.1) | 185/869 (21.3) | <0.001 |
| SAHLSIS (N=1451) | N=805 | N=646 | |
| Age: mean (±SD) | 56.2 (±10.6) | 56.0 (±10.4) | 0.698b |
| Age: median, min, max | 58.8, 18.1, 69.8 | 58.4, 18.6, 70.4 | 0.471c |
| Age: non-missing N | 805 | 646 | |
| Men: frequency/non-missing N (%) | 527/805 (65.5) | 376/646 (58.2) | 0.005 |
| Hypertension: frequency/non-missing N (%) | 464/794 (58.4) | 224/645 (34.7) | <0.001 |
| Diabetes mellitus: frequency/non-missing N (%) | 150/805 (18.6) | 31/644 (4.8) | <0.001 |
| Current smokers: frequency/non-missing N (%) | 307/801 (38.3) | 127/646 (19.7) | <0.001 |
| All subjects in study (N=4692) | N=2599 | N=2093 | |
| Age: mean (±SD) | 67.2 (±12.8) | 66.4 (±12.7) | 0.033b |
| Age: median, min, max | 67.6, 18.1, 101.7 | 67.4, 18.0, 96.4 | 0.045c |
| Age: non-missing N | 2599 | 2030d | |
| Men: frequency/non-missing N (%) | 1471/2599 (56.6) | 1176/2093 (56.2) | 0.790 |
| Hypertension: frequency/non-missing N (%) | 1727/2563 (67.4) | 1056/2091 (50.5) | <0.001 |
| Diabetes mellitus: frequency/non-missing N (%) | 492/2554 (19.3) | 101/2087 (4.8) | <0.001 |
| Current smokers: frequency/non-missing N (%) | 761/2562 (29.7) | 372/2080 (17.9) | <0.001 |
P-values are based on Fisher's exact test if not footnoted otherwise.
Student's t-test (with equal variances assumed).
Mann–Whitney non-parametric test.
Age of ischaemic stroke patient-matched control subjects of whom 63 such subjects from MDC data did not match.
The genotyping success rate for rs12188950 was 98% when considering all data. The genotype distribution in LSR, MDC and SAHLSIS control subject data joined together conformed to Hardy–Weinberg equilibrium (P=0.072). According to a power test, using the parameters alpha=0.05; 1-beta=0.8, we found the entire sample size of our data to be sufficient to detect significant OR estimates when true OR values were below 0.84 or above 1.18. For a subgroup of 382 patients and 321 control subjects (covering the number of participants below 55 years of age), a true OR <0.61 or >1.52 would be required for a significant finding.
Univariate association tests
For the entire study data, we did not detect any statistically significant association between IS risk and allelic variants in rs12188950. Moreover, we did not obtain statistically significant ORs when assessing LSR, MDC and SAHLSIS data separately. Separate assessments of hypertensive participants or participants younger than 55 years of age did not show any results with such significance either. When examining cases defined as IS patients with either LVD, SVD or CE subgroup, we did not find any significant association with rs12188950 (Table 2).23
Table 2. Association between rs12188950 (SNP45) and prevalence of ischaemic stroke for Lund Stroke Register (LSR), Malmö Diet and Cancer study (MDC) and the Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS).
| Population/ Subpopulation | Number of non-missing patients/controls | Number of minor alleles in patients/controlsa | Allelic association test: OR; 95% CI; P-valueb |
|---|---|---|---|
| All patients and controls | |||
| LSR | 920/566 | 257/177 | 0.88; 0.71–1.08; 0.219 |
| MDC | 874/881 | 244/248 | 0.99; 0.82–1.20; 0.923 |
| SAHLSIS | 805/646 | 192/168 | 0.91; 0.73–1.13; 0.396 |
| Total (LSR, MDC, SAHLSIS) | 2599/2093 | 693/593 | 0.93; 0.83–1.05; 0.251 |
| Patients and controls with hypertension | |||
| LSR | 618/319 | 174/94 | 0.95; 0.72–1.24; 0.728 |
| MDC | 645/513 | 176/145 | 0.96; 0.76–1.22; 0.762 |
| SAHLSIS | 464/224 | 116/59 | 0.94; 0.67–1.32; 0.730 |
| Total (LSR, MDC, SAHLSIS) | 1727/1056 | 466/298 | 0.95; 0.81–1.11; 0.521 |
| Age below 55 years | |||
| LSR | 58/44 | 16/13 | 0.92; 0.42–2.04; 1.000 |
| MDC | 31/29 | 10/12 | 0.74; 0.29–1.87; 0.638 |
| SAHLSIS | 293/248 | 53/63 | 0.68; 0.46–1.01; 0.061 |
| Total (LSR, MDC, SAHLSIS) | 382/321 | 79/88 | 0.73; 0.53–1.00; 0.057 |
| IS sub-groups due to TOAST classificationc | |||
| LVD | 161/1212 | 40/345 | 0.85; 0.60–1.22; 0.395 |
| SVD | 339/1212 | 85/345 | 0.86; 0.67–1.11; 0.285 |
| CE | 410/1212 | 123/345 | 1.06; 0.85–1.33; 0.605 |
Abbreviations: CE, cardiembolism; CI, confidence interval; LVD, large vessel disease; OR, odds ratio; SVD,small vessel disease.
Minor alleles T, major alleles C.
CIs computed using Wald approximation of variances, P-values computed using Fisher's exact test. A small divergence may cause conflicting null-hypothesis conclusions.
TOAST classification of 1678 cases and1786 control subjects from LSR and SAHLSIS was performed, MDC is not included.
LR analyses of additive rs12188950 data
From the weighted simple LR analyses we obtained results for LSR, MDC and SAHLSIS after adjustments for heterogeneity caused by dissimilar distributions on sex and age. No significant associations were found (Table 3). These results were approximately similar to the corresponding univariate OR estimates (Table 2).
Table 3. Simple and multiple logistic regression (LR) performing an additive approach for assessing possible association between rs12188950 and ischaemic stroke risk for Lund Stroke Register (LSR), Malmö Diet and Cancer study (MDC) and the Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS).
| Simple LR | Weighted Simple LRa | Weighted Multiple LRa | |||||
|---|---|---|---|---|---|---|---|
| Population | Covariate | OR; 95% CI; P-value | Number of non-missing cases/controls | OR; 95% CI; P-value | Number of non-missing cases/controls | Controlling for IS risk factorsb OR; 95% CI; P-value | Number of non-missing cases/controls |
| LSR | rs12188950 | 0.88; 0.72–1.08; 0.214 | 920/566 | 0.86; 0.70–1.06; 0.154 | 920/566 | 0.88; 0.70–1.10; 0.253 | 856/562 |
| Hypertension | 1.73; 1.37–2.18; <0.001 | ||||||
| Diabetes | 4.18; 2.94–5.96; <0.001 | ||||||
| Current smoker | 2.61; 1.92–3.55; <0.001 | ||||||
| MDC | rs12188950 | 0.99; 0.82–1.20; 0.920 | 874/881 | 0.93; 0.77–1.13; 0.478 | 874/818 | 0.98; 0.80–1.21; 0.873 | 849/795 |
| Hypertension | 2.02; 1.63–2.50; <0.001 | ||||||
| Diabetes | 4.73; 2.86–7.82; <0.001 | ||||||
| Current smoker | 2.45; 1.95–3.07; <0.001 | ||||||
| SAHLSIS | rs12188950 | 0.90; 0.72–1.13; 0.372 | 805/646 | 0.91; 0.73–1.14; 0.419 | 805/646 | 0.86; 0.67–1.09; 0.215 | 793/645 |
| Hypertension | 2.52; 2.00–3.16; <0.001 | ||||||
| Diabetes | 3.86; 2.64–5.66; <0.001 | ||||||
| Current smoker | 3.37; 2.56–4.42; <0.001 | ||||||
| Pooled data | rs12188950 | 0.93; 0.83–1.05; 0.240 | 2599/2093 | 0.90; 0.80–1.02; 0.095 | 2599/2030 | 0.91; 0.80–1.04; 0.159 | 2498/2002 |
| Hypertension | 2.02; 1.77–2.29; <0.001 | ||||||
| Diabetes | 4.40; 3.50–5.53; <0.001 | ||||||
| Current smoker | 2.64; 2.28–3.07; <0.001 | ||||||
Abbreviations: CI, confidence interval; OR, odds ratio.
Weight obtained by age and sex standardization of included populations.
Dichotomous risk factors for ischaemic stroke: hypertension, diabetes mellitus and current smoking.
The weighted multiple LRs, controlling for the dichotomous phenotype risk factors hypertension, diabetes mellitus and current smoking, did not change the results. All of these risk factor covariates were significant with P<0.001, but no significant contribution from rs12188950 could be seen.
Meta-analysis
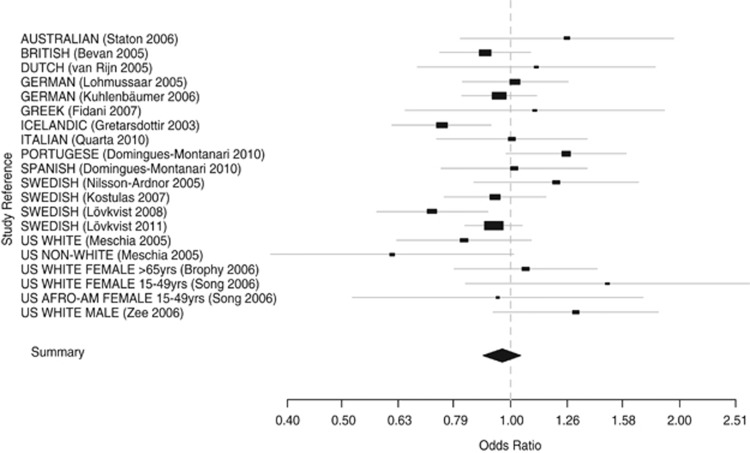
The outcome of the meta-analysis of 10 500 IS patients and 10 102 subjects from 17 published studies (including our previous study) and this study, containing 20 distinguishable populations is visualised in Figure 1. The overall OR of 0.96 (95% CI: 0.89–1.04) indicates no significant overall effect in these 20 populations. The heterogeneity test (P=0.042) shows a slightly significant difference between the ORs of the 20 populations, indicating that the OR estimates from these populations may be caused by other reasons than random variations.
Figure 1.
Random effects (DerSimonian–Laird) meta-analysis of 16 previously published studies and the present study, comprising 20 distinguishable populations assessing the association between the minor (T) allele of rs12188950 and IS in 10 500 IS patients and 10 102 controls. Overall OR: 0.96 (95% CI: 0.89–1.04), test for heterogeneity χ2=30.9 (P=0.0416).
Only two populations showed ORs that significantly suggest allelic association between variants in rs12188950 and IS risk.1, 2 When carrying out a meta-analysis that only comprised the Icelandic and the Swedish populations (five studies including the present study), the OR remained insignificant (OR=0.88; 95% CI: 0.76–1.01; P-value from heterogeneity test=0.047).1, 2, 5, 9
Discussion
In this large multi-centre study we could not detect an association between rs12188950 and IS. This might be in conflict with the results of our previous study and the Icelandic study, although the upper CI limit of 0.91 and 0.92 from these studies, respectively, are close to the estimated OR (0.93) for rs12188950 in this present study.1, 2
Considerations about IS subgroups
None of the TOAST-classified subgroups of IS met the significance level in our analyses. A previous Swedish study by Kostulas et al5 in 2007 showed a relative risk (RR) of 1.43 (to be compared with 1.07 when including all IS causes and transient ischaemic attack) when testing the major (G) allele of rs12188950 against LVD risk. Another study by Kuhlenbäumer et al6 in 2006 found an OR estimate of 1.06 when testing major alleles of rs12188950 against LVD risk among a German population (OR=1.05 when assessing all IS causes). The Icelandic study by Gretarsdottir et al1 showed RR=1.77 when assessing patients with combined carotid and cardiogenic causes of stroke (all IS causes provided an RR estimate of 1.31). From these findings, only the Icelandic RR estimates were significant. However, the tendency towards a protecting effect of minor alleles against IS in the three studies as a whole might be an interesting subject of further studies of LVD subgroups.
Discrepancy between present and previously reported LSR data
Our previously reported study showed a protecting effect against IS that could not be found in our current study, despite similar LSR data collection routines.2 The dataset in the previous study published in 2008 (LSR-I) did not differ considerably from the present LSR data (LSR-II) regarding mean age and proportion of current smokers. Nevertheless, LSR-I comprises a lower frequency of hypertensive controls (33.7%) than LSR-II (56.4%). Patients and controls with diabetes mellitus were also more frequent in LSR-II (29.1% and 8.1%, respectively) compared with that in LSR-I (21.0% and 6.3%, respectively). LSR-I was collected between 2001 and 2004, and the major part of LSR-II was collected between 2004 and 2008.
Meta-analysis findings
Our meta-analysis that included populations from Europe, North America and Australia did not find any overall tendency of IS being affected by rs12188950 minor alleles in neither protective nor augmenting direction. A previously published meta-analysis, with similarities to ours regarding selection of publication, presented a significant overall OR=1.29 (P=0.05) after removal of publications causing heterogeneity.27
The slightly significant heterogeneity test (P=0.042) suggests a non-random effect explaining the difference between the ORs among the 20 compared populations in our meta-analysis. Figure 1 visualises this ambiguity regarding the OR estimates. Dissimilar ethnicities in the studied populations might be one explaining factor. However, other demographic attributes (such as age, sex or occupational status of participants) may also contribute to such an effect. Besides, conclusions regarding possible ethnical differences might be connected with various problems regarding adequate definitions of a particular group of study.28
We found five publications in our PubMed search that presented monomorphic allele representation regarding rs12188950.14, 29, 30, 31, 32 Moreover, we found a Chinese meta-analysis on Asian populations that had excluded rs12188950 from five studies regarding PDE4D gene data because of total absence of individuals with heterozygote allele pairs within that SNP.33 A number of published studies examined SNPs in the PDE4D gene without including rs12188950. Uncommented exclusion of a SNP might cause selection bias in meta-analyses.
Multi-centre data considerations
One main issue when assessing multi-centre data is how to cope with a non-homogenous group. When performing a heterogeneity test of the three subpopulations (LSR, MDC and SAHLSIS) using the algorithm applied in the meta-analysis, we obtained a non-significant P-value of 0.6735. However, although the subpopulations were heterogeneous because of age distribution, we fitted weighted LR models using age- and sex-standardizing stratum weights. We thereby obtained OR estimates that were more similar than those obtained when not using such stratum weights (Table 3). Another source of heterogeneity between LSR, MDC and SAHLSIS is the registration of vascular risk factors (hypertension, diabetes and current smoking). Data for these phenotypes was collected at stroke onset for LSR and SAHLSIS, but at the baseline time of screening for MDC. The MDC data collection regarding these risk factors was therefore performed at an estimated mean time of about 5.5 years before stroke onset.
Conclusion
Our assessment in a large study including 2599 patients and 2093 control subjects did not show any association between rs12188950 and IS. A meta-analysis including 10 500 patients and 10 102 control subjects provided the same result. No association was detected in any of the subgroups. However, a hypothetical protecting effect (although it is non-significant with a P-value of 0.057 in this study) of the rs12188950 minor allele on IS among younger individuals might be subject for further studies.
Acknowledgments
We thank all the subjects as well as the attendant nursing staff, technical advisers and other co-workers involved for their participation in this study. This study was supported by grants from the Swedish Research Council (K2008-65X-14605-06-03, K2011-65X-14605-09-6, K2010-61X-20378-04-3), the Swedish State (ALFGBG-148861), the Swedish Heart and Lung Foundation (20100256), the Yngve Land Foundation for Neurological Research, the Crafoord Foundation, Region Skåne, the Freemasons Lodge of Instruction EOS in Lund, King Gustav V and Queen Victoria's Foundation, Lund University, the Department of Neurology Lund, the Swedish Stroke Association, the Tore Nilsson Foundation and the Emelle Foundation.
The authors declare no conflict of interest.
References
- Gretarsdottir S, Thorleifsson G, Reynisdottir ST, et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet. 2003;35:131–138. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- Lövkvist H, Smith JG, Luthman H, et al. Ischaemic stroke in hypertensive patients is associated with variations in the PDE4D genome region. Eur J Hum Genet. 2008;16:1117–1125. doi: 10.1038/ejhg.2008.62. [DOI] [PubMed] [Google Scholar]
- Bevan S, Porteous L, Sitzer M, Markus HS. Phosphodiesterase 4D gene, ischemic stroke, and asymptomatic carotid atherosclerosis. Stroke. 2005;36:949–953. doi: 10.1161/01.STR.0000162713.06519.41. [DOI] [PubMed] [Google Scholar]
- Brophy VH, Ro SK, Rhees BK, et al. Association of phosphodiesterase 4D polymorphisms with ischemic stroke in a US population stratified by hypertension status. Stroke. 2006;37:1385–1390. doi: 10.1161/01.STR.0000221788.10723.66. [DOI] [PubMed] [Google Scholar]
- Kostulas K, Gretarsdottir S, Kostulas V, et al. PDE4D and ALOX5AP genetic variants and risk for Ischemic Cerebrovascular Disease in Sweden. J Neurol Sci. 2007;263:113–117. doi: 10.1016/j.jns.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Kuhlenbäumer G, Berger K, Huge A, et al. Evaluation of single nucleotide polymorphisms in the phosphodiesterase 4D gene (PDE4D) and their association with ischaemic stroke in a large German cohort. J Neurol Neurosurg Psychiatry. 2006;77:521–524. doi: 10.1136/jnnp.2005.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lõhmussaar E, Gschwendtner A, Mueller JC, et al. ALOX5AP gene and the PDE4D gene in a central European population of stroke patients. Stroke. 2005;36:731–736. doi: 10.1161/01.STR.0000157587.59821.87. [DOI] [PubMed] [Google Scholar]
- Meschia JF, Brott TG, Brown RD, et al. Phosphodiesterase 4D and 5-lipoxygenase activating protein in ischemic stroke. Ann Neurol. 2005;58:351–361. doi: 10.1002/ana.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson-Ardnor S, Wiklund PG, Lindgren P, et al. Linkage of ischemic stroke to the PDE4D region on 5q in a Swedish population. Stroke. 2005;36:1666–1671. doi: 10.1161/01.STR.0000174188.04716.8d. [DOI] [PubMed] [Google Scholar]
- Song Q, Cole JW, O'Connell JR, et al. Phosphodiesterase 4D polymorphisms and the risk of cerebral infarction in a biracial population: the Stroke Prevention in Young Women Study. Hum Mol Genet. 2006;15:2468–2478. doi: 10.1093/hmg/ddl169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton JM, Sayer MS, Hankey GJ, et al. Association between phosphodiesterase 4D gene and ischaemic stroke. J Neurol Neurosurg Psychiatry. 2006;77:1067–1069. doi: 10.1136/jnnp.2006.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn MJE, Slooter AJC, Schut AFC, et al. Familial aggregation, the PDE4D gene, and ischemic stroke in a genetically isolated population. Neurology. 2005;65:1203–1209. doi: 10.1212/01.wnl.0000178744.42953.b7. [DOI] [PubMed] [Google Scholar]
- Fidani L, Clarimon J, Goulas A, et al. Association of phosphodiesterase 4D gene G0 haplotype and ischaemic stroke in a Greek population. Eur J Neurol. 2007;14:745–749. doi: 10.1111/j.1468-1331.2007.01767.x. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Asai S, Sato N, Soma M. Genotype and haplotype association study of the STRK1 region on 5q12 among Japanese – A case-control study. Stroke. 2006;37:69–76. doi: 10.1161/01.STR.0000194961.17292.33. [DOI] [PubMed] [Google Scholar]
- Bevan S, Dichgans M, Gschwendtner A, Kuhlenbaeumer G, Ringelstein EB, Markus HS. Variation in the PDE4D gene and ischemic stroke risk – A systematic review and meta-analysis on 5200 cases and 6600 controls. Stroke. 2008;39:1966–1971. doi: 10.1161/STROKEAHA.107.509992. [DOI] [PubMed] [Google Scholar]
- Dahlberg J, Smith G, Norrving B, et al. Genetic variants in serum and glucocortocoid regulated kinase 1, a regulator of the epithelial sodium channel, are associated with ischaemic stroke. J Hypertens. 2011;29:884–889. doi: 10.1097/HJH.0b013e3283455117. [DOI] [PubMed] [Google Scholar]
- Hallström B, Jönsson AC, Nerbrand C, Petersen B, Norrving B, Lindgren A. Lund Stroke Register: hospitalization pattern and yield of different screening methods for first-ever stroke. Acta Neurol Scand. 2007;115:49–54. doi: 10.1111/j.1600-0404.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- Jood K, Ladenvall C, Rosengren A, Blomstrand C, Jern C. Family history in ischemic stroke before 70 years of age: the Sahlgrenska Academy Study on Ischemic Stroke. Stroke. 2005;36:1383–1387. doi: 10.1161/01.STR.0000169944.46025.09. [DOI] [PubMed] [Google Scholar]
- Olsson S, Jood K, Melander O, et al. Lack of association between genetic variations in the KALRN region and ischemic stroke. Clin Biochem. 2011;44:1018–1020. doi: 10.1016/j.clinbiochem.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Olsson S, Melander O, Jood K, et al. Genetic variant on chromosome 12p13 does not show association to ischemic stroke in 3 Swedish case-control studies. Stroke. 2011;42:214–216. doi: 10.1161/STROKEAHA.110.594010. [DOI] [PubMed] [Google Scholar]
- Smith JG, Melander O, Lövkvist H, et al. Common genetic variants on chromosome 9p21 confers risk of ischemic stroke: a large-scale genetic association study. Circ Cardiovasc Genet. 2009;2:159–164. doi: 10.1161/CIRCGENETICS.108.835173. [DOI] [PubMed] [Google Scholar]
- WHO MONICA Project Principal Investigators The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease). A major international collaboration. J Clinical Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Domingues-Montanari S, Fernandez-Cadenas I, del Rio-Espinola A, et al. Association of a genetic variant in the ALOX5AP with higher risk of ischemic stroke: A Case-Control, Meta-Analysis and Functional Study. Cerebrovasc Dis. 2010;29:528–537. doi: 10.1159/000302738. [DOI] [PubMed] [Google Scholar]
- Quarta G, Stanzione R, Evangelista A, et al. Phosphodiesterase 4D and 5-lipoxygenase activating protein genes and risk of ischemic stroke in Sardinians. Eur J Hum Genet. 2009;17:1448–1453. doi: 10.1038/ejhg.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee RYL, Brophy VH, Cheng S, Hegener HH, Erlich HA, Ridker PM. Polymorphisms of the phosphodiesterase 4D, cAMP-specific (PDE4D) gene and risk of ischemic stroke – a prospective, nested case-control evaluation. Stroke. 2006;37:2012–2017. doi: 10.1161/01.STR.0000230608.56048.38. [DOI] [PubMed] [Google Scholar]
- Yoon D, Park SK, Kang D, Park T, Park JW. Meta-analysis of homogeneous subgroups reveals association between PDE4D gene variants and ischemic stroke. Neuroepidemiology. 2011;36:213–222. doi: 10.1159/000327915. [DOI] [PubMed] [Google Scholar]
- Census, race and science. Nat Genet. 2000;24:97–98. doi: 10.1038/72884. [DOI] [PubMed] [Google Scholar]
- Lin HF, Liao YC, Liou CW, Liu CK, Juo SH. The phosphodiesterase 4D gene for early onset ischemic stroke among normotensive patients. J Thromb Haemost. 2007;5:436–438. doi: 10.1111/j.1538-7836.2007.02350.x. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Kubo M, Yonemoto K, et al. Lack of association between variations of PDE4D and ischemic stroke in the Japanese population. Stroke. 2009;40:1245–1251. doi: 10.1161/STROKEAHA.108.527408. [DOI] [PubMed] [Google Scholar]
- Sun Y, Huang YY, Chen X, et al. Association between the PDE4D gene and ischaemic stroke in the Chinese Han population. Clin Sci(Lond) 2009;117:265–272. doi: 10.1042/CS20080471. [DOI] [PubMed] [Google Scholar]
- Woo D, Kaushal R, Kissela B, et al. Association of phosphodiesterase 4D with ischemic stroke – a population-based case-control study. Stroke. 2006;37:371–376. doi: 10.1161/01.STR.0000198843.72824.0a. [DOI] [PubMed] [Google Scholar]
- Xu XW, Li X, Li JJ, Ou R, Sheng WL. Meta-analysis of association between variation in the PDE4D gene and ischemic cerebral infarction risk in Asian populations. Neurogenetics. 2010;11:327–333. doi: 10.1007/s10048-010-0235-8. [DOI] [PubMed] [Google Scholar]