Abstract
The endocannabinoid system (ECS) tightly controls emotional responses to acute aversive stimuli. Repeated stress alters ECS activity but the role played by the ECS in the emotional consequences of repeated stress has not been investigated in detail. This study used social defeat stress, together with pharmacology and genetics to examine the role of cannabinoid type-1 (CB1) receptors on repeated stress-induced emotional alterations. Seven daily social defeat sessions increased water (but not food) intake, sucrose preference, anxiety, cued fear expression, and adrenal weight in C57BL/6N mice. The first and the last social stress sessions triggered immediate brain region-dependent changes in the concentrations of the principal endocannabinoids anandamide and 2-arachidonoylglycerol. Pretreatment before each of the seven stress sessions with the CB1 receptor antagonist rimonabant prolonged freezing responses of stressed mice during cued fear recall tests. Repeated social stress abolished the increased fear expression displayed by constitutive CB1 receptor-deficient mice. The use of mutant mice lacking CB1 receptors from cortical glutamatergic neurons or from GABAergic neurons indicated that it is the absence of the former CB1 receptor population that is responsible for the fear responses in socially stressed CB1 mutant mice. In addition, stress-induced hypolocomotor reactivity was amplified by the absence of CB1 receptors from GABAergic neurons. Mutant mice lacking CB1 receptors from serotonergic neurons displayed a higher anxiety but decreased cued fear expression than their wild-type controls. These mutant mice failed to show social stress-elicited increased sucrose preference. This study shows that (i) release of endocannabinoids during stress exposure impedes stress-elicited amplification of cued fear behavior, (ii) social stress opposes the increased fear expression and delayed between-session extinction because of the absence of CB1 receptors from cortical glutamatergic neurons, and (iii) CB1 receptors on central serotonergic neurons are involved in the sweet consumption response to repeated stress.
Keywords: social stress, CB1 receptor, anxiety, fear, sucrose consumption, hypothalamo-pituitary-adrenal axis
INTRODUCTION
The cannabinoid type-1 (CB1) receptor, which is the predominant endocannabinoid receptor in neurons, is mainly located in the presynaptic compartment where it negatively impacts neurotransmitter release (Alger, 2002; Piomelli, 2003; Chevaleyre et al, 2006; Ohno-Shosaku et al, 2012). This receptor is found throughout the brain, including in regions/nuclei—such as cortical areas, the basal ganglia, and the hypothalamus—involved in the control of emotional reactivity (Herkenham et al, 1990; Glass et al, 1997; Tsou et al, 1998; Katona et al, 1999; Marsicano and Lutz, 1999). Direct evidence for a tonic role of CB1 receptors in the control of emotionality has been gathered by means of pharmacology and genetics. Thus, the use of CB1 receptor antagonists and of CB1 receptor mutant mice has underlined the prominent role of CB1 receptors on locomotor reactivity, anxiety, and fear responses to the acute exposure to aversive environments (Viveros et al, 2005; Wotjak, 2005; Lafenêtre et al, 2007; Lutz, 2009). However, the tight interactions between the endocannabinoid system (ECS) and stress circuits are not limited to the acute exposure to aversive stimuli. Repeated exposure to homotypic or heterotypic stressors affect in a brain region-dependent manner all components of the ECS (Patel and Hillard, 2008; Hill et al, 2010b; Riebe and Wotjak, 2011). This is true for the concentrations of the major endocannabinoids, namely anandamide (AEA) and 2-arachidonoylglycerol (2-AG), the activities of their respective degrading enzymes, and the mRNA and protein expression of CB1 receptors (Hill et al, 2005; Bortolato et al, 2007; Rademacher et al, 2008; Patel et al, 2009; Reich et al, 2009; Hill et al, 2010a; Zoppi et al, 2011). Moreover, repeated homotypic and heterotypic stress affect the agonist-binding properties of CB1 receptors and/or CB1 receptor-mediated control of neurotransmitter release (Hill et al, 2005; Rossi et al, 2008; Patel et al, 2009; Wamsteeker et al, 2010).
These data indicate that repeated stress alters different components of the ECS, including CB1 receptor-mediated control of neurotransmission. However, whether these alterations extend to the control of emotionality exerted by CB1 receptors has been only scarcely addressed. It has been reported that pretreatment with the CB1 receptor antagonist rimonabant (SR141716) amplifies escape behavior during acute/repeated restraint stress (Patel et al, 2005). Nonetheless, how this observation relates to stress coping is not clear as rimonabant pretreatment may facilitate passive behavior during stress (Steiner et al, 2008). Besides its influence on escape behavior, CB1 receptor blockade further increases the anhedonic consequence of repeated restraint stress, as revealed by sucrose preference tests (Rademacher and Hillard, 2007). Lastly, one recent study indicates that the genetic deletion of CB1 receptors amplifies the anxiogenic consequences of repeated restraint stress, as assessed in the elevated plus-maze (Hill et al, 2011). These observations, which suggest that CB1 receptors have a tonic regulatory role on several aspects of emotionality during repeated stress events, raise three main issues. First, as these data were gathered using one single model of stress, ie, restraint stress, it remains to be investigated whether this link between CB1 receptors and emotionality is present under the same modalities in other stress models. Although restraint is a useful model for stress studies, other stress procedures, including social defeat (Buwalda et al, 2005; Miczek et al, 2008; Golden et al, 2011), have been proposed to better model stress-related psychopathologies in humans. Second, it is unknown whether the control exerted by CB1 receptors on stress-induced anhedonia and anxiety extends to other emotional consequences of repeated stress. As an illustration, it is at the present time unknown whether the well-documented inhibitory effects of repeated stress on fear memory extinction (Rau et al, 2005; Akirav and Maroun, 2007), a cognitive process tightly controlled by CB1 receptors (Marsicano et al, 2002; Suzuki et al, 2004; Chhatwal et al, 2005; Kamprath et al, 2006), is accounted for by stress-induced alterations in the ECS. Lastly, none of the above mentioned studies on the interactions between CB1 receptors and emotionality in repeatedly stressed animals addressed the key question of the neuronal populations through which CB1 receptors exert their input. In this context, the use of mutant lines where CB1 receptors are missing from specific neuronal populations (Marsicano et al, 2003; Monory et al, 2006; Jacob et al, 2009; Lafenêtre et al, 2009; Puighermanal et al, 2009; Bellocchio et al, 2010) can help gathering crucial information on the relationships between the central ECS and stress circuitry.
The goal of this study was to further define the relationships between CB1 receptors and the emotional consequences of repeated stress by addressing the three issues raised above. With regard to the first issue, repeated social defeat was chosen here as a stress model in light of (i) its high ethological value (Buwalda et al, 2005; Miczek et al, 2008), (ii) its relevance to the etiology of human mood disorders (Huhman, 2006; Miczek et al, 2008), and (iii) past evidence that in our hands the social defeat model triggers a vast array of emotional, metabolic, and endocrine changes (Dubreucq et al, 2012). We first ensured that social stress triggered changes in the ECS, as assessed by the analysis of brain tissue levels of AEA and 2-AG in mice acutely or repeatedly submitted to a social defeat protocol. Next, we investigated the respective effects of pharmacological CB1 receptor blockade by rimonabant and of the constitutive deletion of CB1 receptors on the consequences of social stress on food and water intake, sucrose preference, unconditioned anxiety, and cued fear memory. In addition, the important role of CB1 receptors in the control of the activity of the hypothalamo-pituitary-adrenal (HPA) axis in both control and stressed individuals (Steiner and Wotjak, 2008; Hill et al, 2010a) led us to measure the weights of the adrenal glands as an index of chronic HPA axis reactivity to repeated stress. In a last series of experiments, we examined whether CB1 receptors located respectively on cortical glutamatergic neurons, on GABAergic neurons, or on serotonergic neurons exert a control on the aforementioned emotional, metabolic, and endocrine responses to social stress. To achieve this aim, we used conditional mutant lines wherein the CB1 receptor gene was selectively deleted from each of these neuronal populations.
MATERIALS AND METHODS
Animals
The experiments were conducted in strict compliance with European directives and French laws on animal experimentation (authorization number 06369). This study involved 2–3-month-old male C57BL/6N mice purchased from Janvier (Le Genest Saint-Isle, France), 3–12-month-old male CD1 mice purchased from Charles Rivers (L'Arbresle, France), and 2–3-month-old constitutive/conditional male CB1 receptor mutant and wild-type animals bred at the NeuroCentre Magendie. All mice were housed individually 1–2 weeks before experiments with food and water ad libitum under a 12-h light/dark cycle (lights on at 07:00 hours). Wild-type and constitutive CB1 receptor mutant mice (referred to in the text as CB1+/+ and CB1−/−, respectively), wild-type and conditional mutant mice lacking CB1 receptors from cortical glutamatergic neurons (referred to in the text as Glu-CB1+/+ and Glu-CB1−/−, respectively), and wild-type and conditional mutant mice lacking CB1 receptors from GABAergic neurons (referred to in the text as GABA-CB1+/+ and GABA-CB1−/−, respectively), were obtained, maintained, and genotyped/regenotyped, as described previously (Marsicano et al, 2002; Marsicano et al, 2003; Monory et al, 2006; Bellocchio et al, 2010). Conditional mutant mice lacking CB1 receptors from central serotonergic neurons (referred to below as TPH2-CB1−/−) and their wild-type controls (referred to below as TPH2-CB1+/+) were generated through a three-step process. The first step was achieved by crossing homozygous CB1-floxed (CB1f/f) mice (Marsicano et al, 2003) with mice bearing a tamoxifen-inducible Cre-ERT2 recombinase expressed under the regulatory sequences of the mouse tryptophan hydroxylase 2 (Tph2) gene locus (Weber et al, 2009). In a second step, heterozygous Cre-expressing/CB1-floxed mice (CB1TPH2−CreERT2;f/+) were again crossed with CB1f/f to obtain homozygous Cre-expressing/CB1-flox mice (CB1TPH2−CreERT2;f/f). Male mice from step 2 were finally bred with CB1f/f females to generate littermate experimental animals (CB1TPH2−CreERT2;f/f and CB1f/f, referred to as TPH2-CB1−/− and TPH2-CB1+/+, respectively). Genotyping (at 2 weeks of age) and regenotyping (at the end of the experiments) of the Cre transgene were performed by PCR using the primers 5′-CCACTGCGGGCTCTACTTC-3′ (forward) and 5′-TGATGATCTTCTGGCACAGCAG-3′ (reverse), whereas genotyping for the CB1-floxed locus was performed as described (Marsicano et al, 2003). Induction of Cre-mediated recombination was performed by injecting i.p. all mice (including TPH2-CB1+/+ mice) daily for 5 days with 10 mg/ml tamoxifen (Sigma-Aldrich, St Quentin Fallavier, France) dissolved in sesame oil and ethanol (10 : 1) (Imai et al, 2000). All animals, injected when 5–10-week-old, were used at least 3 weeks after the end of tamoxifen treatment. Note that PCR on genomic DNA have confirmed that tamoxifen treatment leads to a specific deletion of the CB1 gene in the dorsal raphe nucleus of TPH2-CB1−/− mice (Bellocchio et al, submitted). All lines were in a mixed genetic background, with a predominant C57BL/6N contribution. For each line, the wild-type animals and the constitutive/conditional mutant animals used in this study were littermates. As illustrated above for the generation of the TPH2-CB1 line, mice from the GABA-CB1 and the Glu-CB1 lines were generated from crossings between CB1Cre;f/f males and CB1f/f females to avoid (i) differences in maternal behavior and (ii) potential germline transmission of the gene deletion in the GABA-CB1 line (Massa et al, 2010).
Social Stress Protocol
Except for the experiments aimed at comparing the effects of acute and repeated social defeats on brain endocannabinoid levels or on cued fear memory one day after conditioning (see below), all experiments involved a daily stress protocol, which began at 16:00 hours, and that was repeated over 7 consecutive days. This protocol consisted of the following three different periods (Dubreucq et al, 2012): (i) placement of the experimental mouse in a wire mesh cylinder inside the home cage of a resident CD1 mouse for 30 min (sensory contacts between mice), (ii) removal of the wire mesh cylinder for 15 min (sensory and physical contacts), a period during which the latency for the first attack and the number of attacks by the resident were scored (note that all mice tested displayed an upright posture, indicating defeat), and (iii) reiteration of (i) for another 30 min, after which the experimental mouse was returned to its home cage (Figure 1a). As already documented, each daily confrontation involved an experimental mouse and a resident mouse that were unknown to each other (Dubreucq et al, 2012). During confrontations, water bottles were removed from all cages, including those housing the unstressed animals. All CD1 residents used in this study were selected 2 weeks after their arrival for their ability to attack an intruder within 20–30 s. These mice were then kept and used for social stress protocols up to 1 year of age.
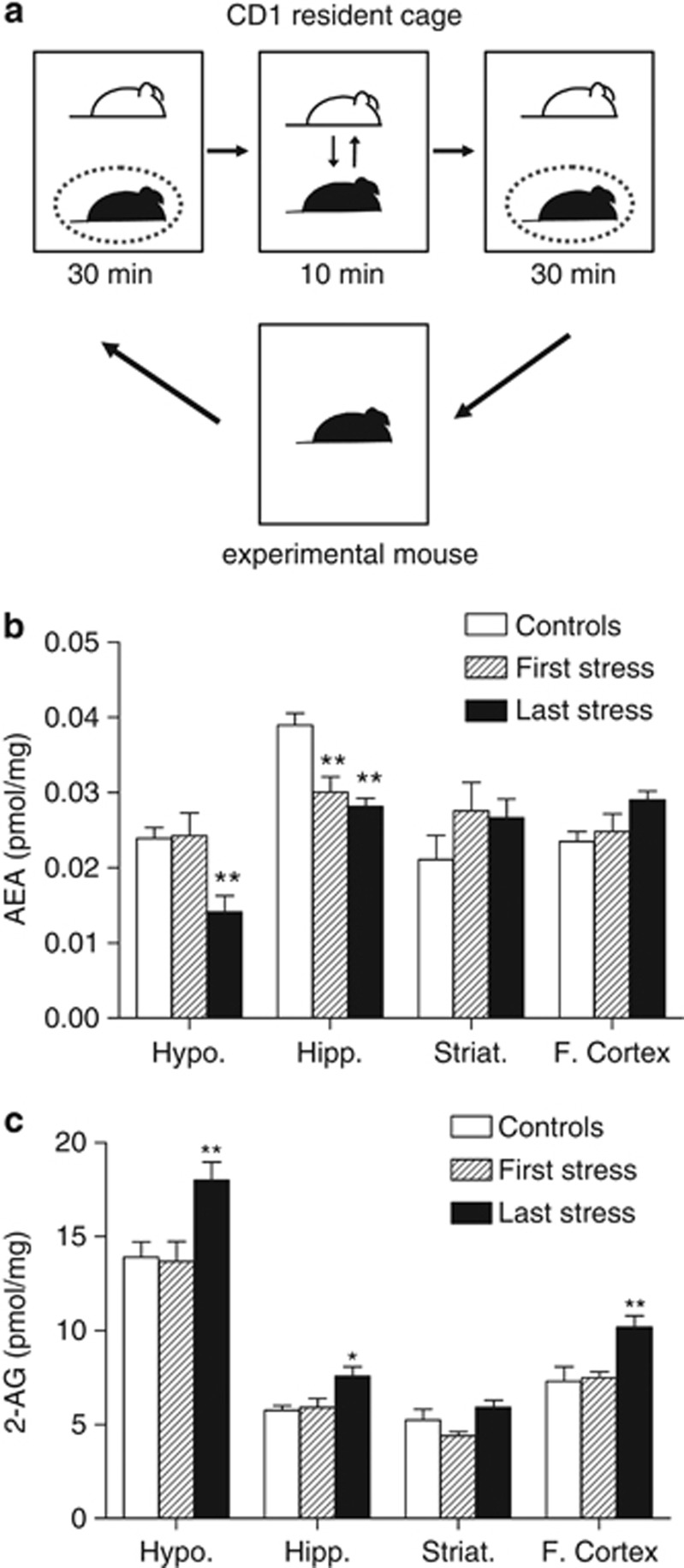
Figure 1.
Social stress protocol and effects of acute/repeated social stress on central endocannabinoid concentrations. Each experimental mouse was placed for 70 min in the home cage of a CD1 resident, with one direct physical confrontation following and preceding phases of sensory contacts (a). Respective effects of the first and the last of seven social stress sessions on hypothalamic (Hypo.), hippocampal (Hipp.), striatal (Striat.), and frontocortical (F. Cortex) anandamide (AEA) (b) and 2-arachidonoylglycerol (2-AG) (c) concentrations in C57Bl/6N mice. Values are the mean±SEM of 5–8 animals. *p<0.05 and **p<0.01 for the effects of acute/repeated stress.
Endocannabinoid Analyses
In one single series of experiments, control mice were killed by cervical dislocation at the same time as mice that had been exposed for the first time or for the seventh time to social stress. Note that stressed mice were killed immediately after the second step of the stress procedure detailed above. The hypothalamus, the frontal cortex, the striatum, and the hippocampus were rapidly dissected out on dry ice and stored at −80 °C for the estimation of AEA and 2-AG concentrations. The extraction, purification, and quantification of AEA and 2-AG from brain areas were performed as previously described (Lafourcade et al, 2011; Lourenço et al, 2011). First, brain areas were homogenized and extracted with chloroform/methanol/Tris-HCl 50 mM pH 7.5 (2 : 1 : 1, v/v) containing internal deuterated standards. The dried lipid extract was pre-purified if necessary by open bed chromatography on silica gel mini-columns, eluted with increasing concentrations of methanol in chloroform. Samples were then subjected to isotope-dilution liquid chromatography-chemical ionization-tandem mass spectrometric analysis (LC-MS/MS). Mass spectral analyses were performed on a TSQ Quantum triple quadrupole instrument (Thermo-Finnigan, San Jose, CA, USA) equipped with an APCI source (atmospheric pressure chemical ionization) and operating in positive-ion mode. A sensitive and specific LC-MS/MS method was developed and validated for endocannabinoid quantification. The amounts of AEA and 2-AG were determined using a calibration curve and expressed as pmol/mg tissue.
General Procedure
This study examined the respective influences of (i) CB1 receptor blockade by rimonabant, (ii) CB1 receptor deletion from the whole body, and (iii) CB1 receptor deletion from either cortical glutamatergic neurons, GABAergic neurons, or serotonergic neurons on several emotional, metabolic, and endocrine consequences of repeated social stress. All control and stressed animals were handled daily throughout the course of the experiments. Except for one series of experiments (which included mice from the CB1, the Glu-CB1, and the GABA-CB1 lines), individual food and water intakes were measured on a daily basis. Within each experimental series, several individuals were randomly tested during the night that followed the seventh stress session for their sucrose intakes in a free choice paradigm (note that these animals underwent a preliminary 1-week habituation period to water and sucrose before stress; see below). On the morning that followed the seventh stress session, mice were first exposed to an elevated plus-maze test. In the afternoon of that same day, mice were then cued fear-conditioned before being tested in fear recall sessions on each of the three following afternoons (ie, 24–72 h after conditioning). One day after the last of these recall sessions, several mice, taken at random, were killed by cervical dislocation and their adrenals dissected out. In one series of experiments aimed at measuring the respective effects of acute and repeated stress on cued fear memory, several mice were exposed for the first time to social defeat when repeatedly stressed mice underwent their seventh stress session. All mice, including controls, were cued fear-conditioned 1 day after stress and tested in a recall session another day later. Investigations were all conducted without any knowledge of genotypes and/or treatments until final analyses.
Rimonabant Administration
Control and stressed C57Bl6/N were daily injected (30 min before each of the seven stress sessions) with the CB1 receptor antagonist rimonabant (3 mg/kg; Sigma-Aldrich) or its vehicle (one drop of Tween 80 in 3 ml of 1.25% dimethylsulphoxide and 0.9% NaCl). Control (ie, unstressed) animals were injected at the same time. All animals were injected on the basis of their individual body weights.
Food and Water Intakes
The individual amounts of food and water consumed were measured each morning, beginning 1 day before the first stress session. With respect to water consumption during the night that followed the last stress, the difference between the respective intakes of animals left with water alone and those of animals exposed to a water/sucrose choice (see below) were low, compared with the total amounts measured during the six preceding days in both animal groups. Accordingly, water intakes of the mice that underwent this choice test were taken into account into the total amount of water consumed through the 7-day protocol. Note that food amounts were not corrected for spillage and that water leaks were avoided by providing 50-ml plastic bottles connected to sippers bearing ball-shaped stoppers (Habitrail, Hagen, France).
Elevated Plus-Maze
The apparatus, made of black Perspex, consisted of four elevated arms (height: 66 cm) 45-cm long and 10-cm wide (Letica, Barcelona, Spain). The arms were arranged in a cross-like disposition, with two opposite arms being enclosed by 50 cm high walls made of grey Perspex, and the two other arms being open. The four arms were connected by a squared central platform (10 × 10 cm). Both the central platform and the open arms were under bright illumination (100–120 lux) whereas the closed arms were under weak illumination (30 lux). Each mouse was placed on the central platform, facing an open arm. The number of visits to, and the time spent on, the open arms and the closed arms were recorded for 5 min, using a videocamera placed above the apparatus, as described previously (Dubreucq et al, 2012). Note that in several instances, mice (three CB1+/+, two CB1−/−, two GABA-CB1+/+, and two GABA-CB1−/−) had to be excluded from the analysis because of peculiar behaviors (full immobility on the central platform or absence of closed arm visits) or falls from open arms.
Cued Fear-Conditioning and Recall
A conditioning box, made of grey Perspex (length: 26 cm, width: 18 cm,and height: 25 cm) with a metal grid floor, was located in a sound-proof chamber (length: 55 cm, width: 60 cm,and height: 50 cm; Imetronic, Pessac, France) in a room adjacent to the housing room. On the conditioning day, each mouse was placed in the conditioning box and left free to explore for 3 min. A sound (1.5 kHz, 60 dB) was then emitted for 20 s, with the last second of tone emission being coupled to one single footshock (0.5 mA). The animal was left in the fear-conditioning box for another minute without any stimulus before being removed from the apparatus, and housed back in its home cage. On the 3 consecutive days (recall tests), the top of each home cage was removed to be covered by a grid, allowing full observation of the mouse in its cage. The home cage was then placed into the sound-proof chamber. After a 3-min pre-tone period, the tone used for conditioning was presented again for a 3-min period. The mouse was then left for another minute in the chamber before removal of the home cage, which was returned back to the housing facility room. The presence of freezing (ie, lack of movements excepted those associated with breathing) was monitored every 20 s during the 3-min exposure to sound on each of the three recall tests, as previously reported (Dubreucq et al, 2010). Freezing behavior was scored by means of a customized EVENTLOG program.
Sucrose Preference
Two 50-ml bottles (see above) filled respectively with water and 2% sucrose were provided throughout the 6 days that preceded the stress protocol to estimate basal sucrose preferences. Each day, the positions of the bottles in the cages were switched as to avoid preference. Mice were then given only water, except during the night that followed the seventh social defeat session, where these mice had also access to a 2% sucrose solution. Water and sucrose amounts were monitored on the basis of weight differences (Dubreucq et al, 2012). When needed, preference ratios were calculated for each individual as the amount of sucrose ingested over the sum of the sucrose and water amounts ingested.
Adrenal Weights
As mentioned previously (Dubreucq et al, 2012), the fat surrounding the glands was visualized using an Olympus SZX10 Stereo microscope (Olympus, Bordeaux, France) and removed for subsequent adrenal weight measurements.
Statistics
All analyses were performed with the GB-Stat software (v10; Dynamic Microsystems, Silver Spring, MD, USA). Comparisons were achieved through Student's t-tests when assessing two-group comparisons, and by means of ANOVAs with/without repeated factors for multiple-group comparisons. Post hoc group comparisons, which were performed using Tukey's multiple comparison test, were achieved only if interactions between main variables were found significant. When necessary, data were log-transformed to reach homogeneity of the variances. In all tests, the significance level was preset to p<0.05.
RESULTS
Acute and Repeated Social Stress Target Central Endocannabinoids
The first and/or the seventh stress sessions decreased AEA levels in the hypothalamus (F(2,15)=6.99; p=0.0071) and hippocampus (F(2,18)=12.57; p=0.0004), but not in the striatum or the frontal cortex (Figure 1b). On the other hand, the last, but not the first, session of social stress increased hypothalamic (F(2,18)=6.62; p=0.007), hippocampal (F(2,17)=3.67; p=0.047), and frontocortical (F(2,18)=5.83; p=0.011) 2-AG levels (Figure 1c).
Pretreatment With a CB1 Receptor Antagonist Prolongs the Stimulatory Effect of Repeated Social Stress on Cued Fear Memory
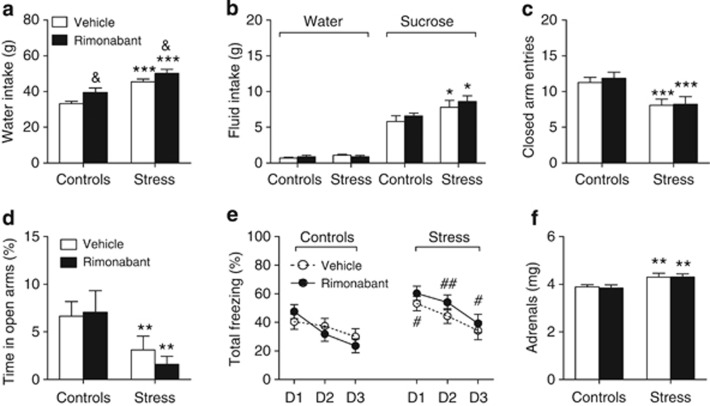
The number of daily attacks during the stress sessions was similar in vehicle- and in rimonabant-pretreated stressed mice (18.20±0.60 and 17.98±0.41, respectively; n=14 in each group). Both social stress (F(1,36)=32.26; p<0.0001) and rimonabant pretreatment (F(1,36)=7.47; p=0.0097) increased water consumption (Figure 2a), but not food intake (data not shown), throughout the 7-day stress protocol. When offered water and sucrose as drinking solutions after the last stress session, the consumption of sucrose, but not that of water, was found to be increased by stress in both vehicle- and rimonabant-pretreated mice (F(1,15)=6.29; p=0.0241; Figure 2b). In the elevated plus-maze, social stress bore hypolocomotor influences, as revealed by the analysis of the number of closed arm entries (F(1,51)=13.92; p=0.0005; Figure 2c). Such an inhibitory influence of stress extended to the percent time spent in the open arms (F(1,51)=7.55; p=0.0084; Figure 2d) while only a trend for an inhibitory effect of stress on the percent number of open arm visits was observed (14.18±2.52% and 12±3.07% in 13 vehicle- and 14 rimonabant-pretreated control mice, respectively, as opposed to 9.13±3.37% and 6.53±2.38% in 14 stressed vehicle-pretreated mice and 14 stressed rimonabant-pretreated mice, respectively). Freezing behavior during cued fear recall sessions (ie, 24–72 h after fear conditioning) was dependent on the recall day (F(2,102)=47.56; p<0.0001); however, whatever the recall day, social stress increased freezing behavior in all mice (F(1,51)=7.47; p=0.0086) (Figure 2e). Moreover, the extent to which the recall session and stress affected freezing behavior was influenced by rimonabant pretreatment (F(2,102)=3.24; p=0.043 for the stress × recall session × pretreatment interaction). Thus, social stress amplified freezing behavior during the first session in vehicle-pretreated mice while leaving unaffected that measured on the following recall sessions (Figure 2e). In contrast, rimonabant pretreatment extended the stimulatory effect of stress on freezing to the last two recall sessions (Figure 2e). These results were confirmed when within-session scores were analyzed; freezing scores were still accounted for by the interaction between stress and the recall session in vehicle-pretreated animals (F(2,50)=3.45; p=0.0395; Supplementary Figure S1a) whereas stress per se amplified freezing scores in rimonabant-pretreated mice (F(1,26)=6.47; p=0.0172; Supplementary Figure S1b). On the other hand, stress did not affect within-session extinction in vehicle- and rimonabant-pretreated mice (Supplementary Figures S1a and b) but increased the initial freezing responses to the cue whatever the mouse group or the recall session considered (F(1,51)=3.45; p=0.0395; Supplementary Figure S1c). Lastly, repeated social stress increased adrenal weight in both vehicle- and rimonabant-preteated mice (F(1,48)=11.61; p=0.0013; Figure 2f).
Figure 2.
Effects of rimonabant pretreatment before each of the seven social stress sessions on the emotional profiles of control and stressed mice. This profile included 7-day water intakes (a), water and sucrose intakes in a free choice paradigm after the seventh stress session (b), closed arm entries (c) and percent time spent in the open arms (d) of an elevated plus-maze, freezing behavior during cued fear recall sessions 24–72 h after conditioning (e), and adrenal weight (f). D1–D3 stand for days 1–3. Values are the mean±SEM of 10–14 animals, except for (b), which refer to 4–5 animals per group. *p<0.05, **p<0.01, and ***p<0.001 for the overall effect of social stress in the ANOVA; &p<0.05 for the overall influence of the pretreatment in the ANOVA; #p<0.05 and ##p<0.01 for the post hoc effects of stress. See text for ANOVAs and additional statistics.
CB1 Receptors are Involved in the Potentiating Effect of Repeated Social Stress on Conditioned Freezing
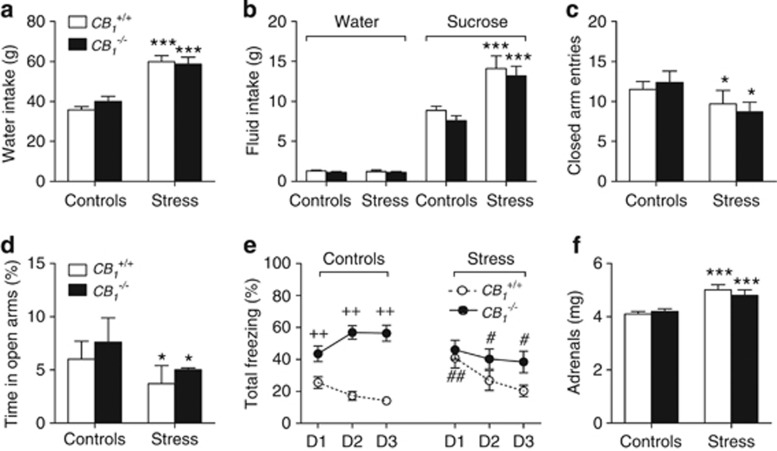
Mice from the CB1+/+ and the CB1−/− groups received an equivalent number of daily attacks throughout the stress protocol (20.02±1.01 and 20.27±1.00, respectively; n=13 in each group). Repeated stress increased water intake in a genotype-independent manner (F(1,43)=60.2; p<0.0005; Figure 3a), this change occurring without any influence of stress and/or genotype on food intake (data not shown). In the sucrose preference test, the genotype bore no effect on the stimulatory impact of repeated stress on sucrose consumption (F(1,28)=27.61; p<0.0001; Figure 3b). Repeated stress, but not the genotype, was endowed with a weak, albeit significant, inhibitory impact on elevated plus-maze behaviors, whether the number of closed arm entries (F(1,47)=4.16; p=0.0469; Figure 3c), the percent time spent on open arms (F(1,47)=4.51; p=0.039; Figure 3d), or the percent number of open arm visits (12.96±2.83% and 11.99±3.27% in 14 control wild-type and 15 mutant mice, respectively, as opposed to 8.21±2.29% and 4.02±2.16% in 11 stressed wild-type and 11 stressed mutant mice, respectively; F(1,47)=5.03; p=0.030) were considered. On the other hand, the genotype, alone (F(1,53)=31.12; p<0.0001) and in combination with either stress (F(1,53)=7.95; p=0.0068) or the recall session (F(2,106)=16.51; p<0.0001) influenced freezing behavior during cued fear memory recall sessions (Figure 3e). Thus, control CB1−/− mice displayed increased freezing behavior, compared with their CB1+/+ littermates, a difference which increased with the number of recall sessions (Figure 3e). Whereas repeated stress amplified the freezing response to the tone in CB1+/+ mice, it weakened that of CB1−/− mice, especially during the last two recall sessions (Figure 3e). Within-session patterns of freezing confirmed that repeated stress stimulated this behavior in CB1+/+ mice (F(1,27)=5.98; p=0.02; Supplementary Figure S1d) whereas the influence of stress depended on the recall day in CB1−/− mice (F(2,52)=7.11; p=0.0019); thus, a decreased freezing response was observed in stressed CB1−/− mice during the last two recall sessions (Supplementary Figure S1e). Initial freezing responses to the tone were dictated by the genotype (F(1,53)=22.43; p<0.001) and by the respective interactions between stress and either the genotype or the recall day (F(1,53)=5.71; p=0.0205 and F(2,106)=7.42; p=0.001; Supplementary Figure S1f). At last, prior repeated stress increased adrenal weight in both genotypes (F(1,40)=19.1; p<0.0001; Figure 3f).
Figure 3.
Influence of the constitutive mutation of cannabinoid type-1 (CB1) receptors on the emotional profiles of control and stressed mice. This profile included 7-day water intakes (a), water and sucrose intakes in a free choice paradigm after the seventh stress session (b), closed arm entries (c) and percent time spent in the open arms (d) of an elevated plus-maze, freezing behavior during cued fear recall sessions 24–72 h after conditioning (e), and adrenal weight (f). D1–D3 stand for days 1–3. Values are the mean±SEM of 11–16 animals, except for (b), which refer to 7–10 animals per group. *p<0.05 and ***p<0.001 for the overall effect of social stress in the ANOVA; #p<0.05 and ##p<0.01 for stress influences within each genotype; ++p<0.01 for the genotype influence in unstressed animals. See text for ANOVAs and additional statistics.
CB1 Receptors on Cortical Glutamatergic Neurons are Involved in the Potentiating Effect of Repeated Social Stress on Conditioned Freezing
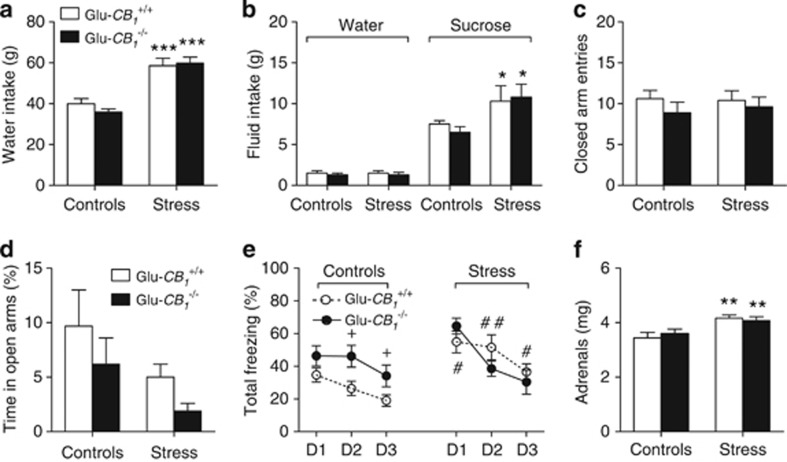
Glu-CB1+/+ and Glu-CB1−/− mice did not differ either in the number of attacks received (21.83±0.53 and 20.50±0.86, respectively; n=12 in each group) or in the amplitude of the dipsogenic effect of repeated stress (F(1,39)=51.69; p<0.0001; Figure 4a). As opposed to water intakes, food intakes proved insensitive to the stress procedure (data not shown). When tested after the last stress session in a sucrose/water choice paradigm, the two genotypes responded in an identical manner to the stimulatory influence of stress on sucrose ingestion (F(1,14)=6.91; p=0.0198; Figure 4b). In the elevated plus-maze, the mouse genotype and/or the stress procedure affected neither the closed arm entries (Figure 4c) nor the percent time spent in the open arms of the elevated plus-maze (albeit trends for negative effects of stress and of the mutation could be noted; see Figure 4d). Conversely, Glu-CB1−/− mice displayed a lower percent number of visits on open arms (11.25±3.41 and 7.18±3.18 in 13 control and 12 stressed animals, respectively) than Glu-CB1+/+ littermates (20.12±4.30 and 14.21±2.73 in 12 control and 11 stressed animals, respectively; F(1,44)=4.71; p=0.036). Stress, alone (F(1,44)=6.47; p=0.0145) or in interaction with either the genotype (F(1,44)=4.17; p=0.047), the recall session (F(2,88)=3.88; p=0.0241) or both (F(2,88)=5.29; p=0.0067), affected freezing behavior during recall (Figure 4e). Indeed, mutant animals displayed increased freezing behavior, compared with their wild-type controls, but that difference vanished with stress because of an increase in freezing in wild-type animals, but not in mutant animals (Figure 4e). Within-session analyses confirmed these results as stress-increased freezing throughout all recall sessions in Glu-CB1+/+ mice (F(1,23)=10.21; p=0.004; Supplementary Figure S2a) whereas it increased freezing behavior only during the first recall session in Glu-CB1−/− mice (F(2,42)=7.95; p=0.0012 for the stress × recall session interaction; Supplementary Figure S2b). Initial freezing responses to the cue were affected by both social stress (F(1,44)=4.08; p=0.0496) and the interaction between stress, genotype, and recall session (F(2,88)=3.56; p=0.0325; Supplementary Figure S2c). Adrenal weight analyses indicated that repeated stress increased that variable in the two genotypes (F(1,37)=13.60; p=0.0007 for the effect of social stress; Figure 4f).
Figure 4.
Influence of the conditional mutation of cannabinoid type-1 (CB1) receptors from cortical glutamatergic neurons on the emotional profiles of control and stressed mice. This profile included 7-day water intakes (a), water and sucrose intakes in a free choice paradigm after the seventh stress session (b), closed arm entries (c) and percent time spent in the open arms (d) of an elevated plus-maze, freezing behavior during cued fear recall sessions 24–72 h after conditioning (e), and adrenal weight (f). D1–D3 stand for days 1–3. Values are the mean±SEM of 9–14 animals, except for (b) that refers to 4–5 animals per group. *p<0.05, **p<0.01, and ***p<0.001 for the overall effect of social stress in the ANOVA; #p<0.05 and ##p<0.01 for stress influences within each genotype; +p<0.05 for the genotype influence in unstressed animals. See text for ANOVAs and additional statistics.
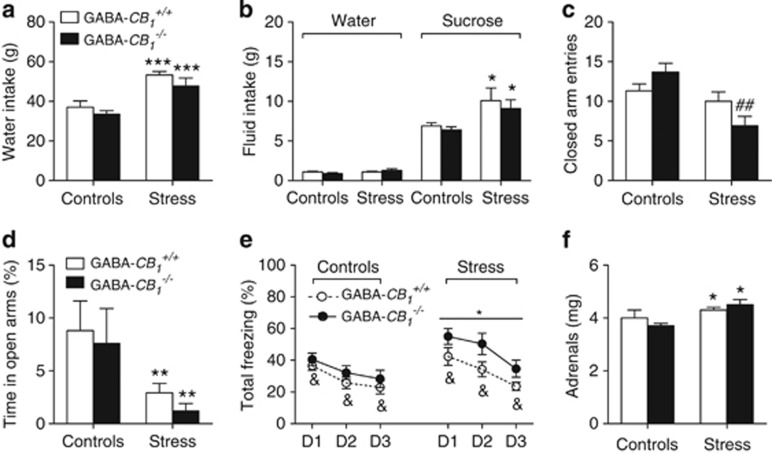
CB1 Receptors in GABAergic Neurons Control the Amplitude of Repeated Social Stress-Induced Decreases in Locomotor Reactivity
GABA-CB1+/+ and GABA-CB1−/− mice did not differ with respect to the number of social attacks (20.05±0.86 and 19.90±0.51, respectively; n=13–14 in each group). The consumption of water throughout the 7-day protocol, but not that of food (data not shown), was increased by social stress in a genotype-independent manner (F(1,39)=25.79; p<0.0001; Figure 5a). Social stress selectively increased sweet consumption in the sucrose test (F(1,23)=7.84; p=0.0102; Figure 5b), and did so in a genotype-independent manner. Locomotor reactivity, as measured by the number of closed arm entries in the elevated plus-maze, was reduced in stressed animals (F(1,46)=13.47; p=0.0006), particularly in GABA-CB1−/− mice (F(1,46)=6.13; p=0.017 for the stress × genotype interaction; Figure 5c). On the other hand, social stress decreased in both genotypes the percent time spent on the open arms (F(1,46)=8.9; p=0.0046; Figure 5d) and the percent number of open arm visits (14.23±2.96% and 10.65±3.39% in 12 control wild-type and 11 mutant mice, respectively, as opposed to 7.83±2.05% and 4.80±2.46% in 13 stressed wild-type and 14 stressed mutant mice, respectively; F(1,46)=4.85; p=0.032 for the influence of stress). Fear recall experiments revealed that either the deletion of CB1 receptors from GABAergic neurons (F(1,50)=5.75; p=0.0203) or prior exposure to social stress (F(1,50)=5.41; p=0.0241) increased freezing responses to the presentation of the cue (Figure 5e). Within-session analyses of freezing scores within each genotype indicated that the stimulatory influence of social stress on freezing behavior was significant in GABA-CB1−/− mice (F(1,25)=4.34; p=0.0476; Supplementary Figure S2e), but not in GABA-CB1+/+ mice (Supplementary Figure S2d). Interestingly, the impact of social stress on freezing in GABA-CB1−/− mice was dependent on the intra-session period of analysis (F(2,50)=4.23; p=0.0201), suggesting that stress delayed within-session extinction of conditioned fear (Supplementary Figure S2d). Social stress, either alone or in association with the genotype, did not affect the initial freezing responses to the cue (Supplementary Figure S2f) but it had a major impact on total freezing behavior in GABA-CB1−/− mice (Figure 5e; Supplementary Figure S2e). Stress increased adrenal weights to similar extents in both genotypes (F(1,33)=2.39; p=0.0111), although a non-significant trend toward a more pronounced effect in GABA-CB1−/− mice, compared with GABA-CB1+/+ mice, was apparent (Figure 5f).
Figure 5.
Influence of the conditional mutation of cannabinoid type-1 (CB1) receptors from GABAergic neurons on the emotional profiles of control and stressed mice. This profile included 7-day water intakes (a), water and sucrose intakes in a free choice paradigm after the seventh stress session (b), closed arm entries (c) and percent time spent in the open arms (d) of an elevated plus-maze, freezing behavior during cued fear recall sessions 24–72 h after conditioning (e), and adrenal weight (f). D1–D3 stand for days 1–3. Values are the mean±SEM of 9–14 animals, except for (b) that refers to 6–7 animals per group. *p<0.05, **p<0.01, and ***p<0.001 for the overall effect of social stress in the ANOVA; &p<0.05 for the overall influence of the genotype in the ANOVA; ##p<0.01 for stress influences within each genotype. See text for ANOVAs and additional statistics.
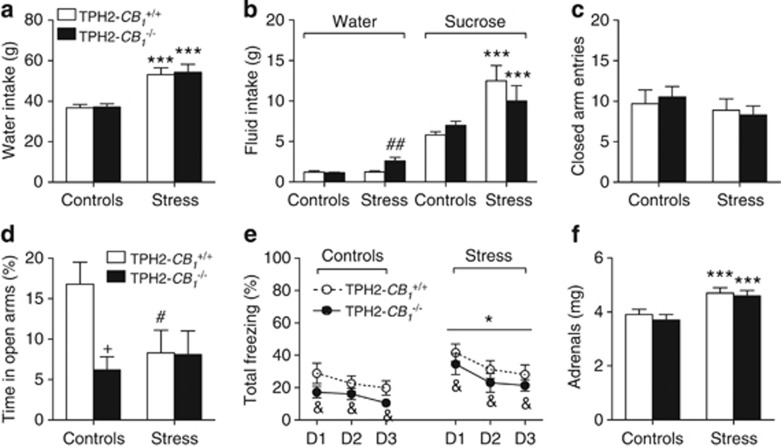
CB1 Receptors on Serotonergic Neurons Mediate the Stimulatory Effects of Repeated Social Stress on Sucrose Preference
The number of social attacks was similar in TPH2-CB1+/+ (21.87±1.19, n=10) and TPH2-CB1−/− (21±1.40, n=11) mice, as was the dipsogenic consequence of stress (F(1,33)=36.31; p<0.0001; Figure 6a). The increased water consumption in stressed mice was not associated with changes in food intake over the 7-day period of analysis (data not shown). In the sucrose preference test, social stress stimulated sucrose intake (F(1,32)=14.00; p=0.0007), as compared with water intake (Figure 6b). Albeit weaker than its impact on sucrose intake, stress also increased water intake in TPH2-CB1−/− mice (F(1,32)=10.61; p=0.0027 for the stress × genotype interaction; Figure 6b). Taken with this last observation, the trend for a decreased sucrose intake in stressed TPH2-CB1−/− mice, compared with stressed TPH2-CB1+/+ (Figure 6b), led us to analyze the respective preference ratios. Hence, the genotype influenced the net impact of stress on preference ratios (F(1,32)=6.54; p=0.0155 for the stress × genotype interaction), with stressed TPH2-CB1−/− mice (75.97±5.96%, n=8) differing from stressed TPH2-CB1+/+ mice (90.22±1.47%, n=9; p<0.05) whereas controls did not differ (81.7±3.13% and 85.69±0.82% in nine TPH2-CB1+/+ mice and in ten TPH2-CB1−/− mice, respectively). In the elevated plus-maze, stressed mice did not significantly differ from their control counterparts, both for their closed arm visits (Figure 6c) and for their percent visits in the open arms (17.30±3.63% and 11.05±2.68% in 9 control wild-type mice and 11 control mutant mice, respectively, as opposed to 13.56±3.98% and 9.68±2.44% in 10 stressed wild-type mice and 11 stressed mutant mice, respectively). At the opposite, the percent time spent in the open arms was lower in TPH2-CB1−/− mice, as compared with TPH2-CB1+/+ mice (F(1,37)=4.60; p=0.0386), whereas stress bore an inhibitory impact on that variable in TPH2-CB1+/+, but not in TPH2-CB1−/−, mice (F(1,37)=4.18; p=0.048 for the stress × genotype interaction; Figure 6d). The deletion of CB1 receptors from central serotonergic neurons decreased (F(1,37)=4.26; p=0.046), whereas social stress increased in a genotype-independent manner (F(1,37)=6.23; p=0.0171), the freezing responses to the presentation of the cue during recall (Figure 6e). The analyses of within-session freezing behaviors in each genotype further indicated that social stress increased the amplitude of freezing in TPH2-CB1−/− mice (F(1,20)=4.42; p=0.0484; Supplementary Figure S2h) whereas its impact in TPH2-CB1+/+ mice did not reach significance because of the heterogeneity of the data (Supplementary Figure S2g). Stress did not affect the initial freezing responses to the cue, although a trend toward a stimulatory impact could be noted in the last recall session (Supplementary Figure S2i). Lastly, a stimulatory effect of social stress on adrenal weight was observed in both genotypes (F(1,18)=17.77; p=0.0005; Figure 6f).
Figure 6.
Influence of the conditional mutation of cannabinoid type-1 (CB1) receptors from serotonergic neurons on the emotional profiles of control and stressed mice. This profile included 7-day water intakes (a), water and sucrose intakes in a free choice paradigm after the seventh stress session (b), closed arm entries (c) and percent time spent in the open arms (d) of an elevated plus-maze, freezing behavior during cued fear recall sessions 24–72 h after conditioning (e), and adrenal weight (f). D1–D3 stand for days 1–3. Values are the mean±SEM of 9–11 animals, except for (b) that refers to 8–10 animals per group. *p<0.05 and ***p<0.001 for the overall effect of social stress in the ANOVA; &p<0.05 for the overall influence of the genotype in the ANOVA; #p<0.05 and ##p<0.01 for stress influences within each genotype; +p<0.05 for the genotype influence in unstressed or stressed animals. See text for ANOVAs and additional statistics.
DISCUSSION
The goal of this study was to examine whether the ECS has a role in the processing of repeated social defeat and in the expression of emotional responses to such a stressor. In addition, this study addressed the question of the neuronal populations through which the ECS modulates these emotional responses to repeated social defeat. We first analyzed the influence of CB1 receptor blockade before each stress session, and compared it with the impact of the constitutive mutation of CB1 receptors. Thereafter, we addressed the possibility that distinct CB1 receptor-expressing neuronal populations exert discrete or even opposite influences in stressed animals that could be masked in constitutive CB1 receptor mutants. To this aim, we analyzed the respective influences of conditioned deletions of CB1 receptors from cortical glutamatergic neurons, from GABAergic neurons, or from serotonergic neurons on the emotional profile of repeatedly stressed individuals. The results show that (i) stress-induced endocannabinoid release modulates the expression of cued fear memory, (ii) CB1 receptors located on serotonergic neurons control unconditioned anxiety and cued fear expression, and mediate the increased sucrose preference observed in repeatedly stressed animals, (iii) CB1 receptors on GABAergic neurons are involved in the locomotor reactivity profile of stressed animals, and (iv) social stress abolishes the deficits in fear extinction of constitutive CB1 receptor mutants, an action that might be linked to the absence of CB1 receptors from cortical glutamatergic neurons.
Treatments with indirect/direct CB1 receptor agonists in stressed animals have helped to tackle the interactions between the ECS and stress circuitry. As an illustration, indirect/direct CB1 receptor stimulation may reduce repeated stress-elicited body weight reductions, anhedonia for sucrose, unconditioned anxiety, and proinflammatory consequences (Bortolato et al, 2007; Zoppi et al, 2011; but see Hill and Gorzalka, 2004). Although these studies provide new routes of therapeutic interventions in the management of stress-related disorders, they do not address the crucial question of the tonic role, if any, exerted by the ECS in the emotional consequences of repeated stress. Neurochemical, biochemical, and electrophysiological tools have indicated that the actions of the ECS are stress sensitive (Patel and Hillard, 2008; Hill et al, 2010b; Riebe and Wotjak, 2011). Whether, in turn, the ECS is involved in the emotional impacts of repeated stress is, however, a matter that has been only sparsely addressed. This issue led us to use the social defeat model of stress and to examine the respective amplitudes of a broad array of responses to repeated stress in mice fully or partly devoid of CB1 receptor activity. However, one prerequisite for the use of social stress in the present study was the need to gather evidence that the ECS is sensitive to that stressor. Indirect support for such an alteration initially stemmed from the report that repeated social defeat impaired the ability of a CB1 receptor agonist to decrease GABA release in the striatum (Rossi et al, 2008). The present analysis of central AEA and 2-AG levels in control and stressed animals provides a direct proof that the ECS is sensitive to a social defeat paradigm. Thus, AEA and 2-AG levels were modified in a brain region-dependent manner in acutely and repeatedly defeated mice. Acute social defeat decreased hippocampal AEA concentrations, but it did not impact on AEA levels in other brain regions or on 2-AG. Exposure to the last of the seven social defeats decreased also hypothalamic AEA levels whereas it increased 2-AG levels in the hypothalamus, hippocampus, and prefrontal cortex. These brain region- and endocannabinoid-dependent changes display similarity with those, respectively, documented in mice submitted to acute and repeated restraint stress sessions (Patel et al, 2005; Rademacher et al, 2008). This observation suggests that the mechanisms leading to these stress-elicited changes in AEA and 2-AG levels may be common to social defeat and restraint. Indeed, there is an experimental support to suggest that hyperactivity of the HPA axis could be such a mechanism (Hill et al, 2010b; Riebe and Wotjak, 2011). The initial observation that repeated, but not acute, exposure to a homotypic stressor increases 2-AG levels in mouse corticolimbic areas, including the basolateral amygdala (BLA), has raised the hypothesis that these increases may contribute to stress habituation (Patel and Hillard, 2008; Patel et al, 2009). Consistently, recent data in repeatedly restrained rats suggested that stress-induced decreases in corticolimbic AEA levels and increases in amygdaloid 2-AG levels are valuable markers of the habituation of the corticotropic axis to restraint (Hill et al, 2010a). These series of results open the question of the relationship between the aforementioned changes in endocannabinoid levels in our repeatedly socially defeated mice and habituation to repeated social stress. The answer to that question is not simple; thus, compared with acutely stressed animals, animals exposed to repeated social stress may show habituation, lack of habituation, or even sensitization according to the variable examined. As an illustration, the amplitude of the hyperthermia and the intensity of ultrasonic vocalization during the sensorial phase (see Figure 1a) of the social stress procedure have been shown to be increased in repeatedly stressed animals, compared with acutely stressed animals (Tornatzky and Miczek, 1994; Bhatnagar et al, 2006). At the opposite, the amplitudes of both the tachycardia and the rise in circulating corticosterone levels associated to the social stress paradigm were found to desensitize progressively with the number of social stress episodes (Tornatzky and Miczek, 1994; Bhatnagar et al, 2006). In our hands, repeatedly defeated mice expressed higher (initial and total) freezing responses to cue presentation 1 day after conditioning, compared with acutely defeated mice and control mice (Supplementary Figure S3). This observation suggests that conditioned freezing behavior sensitized with the number of stress sessions. On the other hand, we have already reported that the dipsogenic response to social stress was not different between the first and the fifth stress session whereas the increased social stress-elicited sucrose preference was observed in repeatedly, but not in acutely, stressed animals (Dubreucq et al, 2012). These observations indicate that the relevance of repeated social stress-induced changes in CNS endocannabinoid levels to stress adaptation is at the present time difficult to determine. Actually, the study of the emotional, metabolic, and endocrine consequences of alterations in AEA and 2-AG synthesis/degradation processes in repeatedly defeated animals could help to solve this issue.
Repeated social defeats increased water consumption in all experiments. This dipsogenic impact of repeated stress, which has been documented in the past using mice exposed either to repeated social defeats (Krishnan et al, 2007; Dubreucq et al, 2012) or to mixed stressors (Strekalova et al, 2006), was not accounted for by changes in food intake. The mechanisms underlying repeated stress-induced dipsogeny as well as its significance in the context of adaptation to stress are unknown. The aforementioned observation that the daily increase in water intake in stressed animals is already maximal after the first social defeat episode (Dubreucq et al, 2012) indicates that repeated stress-induced dipsogeny is a phenotypic response that is held constant after each stress episode. It has been proposed that dipsogeny may belong to a behavioral repertoire that includes anhedonia for sucrose (Strekalova et al, 2006). The present study, wherein social stress in vehicle-injected animals and in wild-type animals triggered both dipsogeny and increased preference for sucrose, does not lend support to this hypothesis. Another finding provided by the present study is that stress-induced dipsogeny may be independent from the ECS as none of the paradigms used to alter CB1 receptor function, including that aimed to block these receptors during stress only, proved effective on that variable.
It is now 30 years that the sucrose consumption/preference test is used to monitor the consequences of stress on hedonic processes, the dysregulation of which is a core symptom in human depression (Katz, 1981). When effective on sucrose consumption/preference, repeated stress is found to reduce sweet consumption in most, but not all, studies (Willner, 2005). The observation that repeated stress, such as chronic mild stress, may increase, rather than decrease, sweet consumption in a minority of studies has been considered a ‘genuine phenomenon' with specific neurobiological grounds (eg, hyperactivity of the mesolimbic dopaminergic system: Willner, 2005). As for chronic mild stress, social stress has been mainly shown to decrease sucrose preference (Krishnan et al, 2007; Becker et al, 2008; Covington et al, 2009; Miczek et al, 2011). However, other reports have concluded that social stress stimulates sucrose preference (Dubreucq et al, 2012) or bears no influence on that variable (Croft et al, 2005; Hollis et al, 2010). That our socially defeated mice increased, rather than decreased, their preference for sucrose, compared with their unstressed counterparts, is noteworthy. Whether the rewarding properties and/or the caloric value of sucrose drive this increase is presently unknown. The use of saccharin, which lacks caloric value, could help to tackle this issue. As indicated above, our observation of an increased preference for sucrose in repeatedly stressed mice has been already reported in animals exposed to chronic mild stress (Willner, 2005) or to other stressors (Dess, 1992; Pecoraro et al, 2004; Leigh Gibson, 2006). Several hypotheses can be proposed to understand this peculiar behavior in our stressed mice. The so-called ‘comfort food hypothesis' (Dallman et al, 2003) postulates that the increased consumption of carbohydrates, due to their rewarding and caloric properties, may help to reduce hyperactivity of the HPA axis and of the sympathetic nervous system in stressed individuals. Stress-induced corticosterone release (in conjunction with insulin release) likely subserves increased carbohydrate consumption in stressed animals (Pecoraro et al, 2005), which is consistent with human observations indicating that, when submitted to psychological stress, women with high levels of cortisol (the human equivalent of rodent corticosterone) show increased sweet consumption, as opposed to individuals with low levels of cortisol (Newman et al, 2007). The second hypothesis is linked to the observation that mice submitted to a mixture of stressors or to social defeats for 4–5 weeks show respectively no change (Rygula et al, 2005) or increased (Strekalova et al, 2006) preference for sucrose (compared with their controls) when tested during the first or the second week of stress, but decreased preference for sucrose when tested thereafter. As already reported above, our social stress model triggers a progressive increase in sucrose preference, ie, sucrose preference is not altered after a single social defeat (Dubreucq et al, 2012). It is therefore possible that our mice might display decreased preference for sucrose if exposed to a higher number of social stress sessions. Lastly, because genetic factors have a key role in the physiological and emotional responses of the individual when confronted to social stress (Berton et al, 1997; Berton et al, 1998), we cannot exclude the hypothesis that the genetic background of our stressed mice had an impact on their sucrose preference.
The key role exerted by the ECS in the regulation of reward circuitry (Maldonado et al, 2006) led us to dissect the role of the ECS on stress-elicited sucrose overconsumption. Mice pretreated with the CB1 receptor antagonist rimonabant before each stress session did not behave differently from vehicle-pretreated stressed mice. This result indicates that stress-elicited sucrose overconsumption was not accounted for by CB1 receptor stimulation during stress. Such a conclusion differs from that gathered in a previous study, where rimonabant pretreatment before each stress session amplified the inhibitory impact of stress on sucrose preference (Rademacher and Hillard, 2007). This discrepancy may be accounted for by numerous methodological differences, compared with the present study, including the stress model (restraint vs social defeat), the impact of that stressor on sucrose preference (inhibition vs stimulation), the duration of each sucrose preference test (1 h vs 12 h), the concentration of sucrose (10% vs 2%), and the metabolic state of the animals (20-h food- and water-deprivation before each test vs non-deprivation). Except for mice lacking CB1 receptors on serotonergic neurons, none of the genetic manipulations of the ECS affected stress-induced overconsumption of sucrose. The observation that the behavior of TPH2-CB1−/− mice did not extend to CB1−/− mice may be considered paradoxical at first glance. Because social stress depends on the behavior of the resident mice, the possibility that TPH2-CB1−/− mice underwent less stress than TPH2-CB1+/+ mice on the one hand, and CB1−/− mice on the other hand, might be considered. However, the observation that our stressed mice all received an equivalent number of daily attacks and displayed upright postures and squealing, which are overt signs of subordination (Miczek et al, 2001), renders this hypothesis unlikely. Actually, that conditional CB1 receptor mutants display phenotypes differing from those measured in CB1−/− mice is not incongruent. Fasting-induced food intake as well as the consumption of palatable food are diminished in CB1−/− mice, but amplified in GABA-CB1−/− mice (Bellocchio et al, 2010), although the majority of CNS CB1 receptors are located on GABAergic neurons (Marsicano and Lutz, 1999; Monory et al, 2006; Bellocchio et al, 2010). This observation suggests that cell type-specific functions of CB1 receptors might be masked by the constitutive deletion of the receptor gene. Taken with these findings, the present study underlines the need to dissect the ECS at the level of the neuronal phenotype to understand its role in brain functions. Our observation that the stress-induced increase in sucrose preference was absent in TPH2-CB1−/− mice, as opposed to TPH2-CB1+/+ mice, indicates that the population of CB1 receptors located on dorsal raphe serotonergic neurons (Häring et al, 2007), albeit discrete, is essential for the expression of that phenotypic response to stress. Interestingly, control (ie unstressed) TPH2-CB1−/− mice did not display any alteration in sucrose preference, compared with their wild-type controls, a finding that was recently replicated using lower (1%) and higher (up to 9%) concentrations of sucrose (data not shown). These results indicate that CB1 receptors on serotonergic neurons are involved in the regulation of sucrose intake under stressful conditions associated with increased sweet consumption, but not under control conditions. The inhibitory role of CB1 receptors on GABA and glutamate neurotransmission (Alger, 2002; Piomelli, 2003; Chevaleyre et al, 2006; Ohno-Shosaku et al, 2012) may indeed extend to serotonergic transmission (Nakazi et al, 2000; but see Gobbi et al, 2005). It is thus expected that under conditions of ECS hyperactivity, the selective lack of CB1 receptors from serotonergic neurons might result in an increased serotonin (5-HT) release, especially in animals exposed to stress, a situation favoring 5-HT neurotransmission (Chaouloff, 1993; Chaouloff, 2000). To the best of our knowledge, neither direct evidence for such an increased release of 5-HT nor its consequences on 5-HT neurotransmission have been documented. The sole information available is based on studies performed on CB1−/− mice, and which reported increased 5-HT release and alterations in pre- and post-synaptic receptor expression and/or function (Mato et al, 2007; Aso et al, 2009). Dorsal raphe neurons project along three ascending pathways to numerous brain locations (including the nucleus accumbens, the prefrontal cortex, the amygdala, the ventral/dorsal hippocampus, and the lateral septum) involved in the regulation of hedonic processes, but also anxiety- and fear-related behaviors (Michelsen et al, 2007). The present observation that CB1 receptors on dorsal raphe serotonergic neurons control sucrose preference in stressed animals, but also open arm behaviors in the elevated plus-maze and cued fear expression (see below), indicates the need for future experiments to examine the functional role of CB1 receptors on 5-HT release in these brain regions.
Comparative analyses of open arm behaviors in the elevated plus-maze (ie, anxiety-related indices; Ramos and Mormède, 1998; Crawley, 2008) between CB1+/+ mice and CB1−/− mice have led to contradictory results (Viveros et al, 2005; Wotjak, 2005; Lafenêtre et al, 2007). The present study reveals that under our experimental settings (see below), neither repeated CB1 receptor blockade nor CB1 receptor mutation in cortical glutamatergic neurons or in GABAergic neurons altered open arm behaviors. Indeed, only trends, such as those observed in Glu-CB1−/− mice, could be noted. The lack of effect of these mutations could be accounted for by the low aversiveness of the anxiety test used here (ie, an experimental condition chosen on purpose to be able to further observe stress-induced increases in anxiety). Indeed, naive (ie, unhandled) CB1−/−, Glu-CB1−/− mice, and GABA-CB1−/− mice all display anxiety when tested in a more aversive context such as a light/dark box (Moustié et al, unpublished data). Taken together, these observations support the hypothesis that CB1 receptors exert a tonic control on anxiety responses when measured under highly aversive conditions (Haller et al, 2004). However, the present study indicates also that such a statement may not apply to all CB1 receptor populations. Thus, under our experimental conditions, we observed that TPH2-CB1−/− mice spent less time in the open arms and tended to visit less frequently the open arms, but not the closed arms (an index of locomotor reactivity: Ramos and Mormède, 1998), compared with TPH2-CB1+/+ mice.
Repeated social defeat has been documented for its anxiogenic impact (Merlo-Pich et al, 1993; Berton et al, 1998; Krishnan et al, 2007). The present study confirms this statement and shows that neither CB1 receptor blockade during stress nor CB1 receptor mutation in the whole body, in cortical glutamatergic neurons, or in GABAergic neurons influenced significantly open arm behaviors in stressed animals. On the other hand, it is unknown whether repeated stress proved inefficient on the percent time spent in the open arms by TPH2-CB1−/− mice because (i) a floor effect was reached because of the mutation and/or (ii) CB1 receptors on serotonergic neurons mediate the anxiogenic impact of repeated stress. Future studies will be required to tackle this particular issue. Besides its anxiogenic impact, a 1-week exposure to repeated stress may also alter the initial (ie, first 5 min) locomotor response to the placement into novel environments (but see Strekalova et al, 2005). As an illustration, 7 daily restraint sessions increased locomotor reactivity in an open field (Ito et al, 2010) whereas 7–10 daily social defeat sessions decreased locomotion in novel activity cages or in an elevated plus-maze (Berton et al, 1998; Rygula et al, 2005; Krishnan et al, 2007). Here, repeated stress decreased locomotor reactivity, as assessed in the elevated plus-maze, and did so only in several mouse groups (eg, vehicle- and rimonabant-injected mice, CB1+/+ and CB1−/− mice, GABA-CB1+/+ and GABA-CB1−/− mice). Of particular interest was the finding that the deletion of CB1 receptors from GABAergic neurons amplified the hypolocomotor effect of stress. This suggests that endocannabinoid release, and then stimulation of these receptors, might help buffering the inhibitory impact of repeated stress on locomotor reactivity.
There is now extensive evidence that CB1 receptors exert a tonic control on conditioned freezing responses to fearful stimuli (Marsicano et al, 2002; Suzuki et al, 2004; Chhatwal et al, 2005; Kamprath et al, 2006). Thus, acute CB1 receptor blockade immediately before the first recall session (Marsicano et al, 2002; Suzuki et al, 2004; Chhatwal et al, 2005) or the genetic deletion of CB1 receptors (Marsicano et al, 2002; Kamprath et al, 2006; Dubreucq et al, 2010) delays conditioned freezing extinction, possibly as a consequence of a dysregulation of habituation processes (Kamprath et al, 2006). The use of conditional CB1 receptor mutants has further suggested that it is the absence of CB1 receptors from cortical glutamatergic neurons that may be responsible for the phenotype observed in constitutive CB1 receptor mutants (Kamprath et al, 2009). In the present study, between-session analyses of freezing behaviors and the comparison between the initial freezing responses to the cue in the control (unstressed) groups revealed that CB1−/− mice displayed increased fear expression and delayed extinction, compared with CB1+/+ mice. On the other hand, within-session analyses of freezing in the two genotypes did not reveal differences in extinction rates, including if analyzed on a daily basis (data not shown). These data, which confirm that between- and within-session extinction processes are independent (Plendl and Wotjak, 2010), differ from previous findings (Marsicano et al, 2002; Kamprath et al, 2006; Plendl and Wotjak, 2010). It is likely that experimental differences between protocols, including recall environments, sound and shock intensities, and day time, underlie our failure to observe within-session extinction of freezing in CB1−/− mice. A deregulation of conditioned fear responses has been observed in acutely and/or repeatedly stressed animals. Indeed, repeatedly stressed rats and mice that were cued fear-conditioned 1 or 7 days after the last stress session displayed an increased fear expression and/or impaired recall of extinction memory, compared with unstressed animals (Izquierdo et al, 2006; Miracle et al, 2006; Garcia et al, 2008). Interestingly, these stress-elicited changes in fear memory were more obvious when unstressed animals had reached extinction and were not accounted for by changes in acquisition during the conditioning sessions (Izquierdo et al, 2006; Miracle et al, 2006; Garcia et al, 2008; but see Rau and Fanselow, 2009). In the present study, between-session analyses of freezing in C57BL/6N mice (including vehicle-injected animals) and in wild-type animals (especially in CB1+/+, Glu-CB1+/+, and TPH2-CB1+/+ mice) confirmed that repeated stress increases fear expression during recall (without changing the immediate freezing response to tone-shock pairing; data not shown). On the other hand, between- and within-session analyses of the effects of social stress in all mice revealed that stress did not affect extinction processes (as indicated by the inability of social stress to alter significantly the daily extinction slopes). Pretreatment with rimonabant before each stress session amplified freezing behavior throughout the three recall sessions, compared with vehicle-pretreated-stressed mice. This result suggests that the release of endocannabinoids that occurs during repeated social stress is involved in the extinction of a fear conditioned by a stimulus different from that used to stress the animals. Interestingly, the absence of CB1 receptors from GABAergic neurons led to a pattern of freezing behavior that resembled that observed in rimonabant-pretreated mice, ie, an amplification of freezing during recall. These results suggest that rimonabant acted mainly through the blockade of that CB1 receptor population. Surprisingly, stressed CB1−/− mice displayed decreased freezing responses to the auditory cue during the last two recall sessions, compared with stressed CB1+/+ mice, and a similar phenotype was observed in stressed Glu-CB1−/− mice, compared with stressed Glu-CB1+/+ mice. Thus, in both mouse lines, prior repeated stress reduced the difference in freezing behavior between wild-type and mutant littermates. Despite some differences between CB1−/− mice and Glu-CB1−/− mice, this observation suggests that the complex recall session-dependent freezing behavior observed in stressed CB1−/− mice lies on changes in the release of glutamate from cortical neurons. The brain regions involved in the fear memory profiles of stressed CB1−/− mice and Glu-CB1−/− mice are unknown at the present time. The BLA might have a key role because CB1 receptors located therein have been recently shown to exert an inhibitory control over fear expression/extinction during late recall (48–72 h after conditioning, corresponding to our last two recall sessions), but not during early recall (ie, 24 h after conditioning, corresponding to our first recall session) (Kamprath et al, 2011).
In summary, this study reveals that (i) CB1 receptors located on serotonergic neurons exert a control on the increased preference for sucrose triggered by repeated stress, (ii) stress-elicited increases in freezing responses to a cue during fear recall sessions are amplified by prior blockade of CB1 receptors before each stress session, an effect that could be mediated by CB1 receptors on GABAergic neurons, (iii) this last population exerts a control on the amplitude of the hypolocomotor reactivity that results from repeated stress, and (iv) repeated stress reverse in a time-dependent manner the increased conditioned freezing behavior observed in animals lacking CB1 receptors from cortical glutamatergic neurons. On the basis of the use of pharmacologically, genetically, and an ethologically relevant model of stress, this study opens new routes of investigation on the role of distinct CB1 receptor populations in the emotional consequences of repeated stress.
Acknowledgments
This work was supported by AVENIR/INSERM (G.M.), EU-FP7 (REPROBESITY, HEALTH-F2-2008-223713, G.M.), European Research Council (ENDOFOOD, ERC-2010-StG-260515, G.M.), NARSAD (2008 Independent Investigator Award to G.M.), Fondation pour la Recherche Medicale (G.M.), l'Agence Nationale pour la Recherche (G.M.), the Aquitaine Region (G.M.), la Délégation Générale pour l'Armement du Ministère de la Défense (S.D.), and the Deutsche Forschungsgemeinschaft (FOR926, B.L.). We thank D. Gonzales and the Genotyping Platform of the NeuroCentre Magendie for mouse genotyping and regenotyping, and D. Bartsch, M. Ekker, T. Lemberger, S.K. Nave, J. Rubinstein, and G. Schütz for providing the Cre-expressing mice.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger E. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Aso E, Renoir T, Mengod G, Ledent C, Hamon M, Maldonado R, et al. Lack of CB1 receptor activity impairs serotonergic negative feedback. J Neurochem. 2009;109:935–944. doi: 10.1111/j.1471-4159.2009.06025.x. [DOI] [PubMed] [Google Scholar]
- Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Lafenêtre P, Cannich A, Cota D, Puente N, Grandes P, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nature Neurosci. 2010;13:281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- Berton O, Aguerre S, Sarrieau A, Mormède P, Chaouloff F. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience. 1998;82:147–159. doi: 10.1016/s0306-4522(97)00282-0. [DOI] [PubMed] [Google Scholar]
- Berton O, Ramos A, Chaouloff F, Mormède P. Behavioral reactivity to social and nonsocial stimulations: a multivariate analysis of six inbred rat strains. Behav Genet. 1997;27:155–166. doi: 10.1023/a:1025641509809. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–34. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Kole MHP, Veenema AH, Huininga M, de Boer SF, Korte SM, et al. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Rev. 1993;18:1–32. doi: 10.1016/0165-0173(93)90005-k. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Serotonin, stress and corticoids. J Psychopharmacol. 2000;14:139–151. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Covington HE, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology. 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of ‘comfort food'. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dess NK. Divergent responses to saccharin vs. sucrose availability after stress in rats. Physiol Behav. 1992;52:115–125. doi: 10.1016/0031-9384(92)90440-d. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Kambire S, Conforzi M, Metna-Laurent M, Cannich A, Soria-Gomez E, et al. Cannabinoid type 1 receptors located on single-minded 1-expressing neurons control emotional behaviors. Neuroscience. 2012;204:230–244. doi: 10.1016/j.neuroscience.2011.08.049. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. 2010;224:106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull R. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal neonatal and adult human brain. Neuroscience. 1997;77:299–331. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Häring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Enhancement of anxiety-like responsiveness to the cannabinoid CB1 receptor agonist HU-210 following chronic stress. Eur J Pharmacol. 2004;499:291–295. doi: 10.1016/j.ejphar.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, McEwen BS. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex. 2011;21:2056–2064. doi: 10.1093/cercor/bhq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci USA. 2010a;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci. 2010b;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology. 2010;211:69–77. doi: 10.1007/s00213-010-1869-9. [DOI] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology. Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Imai T, Chambon P, Metzger D. Inducible site-specific somatic mutagenesis in mouse hepatocytes. Genesis. 2000;26:147–148. doi: 10.1002/(sici)1526-968x(200002)26:2<147::aid-gene15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ito H, Nagano M, Suzuki H, Murakoshi T. Chronic stress enhances synaptic plasticity due to disinhibition in the anterior cingulate cortex and induces hyper-locomotion in mice. Neuropharmacology. 2010;58:746–757. doi: 10.1016/j.neuropharm.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob W, Yassouridis A, Marsicano G, Monory K, Lutz B, Wotjak CT. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes Brain Behav. 2009;8:685–698. doi: 10.1111/j.1601-183X.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, et al. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Plendl W, Marsicano G, Deussing JM, Wurst W, Lutz B, et al. Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behav. 2009;8:203–211. doi: 10.1111/j.1601-183X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Romo-Parra H, Häring M, Gaburro S, Doengi M, Lutz B, et al. Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology. 2011;36:652–663. doi: 10.1038/npp.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Ski A, Käfalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RJ. Animal models and human depressive disorders. Neurosci Biobehav Rev. 1981;5:231–246. doi: 10.1016/0149-7634(81)90004-x. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lafenêtre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res. 2007;56:367–381. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Lafenêtre P, Chaouloff F, Marsicano G. Bidirectional regulation of novelty-induced behavioral inhibition by the endocannabinoid system. Neuropharmacology. 2009;57:715–721. doi: 10.1016/j.neuropharm.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nature Neurosci. 2011;14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- Leigh Gibson E. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Lourenço J, Matias I, Marsicano G, Mulle C. Pharmacological activation of kainate receptors drives endocannabinoid mobilization. J Neurosci. 2011;31:3243–3248. doi: 10.1523/JNEUROSCI.3512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad S, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Massa F, Mancini G, Schmidt H, Steindel F, Mackie K, Angioni C, et al. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci. 2010;30:6273–6281. doi: 10.1523/JNEUROSCI.2648-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato S, Aso E, Castro E, Martín M, Valverde O, Maldonado R, et al. CB1 knockout mice display impaired functionality of 5-HT1A and 5-HT2A/C receptors. J Neurochem. 2007;103:2111–2120. doi: 10.1111/j.1471-4159.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- Merlo-Pich E, Heinrichs SC, Rivier C, Miczek KA, Fisher DA, Koob GF. Blockade of pituitary-adrenal axis activation induced by peripheral immunoneutralization of corticotropin-releasing factor does not affect the behavioral response to social defeat stress in rats. Psychoneuroendocrinology. 1993;18:495–507. doi: 10.1016/0306-4530(93)90043-k. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus – from silver stainings to a role in depression. Brain Res Rev. 2007;55:329–342. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- Newman E, O'Connor DB, Conner M. Daily hassles and eating behaviour: the role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32:125–132. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tanimura A, Hashimotodani Y, Kano M.2012Endocannabinoids and retrograde modulation of synaptic transmission Neuroscientist(in press). [DOI] [PubMed]
- Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur J Neurosci. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Gomez F, Dallman MF. Glucocorticoids dose-dependently remodel energy stores and amplify incentive relativity effects. Psychoneuroendocrinology. 2005;30:815–825. doi: 10.1016/j.psyneuen.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J Neuroscience. 2010;30:4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nature Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Hillard CJ. Interactions between endocannabinoids and stress-induced decreased sensitivity to natural reward. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:633–641. doi: 10.1016/j.pnpbp.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Ramos A, Mormède P. Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev. 1998;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–269. doi: 10.1016/j.bbr.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Wotjak CT. Endocannabinoids and stress. Stress. 2011;14:384–397. doi: 10.3109/10253890.2011.586753. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, et al. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Wanisch K, Monory K, Marsicano G, Borroni E, Bächli H, et al. Impaired cannabinoid receptor type 1 signaling interferes with stress-coping behavior in mice. Pharmacogenomics J. 2008;8:196–208. doi: 10.1038/sj.tpj.6500466. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 2006;17:271–287. doi: 10.1097/00008877-200605000-00008. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Dolgov A, Bartsch D. Stress-induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol. 2005;16:171–180. doi: 10.1097/00008877-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: differential protection by clonidine and metoprolol. Psychopharmacology. 1994;116:346–356. doi: 10.1007/BF02245339. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Wamsteeker JI, Kuzmiski JB, Bains JS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J Neurosci. 2010;30:11188–11196. doi: 10.1523/JNEUROSCI.1046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Böhm G, Hermann E, Schütz G, Schönig K, Bartsch D. Inducible gene manipulations in serotonergic neurons. Front Mol Neurosci. 2009;2:24. doi: 10.3389/neuro.02.024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Wotjak CT. Role of endogenous cannabinoids in cognition and emotionality. Mini Rev Med Chem. 2005;5:559–570. doi: 10.2174/1389557054368763. [DOI] [PubMed] [Google Scholar]
- Zoppi S, Pérez Nievas BG, Madrigal JL, Manzanares J, Leza JC, García-Bueno B. Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology. 2011;36:805–818. doi: 10.1038/npp.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.