Abstract
The altered behavioral effects of morphine, but not most other mu agonists, in mice lacking β-arrestin 2, suggest that this scaffolding protein regulates the signaling cascade of this commonly used analgesic. One of the cascades that could be regulated by β-arrestin 2 is cJun-N-terminal kinase (JNK), which binds with β-arrestin 2 and modulates the analgesic effects of morphine. Using neurons lacking β-arrestin 2 (β-arr2−/−) to examine this interaction, we found that β-arr2−/− neurons show altered intracellular distribution of JNK and cJun, and that morphine, but not fentanyl, increased the nuclear localization of the phosphorylated, therefore activated, form of cJun, a JNK target in dorsal root ganglia neurons. This suggests that deleting β-arrestin 2 affects the JNK cascade. We therefore examined whether some of the behavioral phenotypes of mice lacking β-arrestin 2 could be a result of altered JNK signaling. Indeed, two different JNK inhibitors reversed the enhanced analgesic effect of morphine, a known phenotype of β-arr2−/− mice, to +/+ levels. Both the reduced locomotor effect of morphine and the psychomotor sensitization to repeated morphine administration in β-arr2−/− mice were also returned to +/+ levels by inhibiting JNK. In contrast, the behavioral effects of fentanyl were neither genotype-dependent nor affected by JNK inhibition. Furthermore, a PKC inhibitor had a similar effect as inhibiting JNK in reducing the enhanced analgesic effect of morphine in β-arr2−/− mice to +/+ levels. In summary, removing β-arrestin 2 reveals mu receptor activation of the JNK cascade in a ligand-specific manner explaining several behavioral phenotypes of β-arr2−/− mice.
Keywords: mu opioid receptor, JNK, β-arrestin 2, morphine, analgesia, locomotion
INTRODUCTION
One of the many examples that the ubiquitously expressed arrestins serve as more than terminators of receptor signaling through initiating receptor internalization (Groer et al, 2011; Shenoy and Lefkowitz, 2011; Shukla et al, 2011) is the phenotype of mice lacking β-arrestin 2 (β-arr2−/− Bohn et al, 1999, 2002; Raehal and Bohn, 2011). As morphine does not induce significant internalization of the mu opioid receptor, the effects of morphine in β-arr2−/− mice cannot be explained by this prototypical role of β-arrestin 2. However, some progress has recently been made in revealing several arrestin-dependent, yet internalization-independent, signaling pathways of the mu opioid receptor that explain some, but not all, effects of morphine in mice lacking β-arrestin 2 (Arttamangkul et al, 2008; Dang et al, 2011; Quillinan et al, 2011; Walwyn et al, 2007).
Observations of ligand-specific signaling, or the ability of different ligands to activate specific effectors, were first made in the 1980s (Gee and Yamamura, 1983), but only recently have become an accepted dimension of G-protein-coupled receptor (GPCR) signaling (Reiter et al, 2012; Urban et al, 2007). Such functional selectivity is believed to result from the ability of different agonists of the same GPCR to induce ligand-specific conformational changes of the receptor. These distinct conformations recruit different proteins to the agonist-bound receptor and activate downstream signaling cascades in an agonist-specific manner (Galandrin et al, 2008). The opioid family of GPCRs are no exception to such diversity; ligands of the mu, delta, and kappa opioid receptors are known to activate functionally distinct signaling cascades (Bruchas and Chavkin, 2010; Melief et al, 2010; Peng et al, 2009; Pradhan et al, 2010).
The enhanced analgesic effect of morphine but not many other mu agonists in mice lacking β-arrestin 2 suggests that morphine, in activating a signaling cascade that is regulated by β-arrestin 2, differs from other agonists (Bohn et al, 1999, 2002). As β-arrestin 2 is a multi-functional scaffolding and signal transduction protein (Xiao et al, 2007, 2010), the signaling cascade responsible for such ligand specificity remains unknown. However, the arrestins bind with, and regulate, many components of the map kinase cascade, one of which is the stress-activated protein kinase, cJun-N-terminal kinase (JNK), a kinase that has recently been shown to play an important role in ligand-directed signaling of the mu and kappa opioid receptors (Melief et al, 2010).
As JNK binds with β-arrestin 2, this scaffolding protein may modulate mu receptor activation of the JNK cascade in a ligand-specific manner. Using mice lacking β-arrestin 2 to determine how this scaffolding protein regulates mu receptor signaling, we have found that altered JNK activity explains some of the behavioral phenotypes of mice lacking β-arrestin 2.
MATERIALS AND METHODS
Animals
β-Arrestin 1 (also known as arrestin 2) or β-arrestin 2 (also known as arrestin 3) mice, fully back-crossed into the C57Bl/6 background, were kindly supplied by Dr Lefkowitz (HHMI, University of North Carolina, Chapel Hill, NC). The behavioral experiments used equal numbers of adult male and female β-arrestin (β-arr) 1/2 +/+ and −/− mice that were 81±10 days old, 22.3±3.7 g in weight and bred from heterozygous matings. For the cellular experiments, early postnatal β-arr2+/+ and −/− pups were obtained from homozygous matings, one generation from heterozygous breeders. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and followed institutionally approved animal care and use protocols.
SDS–PAGE and Western Blot Analysis of JNK and Phospho-JNK Proteins Levels
Dorsal root ganglia (DRGs) were collected from all spinal levels of either β-arr2+/+ or −/− adult mice, weighed and homogenized in the following lysis buffer; 20 mM Tris–HCl pH 7.4, 0.32 M sucrose, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 1 mM Na4P2O7, 1 × Halt Protease and Phosphatase Inhibitor Cocktail (Pierce, Thermo Scientific, Rockford, IL), and protein content determined (BCA, Pierce). Protein samples (50 μg/sample) were separated by SDS–PAGE (10% NuPAGE gel; Invitrogen, Carlsbad, CA) and transferred to an Immobilon PVDF membrane (0.45 μm; Millipore, Billerica, MA). After several washes in TBS and a 120 min blocking step (Casein/0.1% Tween-20, Thermo Scientific), the membrane was incubated in anti-JNK or anti-phospho-JNK at 1 : 100 (Thr183/Tyr185), from both Cell Signaling (Danvers, MA) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 2.5 × 10−2 μg/ml, Thermo Scientific), o/n at 4 °C. The membrane was then washed in TBS with 0.1% Tween-20, incubated in Stabilized Peroxidase-conjugated secondary antibodies, anti-rabbit IgG for JNK (1 : 100) or anti-mouse IgG for phospho-JNK (1 : 100), or GAPDH (1 : 10 000) for 120 min at RT (Pierce, Rockford, IL). The labeled protein bands were visualized by chemiluminescence (Super signal Chemiluminescent Substrate, Pierce), and signal intensity (pixels/mm2) quantified (ImageJ, NIH). For each sample, the signal intensity of JNK or phospho-JNK was normalized to that of GAPDH. Statistical analysis: The mean of the three experiments for each of the six samples are expressed as the mean±SEM and the differences between groups determined by one-way ANOVA (Analyse-it Software, Leeds, UK) with significance accepted at p<0.05.
Cell Culture
DRGs from all spinal levels were harvested from early postnatal (p0–1) β-arr2+/+ or −/− pups. After chemical dissociation in trypsin (Invitrogen) and physical dissociation by trituration, 1 × 104 cells were plated on poly-D-lysine (Sigma, St Louis, MO) and laminin (Invitrogen) coated coverslips of MatTek dishes (MatTek, Ashland, MA) as previously described (Walwyn et al, 2007). The cells were maintained for 24–48 h in Neurobasal/B27 media containing NGFs (10 ng/ml) in vitro after which the cells were treated and fixed for immunocytochemistry or used to determine mu agonist inhibition of voltage-dependent Ca2+ channels (VDCCs).
Immunocytochemistry
β-arr2+/+ or −/− cells remained untreated or were treated with morphine (NIDA) or fentanyl (Sigma) for 10 min, washed and fixed. After a series of washes, a 0.3% Triton X100 permeabilization step, and a 10% serum blocking step, anti-JNK3 (1 : 500, Santa Cruz Biotechnology, Santa Cruz, CA) or anti-phosphorylated JNK (anti-phospho-JNK: 1 : 100, Cell Signaling) were added. In a second, matching, set of plates, primary antibodies against cJun (1 : 500, Santa Cruz Biotechnology) and phosphorylated cJun (anti-phospho-cJun, 1 : 250, Santa Cruz Biotechnology) were added. After o/n incubation and further washes, Alexa-555-conjugated donkey anti-goat/mouse (1 : 1000 for phospho-cJun and phospho-JNK; Invitrogen) and Alexa-488-conjugated donkey anti-rabbit/goat (1 : 1000 for cJun and 1 : 400 for JNK; Invitrogen) were added to each plate for 90 min at RT. After a final series of washes, the cells were mounted in Prolong containing 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). Medium-sized DRGs (20–30 μM) were visualized by a × 60 oil immersion objective on a Nikon TE2000 microscope and images were captured by a Retiga 1300Exi CCD camera (QImaging, British Columbia, Canada). All cells were imaged at the mid-nuclear level using DAPI as a guide. Pixel intensity per unit area for each of the three channels (RGB) was quantified by IPLab (version 3.6.5, BioVision Technologies, Exton, PA). Statistical analysis: Data from untreated cells were expressed as mean±SEM of the nuclear/cytosolic signal intensity for >100 cells/sample. The effect of different mu agonists on cJun and phospho-cJun localization was determined by comparing the percent change of the nuclear:cytosolic ratio in the agonist treated vs untreated cells. Differences between groups were determined using two-way ANOVA between groups (Analyse-it Software) and significance accepted at p<0.05.
Analgesia
Thermal nociception was measured by the warm-water tail-immersion assay in which the latency to remove the tail from a 49.5 °C water bath was measured. After a basal response was obtained (average of three to four tests), one of the JNK or PKC inhibitors, or matching vehicle, was injected, another basal measurement was obtained 30 (PKC inhibitor) or 60 (JNK inhibitor) min later and then morphine or fentanyl was administered. The JNK inhibitors used were: SP600125 (SP6; Anthra[1,9-cd]pyrazol-6(2H)-one, 1,9-pyrazoloanthrone, 30 mg/kg i.p., EMD Chemicals, Merck KGaA, Darmstadt, Germany) or BI78D3 (4-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-5-(5-nitrothiazol-2-ylthio)-4H-1,2,4-triazol-3-ol) 25 mg/kg i.p., EMD Chemicals). Both compounds inhibit the activation of all JNK isoforms by binding with either the ATP docking site (SP6; Shin et al, 2002) or the D-domain consensus sequence in JNK to inhibit the docking of upstream kinases (BI78D3; Stebbins et al, 2008). The PKC inhibitor used, Bisindolylmaleimide VIII (Ro-31-7549, EMD Chemicals), inhibits PKCα, βI, βII, γ, ɛ (Wilkinson et al, 1993). Statistical analysis: Data are expressed as the response in seconds (s), and as the mean±SEM of each group. Differences between groups were determined by one- or two-way ANOVA with repeated measures and factorial analysis at each time interval using Stat View (v5). Significance was accepted at p<0.05.
Locomotion
Locomotor activity was measured in 16.7 × 12.7 cm2 boxes by an infrared video-camera and Ethovision video tracking software (v7.1; Noldus, Tacoma). The mice were habituated for 15 min on the first day and, on each of the following 3 days, were given SP6 (30 mg/kg i.p.), or vehicle, 60 min before morphine (10 mg/kg s.c.) or fentanyl (0.2 mg/kg s.c.) and placed in the locomotor boxes. Locomotion was assessed over the following 30 min. Statistical analysis: Data are expressed as mean±SEM and differences between groups determined by one- or two-way ANOVA with repeated measures and factorial analysis at each time interval (Stat View).
Electrophysiology
Voltage-dependent Ca2+ currents were recorded from small–medium-sized DRG neurons 20–30 μm in diameter under whole-cell voltage-clamp conditions as previously described (Tan et al, 2003; Walwyn et al, 2007). Cells were perfused with an external solution containing 10 mM CaCl2, 130 mM tetraethylammonium chloride, 5 mM HEPES, 25 mM d-glucose and 0.25 μM tetrodotoxin at pH 7.35. The patch electrode was filled with an internal solution composed of 105 mM CsCl, 40 mM HEPES, 5 mM d-glucose, 2.5 mM MgCl2, 10 mM EGTA, 2 mM Mg-ATP, and 0.5 mM GTP at pH 7.2. Ca2+ currents were evoked every 10 s by 40-ms voltage steps from −80 to +10 mV using an Axopatch 200B patch-clamp amplifier. Capacitance and series resistance were corrected with the compensation circuitry on the amplifier. Series resistance was compensated by 80–90%. Leak currents were subtracted using a P/6 protocol. Recorded signals were acquired and analyzed using Axon pClamp v8.0 or 9.0 software (Axon Instruments, Foster City, CA). Drug application and desensitization protocols: The JNK inhibitor, SP6, was dissolved in DMSO as a 10-mM stock solution and diluted with the culture medium to a final concentration of 10 μM for application. The effect of SP6 on mu receptor–VDCC inhibition was assessed by a 4-h pre-incubation with SP6 (10 μM). For the desensitization experiments, β-arr2+/+ and −/− DRG neurons were pre-incubated with 1 μM morphine, D-Ala2-MePhe4-Glyol5 Enkephalin (DAMGO), or fentanyl, in the presence or absence of 10 μM SP6 for 4 h, the media removed, and the cells were washed by an ∼100 × solution exchange before being challenged by the full agonist, DAMGO (1 μM). Statistical analysis: Mean Ca2+ current amplitudes were measured (pCLAMP 9.0; Axon Instruments, CA) between 5 and 10 ms after initiating the depolarizing step. Mean current amplitudes were then plotted against time. Stable recordings were fitted by a linear function to compare, by extrapolation, control current amplitude to the current amplitude recorded in the presence of opioid receptor agonists or SP6. Data are expressed as mean±SEM and were compared using ANOVA with a post hoc Tukey's test with significance accepted at p<0.05. Recordings that exhibited marked rundown (>30%) were discarded as were significant outliers, five out of the ∼300+ recordings (Prism v5.03).
RESULTS
DRG Neurons Lacking β-Arrestin 2 Show Altered JNK and cJun Localization
JNK and phospho-JNK expression
As β-arrestin 2 facilitates GPCR activation of the JNK cascade (Willoughby and Collins, 2005), genetically deleting β-arrestin 2 may result in a compensatory adaptation in the expression of JNK and phospho-JNK. This was examined by SDS–PAGE and western blotting in β-arr2−/− and +/+ adult DRGs. No effect of genotype was found. The relative intensities of the protein of interest normalized to that of GAPDH were: JNK; 48 kDa: β-arr2+/+ 0.39±0.05, β-arr2−/−: 0.33±0.05.54 kDa: β-arr2+/+ 0.23±0.06, β-arr2−/−: 0.16±0.04. phospho-JNK; 54 kDa: β-arr2+/+ 0.61±0.13, β-arr2−/−: 0.64±0.04.
The basal intracellular location of JNK and cJun
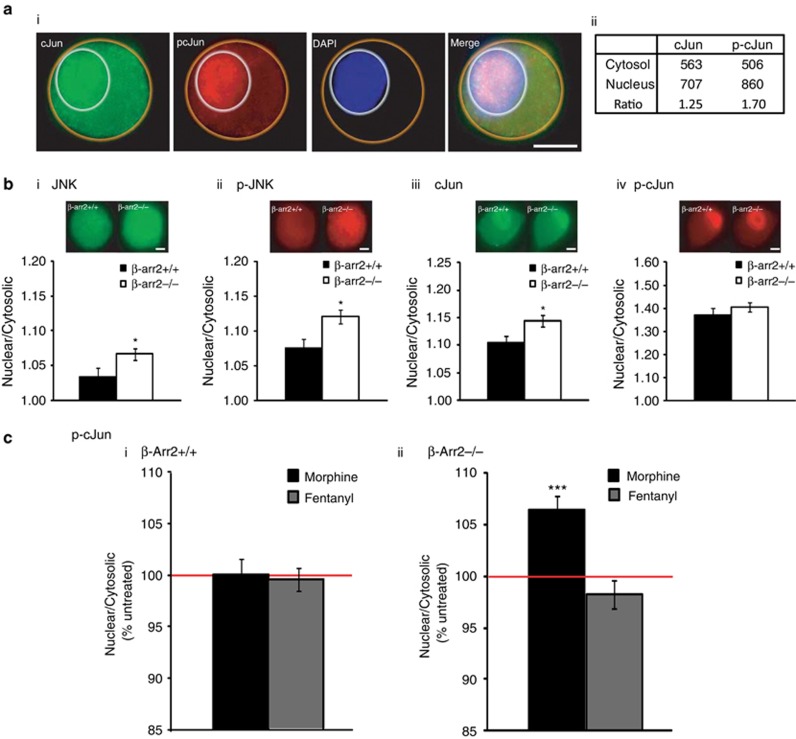
As β-arrestin 2 contains a nuclear export sequence resulting in its constitutive exclusion from the nucleus (Song et al, 2006), β-arr2−/− neurons may show altered nuclear or cytosolic localization of proteins, such as JNK, that bind with β-arrestin 2. We therefore examined the effect of deleting β-arrestin 2 on the relative nuclear:cytosolic ratio of JNK, phospho-JNK, and the major JNK target, cJun, and phospho-cJun. β-arr2−/− DRG neurons showed a basal increase in the nuclear localization of JNK, phospho-JNK, and cJun, but not phospho-cJun (Figure 1b).
Figure 1.
DRG neurons as a model to examine the JNK cascade. DRG neurons from early postnatal mice were used to examine the effect of deleting β-arrestin 2 and different mu receptor agonists on the relative nuclear:cytosolic ratio of JNK and cJun. (a) The relative pixel intensity/unit area of the nuclear or cytosolic labeling for JNK, phospho-JNK, cJun, or phospho-cJun was imaged and quantified in individual neurons. An example of the labeling and quantification for one cell are shown in (ai, aii), respectively. (b) This method was used to quantify the nuclear:cytosolic ratio of JNK, phospho-JNK, cJun, and phospho-cJun, in untreated β-arr2+/+ vs −/− neurons (n>100 neurons/sample). β-arr2−/− neurons showed a relative increase in the nuclear localization of JNK, phospho-JNK, and cJun, (*p<0.05 vs β-arr2+/+), but not phospho-cJun (p=0.75). (c) While morphine treatment (1 μM, 10 min) did not affect the nuclear:cytosolic ratio of phospho-cJun in β-arr2+/+ neurons, this mu agonist did increase phospho-cJun in the nucleus vs cytosol of β-arr2−/− neurons. .***p<0.001 vs untreated. Scale bars=10 μm.
Ligand-induced changes in phospho-cJun location
We next examined whether two mu agonists, morphine and fentanyl, previously shown to activate the JNK cascade (Melief et al, 2010) could alter the intracellular location of phospho-cJun in β-arr2+/+ and −/− neurons. We focused on phospho-cJun for a number of reasons; the activity of this terminal kinase of the JNK pathway is finely regulated by JNK (Hibi et al, 1993) and, importantly, the basal location of phospho-cJun was unaffected by deleting β-arrestin 2. We found that morphine increased the relative intensity of phospho-cJun in the nucleus of β-arr2−/− but had no effect in +/+ neurons. Fentanyl did not alter phospho-cJun localization in +/+ or −/− neurons (Figure 1c). Neither ligand altered the nuclear:cytosolic ratio of cJun (data not shown).
JNK Inhibition Reverses Behavioral Phenotypes of Morphine in β-arr2−/− Mice
Using DRG neurons as a model, we have found altered basal and mu agonist-induced changes in the intracellular location of JNK and/or cJun, suggesting that deleting β-arrestin 2 alters aspects of the JNK cascade. As this cascade can modulate the behavioral effects of mu agonists (Chen et al, 2008; Melief et al, 2010), we next examined whether the behavioral phenotypes of β-arr2−/− mice could be a result of altered JNK activity.
Analgesia
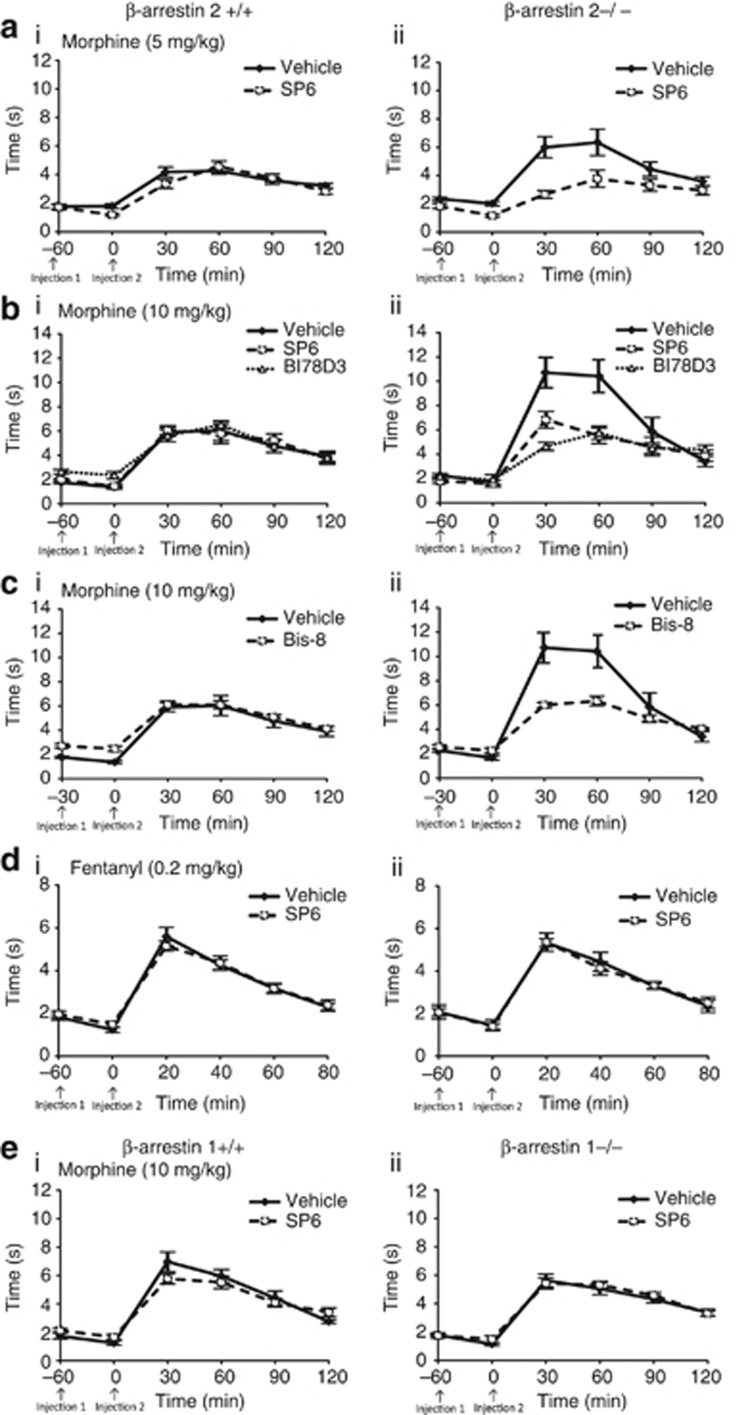
The role of JNK in thermal analgesia was examined by administering one of two JNK inhibitors, SP6 or BI78D3, to β-arr2−/− and +/+ mice, 60 min before morphine (5 and 10 mg/kg s.c.) or fentanyl (0.2 mg/kg s.c.). Thermal analgesia was assessed by the warm-water tail-immersion assay 20–30 min later and at regular intervals thereafter. In β-arr2+/+ mice, neither of the JNK inhibitors altered the analgesic profile of morphine, which showed a comparable dose-response curve to previous publications (Sora et al, 1997; Bohn et al, 2000). In contrast, both SP6 and BI78D3, reversed the enhanced analgesic effect of morphine in β-arr2−/− mice to wild-type levels (Figure 2a and b). The analgesia induced by fentanyl was neither genotype-dependent nor influenced by JNK. In addition, the higher basal analgesia of β-arr2−/− (2.19±0.05 s) vs +/+ (2.03±0.05 s, p<0.05) mice was not affected by either SP6 or BI78D3. As PKC has been shown to activate JNK (Lopez-Bergami et al, 2005; Melief et al, 2010), we next examined whether the effect of morphine in β-arr2−/− mice was also PKC-dependent. The PKC inhibitor, Bisindolylmaleimide VIII, reversed the enhanced effect of morphine in β-arr2−/− mice to wild-type levels (Figure 2c). We also examined whether β-arrestin 1 may be responsible for the effect of JNK in β-arr2−/− mice but found no effect of deleting β-arrestin 1 or inhibiting JNK in the analgesic response to morphine in these mice (Figure 2e).
Figure 2.
The enhanced thermal analgesic effect of morphine in β-arr2−/− mice is reduced to +/+ levels by inhibiting JNK or PKC. The warm-water (49.5 °C) tail-immersion assay was used to assess the analgesic effects of different mu agonists in β-Arr2+/+ and −/− mice and the resultant data are presented as the response (s; y axis) over time (min; x axis). (a, b) β-Arr2−/− mice showed a dose-dependent increase in the thermal analgesic effects of morphine ((a) 5 and (b) 10 mg/kg morphine s.c., 5 mg/kg: p=0.12 vs β-arr2+/+, F1,5=1.773; 10 mg/kg: p<0.001 vs β-arr2+/+, F1,5 13.227), which was reversed by the JNK inhibitor, SP6 (aii) 5 mg/kg: p<0.05 vs vehicle, F1,5=2.422; (bii) 10 mg/kg: p<0.01 vs vehicle, F1,5=4.501). BI78D3 (bii), similarly reversed the enhanced analgesia seen in morphine-treated (10 mg/kg s.c.) β-arr2−/− mice (p<0.001 vs vehicle, F1,5=6.142). Neither JNK inhibitor altered the β-arr2+/+ response ((ai) 5 mg/kg: p=0.06 vs vehicle, F1,5=10.74; (aii) 10 mg/kg: SP6: p=0.94 vs vehicle, F1,5=0.457; BI78D3: p=0.71 vs vehicle, F1,5=0.575) (c) The PKC inhibitor Bisindolylmaleimide VIII (10 mg/kg i.p.) also reversed the enhanced analgesia seen after morphine administration (10 mg/kg s.c.) in β-arr2−/− mice (p<0.001 vs vehicle, F1,5=7.099), while having no effect in β-arr2+/plus; mice (p=0.76 vs vehicle, F1,5=0.937). (d) Mice treated with fentanyl (0.2 mg/kg s.c.) showed no effect of the β-arrestin 2 deletion (p=0.84 vs β-arr2+/+, F1,5=0.403) or JNK inhibition (β-arr2+/+: p=0.59 vs vehicle, F1,5=0.748; β-arr2−/−: p=0.96 vs vehicle, F1,5=0.194). (e) There was also no effect of deleting β-arrestin 1 (p=0.10 vs β-arr2+/+, F1,5=1.883) or JNK inhibition on the morphine response of β-arr1−/− or +/+ mice (SP6: β-arr1+/+: p=0.07 vs vehicle, F1,5=2.089; β-arr1−/−: p=0.80 vs vehicle, F1,5=0.224). Injection 1=JNK or PKC inhibitor, or matching vehicle. Injection 2=morphine or fentanyl.
Psychomotor effects of morphine
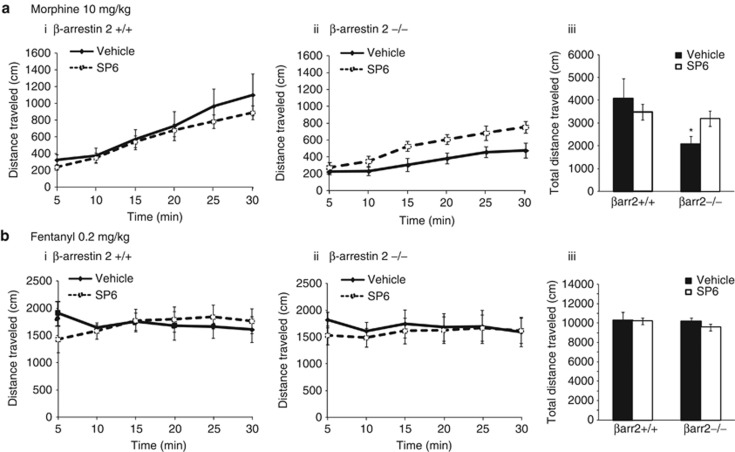
Acute locomotion: Another of the behavioral phenotypes of β-arr2−/− mice is the reduced locomotor effect of morphine (Bohn et al, 2003; Urs et al, 2011). We hypothesized that, similar to the analgesic effect of morphine, this may also be due to altered JNK activity. The JNK inhibitor, SP6, or vehicle, was therefore administered 60 min before assessing the locomotor effects of morphine or fentanyl in β-arr2+/+ and −/− mice. SP6 had no effect in β-arr2 +/+ mice, but SP6-treated −/− mice showed an enhanced locomotor response to morphine matching that of β-arr2 +/+ mice (Figure 3a). By contrast, the locomotor effect of fentanyl was neither genotype nor SP6-dependent (Figure 3b).
Figure 3.
The reduced locomotor effect of morphine in β-arr2−/− mice is returned to +/+ levels by inhibiting JNK. The locomotor effect of different mu agonists in β-arr2+/+ and −/− mice is shown over the duration of the 30-min test in the line graphs with distance traveled (cm; y axis) shown over time (x axis). The total distance traveled (cm; y axis) is shown in the accompanying bar graph. (a) The locomotor effect of morphine was less in β-Arr2−/− mice compared with their wild-type littermates (p<0.01 vs β-arr2+/+, F1,11=14.251). SP6 (30 mg/kg i.p.) increased the effect of morphine in β-arr2−/− mice (p<0.001 vs vehicle, F1,11=19.835) to +/+ levels (p=0.52 vs β-arr2+/+, F1,14=0.429) but had no effect in β-arr2+/+ mice (p=0.46 vs vehicle, F1,14=0.577). (b) Mice treated with fentanyl (0.2 mg/kg s.c.) showed no effect of genotype (p=0.81 vs β-arr2+/+, F1,9=0.061) or JNK inhibition (SP6: β-arr2+/+: p=0.68 vs vehicle, F1,12=0.169; β-arr2−/−: p=0.16 vs Vehicle, F1,11=2.194).
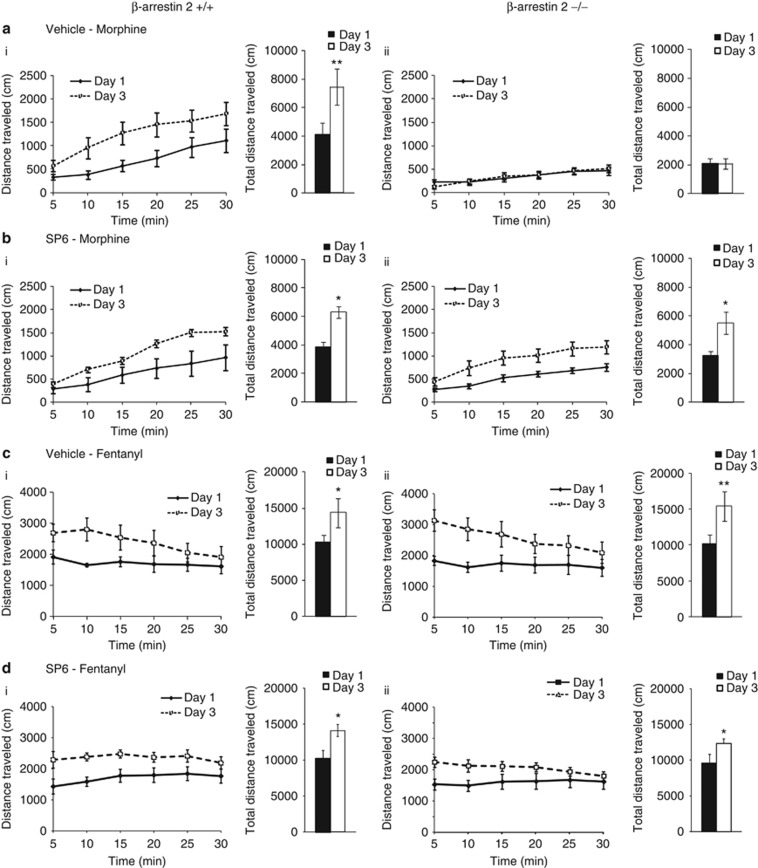
Psychomotor sensitization: As the locomotor effect of a single morphine injection was influenced by both genotype and JNK inhibition, locomotor sensitization induced by repeated morphine administration could similarly be modulated by JNK in β-arr2−/− mice. We found that 3 days of repeated morphine administration resulted in less locomotor sensitization in β-arr2−/− than +/+ mice (10 mg/kg; Figure 4a). This was reversed by SP6, administered 60 min before morphine on each of the 3 test days (Figure 4b). The locomotor sensitization induced by repeated fentanyl administration was not influenced by deleting β-arrestin 2 or inhibiting JNK (Figure 4c and d).
Figure 4.
The reduced psychomotor effect of morphine in β-arr2−/− mice is similarly returned to +/+ levels by inhibiting JNK. These data are presented in the same format as in Figure 2 showing both the distance traveled over time in the line graphs and total locomotion in the accompanying bar graphs (ai) A 3-day protocol of successive morphine (10 mg/kg s.c.) treatment of β-arr2+/+ mice resulted in an enhanced locomotor response to the same dose of morphine by day 3 (p<0.01 day 1 vs day 3, F1,5=16.972). (aii) Conversely, similarly treated β-Arr2−/− mice showed no such increase (p=0.41 day 1 vs day 3, F1,6=0.765). (bi) Administration of SP6 (30 mg/kg i.p.), 60 min before each morphine injection had no effect on the locomotor sensitization in β-arr2+/+ mice (p<0.05 day 1 vs day 3, F1,9=6.866) but reversed the lack of sensitization in the β-arr2−/− mice (p<0.05 day 1 vs day 3, F1,5=6.989) to wild-type levels. (c, d) Mice treated with fentanyl (0.2 mg/kg s.c.) showed locomotor sensitization (p<0.001 day 1 vs day 3, F1,23=63.235), that was unaffected by β-arrestin 2 deletion or SP6 administration (vehicle; p=0.93 day × genotype, F1,23=0.007, SP6; p=0.25 day × genotype × injection, F1,23=1.382). *p<0.05, **p<0.01.
Possible cellular targets of the JNK cascade
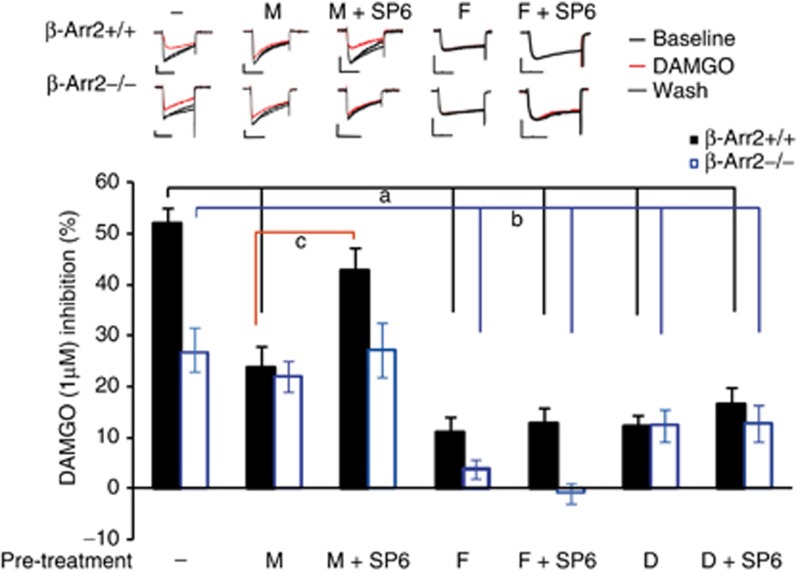
Inhibiting JNK reduced the analgesic efficacy of morphine 30 min after this drug was administered. This suggests that JNK phosphorylation of cytosolic targets, rather than the transcriptional activity of cJun, modulates morphine analgesia. We therefore examined whether opioid coupling with VDCCs, a Gβγ-mediated second messenger cascade, may be influenced by JNK in a ligand-specific manner. We examined both the acute effect of inhibiting JNK on mu receptor–VDCC coupling as well as desensitization of opioid receptor–VDCC inhibition following 4 h of opiate pre-treatment. We found no effect of SP6 pre-treatment on the acute VDCC inhibition induced by morphine, fentanyl or DAMGO in either β-arr2+/+ or −/− neurons (Table 1). However, desensitization induced by each of the ligands revealed both genotype and JNK effects, as shown by a 1-μM test dose of DAMGO. In β-arr2+/+ neurons, the desensitization induced by morphine (4 h, 1 μM) was prevented by SP6 co-incubation. In β-arr2−/− neurons, morphine did not induce significant desensitization, and this was unaffected by SP6 co-incubation. Pre-incubation with either DAMGO or fentanyl resulted in significant desensitization in both β-arr2+/+ and −/− neurons that was not altered by SP6.
Table 1. JNK Inhibition by SP6 has no Effect on Mu Receptor–VDCC Inhibition in β-arr2−/− or +/+ Neurons.
|
Agonist Pre-treatment |
Morphine |
Fentanyl |
DAMGO |
|||
|---|---|---|---|---|---|---|
| − | SP6 | − | SP6 | − | SP6 | |
| β-arr2+/+ | 32.5±4.1 | 37.8±5.2 | 36.3±2.4 | 36.5±2.5 | 44.4±2.9 | 56.0±7.2 |
| β-arr2−/− | 22.0±3.6* | 23.6±2.8* | 26.4±3.1* | 24.4±4.4* | 26.9±3.0** | 23.1±4.4** |
The effect of SP6 incubation (10 μM, 4 h) on Ca2+ channel inhibition (%) by the mu opioid receptor agonists, morphine, fentantyl, and DAMGO (1 μM each) was assessed in early postnatal dorsal root ganglia neurons from β-arr2+/+ and −/− mice.
*, **p<0.05, 0.01 vs β-arr2+/+, same treatment.
DISCUSSION
When compared with other mu agonists, morphine induces less internalization of the mu opioid receptor (Borgland et al, 2003; McPherson et al, 2010; Zheng et al, 2011). This suggests that the prototypical role of β-arrestin 2 in initiating receptor internalization does not explain how the effects of morphine, but not of other mu agonists, are altered in mice lacking β-arrestin 2. Here, we demonstrate that morphine activation of JNK in the absence of β-arrestin 2 explains ligand-specific behavioral phenotypes of β-arr2−/−mice. These findings also demonstrate that the JNK cascade may be activated in a ligand-specific manner, providing further evidence of the role of this member of the map kinase pathway in mediating ligand-directed signaling of the mu receptor.
Compared with other mu agonists such as fentanyl, the relative inability of morphine to cause significant receptor phosphorylation, results in the recruitment of PKC to morphine, but not fentanyl, bound mu receptors (Zheng et al, 2011). As PKC is known to both scaffold with and phosphorylate JNK (Lopez-Bergami et al, 2005; Ping, 2003; Pontrelli et al, 2004), we propose that in wild-type mice, morphine recruitment of PKC activates JNK but that β-arrestin 2 recruitment limits further JNK activation. However, if β-arrestin 2 is absent and is coupled with the inability of morphine to recruit β-arrestin 1 (Groer et al, 2011), sufficient PKC recruitment results in sustained activation of the JNK cascade in β-arr2−/− neurons to affect analgesia. In contrast, other mu agonists, such as fentanyl differ from morphine as they are able to phosphorylate the mu receptor and recruit either β-arrestin 1 or 2 (Groer et al, 2011; Zheng et al, 2011). In the absence of β-arrestin 2, these agonists recruit β-arrestin 1, PKC is not recruited and the JNK cascade is not significantly activated.
β-Arrestin 2 has been shown to bind with JNK3, but not JNK1 or 2, to control GPCR activation of the JNK cascade (McDonald et al, 2000; Miller et al, 2001; Song et al, 2006, 2009; Willoughby and Collins, 2005). Removing β-arrestin 2 may therefore alter both the location and control of JNK3 to allow sustained JNK activation in β-arr2−/− mice. If JNK3 were indeed involved, this is a different isoform to the JNK2-dependent regulation of receptor uncoupling following repeated morphine administration (Melief et al, 2010). In our experiments, acute mu receptor coupling with VDCCs was unaffected by JNK inhibition, suggesting that neither the mu receptor nor Gβγ-coupled Ca2+ channels are downstream targets of the JNK activity unmasked by deleting β-arrestin 2. In contrast, SP6 modulation of mu receptor–VDCC desensitization in β-arr2+/+ neurons shows a JNK dependency (Figure 5) that could be involved in the JNK2 regulation of mu receptor–effector coupling and morphine desensitization in vivo (Melief et al, 2010). This raises an interesting hypothesis that the same isoform, or possibly different JNK isoforms, may regulate different signaling cascades of the mu receptor depending on whether β-arrestin 2 is present or absent and the protocol of morphine application.
Figure 5.
Mu receptor-Ca2+ channel inhibition as a possible cytosolic JNK target. Mu receptor inhibition of voltage-dependent Ca2+ channels (VDCC) was examined in β-arr2+/+ and −/− DRG neurons. Exemplar currents are shown above the bar graph, which summarizes the effect of mu agonist pre-treatment coupled with JNK inhibition on mu receptor–VDCC inhibition, n=6–30 neurons/sample. Although JNK inhibition had no effect on mu receptor–VDCC inhibition in β-arr2−/− or +/+ neurons (Table 1), the desensitization induced by a 4-h pre-treatment of β-arr2+/+ neurons with morphine (1 μM) was prevented by SP6 co-incubation (10 μM, 4 h). In contrast, β-arr2−/− neurons showed no desensitization to morphine and no effect of SP6. Both fentanyl and DAMGO pre-treatment desensitized mu receptor–VDCC inhibition in β-ar2+/+ and −/− neurons but this was not affected by SP6 co-incubation. M=morphine; F=fentanyl; D=DAMGO. a: β-arr2+/+ neurons, p<0.05 vs M, p<0.001 vs F, F + SP6, D, D + SP6. b: β-arr2−/− neurons; p<0.05 vs D, D + SP6, p<0.001 vs F, F + SP6, c: β-arr2+/+ neurons; p<0.05 vs M + SP. Scale bars=40 ms on the x axis and 0.4 nA on the y axis.
The effects of morphine on locomotion and subsequent sensitization in rodents are considered a model of the initial phase of addiction in humans (Robinson and Berridge, 1993; Vanderschuren and Pierce, 2011). This response requires D1 receptors, the phosphoprotein DARPP32 (Becker et al, 2001; Borgkvist et al, 2007; Urs et al, 2011) and ERK (Borgkvist et al, 2008; Urs et al, 2011). We, and others, have shown that the locomotor effect of morphine is blunted in mice lacking β-arrestin 2 (Bohn et al, 2003; Urs et al, 2011). As morphine induces the formation of a β-arrestin 2/ERK complex in D1 neurons, it has been suggested that this complex mediates the hyperlocomotor effect of morphine (Urs et al, 2011). In using pharmacological tools to inhibit JNK in β-arrestin 2−/− mice, we have shown that activation of the JNK cascade in the absence of β-arrestin 2 explains the reduced locomotor response of morphine in these mice. Taken together, these data and those of Caron and colleagues (Urs et al, 2011) suggest that β-arrestin 2 plays complementary roles in this paradigm, one prevents JNK activation whereas the other allows a β-arrestin/ERK complex to form in D1 neurons. We propose that both roles synergize, and are necessary for the characteristic locomotor effect of morphine to occur.
Although others have found that sensitization to morphine is not β-arrestin 2-dependent (Bohn et al, 2003), we have found that this context-specific response to multiple doses of morphine is reduced in mice lacking β-arrestin 2. However, our assay differed in several important ways; we examined the first 30 min after morphine (30 vs 120 min), after 3 rather than 9 days of repeated morphine, and our sensitization protocol was context-associated. The initial loss of such ‘short-term' sensitization in β-arr2−/− mice may therefore not reflect a lack of, but rather an attenuation of the onset of sensitization that may not be detectable in a context-independent manner after an extended protocol of morphine administration. Furthermore, as JNK inhibition recovered the locomotor response at similar relative proportions for each day of the test, this suggests that the JNK cascade does not block morphine sensitization. However, inhibiting JNK allows an initial locomotor response to morphine thus permitting sensitization to develop.
Our findings, and those of several others, show that β-arrestin 2 regulates mu receptor function through several different pathways. For example, the enhanced basal analgesia seen in β-arr2−/− mice results from an enhanced level of constitutively active mu receptors (Lam et al, 2011; Walwyn et al, 2007). Such constitutive activity also explains the reduced VDCC inhibition by mu agonists in β-arr2−/− neurons (Table 1; Figure 5; Lam et al, 2011; Walwyn et al, 2007). The well-known attenuated morphine desensitization and tolerance of the mu receptor in β-arr2−/− mice may result from β-arrestin 2 inhibition of mu receptor resensitization, independent of receptor internalization (Dang et al, 2011; Quillinan et al, 2011). This could explain the lack of desensitization of DAMGO-VDCC inhibition following chronic morphine treatments in β-arr2−/− neurons seen in Figure 5. Our data add yet another mechanism to β-arrestin 2 regulation of mu receptor function. We show that the enhanced morphine-induced analgesia and decreased locomotor response in β-arr2−/− mice is JNK-mediated, demonstrating how β-arrestin 2 normally controls this pathway. It would be of further interest to examine whether this role of β-arrestin 2 in masking JNK activation may underlie the enhanced reward, attenuated tolerance, or indeed other pertinent phenotypes of morphine in mice lacking β-arrestin 2.
Acknowledgments
This work was supported by NIH Grants DA05010 and R24-DA025319. O Egbuta, N Desai, N Mittal, and WM Walwyn are supported in part by Hatos Scholarships. N Mittal was also supported by the Gates Millennium Scholars program. Thanks to the laboratory of Robert Lefkowitz for the β-arrestin-1 and 2 knockout mice.
The authors declare no conflict of interest.
References
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol. 2008;74:972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Kraus J, Peters B, Schroeder H, Schulz S, et al. Loss of locomotor sensitisation in response to morphine in D1 receptor deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:562–568. doi: 10.1007/s002100100404. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, et al. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci. 2003;23:10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Xu F, Gainetdinov RR, Caron MG. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci. 2000;20:9040–9045. doi: 10.1523/JNEUROSCI.20-24-09040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgkvist A, Usiello A, Greengard P, Fisone G. Activation of the cAMP/PKA/DARPP-32 signaling pathway is required for morphine psychomotor stimulation but not for morphine reward. Neuropsychopharmacology. 2007;32:1995–2003. doi: 10.1038/sj.npp.1301321. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Valjent E, Santini E, Herve D, Girault JA, Fisone G. Delayed, context- and dopamine D1 receptor-dependent activation of ERK in morphine-sensitized mice. Neuropharmacology. 2008;55:230–237. doi: 10.1016/j.neuropharm.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- Bruchas M, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology. 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Geis C, Sommer C. Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci. 2008;28:5836–5845. doi: 10.1523/JNEUROSCI.4170-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Chieng B, Azriel Y, Christie MJ. Cellular morphine tolerance produced by betaarrestin-2-dependent impairment of mu-opioid receptor resensitization. J Neurosci. 2011;31:7122–7130. doi: 10.1523/JNEUROSCI.5999-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrin SGN, Oligny-Longpré GV, Bonin HLN, Ogawa K, Galés CL, Bouvier M. Conformational rearrangements and signaling cascades involved in ligand-biased mitogen-activated protein kinase signaling through the β1-adrenergic receptor. Mol Pharmacol. 2008;74:162–172. doi: 10.1124/mol.107.043893. [DOI] [PubMed] [Google Scholar]
- Gee KW, Yamamura HI. Selective anxiolytics: are the actions related to partial ″agonist″ activity or a preferential affinity for benzodiazepine receptor subtypes. Adv Biochem Psychopharmacol. 1983;38:1–9. [PubMed] [Google Scholar]
- Groer CE, Schmid CL, Jaeger AM, Bohn LM. Agonist-directed interactions with specific {beta}arrestins determine MU opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem. 2011;286:31731–31741. doi: 10.1074/jbc.M111.248310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Lam H, Maga M, Pradhan A, Evans CJ, Maidment NT, Hales TG, et al. Analgesic tone conferred by constitutively active mu opioid receptors in mice lacking beta-arrestin 2. Mol Pain. 2011;7:24. doi: 10.1186/1744-8069-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C [corrected] Mol Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, et al. μ-Opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol. 2010;78:756–766. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WE, McDonald PH, Cai SF, Field ME, Davis RJ, Lefkowitz RJ. Identification of a motif in the carboxyl terminus of beta-arrestin2 responsible for activation of JNK3. J Biol Chem. 2001;276:27770–27777. doi: 10.1074/jbc.M102264200. [DOI] [PubMed] [Google Scholar]
- Peng Y, Zhang Q, Arora S, Keenan SM, Kortagere S, Wannemacher KM, et al. Novel delta opioid receptor agonists exhibit differential stimulation of signaling pathways. Bioorg Med Chem. 2009;17:6442–6450. doi: 10.1016/j.bmc.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Ping P. Identification of novel signaling complexes by functional proteomics. Circ Res. 2003;93:595–603. doi: 10.1161/01.RES.0000093221.98213.E0. [DOI] [PubMed] [Google Scholar]
- Pontrelli P, Ranieri E, Ursi M, Ghosh-Choudhury G, Gesualdo L, Paolo Schena F, et al. jun-N-terminal kinase regulates thrombin-induced PAI-1 gene expression in proximal tubular epithelial cells. Kidney Int. 2004;65:2249–2261. doi: 10.1111/j.1523-1755.2004.00644.x. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, et al. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillinan N, Lau EK, Virk M, von Zastrow M, Williams JT. Recovery from mu-opioid receptor desensitization after chronic treatment with morphine and methadone. J Neurosci. 2011;31:4434–4443. doi: 10.1523/JNEUROSCI.4874-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. beta-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Yan C, Boyd D. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim Biophys Acta. 2002;1589:311–316. doi: 10.1016/s0167-4889(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Coffa S, Fu H, Gurevich VV. How does arrestin assemble MAPKs into a signaling complex. J Biol Chem. 2009;284:685–695. doi: 10.1074/jbc.M806124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Raman D, Gurevich EV, Vishnivetskiy SA, Gurevich VV. Visual and both non-visual arrestins in their ″inactive″ conformation bind JNK3 and Mdm2 and relocalize them from the nucleus to the cytoplasm. J Biol Chem. 2006;281:21491–21499. doi: 10.1074/jbc.M603659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, et al. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins JL, De SK, Machleidt T, Becattini B, Vazquez J, Kuntzen C, et al. Identification of a new JNK inhibitor targeting the JNK-JIP interaction site. Proc Natl Acad Sci USA. 2008;105:16809–16813. doi: 10.1073/pnas.0805677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Groszer M, Tan AM, Pandya A, Liu X, Xie CW. Phosphoinositide 3-kinase cascade facilitates mu-opioid desensitization in sensory neurons by altering G-protein-effector interactions. J Neurosci. 2003;23:10292–10301. doi: 10.1523/JNEUROSCI.23-32-10292.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent [beta]-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology. 2011;36:551–558. doi: 10.1038/npp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2011;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J Neurosci. 2007;27:5092–5104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294 (Part 2:335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby EA, Collins MK. Dynamic interaction between the dual specificity phosphatase MKP7 and the JNK3 scaffold protein beta-arrestin 2. J Biol Chem. 2005;280:25651–25658. doi: 10.1074/jbc.M501926200. [DOI] [PubMed] [Google Scholar]
- Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, et al. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villen J, et al. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc Natl Acad Sci USA. 2010;107:15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chu J, Zhang Y, Loh HH, Law PY. Modulating micro-opioid receptor phosphorylation switches agonist-dependent signaling as reflected in PKCepsilon activation and dendritic spine stability. J Biol Chem. 2011;286:12724–12733. doi: 10.1074/jbc.M110.177089. [DOI] [PMC free article] [PubMed] [Google Scholar]