Abstract
Using a combination of linkage mapping and massively parallel sequencing of the X-chromosome exome, we identified an 18-bp deletion in exon 8 of the oral-facial-digital syndrome type 1 (OFD1) gene in a family with X-linked Joubert syndrome (JBTS10). The deletion results in an in-frame deletion of six amino acids. New features not noted in the two previously reported cases of X-linked Joubert syndrome include the presence of polycystic kidney disease, polymicrogyria and hydrocephalus. Our study further underlines the power of genetic mapping combined with massively parallel sequencing as a powerful tool for novel disease gene and mutation discovery.
Keywords: OFD1, X-linked Joubert, X-linked intellectual disability, massively parallel sequencing
Introduction
The oral-facial-digital syndromes (OFDSs) are a group of nine disorders whose features include midline oral clefts (lip, palate and tongue), nodules (hamartomas) on the tongue, and digital abnormalities, including brachydactyly, syndactyly and polydactyly. Both polycystic kidney disease and central nervous system anomalies including hydrocephalus and cerebellar anomalies are reported in some cases. OFDS type 1 (OFD1) is the X-linked dominant form associated with male lethality and the presence of polycystic kidneys. Mutations in the OFD1 gene (MIM 311200) were first identified in 2001.1
Joubert syndrome is characterized by the presence of cerebellar vermis hypoplasia, hypotonia, ataxia, oculomotor apraxia and irregular respiration with tachypnea followed by apnea in the neonatal period. Abnormalities may be present in other organs including the kidney, liver and eye. A key neuro-imaging finding is the molar tooth sign (MTS) reflecting a deepening of the interpeduncular fossa at the level of the isthmus and upper pons, elongation, thickening and mal-orientation of the superior cerebellar peduncles and cerebellar vermis hypoplasia. The MTS is not absolutely pathognomonic of this disorder and may be seen in other conditions including OFD type VI.2 With advances in our molecular understanding of these conditions, it has become apparent that some of these disorders are allelic.
Coene et al3 reported mutations in the OFD1 gene in a family with X-linked Joubert syndrome (JBTS10) (MIM 300804) and in an isolated male with Joubert syndrome. Clinical features included severe intellectual disability with absent speech and severe motor impairment, MTS on imaging, juvenile retinitis pigmentosa in the familial case and bilateral polydactyly. Females were unaffected. For the first time, the OFDS phenotype and JS phenotype was shown to be allelic. More recently, mutations in TMEM216 (MIM 613277) have been identified in patients with JS and OFD type VI, again illustrating the overlap of these phenotypes.4 We report a new family with X-linked Joubert syndrome in which linkage mapping combined with massively parallel sequencing of the X-chromosome exome revealed an in-frame 18-bp deletion within exon 8 of the OFD1 gene. In addition, we extend the phenotype associated with mutations in OFD1.
Subjects and methods
Two distant male maternal cousins V-3 and V-9 now aged 7 and 11 years, respectively, who were related through six female relatives (Figure 1), were independently identified to have common neuroradiology and developmental features. V-3 was noted on second trimester antenatal morphology scans to have macrocephaly with non-progressive ventriculomegaly. He was delivered at term by a breech normal vaginal delivery with a birth weight of 3520 g (50th centile) and head circumference of 39.5 cm (>97th centile). Motor milestones were delayed, sitting independently at 12 months and walking independently at 5 years of age. He had no focal neurology signs, nystagmus or intention tremor. He had hypermetropia and an intermittent esotropia requiring visual correction. There was a large discrepancy between his receptive and expressive language skills. At 6 years of age, he was non-verbal, but was able to sign, use an assisted communication device and read some age appropriate material.
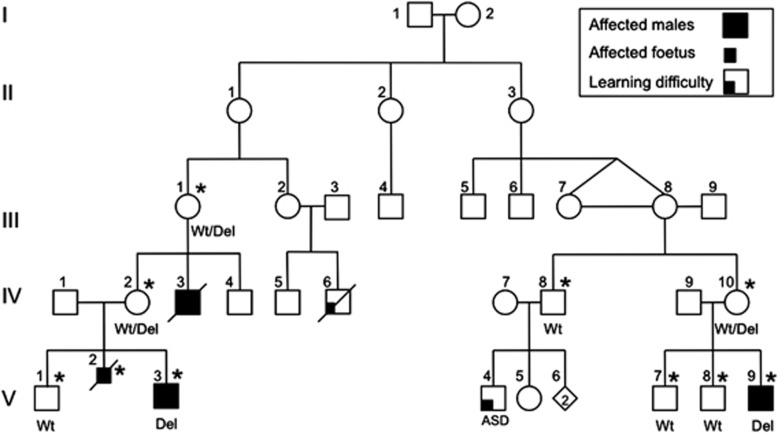
Figure 1.
Family pedigree. ASD, autism spectrum disorder. *Indicates DNA available for linkage analysis. Genotype data are given as mutation and/or wild type.
V-9 was delivered by cesarean section at 39 weeks, with a birth weight of 3485 g (50th centile) and head circumference of 36 cm (75th centile). He was macrocephalic by 4 months, and had asymmetric spasticity in his upper limbs worse on the left side at 6 months. He sat independently at 33 months and walked with ankle-foot orthoses after 5 years. He had single words at 20 months and at 10 years of age spoke in short phrases with significant dysarthria and dyspraxia. Recent developmental assessments showed borderline/low non-verbal cognitive skills. EEG studies persistently showed epileptiform activity with a right centrotemporal focus. He had febrile seizures from 2.5 years, an afebrile focal dyscognitive seizure at 4.5 years followed by infrequent generalized tonic-clonic seizures treated with carbamazepine.
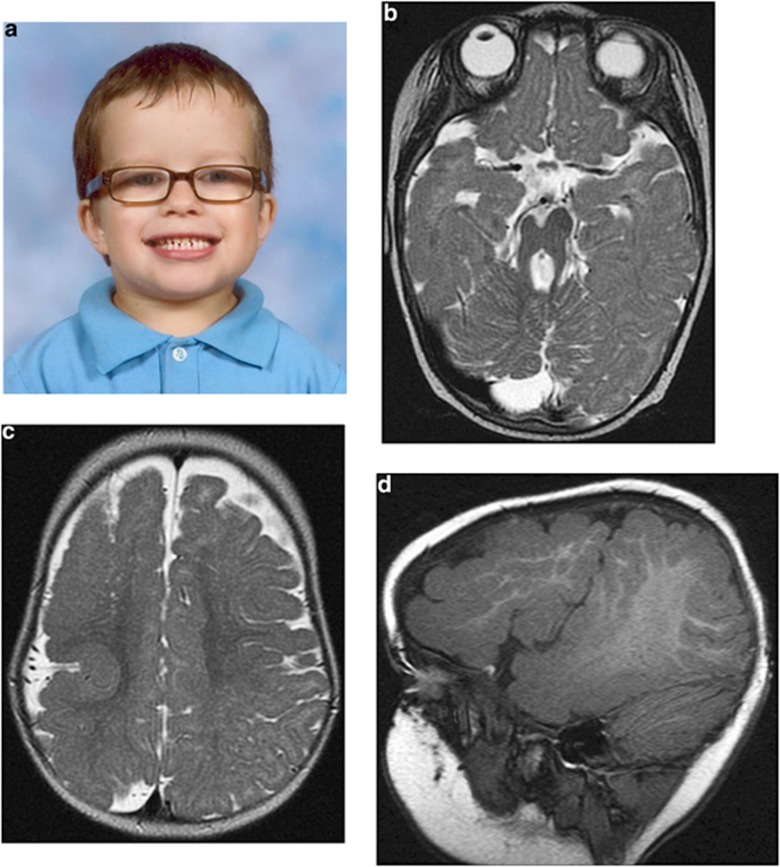
Both boys had macrocephaly (>97th centile) with frontal bossing, V-9 has mild down- sloping palpebral fissures with epicanthic folds and V-3 deep-set eyes with infra-orbital creases (Figure 2a). Neither had digital anomalies or oral abnormalities including dental abnormalities, clefting or frenulae. There was no evidence of retinitis pigmentosa on formal ocular examination in either boy at 5 years. A renal ultrasound in V-3 identified renal cystic disease at 5 years of age. His renal function has subsequently deteriorated, and he is awaiting transplantation. V-9 had increased echogenicity of his kidneys at 6.5 years without obvious cysts or renal impairment. Neither boy had cardiac abnormalities Neuro-imaging showed a number of similarities including horizontal cerebellar peduncles, an enlarged cisterna magna and a MTS (Figure 2b). V-9 also had extensive polymicrogyria of the right frontal and temporal lobes (Figures 2c and d).
Figure 2.
Clinical data. (a) Photograph of V-3 – note prominent forehead and macrocephaly. (b) MRI scan V-9. Axial T2 image showing the MTS with a small right cerebral peduncle (RCP). (c) MRI scan V-9. Axial T2 image showing extensive right hemispheric polymicrogyria with sulcation abnormalities. (d) MRI scan V-9. T1 reformatted sagittal image through the lateral right hemisphere showing extensive polymicrogyria.
The family history included two further affected males whose cerebral abnormalities may have been manifestations of the X-linked Joubert syndrome. A male sibling V-2 was identified on antenatal scan and at postmortem to have significant hydrocephalus at 19 weeks gestation. Antenatal scans suggested a posterior fossa abnormality, but macroscopically this could not be confirmed at postmortem. A maternal uncle IV-3 died after birth of complications relating to hydrocephalus and a cyanotic congenital heart anomaly.
Results
Based on the pedigree structure, sequencing of a number of candidate genes on the X chromosome including OPHN1, L1CAM and AP1S2 was performed, without identification of a causative mutation. When we began our study OFD1 had not been identified as the cause of X-linked Joubert syndrome. Subsequent X chromosome linkage analysis was carried out (ABI Prism (Melbourne, Victoria, Australia) Linkage Mapping set HD) on available samples, including three obligate carriers, two affected and four unaffected males. Maximum two point LOD scores of 2.27 at θ=0 were achieved for DXS987 and DXS7593, with recombinant events detected at DXS7108 and DXS1061, localizing the gene to Xp22.2–21.3.3 (hg18, chrX:10 052 226–27 452 338; linkage data not shown) (Figure 3a).
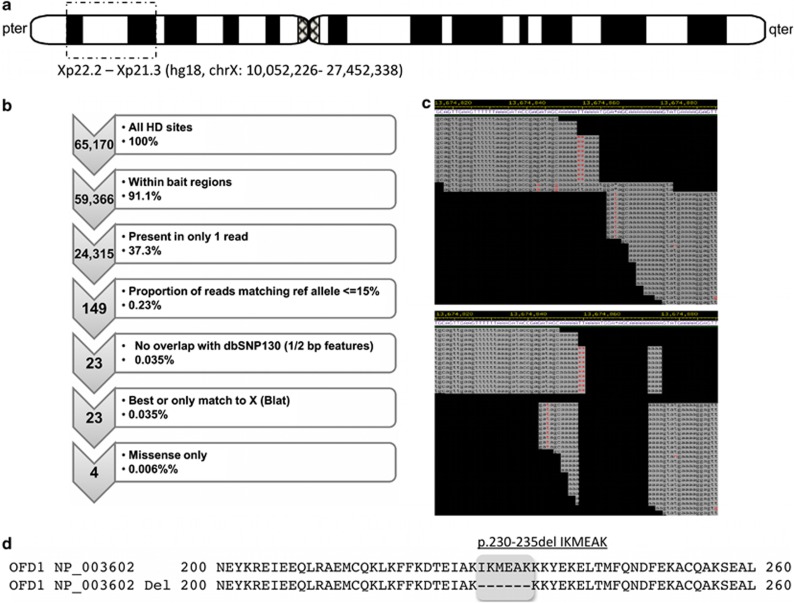
Figure 3.
Identification of the OFD1 gene 18-bp deletion. (a) The disease gene in this family has been mapped to an Xp22.2–Xp21.3 interval using traditional linkage mapping. (b) Following X-chromosome exome capture and massively parallel sequencing the variants (referred to as highly discrepant, or HD sites) were filtered independently of the linkage interval. (c) Relaxing the stringency of filtering lead us to the identification of a region in OFD1, which upon further adjustment revealed an 18-bp deletion within exon 8 of OFD1. (d) This deletion was subsequently predicted to result in an in-frame deletion of six amino acids without the introduction of a missense change.
In the absence of an obvious candidate gene within the linkage interval, massively parallel sequencing of the X-chromosome exome (Agilent, Melbourne, Victoria, Australia) was performed (Illumina GA II; Geneworks, Adelaide, South Australia, Australia) on the proband's (V-3) whole blood isolated DNA (see Supplementary Information). A mean of 84.3-fold coverage was achieved in bait regions (median 78), and 86.9% of bait bases were captured at a coverage depth ≥10. A total of 23 discrepant sites met filtration criteria, all of which demonstrated the only or highest quality alignments to chromosome X in UCSC blat searches. Of these 23 sites, 4 yielded missense sequence changes (Figure 3b). These changes included p.V328I in MAGEB10, p.F101C in SSX1, p.R47W in TIMM17B and p.L15Q in TREX2 (Supplementary Table 1). None of these changes localized either within or in the proximity of our family's linkage interval. As such the stringency of the filtration criteria was relaxed to include sequence discrepancies present at lower frequencies (≥30% reads). This approach generated a total of 135 sites for investigation, each of which was examined manually in Consed. Among these 135 sites, 1 was found to be consistent with an 18-bp deletion in the eighth exon of the OFD1 gene (Figures 3c and d). This previously unreported deletion results in a six amino-acid deletion without a frameshift. The deletion was confirmed by Sanger sequencing (Supplementary Figure 1) and segregated completely with the phenotype (Figure 1).
Discussion
The phenotypic spectrum associated with OFD1 mutations has been recently extended. The spectrum includes a syndrome including macrocephaly, severe mental retardation, ciliary dyskinesia leading to recurrent respiratory infections and in some cases polydactyly5 (also referred to as Simpson–Golabi–Behmel type 2), an X-linked Joubert phenotype and the classical OFD1 pattern with male lethality.3 This case confirms the reported association of specific OFD1 mutations with X-linked Joubert syndrome. Unlike previous reported cases of males with an OFD1 mutation, our patients show relatively well-preserved non-verbal cognitive abilities. This does not seem uncommon in patients with Joubert syndrome, with their receptive language skills often being advanced and gross motor skills often being the weakest domain.6
The extended anomalies seen in this family include polycystic kidney disease, hydrocephalus, polymicrogyria and possibly congenital heart disease. Hydrocephalus is reported in a minority of females with OFD1, as is cerebellar hypoplasia and Dandy Walker malformation,7, 8 but the spectrum of anomalies can extend to cerebral dysgenesis with evidence of a severe neuronal migration abnormality in one female fetus.9 A recent review suggests that the presence of polycystic kidneys is positively associated with structural neurological abnormalities in OFD1 patients.7 Functionally, in both the mouse and zebrafish more complete loss of OFD1 function has been shown to be associated with abnormal ciliary function, with an extreme manifestation being hydrocephalus and laterality defects including cardiac anomalies.10, 11 The congenital heart lesion and hydrocephalus reported in IV-3 is compatible with the animal model phenotype for OFD1 loss of function.
It has been suggested that the phenotype associated with OFD1 mutations is dependent on the location of the mutation and how it impacts on nuclear localization and binding with Lebercilin.3 Until this report, it was thought that mutations proximal to exon 17 would always be associated with male lethality or females with an OFD1 phenotype. The mutations identified in patients with OFD1 are predominantly truncating, with the few reported missense mutations clustering in a LisH motif in exon 3.8, 12 To our knowledge, mutations in exon 8 have all been splice site or frameshift. Our family with a six amino-acid in-frame deletion in exon 8 demonstrates that the relationship between location and phenotype is more complex than initially hypothesized. We suspect this in-frame deletion in a male must reduce OFD1 expression, to a lower level than in a female who is heterozygous for a nonsense mutation, but is not associated with male lethality.
This family is the third family with an X-linked Joubert phenotype to be reported with an OFD1 mutation. The case extends the spectrum associated with OFD1 mutations and illustrates that a proximally placed mutation that is non-truncating will not always result in the classical OFD1 phenotype. The small linkage interval allowed us to rigorously analyze next-generation sequencing data and by softening our search criteria, to identify the 18-bp deletion, which could otherwise be overlooked. With the ongoing advancement of the massively parallel sequencing technologies and analysis tools13 and paired-end sequencing in particular, such deletions will be a lot easier to identify.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Ferrante MI, Giorgio G, Feather SA, et al. Identification of the gene for oral-facial-digital type I syndrome. Am J Hum Genet. 2001;68:569–576. doi: 10.1086/318802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Keeler LC, Parisi MA, et al. Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet A. 2004;125A:125–134. doi: 10.1002/ajmg.a.20437. [DOI] [PubMed] [Google Scholar]
- Coene KL, Roepman R, Doherty D, et al. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am J Hum Genet. 2009;85:465–481. doi: 10.1016/j.ajhg.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Logan CV, Mougou-Zerelli S, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet. 2010;42:619–625. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budny B, Chen W, Omran H, et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum Genet. 2006;120:171–178. doi: 10.1007/s00439-006-0210-5. [DOI] [PubMed] [Google Scholar]
- Hodgkins PR, Harris CM, Shawkat FS, et al. Joubert syndrome: long-term follow-up. Dev Med Child Neurol. 2004;46:694–699. doi: 10.1017/s0012162204001161. [DOI] [PubMed] [Google Scholar]
- Saal S, Faivre L, Aral B, et al. Renal insufficiency, a frequent complication with age in oral-facial-digital syndrome type I. Clin Genet. 2010;77:258–265. doi: 10.1111/j.1399-0004.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- Thauvin-Robinet C, Cossée M, Cormier-Daire V, et al. Clinical, molecular, and genotype-phenotype correlation studies from 25 cases of oral-facial-digital syndrome type 1: a French and Belgian collaborative study. J Med Genet. 2006;43:54–61. doi: 10.1136/jmg.2004.027672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauvin-Robinet C, Lesca G, Aral B, et al. Cerebral dysgenesis does not exclude OFD1 syndrome. Am J Med Genet Part A. 2011;155A:455–457. doi: 10.1002/ajmg.a.33812. [DOI] [PubMed] [Google Scholar]
- Ferrante MI, Zullo A, Barra A, et al. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38:112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- Ferrante MI, Romio L, Castro S, et al. Convergent extension movements and ciliary function are mediated by ofd1, a zebrafish orthologue of the human oral-facial-digital type 1 syndrome gene. Hum Mol Genet. 2009;18:289–303. doi: 10.1093/hmg/ddn356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prattichizzo C, Macca M, Novelli V, et al. Oral-Facial-Digital Type I (OFDI) Collaborative Group. Mutational spectrum of the oral-facial-digital type I syndrome: a study on a large collection of patients. Hum Mutat. 2008;29:1237–1246. doi: 10.1002/humu.20792. [DOI] [PubMed] [Google Scholar]
- Bamshad MJ, Ng SB, Bigham AW, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.