Abstract
Numerous epidemiological studies confirm that the prevalence of obesity and the cardiorenal metabolic syndrome (CRS) is extraordinarily high and that the rates have increased dramatically in the last three decades. In addition, epidemiological data demonstrate that obesity, the CRS, and diabetes are inextricably linked and are all associated with an increased incidence of a number of solid tissue cancers. The mechanisms for this association have been examined, including, but not limited to, higher levels of insulin and free levels of insulin-like growth factor and insulin resistance in obesity and the CRS. Mortality, morbidity, and the associated health care costs which are the link between obesity, the CRS, and diabetes are just beginning to be examined. In addition, we review the advantages of implementing lifestyle and surgical changes to modify obesity, lessening the development of the CRS, diabetes, and associated cancers. Epidemiological data regarding the general mechanisms of the pathogenesis of cancers associated with obesity, the CRS, and diabetes (specifically colon, pancreas, esophageal, liver, breast, prostate, thyroid, and renal carcinomas) are reviewed. The mechanisms by which obesity and other components of the CRS contribute to the pathogenesis of these cancers, such as hormone alterations and insulin- and insulin-like growth factor-dependent pathways of tumor pathogenesis, include the attending roles of inflammation and oxidative stress. Emphasis has been placed on obesity as a modifiable risk factor which, when addressed, provides a reduction in the rate of cancer deaths. In a second part to be published in the next issue of this journal, the relationship between diabetes and cancer will be reviewed in detail.
Key Words: Insulin resistance, Cancer, Inflammation, Oxidative stress
Diabetes Mellitus
The prevalence of diabetes mellitus (DM) has increased substantially over the past several decades, in large part due to the growing epidemic of obesity. Approximately 8% of the US population has diabetes, of which more than 90% of cases are classified as type 2 diabetes mellitus (T2DM), with insulin resistance as the major underlying pathophysiology. Obesity has a strong association with insulin resistance, hyperinsulinemia, and glucose intolerance. Adiposity, both subcutaneous and visceral, has been proposed to contribute to insulin resistance, eventually leading to T2DM. Strong evidence exists for the increased glucose intolerance and incidence of T2DM with increasing weight gain in both men and women [1, 2, 3]. Diabetes affects 25.8 million people in the United States, of which 18.8 million cases are diagnosed and 7 million are undiagnosed [1]. National databases, such as the National Health and Nutrition Examination Survey (NHANES), have suggested a greater than fourfold increase in the diabetes prevalence over the past three decades. Given the marked increase in childhood obesity, there is concern that the prevalence of diabetes will continue to increase substantially [1, 3]. According to the NHANES, based on fasting glucose or hemoglobin A1c levels, 35% of adults aged 20 years or older and 50% of adults aged 65 years or older had pre-diabetes in the United States in 2005–2008. Applying this percentage to the entire US population in 2010 yields an estimated 79 million American adults aged 20 years or older with pre-diabetes [1, 3, 4].
DM is clearly an overwhelming pandemic with deathly consequences. The risk of death among people with diabetes is twice that of people of a similar age without diabetes. Adults with diabetes have heart disease death rates that are two to four times higher than those of adults without diabetes, and the risk for stroke is also two to four times higher among people with diabetes [1]. T2DM doubles the risk of all-cause mortality [4] and is also the leading cause of end-stage renal disease, blindness, and non-traumatic amputations [5]. In a recent analysis of 97 prospective studies (820,900 people) calculating the hazard ratio (HR) for cause-specific deaths according to diabetes status in addition to vascular disease, diabetes has been associated with substantial premature death from several cancers, infectious diseases, and degenerative disorders [6].
Obesity
Obesity rates have mirrored diabetes rates and have also trended upward significantly since the 1960s. Overweight is defined as a body mass index (BMI) of 25–29.9, obesity as a BMI >30, and severe obesity as a BMI >40 (or ≥35 in the presence of comorbidities) [7]. Currently, approximately 30% of adults in the United States are obese and 34.4% are overweight. Therefore, over 65% of Americans are overweight or obese [8]. The NHANES, which provides the opportunity to track trends for the prevalence of obesity in the United States by collecting data on height and weight since 1960, shows significant increases in obesity in all sex and age groups. The most striking increases occurred in the 1980s and 1990s. The prevalence of obesity among adults aged 20–74 years increased by 7.9% in men and by 8.9% in women between 1976–1980 and 1988–1994 and, subsequently, by 7.1% in men and by 8.1% in women between 1988–1994 and 1999–2000. The most current data from 2007–2008 suggest that the prevalence rates may have entered a period of relative stability, perhaps with only small increases in obesity. However, the prevalence rates continue to exceed 30% in most sex and age groups [7, 9, 10]. Worldwide, approximately 1 billion people are overweight and 475 million are obese [11]. Excess body weight leads to considerable morbidity, premature mortality, and impaired quality of life. It accounts for 2–8% of all health care costs in developed nations and is rapidly increasing in developing countries [12, 13].
Of the many health conditions associated with obesity, the cardiorenal metabolic syndrome (CRS) sequelae, including cardiovascular and kidney disease, are among the most prevalent ones. When compared to individuals who have a normal BMI, the 10-year risk of developing diabetes increases approximately 20-fold in persons with a BMI ≥35 [14]. In the Prospective Studies Collaboration analysis [15], in individuals within the upper BMI range (25–50), each 5-kg/m2 increase in BMI was associated with a significant increase in mortality from each of the following disorders: ischemic heart disease (HR 1.39) and stroke (HR 1.39), diabetes (HR 2.16), non-neoplastic chronic kidney disease (HR 1.59), and neoplastic disease (HR 1.10). The association between BMI and mortality was significant for several types of cancer, including liver, kidney, breast, endometrial, prostate, colon, and respiratory diseases (HR 1.20) [15].
Obesity among Diabetics
A recent trial analyzing BMI and waist circumference among adults with and without T2DM obtained by the NHANES over the 30-year period from 1976 to 2006 revealed that approximately two-thirds of the US population with T2DM and approximately one-third of the population without diabetes are now obese. What is most concerning is the rapid rise in the prevalence of class III obesity, which has more than doubled over the past two decades. Among adults with T2DM, class III obesity is now present in 1 out of every 5 adults. Among non-Hispanic blacks with T2DM, 1 out of every 3 adults now has class III obesity [16]. In another recent meta-analysis combining 12 fairly homogeneous, high-quality studies, the relative risk of developing T2DM for obese persons, compared to those with normal weight, was sevenfold. The relative risk for overweight individuals was almost threefold [17].
Obesity, Diabetes, and the CRS
Obese adults with T2DM are more likely to suffer from other diabetes-related comorbidities such as end-stage kidney disease. A Swedish population-based case-control study reported that a BMI ≥35 at any point during a person's lifetime increased the odds of diabetic nephropathy by over sevenfold compared to maintaining a lifetime BMI of 25. Other complications include sleep-disordered breathing, non-alcoholic steatohepatitis, and osteoarthritis [16].
The Link between Obesity, Diabetes, and the CRS
The link between diabetes and obesity is well known to medical professionals. In fact, the term ‘diabesity’ was famously coined by several investigators in the 1970s to highlight the close relationship between T2DM and obesity. These investigators [18, 19] demonstrated that young men with no family history of diabetes, when overfed for 6 months, underwent a BMI increase to 28.0, alongside reversible rises in levels of fasting insulin, glucose, and triglycerides as well as impaired glucose tolerance [18, 19]. Around 90% of T2DM patients have a BMI >23.0, with the risk of diabetes being greatly increased by a family history of diabetes or gestational diabetes and early weight gain.
Cohort studies of diabetes incidence have provided a rich body of literature [20, 21, 22, 23, 24, 25] on the association of BMI and diabetes and have shown, for example, that about half of incident diabetes cases have a BMI >30 and that one-fourth to one-sixth have a BMI >35 [24, 25]. Interestingly, both elevated insulin levels and BMI have been shown to be independent predictors of cardiovascular disease [26].
In the Nurses’ Health Study [21],the risk of developing T2DM was shown to correlate with an increasing BMI in women. In this study, a cohort of over 100,000 nurses was followed over a 14-year period. Based on their findings, women with a BMI of 24.0–24.9 had five times the risk of developing T2DM compared to women with a BMI <22. The risk of T2DM in women with a BMI >31 and >35 was increased further to 40 times and 93 times, respectively [21]. The lowest risk was associated with a BMI <22 (slightly lower than in men from the Health Professionals Study) [27].
Weight gain after the age of 18 years in women and after the age of 20 years in men also increases the risk of T2DM. The Nurses’ Health Study, for example, compared women with stable weight (those who gained or lost <5 kg) after the age of 18 years to women who gained weight [21, 27]. Those who had gained between 5.0 and 7.9 kg had a relative risk of diabetes of 1.9; this risk increased to 2.7 for women who gained between 8.0 and 10.9 kg. Similar findings were noted for men in the Health Professionals Study [21, 27]. Men with a BMI >35 had 42 times the risk of developing T2DM compared to men with a BMI <23 [28]. Additionally, in men, both BMI and absolute weight gain at the age of 21 years were reported to be independent risk factors for T2DM [27, 28]. Thus, the excess risk for diabetes with even modest weight gain is substantial [28]. It has been reported that weight gain in men and women during early adulthood (between ages 25 and 40 years) was associated with an increased risk of diabetes as opposed to weight gain in late adulthood (between ages 40 and 55 years). Furthermore, in those individuals who gained weight in early and late adulthood, the relative risk for T2DM was more than 14 times that of individuals who maintained their BMI [29].
The relationship between weight gain and diabetes appears to be relatively consistent among different ethnicities. In a cross-sectional study, the risk of T2DM increased in both obese African-American and Caucasian adults from the United States [30]. Weight gain preceded the onset of diabetes. For example, among Pima Indians (a group with a particularly high incidence of T2DM), body weight gradually increased by 30 kg (from 60 to 90 kg) in the years preceding the diagnosis of diabetes [31, 32].
Obesity and Insulin Resistance
A strong association has been observed between obesity and insulin resistance. Obesity includes both subcutaneous and visceral adiposity [33]. Increased adipose tissue in obese individuals is important because it correlates with higher insulin levels and insulin resistance. It has been postulated that with increased abdominal adiposity there is greater lipolytic activity leading to higher concentrations of free fatty acids (FFA) [34]. Plasma FFA concentrations are high in obese patients. A high plasma FFA concentration is a risk factor for T2DM [relative risk (RR) 2.3] [35], may inhibit insulin secretion, and can inhibit insulin-stimulated glucose uptake in patients with T2DM [36]. In response to the increased circulating FFA, the liver increases synthesis of triglycerides and is unable to breakdown insulin proficiently, resulting in hyperinsulinemia [37].
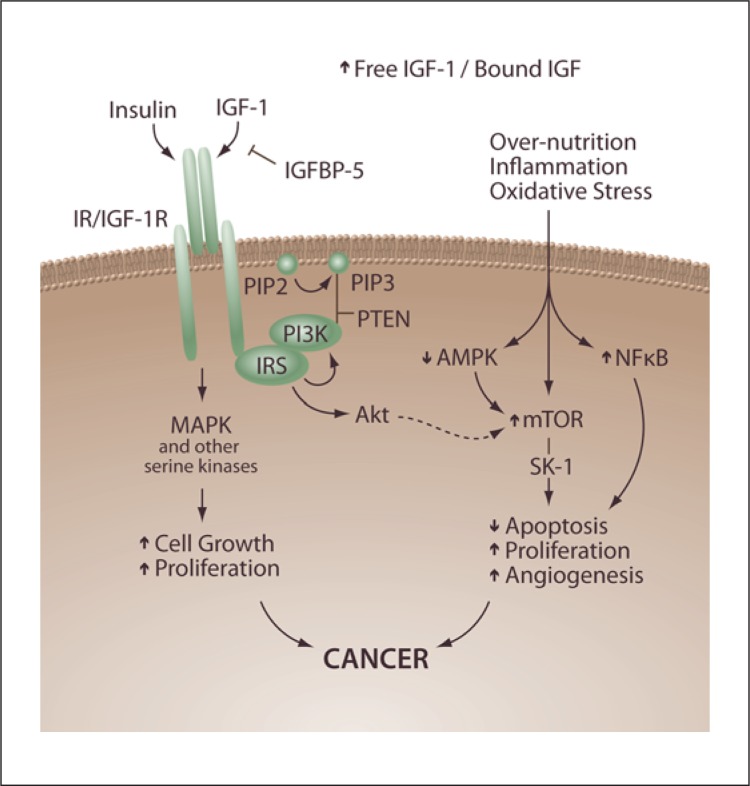
In overweight/obesity and the CRS, adipocytes expand and enlarge; further, they do not only release fatty acids but also adipokines, which are inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, plasminogen activator inhibitor-1, retinol-binding protein, visfatin, and other adipose-derived hormones such as adiponectin, obestatin, and leptin. These substances are known to increase insulin resistance. TNF-α, for instance, can increase the risk of DM development through the inactivation of the insulin receptor substrate-1 (IRS-1) which results in insulin resistance [38, 39, 40, 41, 42] (fig. 1).
Fig. 1.
Overnutrition and insulin resistance in the pathogenesis of cancer. In obesity, an increased release of FFA from adipose tissue and TNF-α as well as a reduced release of adiponectin lead to the development of insulin resistance and compensatory hyperinsulinemia. Increased insulin levels upregulate the hepatic synthesis of IGF-1. These IGF-1 effects are mediated through several downstream signaling pathways, including the PI3K-Akt system. Insulin and IGF-1 signal through the insulin and IGF-1 receptors, respectively, promote cellular proliferation, and inhibit apoptosis in many tissue types. These effects might contribute to tumorigenesis.
Intensive lifestyle interventions and metformin have been shown to decrease markers of inflammation [43]; additionally, among patients with rheumatoid arthritis or psoriasis, the use of anti-inflammatory disease-modifying anti-rheumatic drugs, such as inhibitors of TNF and hydroxychloroquine, is associated with a lower incidence of diabetes than the use of other agents [44]. Furthermore, decreased insulin binding has been suggested as a key feature of insulin resistance in obesity [45]. Obese individuals display hyperinsulinemia during fasting and after a glycemic stimulus; however, despite elevated levels of insulin, they are unable to completely compensate for insulin resistance within peripheral tissues [46]. Ultimately, this leads to an impaired ability to uptake glucose and suppress hepatic glucose production. Thus, both hyperinsulinemia and insulin resistance have been linked to glucose intolerance and other metabolic abnormalities including the metabolic syndrome [34].
The CRS
The CRS, which links abdominal obesity and other cardiovascular and kidney risk factors, was first described in the American Diabetes Association Banting lecture in 1988 [47]. The initial description explored the method by which adipose tissue, skeletal muscle, and liver become resistant to the effects of insulin, the subsequent hyperglycemic drive, and compensatory hyperinsulinemia. Eventually amidst increasing demands for insulin, cell failure occurs, blood glucose rises unchallenged, and T2DM develops. Other organs of the body, such as the ovaries, kidneys, and brain, react badly to raised insulin levels and were described as ‘innocent bystanders’ of the hyperinsulinemic state [48, 49]. According to the International Diabetes Federation, obesity is the single characteristic which must be present for an individual to be categorized as having the metabolic syndrome [18]. A meta-analysis of 21 studies [9] demonstrated that the metabolic syndrome is associated with an increase in the risk of death from cardiovascular disease [RR 1.4; 95% confidence interval (CI) 1.2–1.6] and mortality (RR 1.7; 95% CI 1.3–2.4).
Prevention
The primary benefit of weight loss in patients with T2DM is improved glycemic control [50, 51]. Furthermore, weight loss may reduce fasting hyperinsulinemia, hepatic glucose production, and insulin resistance [51, 52, 53]. In a randomized controlled trial of middle-aged men, weight loss decreased fasting glucose and improved insulin levels compared to aerobic exercise [54]. The same authors suggest that actual weight loss is more important to improving glucose tolerance and should be an essential element of any interventional strategy. Others reported that weight loss significantly reduced the risk for T2DM during a 14-year follow-up in women [21]. Impressively, based on their findings, a weight loss of 5.0 kg reduced the risk of T2DM by 50%. In those with existing impaired glucose tolerance, moderate weight loss combined with physical activity may delay or prevent T2DM [55]. Recent randomized controlled trials have demonstrated that prevention or delay of T2DM is feasible in high-risk populations. A meta-analysis of 8 studies [56] involving 4,573 patients reported that lifestyle modification reduced the incidence of T2DM in patients at risk for T2DM by 63% (95% CI 49–79).
The largest of these trials involving lifestyle impact on diabetes was the Diabetes Prevention Program (DPP) [57] – a 4-year (mean follow-up 2.8 years) randomized controlled trial involving 3,234 individuals with pre-diabetes. Patients were randomly assigned to placebo, metformin, or intensive lifestyle modification. The lifestyle modification intervention involved a 16-session core curriculum delivered by a multidisciplinary team over 24 weeks, which was followed by a long-term maintenance program. Weight losses of 7%, dietary fat intake reductions to less than 25% of total calories, an overall caloric intake of 1,200–1,800 kcal/day, and at least 150 min of physical activity per week were recommended. Weight losses of 0.1 kg in the placebo group, 2.6 kg in the metformin group, and 5.6 kg in the lifestyle modification group were achieved. Compared with placebo, the incidence of T2DM was reduced by 56% (from 11 to 4.8%; 95% CI 48–66) with intensive lifestyle modifications and by 29% (from 11 to 7.8%; 95% CI 17–43) with metformin therapy.
The 3-year cost-effectiveness of the intervention as it was delivered in the DPP was USD 50,000/quality-adjusted life year (QALY) and USD 27,000/QALY in the modified form (primarily assuming that the intervention would be given in groups of ten rather than on an individual basis) [58]. The long-term (30-year) cost-effectiveness for the lifestyle intervention varied between USD 8,800/QALY and USD 63,000/QALY depending on the type of economic model used and the underlying assumptions regarding downstream complication rates [59].
Non-diabetic patients with glucose intolerance were randomized to lifestyle modifications, metformin treatment, or placebo, and a reduced incidence of T2DM was reported in those treated with lifestyle modifications at 2.8 years [55]. According to one group of investigators, improving insulin resistance with weight loss is related to abdominal visceral adiposity [60]. This group reported a 40% decrease in visceral adipose tissue following weight loss. The strong association between abdominal adiposity and insulin resistance supports weight loss as a primary strategy to treat T2DM [61]. Furthermore, according to the 4-year results of the Look AHEAD trial, weight loss markedly improved diabetes control in a large patient cohort [62]. Weight loss was correlated with lower glycated hemoglobin levels in patients treated with intensive lifestyle modifications.
Surgery
Bariatric surgery is recommended by the National Institute for Health and Clinical Excellence (NICE) as a treatment option for adults with obesity if all of the following criteria are fulfilled: the person has a BMI ≥40 or between 35 and 40 as well as another significant disease (for example, T2DM or high blood pressure) that could be improved if they lost weight; all appropriate non-surgical measures have been tried but have failed to achieve or maintain adequate, clinically beneficial weight loss for at least 6 months; the person has been receiving or will receive intensive management in a specialist obesity service; the person is generally fit for anesthesia and surgery, and the person commits to the need for long-term follow-up. Bariatric surgery is also recommended by the NICE as a first-line option for adults with a BMI >50, in whom surgical intervention is considered appropriate [18, 63].
The goal of an effective weight management strategy remains a long-term sustainable weight loss. A subsequent goal of weight management therapy in patients with T2DM is the discontinuation of diabetic medications and improved glycemic control. The role of bariatric surgery has increased over the last two decades as a result of these goals. Bariatric surgery encompasses a range of surgical procedures, broadly classified as either restrictive or malabsorptive. Restrictive procedures include laparoscopic gastric banding and laparoscopic sleeve gastrectomy (LSG), both designed to decrease the quantity of intake by reducing the available gastric volume [64]. In the LSG technique, an inflatable silicone band with a balloon at the inner margin of the ring is placed around the upper part of the stomach. The balloon is connected via a tube to a subcutaneous port situated under the lower rib margin or xiphisternum. Postoperatively, band adjustments to alter the stoma diameter can be made at outpatient follow-up [18].
Malabsorptive bariatric procedures including roux-en-Y gastric bypass (RYGB) are designed to decrease the absorption of nutrients ingested. A RYGB involves dividing the upper part of the stomach to create a small 20-ml pouch as well as the creation of an anastomosis between this pouch and the jejunum, thereby bypassing most of the stomach, duodenum, and about 3 feet of the upper small intestine, rendering them obsolete in processing food [64, 65]. In a systematic review of over 1,846 patients, a T2DM resolution rate of 76% followed bariatric surgery. Resolution was defined as a normalization of laboratory values (i.e. fasting plasma glucose) or discontinuation of medications. Bariatric procedures including RYGB produced impressive T2DM resolution rates of 84 and 97%, respectively. The cause-specific mortality in the surgery group was decreased by 56% for coronary artery disease, by 92% for diabetes, and by 60% for cancer [66]. After a mean follow-up of 7.1 years, in the surgery group, 171 deaths from disease were prevented per 10,000 operations – a net reduction of 136 if other deaths are taken into account [66]. The mortality rate reported by one group was 9% compared to 28% in medically treated controls, which was attributed to a decrease in cardiovascular-related deaths [67].
Restrictive procedures such as LSG have also been shown to be effective in producing weight loss and T2DM resolution. Two-thirds of obese patients had resolution of T2DM following LSG, with an improvement in the majority of the other patients [68]. Plasma glucose levels were reduced from 181 to 119 mg/dl in these patients. The Swedish Obesity Subjects (SOS) study also demonstrated that the 2-year recovery rates from diabetes were higher in the group treated with bariatric surgery [69]. Laparoscopic sleeve banding has been shown to improve T2DM compared to lifestyle changes in a randomized controlled trial [70]. The results are impressive: weight loss is slower than with bypass but equally effective at 4 years, given vigorous postoperative follow-up. The SOS study demonstrated only an average 20% [21] weight loss with the gastric band, but the versions used were early non-adjustable versions [71]. The resolution rate for patients with T2DM is not as high as with gastric bypass; however, an Australian paper reported a 73% remission rate in newly diagnosed diabetic patients compared to 13% in the control group [64].
In conclusion, obesity, the CRS, and diabetes are inevitably linked and, since obesity is clearly a modifiable risk factor, the high prevalence rates of obesity, the CRS, and diabetes can be altered, thereby preventing numerous deaths, associated health care conditions, and costs.
Obesity, the CRS, and Cancer
Obesity is one of the greatest pandemics of our time, and the direct relationship to morbidity and mortality is well known to medical professionals. Despite this knowledge, however, the prevalence of obesity is astounding. There are currently 475 million obese individuals worldwide, and the WHO predicts that by 2015 approximately 2.3 billion adults will be overweight and more than 700 million will be obese [11]. Much of the emphasis on obesity-related complications has included diabetes, cardiovascular disease, hypertension, hyperlipidemia, renal disease, and stroke; however, the striking association to a variety of cancers has often been overlooked. It has been estimated that 15–20% of all cancer deaths in the United States can be attributed to being overweight or obese [72, 73].
In a recent study, an estimated 33,966 new cancers (4% of all estimated cancers) in males and 50,535 (7% of all estimated cancers) in females or 6% of all new cancers diagnosed in 2007 may potentially be attributable to obesity [74]. In a landmark epidemiological study, those with a BMI ≥40 had death rates from cancer that were 52% (for men) and 62% (for women) higher than the rates in men and women with normal weight [72]. In a systematic review and meta-analysis from the Comparative Risk Assessment Project evaluating data on 7 million deaths from cancer worldwide, 2.43 million were attributable to potentially modifiable risk factors, including being overweight or obese [75].
Which Cancers Are Associated with Obesity?
In 2002, the International Agency for Research on Cancer's Handbook on Weight Control and Physical Activity concluded that being overweight or obese is related to cancers of the colon, endometrium, kidney, pancreas, and esophagus (adenocarcinomas) as well as to postmenopausal breast cancer [75]. Since that report, continuing epidemiological investigation has suggested that other cancers are also related to being overweight or obese. In addition to those listed above, the 2007 report by the World Cancer Research Fund (WCRF) Panel on Food, Nutrition, Physical Activity, and the Prevention of Cancer determined that there was convincing evidence for an association of cancers of the pancreas, colon, and rectum and a probable association with cancers of the gall bladder as well [76]. In addition, the authors found probable evidence that abdominal fatness, in particular, increases the risk for pancreatic, endometrial, and postmenopausal breast cancer [77].
In a meta-analysis of 141 studies that included 282,137 cancer cases, a 5-kg/m2 increase in BMI in men was associated with esophageal, colon, thyroid, and renal cancers [76]. In women, a 5-kg/m2 increase was associated with endometrial, gallbladder, esophageal, and renal cancers. Associations with leukemia and thyroid, postmenopausal breast, pancreas, and colon cancers as well as with non-Hodgkin's lymphoma were similar in studies from North America, Europe, Australia, and the Asia-Pacific region, with the exception of stronger associations between BMI and breast cancer in the Asia-Pacific populations [76].
The strong epidemiological data presented above suggest that obesity is one of the causes of cancer. However, these data cannot prove cause-effect relationships and may be confounded by selection bias. There are also limited data clarifying the underlying mechanisms for this association. It is possible that the increased production of estrogens by adipose tissue stromal cells, together with the decrease of sex steroid-binding globulin, is responsible for the increased risk of endometrial and, perhaps, breast cancer. Insulin resistance and increased free levels of insulin-like growth factor-1 (IGF-1) may play a role in cancer formation (fig. 1, 2) [78, 79, 80].
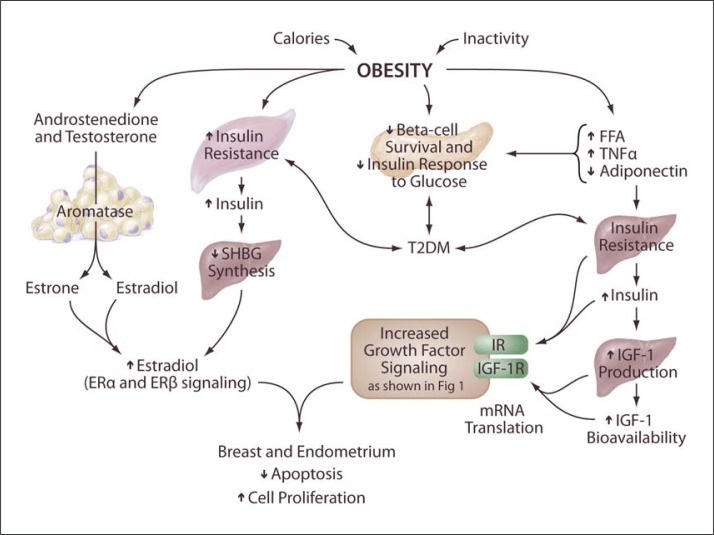
Fig. 2.
Obesity-promoting insulin- and IGF-1-dependent pathways of tumorigenesis. There is increased aromatase activity in adipose tissues. Therefore, in obese individuals, there is typically an increased conversion of the androgens androstenedione and testosterone into the estrogens estrone and estradiol by aromatase enzyme. In parallel, obesity leads to hyperinsulinemia, which in turn causes a reduction in the hepatic synthesis and circulating levels of SHBG. The combined effect of the increased formation of estrone and estradiol along with the reduced levels of SHBG leads to an increase in the bioavailable fractions of estradiol that can diffuse to target cells, and, in some tissues, for example breast epithelium and endometrium, they promote cellular proliferation and inhibit apoptosis.
Insulin-/IGF-1-Dependent Pathway of Tumor Pathogenesis
In obesity, an increased release of FFAs from adipose tissue and TNF-α as well as a reduced release of adiponectin and reduced generation of 5′-adenosine monophosphate-activated protein kinase (AMPK) lead to the development of insulin resistance and compensatory, chronic hyperinsulinemia (fig. 1). Increased insulin levels upregulate the hepatic synthesis of IGF-1, and the growth effects of IGF-1 are mediated through several downstream signaling networks, including the phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) system. Insulin and IGF-1 signal through the insulin and IGF-1 receptors, respectively, to promote cellular proliferation and inhibit apoptosis in many tissue types. These effects might contribute to tumor pathogenesis [80, 81, 82].
Obesity and Hormone Production
Adipose tissue produces aromatase enzymes. Therefore, in obese individuals, there is typically an increased conversion of the androgens androstenedione and testosterone into the estrogens estrone and estradiol by these aromatase enzymes. In parallel, obesity leads to hyperinsulinemia, which in turn causes a reduction in the hepatic synthesis and circulating levels of sex hormone-binding globulin (SHBG). The combined effect of the increased formation of estrone and testosterone along with the reduced levels of SHBG leads to an increase in the bioavailable fractions of estradiol that can diffuse to target cells, and, in some tissues, for example breast epithelium and endometrium, they promote cellular proliferation and inhibit apoptosis (fig. 2) [82].
The Role of Inflammation and Oxidative Stress
Obesity is associated with systemic low-grade inflammation, which has been suggested to have an important role in the pathogenesis of some disorders such as insulin resistance, atherosclerosis, and cancer. Adipose tissues release a variety of factors, including cytokines (IL-6 and IL-1) and TNF-α, chemokines [monocyte chemotactic protein 1 (MCP-1)] and adipokines [haptoglobin, leptin, visfatin, resistin, and vascular endothelial growth factor (VEGF)], and the reduction of anti-inflammatory adipokines (e.g. adiponectin, IL-10, IL-1) [83, 84].
TNF-α is involved in carcinogenesis, and recent studies have suggested that this is because of its ability to activate nuclear factor-kappa B (NFκB), which promotes cell survival [85, 86]. Also, TNF-α appears to contribute to the development of the tissue architecture necessary for tumor growth and metastasis [87, 88]. It also induces other cytokines, angiogenic factors, and matrix metalloproteinases (MMPs) and thus drives the increased growth and survival of tumor cells [89]. IL-6 is an important regulator of immune cell growth and differentiation. Recent studies have demonstrated that IL-6 regulates chronic inflammation, which can create a cellular microenvironment beneficial to cancer growth [90]. High circulating IL-6 concentrations in obesity correlated with overall cancer death and increased risk of cancer precursor lesions [91]. The activation of the IL-6 complex activates Janus kinases (JAK) and the signal transducer and activator of transcription 3 (STAT3) pathways, which regulate cell proliferation and apoptosis [92].
Adiponectin is a hormone mainly secreted by adipose tissue. The most important functions of adiponectin are anti-atherogenic, anti-inflammatory, and insulin-sensitivity effects. In contrast to other adipokines, circulating levels of adiponectin are negatively associated with obesity, BMI, visceral fat accumulation, and insulin resistance [93]. Several case-control studies have observed that serum adiponectin levels were significantly decreased in breast cancer patients [94]. Moreover, in vitro studies have demonstrated that adiponectin treatment suppressed cell proliferation and caused cell growth arrest and apoptosis in breast cancer cells [95]. Adiponectin has been shown to inhibit endothelial NFκB signaling and to markedly reduce TNF-α production in cultured macrophages [96].
Colon Cancer and Obesity
Obesity has been consistently associated with a higher risk of colorectal cancer in men and women in both case-control and cohort studies [97, 98, 99, 100]. A meta-analysis performed in 2007 examining 30 prospective studies demonstrated that there was an increased risk of colon cancer with increasing waist circumference (per 10-cm increase) in both men and women and with increasing waist-hip ratio in both men and women. BMI was positively associated with rectal cancer in men but not in women [100].
For colon cancer, growing evidence points to insulin pathways mediating the effect of increased BMI and associated cancer risk [101]. Studies of blood glucose levels and colon cancer have shown a direct relation between higher glucose level and the subsequent risk [102]. Providing further biologic rationale, C-peptide [103], a marker of insulin production, has also shown this positive relationship, and animal models using insulin injection versus saline have demonstrated a significantly higher incidence of colon cancer among those animals injected with insulin [104]. The PI3K/Akt pathway stimulation of mTOR and serine 6-kinase (S6K-1) likely mediates the downstream growth effects of insulin and IGF-1, and is one of the pathways most commonly altered in tumors [105]. Drugs which target this pathway are interesting possibilities for the treatment of cancers which are promoted by signaling through this pathway (fig. 1) [106].
Pancreatic Cancer and Obesity
Obesity has also been linked to other types of cancer, although overall the amount of studies or data available is still limited. Several recent studies have suggested that a high BMI may be associated with approximately doubling the risk for pancreatic cancer in men and women [107]. Moreover, a recent meta-analysis supports a positive relationship between BMI and risk of pancreatic cancer [108]. Two further studies have found some evidence for a positive association with waist circumference in men but not in women [109, 110].
Esophageal Cancer and Obesity
Obesity is associated with a threefold increase in the risk for adenocarcinoma of the esophagus [107]. The link between obesity and risk of esophageal cancer has recently been confirmed by a quantitative meta-analysis that included 12 case-control studies and 2 cohort studies [111]. Obesity is associated with gastroesophageal reflux, and frequent reflux is, in turn, very strongly associated with esophageal adenocarcinoma [107].
Liver Cancer and Obesity
Obesity has been established as a significant risk factor for liver diseases. A large prospective mortality study demonstrated that a high BMI was significantly associated with higher rates of liver cancer-related deaths. Compared to patients with a normal BMI, the relative risk of mortality from liver cancer was 1.68 times higher in women and 4.52 times higher in men with a BMI >35 [72]. Similarly, data obtained from the United Network of Organ Sharing (UNOS) database on all liver transplants carried out in the United States from 1991 to 2000 showed that the overall incidence of hepatocellular carcinoma (HCC) in patients undergoing liver transplantation was 3.4%, with a slightly higher prevalence among obese patients with an incidence of 4.0%. Moreover, in this study, obesity was confirmed to be an independent risk factor for HCC in patients with alcoholic cirrhosis (odds ratio 3.2) and cryptogenic cirrhosis (odds ratio 11.1) [112]. Obesity has definitively been established as a risk factor for the development of HCC. It is likely that this association underlies the progression from non-alcoholic fatty liver disease to cirrhosis, but it remains unclear whether cirrhosis is a necessary prerequisite for the development of HCC [113]. Animal models of non-alcoholic fatty liver disease support the hypothesis that obesity-related metabolic abnormalities, rather than cirrhosis, initiate the hepatic neoplastic process during obesity [114].
Breast Cancer and Obesity
Many epidemiological studies since the 1970s have shown an association between obesity and risk of breast cancer. Early studies established that the association between body size and risk of breast cancer varied based on the menopausal status, and that heavier women were at increased risk of developing postmenopausal, but not premenopausal, breast cancer [115]. Obesity has been consistently shown to increase the rates of breast cancer in postmenopausal women by 30–50% by increasing serum concentrations of free estradiol [116, 117]. Both BMI and weight gain are more strongly related to the risk of breast cancer among postmenopausal women who have never used hormone replacement therapy, compared with women who have used hormones [118]. Furthermore, abdominal adiposity has been found to be positively associated with a higher risk of breast cancer in postmenopausal women, this relationship being stronger among those without hormone replacement therapy than among those with hormone replacement therapy [119]. Several hypotheses have been proposed to explain this effect, including alterations in sex hormones, growth factors, cytokines, and insulin and/or IGFs, but there are numerous mechanisms at play and many are not fully understood (fig. 1, 2) [120].
Endometrial Cancer and Obesity
There is convincing and consistent evidence from both case-control and cohort studies that being overweight or obese is strongly associated with endometrial cancer [72]. In fact, the risk of developing endometrial cancer is about two to four times higher in obese women than in lean women [72], and about 40% of endometrial cancer incidence has been estimated to be attributable to excess body weight [121]. As with breast cancer, the potential mechanism for the increased risk of endometrial cancer associated with obesity is the increase in circulating estrogens [72]. Many studies have shown large increases in the endometrial cancer risk among postmenopausal women who take unopposed estrogen (i.e. estrogen in the absence of progesterone) [122].
Thyroid Cancer and Obesity
The prevalence of thyroid cancer has risen dramatically since the 1980s [123] along with the prevalence of obesity [1]. Several studies have shown an association between thyroid cancer and obesity. In a meta-analysis of 141 studies that included 282,137 cancer cases, a 5-kg/m2 increase in BMI in men was associated with thyroid cancer [76]. In addition, a recent pooled analysis of 5 prospective studies examined the association between BMI and thyroid cancer risk of 413,979 women and 434,953 men. Over a median follow-up of 10.3 years, 768 women and 388 men were diagnosed with thyroid cancer. The risk of thyroid cancer was greater with each 5-kg/m2 increase in BMI (in women, HR 1.16; 95% CI 1.08–1.24; in men, HR 1.21; 95% CI 0.97–1.49) [123].
Renal Cancer and Obesity
An analysis of the association of body size, lifestyle, and medical conditions with renal cell cancer risk was performed among 161,126 participants of the Hawaii-Los Angeles Multiethnic Cohort (1993–2002). After 8.3 years of follow-up, 347 renal cell cancer cases (220 men, 127 women) were identified. The renal cell cancer risk increased with increasing BMI in men and women. The RR associated with being obese compared with being lean was 1.76 [124]. A meta-analysis of 141 studies also found an association between renal cancer and increased BMI in men and woman [76].
Obesity, Cancer, and Death
Obesity also increases the likelihood of dying from cancer. A 16-year prospective study of >900,000 men and women in the United States found a RR of death from cancer of 1.5 for men and 1.6 for women in the group with a BMI >40 versus the group with a BMI of 18.5–24.9. For both men and women, increasing BMI was associated with higher death rates due to cancers of the esophagus, colon and rectum, liver, gallbladder, pancreas, and kidney as well as due to non-Hodgkin's lymphoma and multiple myeloma. Men were also at increased risk for death from stomach or prostate cancer, while women were at increased risk of death from cancers of the breast, cervix, uterus, or ovary. On the basis of these data, the authors estimated that being overweight or obese could account for 14% of all cancer deaths in men and for 20% in women in the United States [72].
Effects of Weight Gain and Loss
Loss of weight and weight maintenance are associated with a decreased risk of cancer. There has been a documented 50% reduction in the risk for breast cancer among women who lost ≥10 kg after the menopause and kept it off. Menopause is a time in life when obesity clearly increases the risk for breast cancer, so it is important for women to be cognizant of their weight at this time. This lower incidence of postmenopausal breast cancer follows the decline in circulating estrogen after weight loss [125]. More recent evidence confirms that weight gain in adulthood appears to increase the risk for colon cancer. In a case-control study in Canada, men who gained more than 21 kg after the age of 20 years had a 60% higher risk for colorectal cancer than men who had gained just 1–5 kg [126, 127].
Physical Activity and Weight Loss
The majority of evidence points to weight at diagnosis as the major lifestyle risk for poor breast cancer outcomes (and poorer quality of life), with growing evidence that weight gain after diagnosis exacerbates the risk [128] – a result seen most clearly among women who were lean at the time of diagnosis or non-smokers. Non-smoking women with an increase in their BMI of more than 2 kg/m2 after a breast cancer diagnosis had a RR of 1.64 (95% CI 1.07–2.51) for death during 9 years of follow-up, compared with woman with a stable weight. Insulin/IGF-1 pathways have been suggested as one mechanism for this effect [129]. Weight gain following diagnosis may be particularly problematic because research suggests that it is largely an increase in fat mass and not muscle mass [130]. Furthermore, evidence shows that physical activity after diagnosis reduces the risk for breast cancer recurrence [131], and intervention trials of diet and physical activity have shown longer disease-free survival times among the intervention group, whose members lost substantially more weight than the control group [132]; these results point to the importance of energy balance in predicting progression of breast cancer.
Chemotherapy
In addition to the impact of obesity on disease incidence and progression, concern has been raised regarding the potential for chemotherapy dosing to be poorly matched to weight in heavier cancer patients. The narrow therapeutic index associated with chemotherapeutic drugs prompts a rational concern on the part of the medical oncologist that high doses of chemotherapy required by very obese individuals will result in excess toxicity. Research in this area has suggested that obese patients are frequently treated at lower chemotherapy dose intensities than the non-obese patients. Paradoxically, studies have not demonstrated greater chemotherapy-related toxicity in obese patients treated at full dose intensities [133].
Obesity may also interfere with the ability to deliver other forms of treatment. Wong et al. [134] found that there was a shift in the delivery of external beam radiation in obese patients, resulting in the target location not receiving the full dose. In addition, research has suggested that, among men undergoing prostatectomy, surgical margins may not be as clean in obese men and that they may have fewer nerve bundles preserved [135]. The quality of life among cancer patients and those free from cancer is reduced by a higher BMI. Limited data suggest that weight loss is associated with improved quality of life. More substantial data indicate that an increase in physical activity among cancer survivors leads to significant increases in their quality of life [136].
Some of the best evidence indicating that weight loss can reduce the risk for cancer comes from recent studies in bariatric surgery patients. Two large cohort studies suggest that large weight loss from bariatric surgery reduces the risk for cancer death [67, 71]. The mean weight loss 15 years after surgery was in the range of 14–27% in a Swedish patient population [71]. In a US patient sample, cancer death rates, excluding prevalent cancers, were 38% lower (HR 0.62; 95% CI 0.61–0.74) in patients undergoing RYGB than in BMI-matched controls, with some indication that the reduction in the death rate was stronger in men than in women. The cancer death rate reduction was larger when including prevalent cases of cancer at baseline (HR 0.40; 95% CI 0.25–0.65) [67].
In summary, the role of obesity, the CRS, and diabetes in contributing to an increased incidence of cancer has been reviewed. In addition, adipokines, sex and growth hormones, insulin-/IGF-1-dependent growth pathway inflammation, and oxidative stress have been explored as factors promoting cancer. Emphasis has been placed on obesity as a modifiable risk factor which, when addressed, provides a reduction in the cancer death rate. Finally, the correlation between DM and cancer was presented, including the associated cancers and the mechanisms linking obesity, the CRS, and DM in the pathogenesis of solid tumors.
Acknowledgments
The authors thank Brenda Hunter for editing the manuscript. Research in the Sowers and Whaley-Connell laboratories is supported by NIH (R01 HL73101 and R01 HL107910-01) and Veterans Affairs Merit System 0018 (J.R.S.).
References
- 1.Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, US Department of Health and Human Services, Centers for Disease Control and Prevention, 2011.
- 2.Cooney K, Gruber S. Hyperglycemia, obesity, and cancer risks on the horizon. JAMA. 2005;293:235–236. doi: 10.1001/jama.293.2.235. [DOI] [PubMed] [Google Scholar]
- 3.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 4.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 5.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32:S1–S201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J, Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose and risk of cause specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics Health, United States, 2008: With Chartbook. Hyattsville, 2009. http://www.cdc.gov/nchs/data/hus/hus08.pdf (accessed January 3, 2012).
- 8.National Center for Health Statistics Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, 2011. http://www.cdc.gov/nchs/data/hus/hus10.pdf (accessed January 3, 2012). [PubMed]
- 9.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 10.World Cancer Research Fund/American Institute for Cancer Research (AICR) Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington: AICR; 2007. [Google Scholar]
- 11.International Obesity Task Force Global obesity prevalence in adults. www.iaso.org/iotf/obesity/obesitytheglobalepidemic (accessed January 9, 2011).
- 12.Haslam D, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 13.Birmingham CL, Muller JL, Palepu A, Spinelli JJ, Anis AH. The cost of obesity in Canada. CMAJ. 1999;160:483–488. [PMC free article] [PubMed] [Google Scholar]
- 14.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 15.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R, Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer H, Cao G, Dugas L, Luke A, Cooper R, Durazo-Arvizu R. Increasing BMI and waist circumference and prevalence of obesity among adults with Type 2 diabetes: the National Health and Nutrition Examination Surveys. J Diabetes Complications. 2010;24:368–374. doi: 10.1016/j.jdiacomp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89:309–319. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Haslam D. Obesity and diabetes: the links and common approaches. Prim Care Diabetes. 2010;4:105–112. doi: 10.1016/j.pcd.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Wannamethee SG, Shaper AG. Weight change and duration of overweight and obesity in the incidence of type 2 diabetes. Diabetes Care. 1999;22:1266–1272. doi: 10.2337/diacare.22.8.1266. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care. 2005;28:1599–1603. doi: 10.2337/diacare.28.7.1599. [DOI] [PubMed] [Google Scholar]
- 21.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, Haffner SM, Pettitt DJ, Sorkin JD, Muller DC, Collins VR, Hamman RF. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701–710. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997;146:214–222. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- 24.Leibson CL, Williamson DF, Melton LJ, 3rd, Palumbo PJ, Smith SA, Ransom JE, Schilling PL, Narayan KM. Temporal trends in BMI among adults with diabetes. Diabetes Care. 2001;24:1584–1589. doi: 10.2337/diacare.24.9.1584. [DOI] [PubMed] [Google Scholar]
- 25.Burke JP, Williams K, Narayan KM, Leibson C, Haffner SM, Stern MP. A population perspective on diabetes prevention: whom should we target for preventing weight gain? Diabetes Care. 2003;26:1999–2004. doi: 10.2337/diacare.26.7.1999. [DOI] [PubMed] [Google Scholar]
- 26.Wing RR, Bunker CH, Kuller LH, Matthews KA. Insulin, body mass index, and cardiovascular risk factors in premenopausal women. Arteriosclerosis. 1989;9:479–484. doi: 10.1161/01.atv.9.4.479. [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 28.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 29.Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2006;84:427–433. doi: 10.1093/ajcn/84.1.427. [DOI] [PubMed] [Google Scholar]
- 30.Cohen SS, Signorello LB, Blot WJ. Adult weight gain and diabetes among African American and white adults in southeastern US communities. Prev Med. 2009;49:476–481. doi: 10.1016/j.ypmed.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felber JP. From obesity to diabetes. Pathophysiological considerations. Int J Obes Relat Metab Disord. 1992;16:937–952. [PubMed] [Google Scholar]
- 32.Knowler WC, Pettitt DJ, Saad MF, Charles MA, Nelson RG, Howard BV, Bogardus C, Bennett PH. Obesity in the Pima Indians: its magnitude and relationship with diabetes. Am J Clin Nutr. 1991;53:1543S–1551S. doi: 10.1093/ajcn/53.6.1543S. [DOI] [PubMed] [Google Scholar]
- 33.Spiegelman D, Israel RG, Bouchard C, Willett WC. Absolute fat mass, percent body fat, and body-fat distribution: which is the real determinant of blood pressure and serum glucose? Am J Clin Nutr. 1992;55:1033–1044. doi: 10.1093/ajcn/55.6.1033. [DOI] [PubMed] [Google Scholar]
- 34.Rexrode KM, Manson JE, Hennekens CH. Obesity and cardiovascular disease. Curr Opin Cardiol. 1996;11:490–495. doi: 10.1097/00001573-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38:1213–1217. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- 36.Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest. 1995;96:1261–1268. doi: 10.1172/JCI118160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 38.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G, Atherosclerosis Risk in Communities Study Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 39.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 40.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 41.de Rekeneire N, Peila R, Ding J, Colbert LH, Visser M, Shorr RI, Kritchevsky SB, Kuller LH, Strotmeyer ES, Schwartz AV, Vellas B, Harris TB. Diabetes, hyperglycemia, and inflammation in older individuals: the health, aging and body composition study. Diabetes Care. 2006;29:1902–1908. doi: 10.2337/dc05-2327. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Du YY, Lin YF, Chen YT, Yang L, Wang HJ, Ma D. The cell growth suppressor, mir-126, targets IRS-1. Biochem Biophys Res Commun. 2008;377:136–140. doi: 10.1016/j.bbrc.2008.09.089. [DOI] [PubMed] [Google Scholar]
- 43.Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, Marcovina S, Mather K, Orchard T, Ratner R, Barrett-Connor E, Diabetes Prevention Program Research Group Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525–2531. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 45.Harrison LC, Martin FI, Melick RA. Correlation between insulin receptor binding in isolated fat cells and insulin sensitivity in obese human subjects. J Clin Invest. 1976;58:1435–1441. doi: 10.1172/JCI108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonadonna RC, Groop L, Kraemer N, Ferrannini E, Del Prato S, DeFronzo RA. Obesity and insulin resistance in humans: a dose-response study. Metabolism. 1990;39:452–459. doi: 10.1016/0026-0495(90)90002-t. [DOI] [PubMed] [Google Scholar]
- 47.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 48.An Interview with Gerald Reaven Syndrome X: The Risks of Insulin Resistance. Gerald Reaven, MD, Professor Emeritus (Active) of Medicine at Stanford University. http://www.cacr.ca/informationforpublic/archivedissues/2000s/0009Reaven.pdf (accessed January 4, 2012).
- 49.Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, Nadeau A, Després JP. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–184. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 50.Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian AD, Purnell JQ, Wheeler M, American Diabetes Association Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2003;26:S51–S61. doi: 10.2337/diacare.26.2007.s51. [DOI] [PubMed] [Google Scholar]
- 51.Franz MJ. The glycemic index: not the most effective nutrition therapy intervention. Diabetes Care. 2003;26:2466–2468. doi: 10.2337/diacare.26.8.2466. [DOI] [PubMed] [Google Scholar]
- 52.Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994;17:30–36. doi: 10.2337/diacare.17.1.30. [DOI] [PubMed] [Google Scholar]
- 53.Henry RR, Wallace P, Olefsky JM. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986;35:990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- 54.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995;274:1915–1921. doi: 10.1001/jama.1995.03530240025035. [DOI] [PubMed] [Google Scholar]
- 55.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orozco LJ, Buchleitner AM, Gimenez-Perez G, Roqué I Figuls M, Richter B, Mauricio D. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;3:CD003054. doi: 10.1002/14651858.CD003054.pub3. [DOI] [PubMed] [Google Scholar]
- 57.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diabetes Prevention Program Research Group Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26:2518–2523. doi: 10.2337/diacare.26.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engelgau MM. Trying to predict the future for people with diabetes: a tough but important task. Ann Intern Med. 2005;143:301–302. doi: 10.7326/0003-4819-143-4-200508160-00011. [DOI] [PubMed] [Google Scholar]
- 60.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete F. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 61.Abate N, Garg A, Peshock RM, Stray-Gundersn J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996;45:1684–1693. doi: 10.2337/diab.45.12.1684. [DOI] [PubMed] [Google Scholar]
- 62.Look AHEAD Research Group. Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Institute for Health and Clinical Excellence 2002/041 NICE issues guidance on surgery for morbid obesity. NICE 2002/041 Issued: 19th July 2002. Press Release. www.nice.org.uk/guidance/index.jsp?action=article&o=32423
- 64.Gill RS, Sharma AM, Gill SS, Birch DW, Karmali S. The impact of obesity on diabetes mellitus and the role of bariatric surgery. Maturitas. 2011;69:137–140. doi: 10.1016/j.maturitas.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 66.Adams TD, Gres RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, LaMonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 67.MacDonald KG, Jr, Long SD, Swanson MS, Brown BM, Morris P, Dohm GL, Pories WJ. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg. 1997;1:213–220. doi: 10.1016/s1091-255x(97)80112-6. [DOI] [PubMed] [Google Scholar]
- 68.Gill RS, Birch DW, Shi X, Sharma AM, Karmali S. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707–713. doi: 10.1016/j.soard.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Sjöström CD, Lissner L, Wedel H, Sjöström L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–484. doi: 10.1002/j.1550-8528.1999.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 70.Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 71.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM, Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 72.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 73.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Polednak AP. Estimating the number of U.S. incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-related cancers. Cancer Detect Prev. 2008;32:190–199. doi: 10.1016/j.cdp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 75.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M, Comparative Risk Assessment collaborating group (Cancers) Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 76.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 77.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 78.McTiernan A. Obesity and cancer: the risks, science, and potential management strategies. Oncology. 2005;19:871–881. [PubMed] [Google Scholar]
- 79.Otake S, Takeda H, Suzuki Y, Fukui T, Watanabe S, Ishihama K, Saito T, Togashi H, Nakamura T, Matsuzawa Y, Kawata S. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res. 2005;11:3642–3646. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 80.Yakar S, Leroith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407–420. doi: 10.1016/j.cytogfr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 81.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 82.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 83.Maccio A, Madeddu C, Mantovani G. Adipose tissue as target organ in the treatment of hormone-dependent breast cancer: new therapeutic perspectives. Obes Rev. 2009;10:660–670. doi: 10.1111/j.1467-789X.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 84.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 85.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 86.Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, Gould D, Ayhan A, Balkwill F. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tomita Y, Yang X, Ishida Y, Nemoto-Sasaki Y, Kondo T, Oda M, Watanabe G, Chaldakov GN, Fujii C, Mukaida N. Spontaneous regression of lung metastasis in the absence of tumor necrosis factor receptor p55. Int J Cancer. 2004;112:927–933. doi: 10.1002/ijc.20493. [DOI] [PubMed] [Google Scholar]
- 88.Balkwil F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 89.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 90.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 91.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 92.Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J, Vermeulen J, Singh N, Avril N, Cummings J, Rexhepaj E, Jirström K, Gallagher WM, Brennan DJ, McNeish IA, Balkwill FR. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–6096. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 94.Schäffler A, Schölmerich J, Buechler C. Mechanisms of disease: adipokines and breast cancer – endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab. 2007;3:345–354. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- 95.Kang JH, Lee YY, Yu BY, Yang BS, Cho KH, Yoon DK, Roh YK. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res. 2005;28:1263–1269. doi: 10.1007/BF02978210. [DOI] [PubMed] [Google Scholar]
- 96.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 97.Lee IM, Paffenbarger RS., Jr Quetelet's index and risk of colon cancer in college alumni. J Natl Cancer Inst. 1992;84:1326–1331. doi: 10.1093/jnci/84.17.1326. [DOI] [PubMed] [Google Scholar]
- 98.MacInnis RJ, English DR, Hopper JL, Haydon AM, Gertig DM, Giles GG. Body size and composition and colon cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2004;13:553–559. [PubMed] [Google Scholar]
- 99.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjønneland A, Halkjaer J, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Guernec G, Bergmann MM, Linseisen J, Becker N, Trichopoulou A, Trichopoulos D, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, Bueno-de-Mesquita HB, Boshuizen HC, Van Guelpen B, Palmqvist R, Berglund G, Gonzalez CA, Dorronsoro M, Barricarte A, Navarro C, Martinez C, Quirós JR, Roddam A, Allen N, Bingham S, Khaw KT, Ferrari P, Kaaks R, Slimani N, Riboli E. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:920–931. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 100.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 101.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–s842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 102.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005;14:850–855. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 103.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 104.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 105.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, Roberts TM, Shivdasani RA. Targeting P13K signaling as a therapeutic approach for colorectal cancer. Gastroenterology. 2011;141:50–61. doi: 10.1053/j.gastro.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 107.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog. 2012;51:53–63. doi: 10.1002/mc.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer. 2007;120:1993–1998. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 109.Larsson SC, Permert J, Hakansson N, Naslund I, Bergkvist L, Wolk A. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer. 2005;93:1310–1315. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sinner PJ, Schmitz KH, Anderson KE, Folsom AR. Lack of association of physical activity and obesity with incident pancreatic cancer in elderly women. Cancer Epidemiol Biomark Prev. 2005;14:1571–1573. doi: 10.1158/1055-9965.EPI-05-0036. [DOI] [PubMed] [Google Scholar]
- 111.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15:872–878. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 112.Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–155. doi: 10.1053/jhep.2002.33713. [DOI] [PubMed] [Google Scholar]
- 113.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 114.Yang S, Lin HZ, Hwang J, Chacko VP, Diehl AM. Hepatic hyperplasia in noncirrhotic fatty livers: is obesity-related hepatic steatosis a premalignant condition? Cancer Res. 2001;61:5016–5023. [PubMed] [Google Scholar]
- 115.International Agency for Research on Cancer IARC Handbooks of Cancer Prevention. Weight Control and Physical Activity. International Agency for Research on Cancer, Lyon, vol. 6, 2002.
- 116.Trentham-Dietz A, Newcomb PA, Egan KM, Titus-Ernstoff L, Baron JA, Storer BE, Stampfer M, Willett WC. Weight change and risk of postmenopausal breast cancer (United States) Cancer Causes Control. 2000;11:533–542. doi: 10.1023/a:1008961931534. [DOI] [PubMed] [Google Scholar]
- 117.Trentham-Dietz A, Newcomb PA, Nichols HB, Hampton JM. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res Treat. 2007;105:195–207. doi: 10.1007/s10549-006-9446-y. [DOI] [PubMed] [Google Scholar]
- 118.Begum P, Richardson CE, Carmichael AR. Obesity in post menopausal women with a family history of breast cancer: prevalence and risk awareness. Int Semin Surg Oncol. 2009;6:1. doi: 10.1186/1477-7800-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev. 2003;4:157–173. doi: 10.1046/j.1467-789x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 120.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 121.Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91:421–430. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 122.Key TJ, Pike MC. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer. 1988;57:205–212. doi: 10.1038/bjc.1988.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, Schairer C, Schatzkin A, Shikany JM, Berrington de González A. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20:464–472. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007;166:932–940. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 125.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 126.Campbell PT, Cotterchio M, Dicks E, Parfrey P, Gallinger S, McLaughlin JR. Excess body weight and colorectal cancer risk in Canada: associations in subgroups of clinically defined familial risk of cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1735–1744. doi: 10.1158/1055-9965.EPI-06-1059. [DOI] [PubMed] [Google Scholar]
- 127.Russo A, Franceschi S, La Vecchia C, Dal Maso L, Montella M, Conti E, Giacosa A, Falcini F, Negri E. Body size and colorectal-cancer risk. Int J Cancer. 1998;78:161–165. doi: 10.1002/(sici)1097-0215(19981005)78:2<161::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 128.Fine JT, Colditz GA, Coakley EH, Moseley G, Manson JE, Willett WC, Kawachi I. A prospective study of weight change and health-related quality of life in women. JAMA. 1999;282:2136–2142. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 129.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 130.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 131.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 132.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, Goodman MT, Giuliano AE, Karanja N, McAndrew P, Hudis C, Butler J, Merkel D, Kristal A, Caan B, Michaelson R, Vinciguerra V, Del Prete S, Winkler M, Hall R, Simon M, Winters BL, Elashoff RM. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 133.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 134.Wong JR, Gao Z, Merrick S, Wilson P, Uematsu M, Woo K, Cheng CW. Potential for higher treatment failure in obese patients: correlation of elevated body mass index and increased daily prostate deviations from the radiation beam isocenters in an analysis of 1,465 computed tomographic images. Int J Radiat Oncol Biol Phys. 2009;75:49–55. doi: 10.1016/j.ijrobp.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 135.Freedland SJ, Isaacs WB, Platz EA, Terris MK, Aronson WJ, Amling CL, Presti JC, Jr, Kane CJ. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005;23:7546–7554. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 136.Speed-Andrews AE, Courneya KS. Effects of exercise on quality of life and prognosis in cancer survivors. Curr Sports Med Rep. 2009;8:176–181. doi: 10.1249/JSR.0b013e3181ae98f3. [DOI] [PubMed] [Google Scholar]