Abstract
We discuss the current state of knowledge related to the pathogenesis of myocardial stunning as well as the potential mechanisms responsible for the clinical presentation of myocardial stunning in hemodialysis patients. We suggest future research areas for this critical and clinically important condition in this high-risk patient population. In consideration of acute and chronic changes secondary to dialysis, especially in patients with risk for coronary artery disease, the prevalence of myocardial stunning and its role in the natural history of these patients’ disease progression is considered. We propose a paradigm: that the majority of the pathophysiologic mechanisms by which hemodialysis may induce myocardial stunning falls into two categories with (1) vascular and/or (2) metabolic contributions. In order to prevent eventual myocardial hibernation, myocardial remodeling, scarring, and loss of contractile function with aberrant electrical conductivity that could lead to sudden death, it is imperative to identify the risk factors associated with myocardial stunning during hemodialysis. Further understanding of these mechanisms may lead to novel clinical interventions and pharmacologic therapeutic agents.
Key Words: Regional wall motion abnormality, Myocardial stunning and hibernation, Ultrafiltration
Introduction
There are more than 500,000 people living in the United States with end-stage renal disease (ESRD). The prevalence of coronary artery disease (CAD) in patients with dialysis contributes significantly to elevated mortality and CAD prevalence. Although increased mortality in this patient population was considered to be secondary to increased cardiovascular events, the reduction of typical cardiovascular risk factors (hyperlipidemia, hypertension, obesity) has not had the expected reduction in mortality in this patient population. Additionally, the majority of cardiovascular deaths in hemodialysis (HD) patients are secondary to sudden cardiac death, rather than myocardial infarction [1, 2], with the incidence of cardiac arrest being 100 times higher in the dialysis population than in the general population [3].
Recently, several investigations proposed that, in some patients undergoing HD, there is myocardial stunning that occurs during the episode of HD [4, 5, 6, 7]. These patients have higher mortality and morbidity rates and tolerate HD less well than their counterparts without myocardial stunning during HD. Interestingly, patients on peritoneal dialysis do not appear to have an increased risk of myocardial stunning, despite changes in systemic hemodynamics [8]. This review will attempt to address the state of our understanding of potential mechanisms responsible for HD-induced myocardial stunning and propose several potential therapeutic approaches which may warrant further clinical investigation.
Pathophysiology of Myocardial Stunning: Etiology, Mechanisms, and Therapeutics
Braunwald and Kloner [9] originally described myocardial stunning as ‘delayed recovery of regional myocardial contractile function after reperfusion despite the absence of irreversible damage and despite restoration of normal flow’. Since that original description, transient episodes of myocardial ischemia leading to prolonged left ventricular (LV) dysfunction were characterized and established as a cause of heart failure [9]. The clinical importance of myocardial stunning resulted in several studies that describe the mechanisms involved in myocardial stunning, including loss of high-energy phosphates, impaired microvascular perfusion, impaired sympathetic neural responses, reactive oxygen species, leukocyte activation, and disturbances in calcium homeostasis [10]. At the cellular level and through animal studies, it has been demonstrated that reactive oxygen species, thrombosis, ischemia/reperfusion injury, microvascular dysfunction, and channelopathies may contribute to myocardial stunning [11, 12, 13]. There remains some controversy with respect to clinical significance and inclusion of all of these mechanisms; however, the two main metabolic pathways leading to myocardial stunning include calcium overload and reactive oxygen species (ROS) generation.
Free radicals produced during reperfusion contribute to myocardial stunning, with the duration of ischemia determining the magnitude of free radical formation. With free radical formation, the generation of reactive oxygen species occurs, and they are then available to interact with lipids within cell membranes or with other cellular components such as calcium to promote myocardial stunning. These reactions activate calcium-dependent protease activity and consequently troponin I proteolysis. These factors present during ischemia and early reperfusion act to damage the sarcoplasmic reticulum and contractile machinery and, thereby, result in impaired LV function [14, 15]. Specifically, it has been proposed that a stunned myocardium could be the result of reparative processes after ischemia, with the generation of ROS and slowed resynthesis of contractile proteins as well as a decreased calcium responsiveness leading to LV dysfunction. Several other studies also emphasize the central mechanism of metabolism in myocardial stunning [16]. Prostacyclin and angiotensin-converting enzyme inhibition and the subsequent increase of bradykinin can modulate and protect against the severity of stunning.
Metabolic and cellular contributions from ROS and calcium homeostasis also have some overlap with the vascular dysfunction that is likely to occur with myocardial stunning. More specifically, the vascular elements that contribute to myocardial stunning are likely dependent on the endothelium and lead to microvascular dysfunction. Extravascular influences also include compressive forces from edema. These factors can impair blood flow regulation and directly affect contractile function. In addition, vascular dysfunction can negatively impact myocardial ventricular wall energetics and mechanics. Wall stress likely increases myocardial energy expenditure and then introduces a load that negatively influences myocardial mechanics. Certainly, it is the interplay of metabolic demand and myogenic supply and function that is of central importance in the development of stunning, particularly in the HD population.
Cardiac Physiology of HD
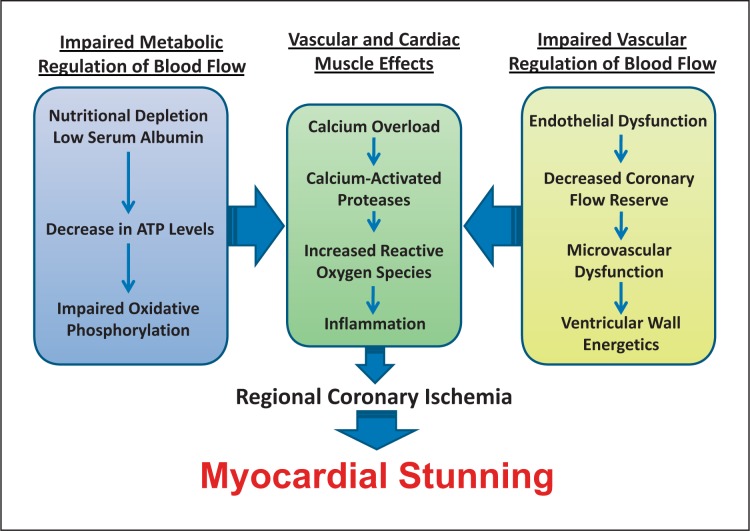
HD exerts hemodynamic changes, with the most deleterious effect of hypotension during dialysis. Hypotension during dialysis in patients with an already increased risk for CAD has been shown to precipitate myocardial ischemia [17]. Interestingly, the development of ST-segment depression during HD is independent of angiographic evidence of CAD [18]. Cardiac troponin T levels are often elevated in dialysis patients, and these elevated levels predict increased mortality [6, 19, 20]. Indeed, chronic kidney disease (CKD) is an independent risk factor for acute myocardial infarction and stable angina in patients with CAD [21]. Myocardial stunning can occur in the HD population even in the absence of CAD. In figure 1, a schematic of contributing factors is illustrated, with a specific focus to the known pathophysiology of myocardial stunning and the potential hemodynamics of HD that may contribute to the increased prevalence of myocardial stunning in this population.
Fig. 1.
Hemodialysis-induced myocardial stunning: our paradigm of myocardial stunning in the HD population proposes several metabolic/cellular factors as well as vascular factors that lead to impaired calcium signaling, oxidative stress and myocardial inflammation, predisposing to regional coronary ischemia and myocardial stunning.
With intravascular volume shifts, it is perceivable that the LV volume, pressure, shape, and morphology are in a load-dependent state, increasing myocardial energy demand and negatively impacting myocardial mechanics. These alterations might contribute to the increased peak systolic and diastolic wall stress, similar to the wall stress seen in patients with severe CAD. With even temporary hemodynamic overload, ventricular dilation and increased wall stress could induce and release numerous agents such as angiotensin II, endothelin-1, interleukin-6, tumor necrosis factor-α, insulin-like growth factor-1, and atrial natriuretic peptide.
Inflammation is well known to occur at increased and deleterious levels in the CKD population. This inflammation is likely to contribute to pathologic changes in the HD patient, predisposing and contributing to myocardial stunning. The inflammatory processes that are most likely to contribute to HD-induced myocardial stunning in CKD patients include atherosclerosis, altered endothelial permeability, high pro-inflammatory cytokine content in the myocardium and systemic circulation, nitric oxide-dependent negative inotropy as well as myocardial inflammatory cell infiltration. These factors might directly contribute to hemodynamic overload and myocardial wall stress, predisposing to myocardial stunning (fig. 1).
Along the spectrum of inflammation and endothelial dysfunction, the pathophysiology of acute thrombosis and inflammation is implicated in CKD: ADP-induced platelet aggregation and dysfunction is seen in patients with moderate-to-severe CKD [22]. Certainly, aspirin reduces the incidence of major cardiovascular events and death in patients with CKD [23]. Vascular calcium is associated with an increased risk of coronary events in patients with or without CKD. However, vessel calcification is rapidly progressive after the initiation of chronic dialysis. CKD patients appear to have characteristic medial calcification, in addition to the classical atherosclerotic intimal and subintimal vascular calcification [24, 25]. Although vascular calcification is characterized in this population, myocardial calcification is less characterized and may be a suitable field for study with the advent of advanced cardiac imaging modalities for elucidating the underlying mechanisms. Myocardial calcification is associated with fibrosis, reduced LV compliance, and diastolic dysfunction; it is feasible that these changes could predispose a HD patient undergoing rapid volume changes to pulmonary edema and hypotension [7]. Certainly, the well-known ‘calcium hypothesis’ in the pathogenesis of myocardial stunning due to calcium overload, calcium-activated proteases, and the generation of ROS can directly be linked to the increased prevalence of myocardial stunning in the HD population.
Clinical Data on HD-Induced Myocardial Stunning
HD patients are at increased risk for myocardial ischemia due to the high prevalence of LV hypertrophy, hypotension during HD, coronary artery atherosclerosis, and reduced coronary flow reserve [26, 27, 28, 29, 30, 31]. However, several other factors are likely to contribute to myocardial stunning in the HD population. It is interesting to note that even pediatric patients undergoing HD are found to experience myocardial stunning, suggesting a characteristic cardiovascular phenotype in HD patients that predisposes to significant demand ischemia and myocardial stunning [32], even in the absence of CAD. Indeed, positron-emission tomography measuring myocardial blood flow during HD in patients without angiographically significant CAD confirms HD-induced segmental LV dysfunction correlating with the matched reduction in myocardial blood flow [5]. HD-induced myocardial stunning is common and could be the predisposing element leading to the increased prevalence of heart failure and increased mortality in CKD patients with ESRD [7].
In a multivariate analysis, age, predialysis cardiac troponin T levels, hypotension during dialysis, and ultrafiltration volumes independently predicted the occurrence of HD-induced cardiac injury. Fluid removal of even 1 liter over the standard 4-hour HD session conferred a 5 times greater risk of developing myocardial stunning, with 2 liters conferring a 26 times greater risk. The effects of ultrafiltration volume are hypothesized to be secondary to the potential hemoconcentration with microcirculatory shear stress and reduced perfusion, leading to an exacerbation of myocardial ischemia [6]. Presumably, HD patients have a higher incidence of cardiovascular events secondary to the presence of risk factors for microvascular and endothelial dysfunction: advanced age, increased inflammation, atherosclerosis, hyperlipidemia, and hypertension [33, 34, 35]. The basic mechanisms of myocardial stunning have been previously evaluated in the CAD population; however, only a few studies exist that evaluate the mechanisms of myocardial stunning in HD patients, with most of the data from McIntyre's group in the United Kingdom and consisting of small observational studies with great clinical utility [7, 36].
McIntyre's group first studied the effect of hypotension during dialysis and found that, when standard bicarbonate HD was compared with a biofeedback technique that responded to significant declines in relative blood volume by temporarily reducing the ultrafiltration rate and increasing dialysate conductivity, there was a 1.8-fold increased risk of myocardial stunning in the standard HD group [37]. Frequency of HD was also analyzed as a second therapeutic variable. To avoid large shifts in volume and associated hemodynamic instability, dialysis intervals of less than or equal to 1 day are suggested. Evidence shows a lower incidence of myocardial stunning with frequent HD versus the longer 2-day dialysis intervals [38, 39]. Daily HD treatment also improved LV mass and quality of life [40, 41].
Many CKD patients do not have increased mortality from myocardial infarction but instead suffer from sudden cardiac death. There is much work needed in order to determine whether HD itself is pro-arrhythmogenic [4]. Although dialysis can increase QTc intervals and does show arrhythmia on Holter monitoring, the links between these ECG changes and sudden cardiac death are unknown in the HD population. Perhaps channelopathies in the HD population, in the setting of myocardial stunning, warrant further investigation to elucidate the possible mechanisms of arrhythmia and sudden death.
Several inflammatory states could also contribute to the clinical prevalence of myocardial stunning. Potential contributing factors in diabetic patients include high levels of glucose exposure and systemic absorption of glucose degradation products directly toxic to cardiac myocytes, elevated tissue and serum advanced glycation end-products with deleterious effects on vascular function, and hypokalemia increasing the arrhythmogenic potential.
HD patients also have increased inflammation due to mucosal ischemia and ultrafiltration; this can cause a reduction in splanchnic blood volume in the absence of systemic hypotension [42, 43, 44]. Endotoxin derived from intestinal bacteria can have deleterious effects on the cardiovascular system, driving systemic inflammation, oxidative stress, and atherosclerosis. Endotoxin is elevated in early-stage CKD patients and is likely to be secondary to altered gut permeability [45]. Furthermore, circulating endotoxin was found to be grossly elevated in stage 3–5 CKD patients, with a further increase in endotoxemia in the HD population, also correlating with the increased severity of myocardial stunning (r = 0.44, p = 0.035) [46]. Endotoxin contamination of dialysis water has also been recognized as a contributing cause of cardiovascular instability during dialysis [47].
Therapeutic Options: More Questions than Answers
Today's dialysis patients are frequently diagnosed with disease that is relevant to cardiorenal syndrome [48, 49]. However, treatment and management of these patients are often compartmentalized and followed individually by both cardiology and nephrology specialty providers. CAD is frequently present in patients with ESRD and has a high likelihood of contributing to these patients’ morbidity and mortality. To better treat these patients, a thorough understanding of the physiological changes occurring during dialysis as well as of the potential pathophysiology that can occur in dialysis patients is essential (table 1).
Table 1.
Directions for future investigation and clinical implementation
| Peritoneal dialysis versus HD |
| Antioxidants/anti-inflammatory agents |
| Remote preconditioning |
HD protocol
|
To begin, an analysis of CAD risk factors and treatment of risk shows some benefit for but without total recovery of the incidence of myocardial stunning. A systematic review of the effect of beta-blockers in patients with CKD has shown all-cause mortality did decrease with beta-blocker use. However, adverse effects such as bradycardia, hypotension, and hyperkalemia were also increased [50].
Interestingly, dialysis-induced myocardial stunning can be partially abrogated by altering the HD process. Native arteriovenous fistulas (AVF) have an improved long-term outcome compared with central catheter HD, with higher AVF blood flows (Qa >1,000 ml/min) proving less cardiac injury than lower blood flows [51]. This was independent of the cardiac index, systolic blood pressure, AVF position, or other patient descriptive factors. Korsheed et al. [51] hypothesize that some of the beneficial effect of AVFs may be secondary to distal relative ischemia at the higher flow, either at rest or during HD, resulting in a remote preconditioning [52, 53] effect providing a degree of protection to the heart. Several other preconditioning stimuli (adenosine, NO, CO, H2S, ethanol, etc.) have been shown to be protective against myocardial ischemia and stunning in animal studies [54, 55, 56], and it would be interesting to determine whether pharmacologic preconditioning would have a similar beneficial effect in the HD population.
Temperature control could also decrease inflammation, and a randomized crossover study compared standard dialysis with a temperature of 37°C to standard dialysis using cooled dialysate of 35°C, and found lower blood pressure values, a 3.8-fold increase in myocardial stunning, and an impaired regional systolic LV function during HD at 37°C [37]. A succeeding study found that dialysis at approximately tympanic temperature provides maximal cardioprotection while avoiding the issues of patient tolerability of cold dialysate [57].
With so many potential mechanisms responsible for myocardial stunning in the HD population, the most exciting avenues for future research are yet unexplored. Specifically, calcium homeostasis, ROS, endothelial permeability, microvascular function, cytokine release, inflammatory infiltrates, and systemic circulating levels of biomarkers of classic inflammatory mediators are just a few research possibilities yet unchartered.
Summary
The cardiorenal syndrome is characterized by the interplay between renal and cardiac disease. These patients are among the most difficult with regard to treatment and management [34]. Cardiovascular morbidity and mortality in HD patients are an overwhelming burden in the care of ESRD patients in the United States; yet, data analyzing the mechanisms of such hemodynamic and cardiovascular complications are lacking. Altering HD protocols and optimizing cardiovascular risk and cardiac functional capacity with pharmacologic therapy may help improve the morbidity and mortality of the HD population. Mortality in CKD is also proportional to LV ejection fraction, with the concomitant presence of heart failure and CKD creating a ‘perfect storm’ for the patient [58]. Congestive heart failure development and progression are considered two of the major causal factors to the mortality seen in dialysis patients [59]. The most likely clinical explanation for the increased mortality seen in HD patients is myocardial stunning. Clearly, there is need for further mechanistic studies of myocardial stunning in the HD population. Translational clinical research and multicenter clinical trials are needed to further elucidate the mechanisms of myocardial stunning in HD and have the ability to enhance the treatment and clinical management of the cardiorenal patient.
Disclosure Statement
The authors have no conflict of interest to disclose.
Acknowledgments
M.Y.Z.'s research is supported by the American Heart Association (AHA 10POST3870022) and a Myears Fellowship, and K.C.D.'s research is supported by the National Institutes of Health (HL077566, HL085119).
References
- 1.Herzog CA. Sudden cardiac death and acute myocardial infarction in dialysis patients: perspectives of a cardiologist. Semin Nephrol. 2005;25:363–366. doi: 10.1016/j.semnephrol.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Cannizzaro LA, Piccini JP, Patel UD, Hernandez AF. Device therapy in heart failure patients with chronic kidney disease. J Am Coll Cardiol. 2011;58:889–896. doi: 10.1016/j.jacc.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Herzog CA. Cardiac arrest in dialysis patients: approaches to alter an abysmal outcome. Kidney Int Suppl. 2003;84:S197–S200. doi: 10.1046/j.1523-1755.63.s84.17.x. [DOI] [PubMed] [Google Scholar]
- 4.Selby NM, McIntyre CW. The acute cardiac effects of dialysis. Semin Dial. 2007;20:220–228. doi: 10.1111/j.1525-139X.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, Camici PG. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breidthardt T, McIntyre CW. Dialysis-induced myocardial stunning: the other side of the cardiorenal syndrome. Rev Cardiovasc Med. 2011;12:13–20. doi: 10.3909/ricm0585. [DOI] [PubMed] [Google Scholar]
- 8.Selby NM, McIntyre CW. Peritoneal dialysis is not associated with myocardial stunning. Perit Dial Int. 2011;31:27–33. doi: 10.3747/pdi.2010.00007. [DOI] [PubMed] [Google Scholar]
- 9.Braunwald E, Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- 10.Duncker DJ, Schulz R, Ferrari R, Garcia-Dorado D, Guarnieri C, Heusch G, Verdouw PD. ‘Myocardial stunning’ remaining questions. Cardiovasc Res. 1998;38:549–558. doi: 10.1016/s0008-6363(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 11.Kersten JR, Brooks LA, Dellsperger KC. Impaired microvascular response to graded coronary occlusion in diabetic and hyperglycemic dogs. Am J Physiol. 1995;268:H1667–H1674. doi: 10.1152/ajpheart.1995.268.4.H1667. [DOI] [PubMed] [Google Scholar]
- 12.Embrey RP, Brooks LA, Dellsperger KC. Mechanism of coronary microvascular responses to metabolic stimulation. Cardiovasc Res. 1997;35:148–157. doi: 10.1016/s0008-6363(97)00096-5. [DOI] [PubMed] [Google Scholar]
- 13.Ammar RF, Jr, Gutterman DD, Brooks LA, Dellsperger KC. Impaired dilation of coronary arterioles during increases in myocardial O(2) consumption with hyperglycemia. Am J Physiol Endocrinol Metab. 2000;279:E868–E874. doi: 10.1152/ajpendo.2000.279.4.E868. [DOI] [PubMed] [Google Scholar]
- 14.Gao WD, Atar D, Liu Y, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997;80:393–399. [PubMed] [Google Scholar]
- 15.Xu KY, Zweier JL, Becker LC. Hydroxyl radical inhibits sarcoplasmic reticulum Ca(2+)-ATPase function by direct attack on the atp binding site. Circ Res. 1997;80:76–81. doi: 10.1161/01.res.80.1.76. [DOI] [PubMed] [Google Scholar]
- 16.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 17.Zuber M, Steinmann E, Huser B, Ritz R, Thiel G, Brunner F. Incidence of arrhythmias and myocardial ischaemia during haemodialysis and haemofiltration. Nephrol Dial Transplant. 1989;4:632–634. [PubMed] [Google Scholar]
- 18.Mohi-ud-din K, Bali HK, Banerjee S, Sakhuja V, Jha V. Silent myocardial ischemia and high-grade ventricular arrhythmias in patients on maintenance hemodialysis. Ren Fail. 2005;27:171–175. [PubMed] [Google Scholar]
- 19.Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation. 2005;112:3088–3096. doi: 10.1161/CIRCULATIONAHA.105.560128. [DOI] [PubMed] [Google Scholar]
- 20.Peacock WFt, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Bansal N, Chandra M, Lathon PV, Fortmann SP, Iribarren C, Hsu CY, Hlatky MA. Chronic kidney disease and risk for presenting with acute myocardial infarction versus stable exertional angina in adults with coronary heart disease. J Am Coll Cardiol. 2011;58:1600–1607. doi: 10.1016/j.jacc.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angiolillo DJ, Bernardo E, Capodanno D, Vivas D, Sabate M, Ferreiro JL, Ueno M, Jimenez-Quevedo P, Alfonso F, Bass TA, Macaya C, Fernandez-Ortiz A. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. doi: 10.1016/j.jacc.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Jardine MJ, Ninomiya T, Perkovic V, Cass A, Turnbull F, Gallagher MP, Zoungas S, Lambers Heerspink HJ, Chalmers J, Zanchetti A. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol. 2010;56:956–965. doi: 10.1016/j.jacc.2010.02.068. [DOI] [PubMed] [Google Scholar]
- 24.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 25.Gross ML, Meyer HP, Ziebart H, Rieger P, Wenzel U, Amann K, Berger I, Adamczak M, Schirmacher P, Ritz E. Calcification of coronary intima and media: immunohistochemistry, backscatter imaging, and x-ray analysis in renal and nonrenal patients. Clin J Am Soc Nephrol. 2007;2:121–134. doi: 10.2215/CJN.01760506. [DOI] [PubMed] [Google Scholar]
- 26.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65:2380–2389. doi: 10.1111/j.1523-1755.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 28.Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36:286–290. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 29.Bos WJ, Bruin S, van Olden RW, Keur I, Wesseling KH, Westerhof N, Krediet RT, Arisz LA. Cardiac and hemodynamic effects of hemodialysis and ultrafiltration. Am J Kidney Dis. 2000;35:819–826. doi: 10.1016/s0272-6386(00)70250-2. [DOI] [PubMed] [Google Scholar]
- 30.Tok D, Gullu H, Erdogan D, Topcu S, Ciftci O, Yildirim I, Muderrisoglu H. Impaired coronary flow reserve in hemodialysis patients: a transthoracic Doppler echocardiographic study. Nephron Clin Pract. 2005;101:c200–c206. doi: 10.1159/000087579. [DOI] [PubMed] [Google Scholar]
- 31.Ragosta M, Samady H, Isaacs RB, Gimple LW, Sarembock IJ, Powers ER. Coronary flow reserve abnormalities in patients with diabetes mellitus who have end-stage renal disease and normal epicardial coronary arteries. Am Heart J. 2004;147:1017–1023. doi: 10.1016/j.ahj.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Hothi DK, Rees L, Marek J, Burton J, McIntyre CW. Pediatric myocardial stunning underscores the cardiac toxicity of conventional hemodialysis treatments. Clin J Am Soc Nephrol. 2009;4:790–797. doi: 10.2215/CJN.05921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Botdorf J, Chaudhary K, Whaley-Connell A. Hypertension in cardiovascular and kidney disease. Cardiorenal Med. 2011;1:183–192. doi: 10.1159/000329927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan EJ, Dellsperger KC. Update on cardiorenal syndrome: a clinical conundrum. Adv Perit Dial. 2011;27:82–86. [PubMed] [Google Scholar]
- 35.US Renal Data System 2010 Annual Data Report Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2010.
- 36.Dorairajan S, Chockalingam A, Misra M. Myocardial stunning in hemodialysis: what is the overall message? Hemodial Int. 2010;14:447–450. doi: 10.1111/j.1542-4758.2010.00495.x. [DOI] [PubMed] [Google Scholar]
- 37.Selby NM, Burton JO, Chesterton LJ, McIntyre CW. Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol. 2006;1:1216–1225. doi: 10.2215/CJN.02010606. [DOI] [PubMed] [Google Scholar]
- 38.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–1107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 39.Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning) Clin J Am Soc Nephrol. 2011;6:1326–1332. doi: 10.2215/CJN.05200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 41.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diebel L, Kozol R, Wilson RF, Mahajan S, Abu-Hamdan D, Thomas D. Gastric intramucosal acidosis in patients with chronic kidney failure. Surgery. 1993;113:520–526. [PubMed] [Google Scholar]
- 43.Yu AW, Nawab ZM, Barnes WE, Lai KN, Ing TS, Daugirdas JT. Splanchnic erythrocyte content decreases during hemodialysis: a new compensatory mechanism for hypovolemia. Kidney Int. 1997;51:1986–1990. doi: 10.1038/ki.1997.270. [DOI] [PubMed] [Google Scholar]
- 44.Jakob SM, Ruokonen E, Vuolteenaho O, Lampainen E, Takala J. Splanchnic perfusion during hemodialysis: evidence for marginal tissue perfusion. Crit Care Med. 2001;29:1393–1398. doi: 10.1097/00003246-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Goncalves S, Pecoits-Filho R, Perreto S, Barberato SH, Stinghen AE, Lima EG, Fuerbringer R, Sauthier SM, Riella MC. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant. 2006;21:2788–2794. doi: 10.1093/ndt/gfl273. [DOI] [PubMed] [Google Scholar]
- 46.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S, Owen PJ, Lai KB, Li PK. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:133–141. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raij L, Shapiro FL, Michael AF. Endotoxemia in febrile reactions during hemodialysis. Kidney Int. 1973;4:57–60. doi: 10.1038/ki.1973.79. [DOI] [PubMed] [Google Scholar]
- 48.Chan EJ, Dellsperger KC. Cardiorenal syndrome: the clinical cardiologists’ perspective. Cardiorenal Med. 2011;1:13–22. doi: 10.1159/000322820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronco C. The cardiorenal syndrome: basis and common ground for a multidisciplinary patient-oriented therapy. Cardiorenal Med. 2011;1:3–4. doi: 10.1159/000323352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badve SV, Roberts MA, Hawley CM, Cass A, Garg AX, Krum H, Tonkin A, Perkovic V. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:1152–1161. doi: 10.1016/j.jacc.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 51.Korsheed S, Burton JO, McIntyre CW. Higher arteriovenous fistulae blood flows are associated with a lower level of dialysis-induced cardiac injury. Hemodial Int. 2009;13:505–511. doi: 10.1111/j.1542-4758.2009.00384.x. [DOI] [PubMed] [Google Scholar]
- 52.Lavi S, Lavi R. Conditioning of the heart: from pharmacological interventions to local and remote protection: possible implications for clinical practice. Int J Cardiol. 2011;146:311–318. doi: 10.1016/j.ijcard.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 54.Peart J, Headrick JP. Intrinsic A(1) adenosine receptor activation during ischemia or reperfusion improves recovery in mouse hearts. Am J Physiol Heart Circ Physiol. 2000;279:H2166–H2175. doi: 10.1152/ajpheart.2000.279.5.H2166. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi T, Kamada K, Dayton C, Gaskin FS, Yusof M, Yoshikawa T, Carter P, Korthuis RJ. Role of eNOS-derived NO in the postischemic anti-inflammatory effects of antecedent ethanol ingestion in murine small intestine. Am J Physiol Heart Circ Physiol. 2007;292:H1435–H1442. doi: 10.1152/ajpheart.00282.2006. [DOI] [PubMed] [Google Scholar]
- 56.Yusof M, Kamada K, Kalogeris T, Gaskin FS, Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol. 2009;296:H868–H876. doi: 10.1152/ajpheart.01111.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jefferies HJ, Burton JO, McIntyre CW. Individualised dialysate temperature improves intradialytic haemodynamics and abrogates haemodialysis-induced myocardial stunning, without compromising tolerability. Blood Purif. 2011;32:63–68. doi: 10.1159/000324199. [DOI] [PubMed] [Google Scholar]
- 58.Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE. The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol. 2009;53:2129–2140. doi: 10.1016/j.jacc.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 59.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, Wetzels JF, Rosendaal FR, Dekker FW. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]