Abstract
Hypothesis
It is possible to implant a stimulating electrode array in the semicircular canals without damaging rotational sensitivity or hearing. The electrodes will evoke robust and precisely controlled eye-movements
Background
A number of groups are attempting to develop a neural prosthesis to ameliorate abnormal vestibular function. Animal studies demonstrate that electrodes near the canal ampullae can produce electrically-evoked eye movements. The target condition of these studies is typically bilateral vestibular hypofunction. Such a device could potentially be more widely useful clinically, and would have a simpler roadmap to regulatory approval if it produced minimal or no damage to the native vestibular and auditory systems.
Methods
An electrode array was designed for insertion into the bony semicircular canal adjacent to the membranous canal. It was designed to be sufficiently narrow so as to not compress the membranous canal. The arrays were manufactured by Cochlear Ltd and linked to a Nucleus Freedom receiver/stimulator. Seven behaviorally-trained rhesus macaques had arrays placed in two semicircular canals using a transmastoid approach and “soft-surgical” procedures borrowed from Hybrid cochlear implant surgery. Postoperative vestibulo-ocular reflex was measured in a rotary chair. Click-evoked auditory brainstem responses were also measured in the seven animals using the contralateral ear as a control.
Results
All animals had minimal postoperative vestibular signs and were eating within hours of surgery. Six out of six animals tested had normal postoperative sinusoidal gain. Six out of seven animals had symmetric postoperative velocity-step responses toward and away from the implanted ear. The one animal with significantly asymmetric velocity-step responses also had a significant sensorineural hearing loss. One control animal which underwent canal-plugging had substantial loss of the velocity-step response toward the canal-plugged ear. In five animals, intraoperative electrically-evoked vestibular compound action potential (ECAP) recordings facilitated electrode placement. Postoperatively, electrically evoked eye-movements were obtained from electrodes associated with an ECAP waveform. Hearing was largely preserved in six animals and lost in one animal.
Conclusions
It is possible to implant the vestibular system with prosthetic stimulating electrodes without loss of rotational sensitivity or hearing. Since electrically-evoked eye-movements can be reliably obtained with the assistance of intraoperative electrophysiology, it is appropriate to consider treatment of a variety of vestibular disorders using prosthetic electrical stimulation. Based on these findings, and others, a feasibility study for the treatment of human subjects with disabling Meniere’s disease has begun.
Introduction
Vestibular dysfunction affects a large and growing population and poses a major public health problem due to disability and lost productivity. Current therapy for these disorders, while helpful, is suboptimal. It consists primarily of ablative therapies for disorders, such as Meniere’s disease, characterized by paroxysmal attacks of vertigo, and physical therapy, or vestibular rehabilitation, for those disorders producing chronic imbalance. The latter symptom can be produced by either unilateral or bilateral vestibular loss but bilateral disease, in particular, condemns those affected to a lifetime of postural instability and oscillopsia.
The tremendous success of the cochlear implant in treating deafness has led neurotologists, vestibular scientists, the medical device industry and the NIDCD to question whether a vestibular implant might be an effective treatment for a variety of vestibular disorders. Such a device would electrically stimulate the diseased vestibular labyrinth to provide missing or abnormal afferent signals in a manner comparable to how a cochlear implant stimulates the cochlea. Our team has developed and studied a semicircular canal implant in the non-human primate. A device, suitable for human use, has been designed in collaboration with Cochlear Ltd, fabricated and implanted in seven rhesus monkeys. These studies will give us the necessary physiologic information to proceed with similar studies in human subjects.
Background
Despite great advances in our understanding of the vestibular system from basic research, treatment of vestibular disorders remains based on therapies developed decades ago. With the exception of vestibular rehabilitation and surgery for superior semicircular canal dehiscence, most drugs and surgical therapies have not changed substantially for over 25 years. Vestibular disorders have been the subject of meetings, seminars, publications, and research grants. These activities have reflected and supported substantial increases in knowledge about the effects of various diseases on balance, and in particular, the effects of aging. Nonetheless, there have been few significant new therapies translated to clinical practice. For example, we still rely on destructive procedures to correct the irreversibly diseased inner ear. Unlike the auditory system, where there are now developing notions of factors governing vulnerability to the major causes of deafness, most of the common vestibular disorders – e.g. Meniere’s disease and vestibular neuronitis – remain poorly understood, etiologically and clinically.
A new direction in the treatment of vestibular disorders has recently emerged. First, with the successful introduction of implantable cochlear prostheses for hearing loss (1–3) work has begun in earnest on an implantable vestibular prosthesis of similar design. The device would, in theory, provide an analog of the missing vestibular information by stimulating intact nerves that are no longer receiving input from vestibular hair cells (4–13). Although this strategy holds great promise, such a device presents many challenges in its design and implementation. It is clear, however, that many vestibular patients could potentially benefit from the introduction of such a device. It has also been clear for nearly fifty years that the vestibular system can be electrically activated (14).
While true prosthetic replacement of vestibular function will require a velocity sensor for head motion, the pathophysiology of Meniere’s disease may not require such a sensor. Meniere’s attacks are believed to result from a sudden loss of spontaneous afferent activity in the involved ear due to depolarization block from admixture of endolymph and perilymph associated with ruptures of the membranous labyrinth (15). Eye-movements recorded during Meniere’s attacks are usually consistent with such a loss of spontaneous activity (16). A prosthesis could potentially halt these symptoms by replacement of absent spontaneous activity with unmodulated electrically-evoked afferent activity. Even if attacks are associated with increased spontaneous activity, a prosthesis could potentially override that activity via appropriate signal processing to generate controlled refractoriness in the stimulated neurons. Such a “vestibular pacemaker” would not require a sensor as the subject would simply turn the device on at the onset of symptoms, or possibly leave it on chronically during periods of frequent attacks (8). Thus subjects could potentially benefit from the procedure while providing the groundwork for treatments of vestibulopathic disorders that would require a modulated signal driven by head motion. Critical questions prior to the initiation of any human trials relate to the safety of the device to native vestibular and auditory function, and its efficacy in directly activating the vestibular nerve. This paper begins to address these questions by introducing our device and surgical techniques, providing a preliminary demonstration of its safety and functionality.
Materials and Methods
Device Design
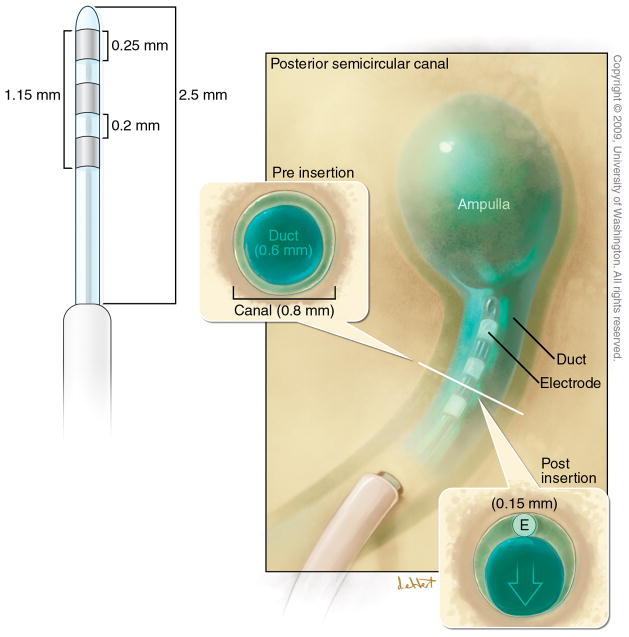
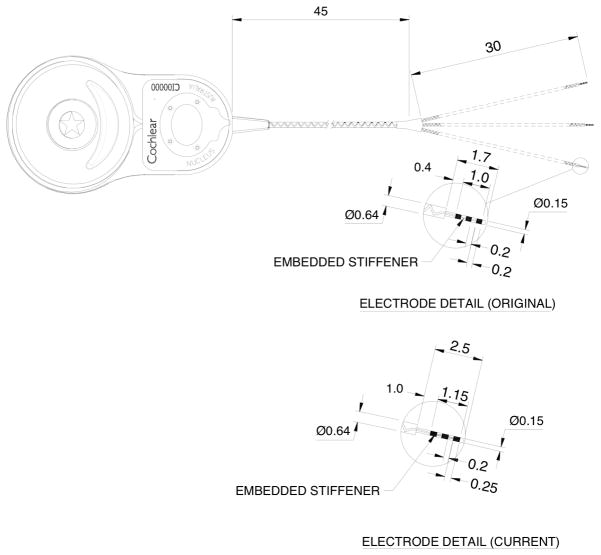
Rhesus temporal bones were dissected in order to generate a feasible device design for chronic implantation. The design was intended to permit stimulation of the three semicircular canals and electrode placement within each bony canal without compression of the membranous canal. Due to the highly conserved anatomy between human and rhesus, a device that meets these criteria for rhesus, would exceed the specification for human due to the larger size of the human labyrinth (17). Our initial device design is illustrated in Figures 1 and 2. It consists of Cochlear’s FDA-approved receiver-stimulator linked to a custom trifurcating array of nine electrodes. Our intent was to mimic established “soft-surgery” techniques used in the implantation of Nucleus Hybrid cochlear implants (18,19), as well as hearing-preservation vestibular surgery such as posterior and superior canal ablations (20–22). Early experience with a 1.7 mm array with .2 mm electrode pads demonstrated that both device stability and electrode impedance could be improved with a 2.5 mm length and .25mm electrode pads. The .25mm pads have an impedance of approximately 10 kOhm in the operating room after placement within the labyrinth. Three electrode pads are included on each array to permit bipolar or monopolar stimulation as well as recordings of vestibular electrically-evoked compound action potentials (23).
Figure 1.
The UW/Cochlear Ltd vestibular implant. The Cochlear Ltd “Freedom” cochlear implant receiver/stimulator has been linked with electrode arrays designed for the vestibular system. The original design was modified to better stabilize the electrode arrays in the labyrinth and decrease the electrode impedance.
Figure 2.
The electrode arrays are designed to be minimally disruptive to normal function in both non-human primates and humans. The three semicircular canals are each implanted with a three-electrode array (insert).
Software control
The implant was controlled using custom software based on subroutines (NIC-2) provided by Cochlear, Ltd that drive the radio-frequency link to the implant. This interface allows us to deliver arbitrary stimuli to each of the implanted semicircular canals under computer control. The system is currently for laboratory use only as it requires a computer connection but has also been used in the clinical vestibular laboratory for mapping of human subjects. It has also been modified to use other low-level code for the Cochlear device so that human subjects can wear portable speech processors to provide appropriate stimuli outside of the laboratory. Extensive software modification was necessary for several reasons, one of which is that vestibular stimulation with pulse trains typically requires much lower pulse rates than can be produced with clinical cochlear implant software.
Surgery
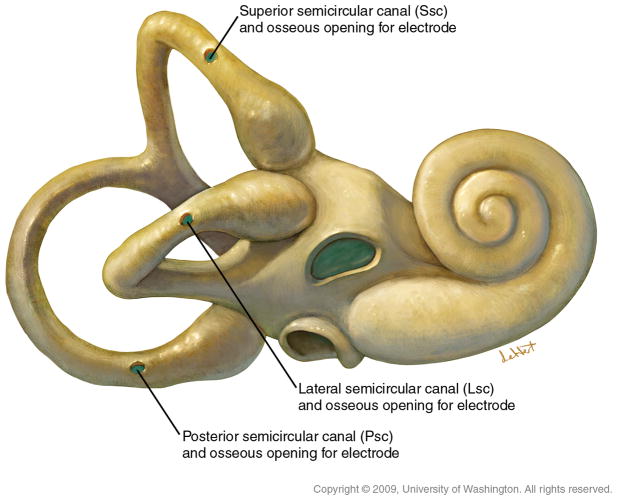
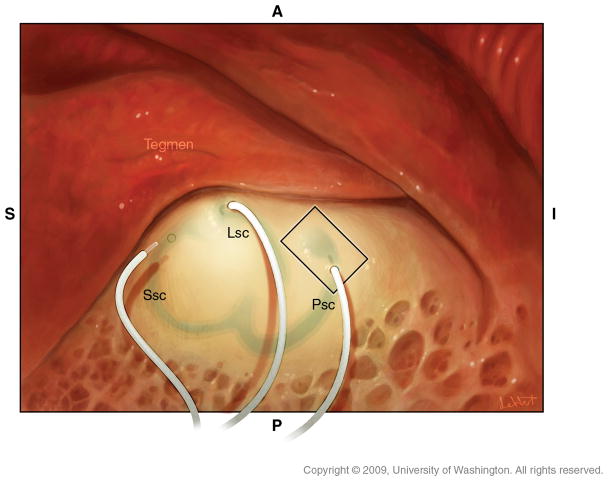
Our primate surgeries are performed in a manner virtually identical to human otologic surgery. All protocols were approved by the UW IACUC and the Washington National Primate Research Center. Six of the seven animals underwent at least two surgeries, as the initial procedure was unsuccessful in reliably producing electrically-evoked responses. There were two reasons for this: First, initial surgical techniques were inadequate to secure the electrode arrays in the labyrinth and the animals dislodged them postoperatively. Second, the initial surgery was performed without electrophysiologic guidance and electrodes were placed too far from the ampulla. Revision surgeries documented the dislodged electrodes and most were performed with electrophysiologic guidance. Such guidance permitted optimization of electrode placement (23) so that we are now able to reliably place electrodes in a single surgery. Under general oral-endotracheal anesthesia, the animal is prepped and draped in a sterile manner. A post-auricular incision is made with a #15 blade and carried down to the temporalis with a Bovie. A Palva flap is raised exposing the mastoid cortex. A sub-perisosteal pocket is created for the receiver/stimulator posterosuperior to the external auditory canal. A simple mastoidectomy is performed exposing the incus and the target portions of the vestibular labyrinth (Figure 3). Each of the three semicircular canals can then be blue-lined with a 1mm diamond burr, fenestrated with an angled pick and electrodes inserted as shown using a jeweler’s forcep after securing the receiver-stimulator in its sub-perisosteal pocket (Figure 4). Care is taken to avoid suctioning perilymph from the canals after their fenestration and electrodes are inserted parallel to the trajectory of the canal so as to avoid penetrating the membranous labyrinth (Figure 5). Due to the small size of the rhesus mastoid, most of our animals had implantation of only the lateral and posterior canals. One animal had the lateral and superior canals implanted. With the larger size of the human mastoid we expect to routinely implant all three canals. Similarly, due to the lack of adequate space in the rhesus mastoid, no fascia has been used to seal the labyrinthotomy though this would be recommended for human surgery. Intraoperative electrophysiology is used to determine optimal electrode positioning in the canals and is described more fully in the Results section and in (23).
Figure 3.
The semicircular canals are “blue-lined” and fenestrated near their respective ampullae.
Figure 4.
The electrode arrays are inserted into the canal fenestrations with a trajectory following the curvature of the canal.
Figure 5.
The electrode array is positioned parallel to the membranous canals so that they are not compressed.
Rotational testing
Natural stimulation was provided by en-bloc rotation in a 3-dimensional rotator. The rotator moved in multiple axes. The yaw rotation axis was a vertical axis aligned with the midline of the interaural axis with the animal rotated 20 deg nose down from the stereotaxic plane. The yaw axis was therefore aligned with the direction of gravity and the contributions from the otolith endorgans to yaw rotation were not excluded. Eye movements were recorded with implanted single scleral coils linked to a head-mounted connector (24). They were calibrated behaviorally with the animals trained to orient to fixed targets and a single eye was measured. There were two field coils used, both CNC. One system was a Robinson system in both dimensions and the other was Robinson in the vertical dimension and a phase angle in the horizontal dimension. The theoretical resolution of the coil system is 6 seconds of arc. The sampling rate was 1 kHz. The raw coil data were digitized using custom software developed in Spike II (CED), and analyzed offline. Sinusoidal en-bloc rotational vestibular testing was performed in the plane of the implanted lateral canal in all animals at a fixed amplitude of +- 10 deg. at rotational frequencies of 0.01 to 1.4 Hz. Desaccaded eye and chair velocity were accumulated over at least 10 cycles of rotation, and fit with a least squares approximation to a sign wave at the frequency of chair rotation. The gain, phase and symmetry of eye velocity relative to chair velocity were calculated. En-bloc velocity-step vestibular testing was performed in four consecutive velocity steps of 1000 deg/s/s acceleration followed by a constant velocity of 0 or 100 deg/s for 45 seconds. The gain of the first slow phase was calculated as the ratio of average eye velocity to step velocity, and the decaying slow phase eye velocity was fit with a single exponential function to determine the time constant of the decay.
Auditory Brainstem Response
Auditory brainstem responses were recorded under sedation with a mixture of ketamine (7–14 mg/kg) and xylazine (0.6–1.2 mg/kg). Signals were recorded from subdermal electrodes placed at the midline scalp just above the brows (+), ipsilateral mastoid (−), and contralateral mastoid (ground), and amplified using a gain of 10,000 at a bandwidth of 100–3000 Hz. Acoustic condensation clicks (100 ms phase, repeated 300–500 times at a rate of 10/sec) were generated with a PC sound card controlled by custom software and delivered via infant-size earphones inserted into the external canal. Thresholds were obtained by visual inspection of the waveforms and comparison with the contralateral ear, except in one animal where the contralateral ear had been deafened by an unrelated procedure after vestibular testing had been completed but before auditory brainstem responses had been obtained. In this animal (animal C in Table 1), thresholds were compared with the average results seen in the other animals’ unimplanted ears.
Table 1.
Postoperative click ABR threshold difference and velocity-step gains and time constants in the seven animals. Results are obtained after revision surgery in animals A-F and after primary surgery in animal G. Normal velocity-step gain in our laboratory is greater than or equal to 0.4 with a time constant longer than 10 seconds. A control animal that underwent a canal plugging procedure had a velocity-step gain of .35 and a time constant of 8.34 seconds as well as an obvious asymmetry in the responses toward and away from the involved ear (see Figure 7 panel A).
| Animal | Implanted canals | Hearing (implanted vs unimplanted) | Horizontal velocity-step gain/time constant |
|---|---|---|---|
| A | Lateral, Posterior | +15 dB | 0.78/16.0s |
| B | Lateral, Posterior, Superior | +5 dB | 0.93/13.3s |
| C | Lateral, Posterior | +26 dB | 1.11/12.5s |
| D | Lateral, Posterior | +70 dB | 0.52/7.6 s |
| E | Lateral, Posterior | +5 dB | 0.62/50s |
| F | Lateral, Posterior | −5 dB | 0.75/18.0s |
| G | Lateral, Posterior | +10 dB | 0.69/12.5s |
Behavioral training and related methods
Prior to undergoing testing or any surgical procedures, animals are behaviorally trained. Animals were trained to track point targets in an otherwise dark enclosure for an applesauce reward. The animals were seated 20 deg nose down from the stereotaxic plane in a primate chair. To assess the vestibulo-ocular reflex, VOR, the primate chair was rotated about a vertical axis which bisected the interaural axis. During rotation, the fixation stimulus was extinguished so that the animals were in complete darkness. They received ad-libidum reward during rotation. The rotations were presented in sets of 10 sinusoids or 4 velocity steps. Training endpoint is when the animals can track targets consistently within a +/−2 degree reward window for one hour. These procedures are extensively described elsewhere (25).
Results
Intraoperative electrophysiology
Precise electrode placement near the ampullae of the semicircular canals is critical for robust activation of eye movements. Electrode placement that is too shallow, or too deep, results in weak or absent vestibular responses. Fortunately a reliable intraoperative tool for assisting in optimal electrode placement has been developed as a result of our initial, unsuccessful procedures (23). We have demonstrated that, like the cochlear nerve, the vestibular afferents produce an electrically-evoked compound action potential (ECAP) that can be recorded from the implant using standard clinical Neural Response Telemetry software. When high quality responses can be recorded, robust electrically-evoked eye movements are obtained. When there is no ECAP, minimal or no eye movement is obtained from stimulation. Examples of such intraoperative recordings are illustrated in Figure 6 and detailed methods for obtaining them are provided in (23). The ability to measure these potentials has been critical to our success in obtaining high-velocity electrically-evoked eye movements. These potentials’ strong dependence on precise electrode position relative to the ampullae, their correlation with evoked eye-movements, and their latency, all demonstrate that they arise from direct electrical activation of the vestibular nerve and are not driven by hair-cell mediated activity (23).
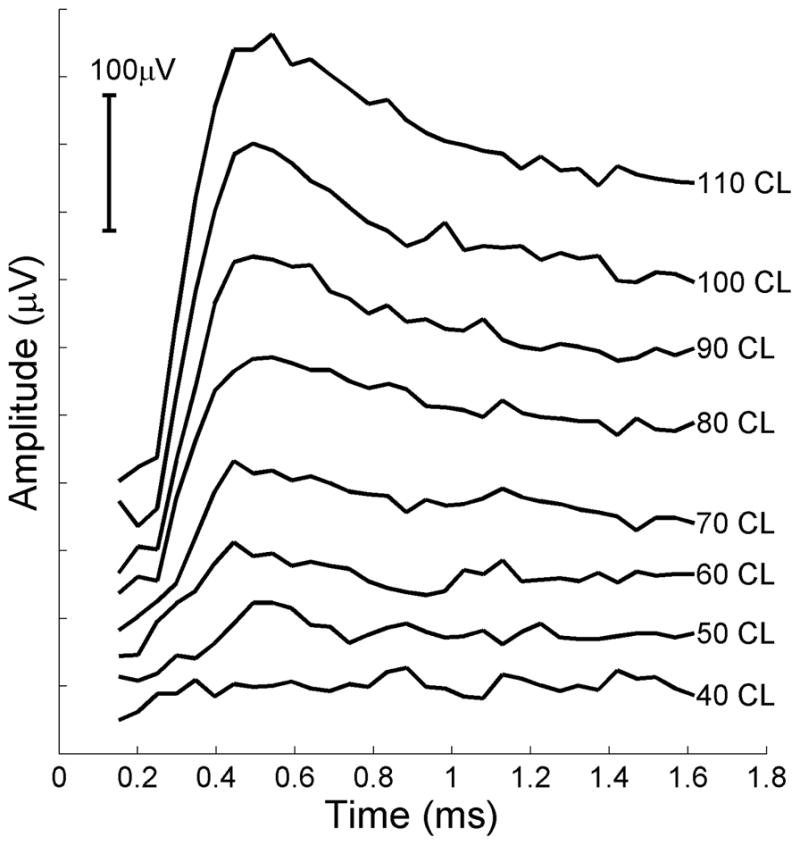
Figure 6.
Intraoperative electrically evoked compound action potentials (ECAPs) measured in lateral semicircular canal of a rhesus monkey. An amplitude series is shown where CL refers to clinical current levels in the Nucleus Freedom implant. Current levels are logarithmically related to microamperes where 40 CL is 36 microamperes. 110 CL is 127.6 microamperes. Potentials were obtained using standard clinical Neural Response Telemetry™ for this device. Stimuli were delivered in monopolar mode between one intralabyrinthine electrode and a distant return electrode. Recordings obtained from a second intralabyrinthine electrode in the same canal. Pulse width, 50 μs/phase. See (23) for further details.
Preservation of rotational sensitivity
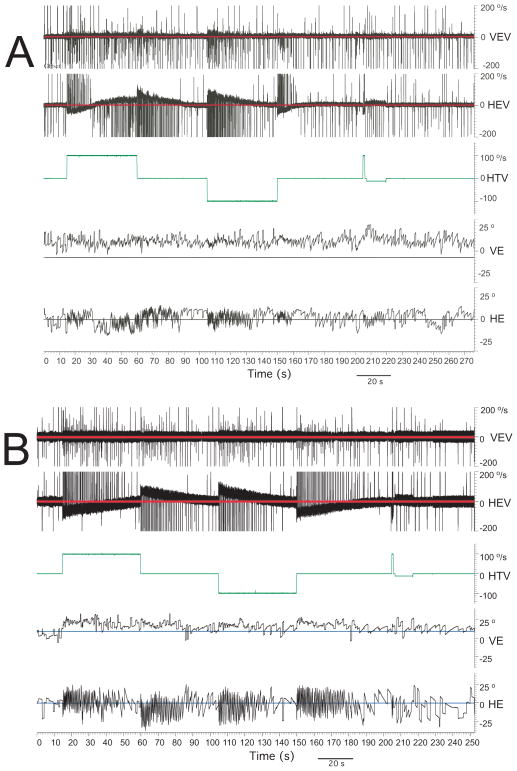
The animals have not undergone prior destructive procedures to the labyrinth so as to allow determination of injury secondary to our electrode placements. All seven animals had minimal vestibular signs postoperatively, consisting of mild imbalance lasting about an hour, and were eating within a few hours of surgery. Three of six animals tested had both pre- and postoperative sinusoidal rotational testing. At the standard test frequency of 0.5 Hz, there was little change in either the gain or phase of the response, with gain changes of +0.03, 0.0, and −0.05, and phase changes of +2 deg, +1 deg, and +1 deg. The mean post operative gain at 0.5 Hz for all 6 animals tested was 0.78, and the mean phase (eye re head velocity) was 178.3 deg. In two animals that showed some post-operative hearing loss (> 20 dB), the gain of the sinusoidal VOR was 0.63 and 0.67, and the phase was 172 and 177 deg at 0.5 Hz. Neither animal had preoperative sinusoidal vestibular testing. Six out of seven animals had symmetric velocity-step responses after revision implant surgery. The one animal (Animal D) with the most significant hearing loss had asymmetric responses with a gain of 0.52 and a time constant of 7.6 seconds for steps toward the implanted and consequently deafened ear. Figure 7 illustrates horizontal step rotational testing in one of our animals after a revision implant in the left horizontal semicircular canal (Panel B) compared to a control animal that had undergone intentional canal-plugging of that canal (Panel A). It demonstrates that velocity-step rotational responses toward the implanted ear can be preserved after surgical placement of our electrode array twice. Such responses are substantially eliminated with canal-plugging hence the device clearly can maintain these responses. The 0.15 mm diameter of the intralabyrinthine component of the electrode array was intended to be sufficiently small to avoid damaging the membranous semicircular canal and these measures suggest that this design criterion has been met. (See Table 1)
Figure 7.
Velocity step vestibular testing in two monkeys in the plane of the horizontal canal. Traces, from top to bottom in each panel, are vertical eye velocity, horizontal eye velocity, chair velocity, vertical eye position, and horizontal eye position. Upwards is to the right and downwards is to the left. A) Unilateral left horizontal canal-plugged monkey, B) Monkey implanted with our device in the left horizontal canal.
Preservation of hearing
The seven animals have undergone click-evoked auditory brainstem response testing after primary or revision surgery using the contralateral ear as a control. Five of these seven animals had symmetric ABR thresholds (<15 dB loss in the implanted ear compared to control). One had a 26 dB loss on the surgical side that also showed otoscopic evidence of inflammation and negative middle-ear pressure. This animal underwent canal-plugging prior to ABR testing, in addition to having had two prior implant surgeries. Its vestibular function was preserved after the second implant surgery and lost, as expected, after canal plugging. One animal had a 70 dB shift in threshold in the surgical ear and also lost its vestibular sensitivity after revision surgery. Vestibular sensitivity had been preserved after the first surgery (see Table 1).
Electrically-evoked eye movements
Slow-phase eye movements were elicited by implant channels from which a clear vestibular ECAP could be recorded (23). Eye-movement velocity varied as a function of stimulus frequency or amplitude (26). Stimulation evoked responses primarily within the plane of the stimulated canal. In some animals, lateral canal stimulation evokes some vertical plane eye movement presumably via current spread to the superior canal ampulla that is anatomically close. An example of such stimulation is shown in Figure 8. At high levels, responses are larger than those that can be evoked with rotational stimuli in normal animals suggesting that the origin of the responses is direct electrical activation of the afferents rather than via hair cell mechanisms.
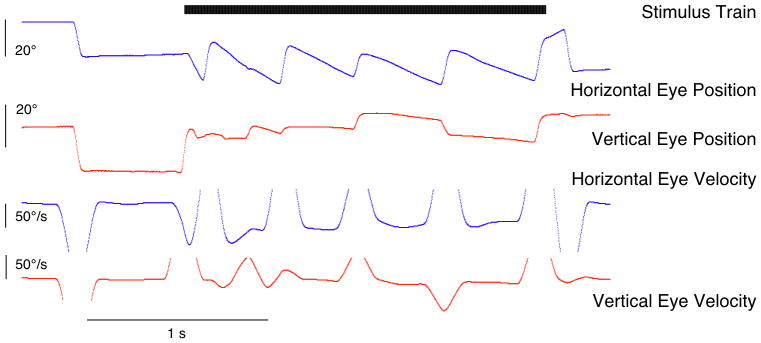
Figure 8.

Electrically-evoked eye-movements. Stimulus is a 600 pps biphasic pulse-train of two second duration delivered in monopolar mode to an electrode in the lateral canal. Pulses are 100 microseconds per phase with an 8 microscond interphase gap. Note that while slow-phase movements are primarily horizontal some vertical motion is produced presumably due to current spread to the superior canal.
Discussion
Mechanisms of labyrinthine injury
There appear to be five possible mechanisms whereby this device could result in loss of vestibular function. The most obvious two are compression or violation of the membranous canal during electrode insertion. Another possibility would be damage to the ampulla if the canal fenestration is made too closely to this structure. A third is through suctioning of perilymph which is known to result in loss of hearing sensitivity in similar procedures performed on the cochlea. Lastly infection or inflammation of the canal is a significant possibility. These animals are fitted not just with their implant device, but also percutaneous head lugs, eye-coil connectors, and neurophysiologic recording chambers. Very little scalp protects the device that certainly could wick infection to the labyrinth. It is also well known that cochlear implant electrode arrays become encased in a fibrous sheath and there is little space for such a sheath in the semicircular canals without compressing the membranous labyrinth. Having said that, all seven animals showed no evidence of such injury after their first implant surgery, and five of the six who underwent revision implant surgery continued to manifest normal function.
Clinical and scientific significance
The ability to surgically implant electrodes into the vestibular labyrinth without causing hearing or vestibular loss offers the potential to treat a variety of vestibular disorders. We are proceeding with a clinical feasibility study of using our device for the treatment of Meniere’s disease given that risk to hearing or vestibular function is a normal attribute of present therapies. If however we are able to replicate our animal safety profile in human subjects, then the potential to treat many other vestibular disorders will be enormous. Most patients suffering from bilateral vestibular hypofunction, or failure to compensate for a unilateral loss have normal hearing. In addition, it would be desirable to avoid further vestibular loss in a patient for whom a partial fixed loss is already causing symptoms. Hence any proposed treatment must have a strong track record for hearing preservation to be broadly acceptable in the management of vestibular disease. The ability to preserve vestibular function would potentially make it even more attractive. Based on our animal data, our electrode design and associated surgical techniques appear promising. While animal studies such as those described here will remain critical in the efforts to develop such a vestibular prosthesis, the clinical trials those animal studies have now facilitated will determine which vestibular disorders are potentially amenable to treatment with a neurostimulator. The rhesus model, with its close anatomical relationship to humans, allows leveraging the wealth of pertinent vestibulo-ocular neurophysiological knowledge and techniques that are indispensable for such translational research. In a manner analogous to the role of cochlear implants in hearing science, the clinical application of vestibular neurostimulation could also possibly introduce new methods for basic research on the vestibular system.
Acknowledgments
This work was supported by NIDCD contract N01-DC-6-005 (JOP), the Wallace H. Coulter Foundation (JTR/JOP), and Cochlear, Ltd (JTR). JTR is a paid consultant for Cochlear, Ltd. Melissa Marshburn, RN assisted with the implant surgeries. The Anspach Effort, Inc. provided surgical drills. Colin Irwin from Cochlear, Ltd provided critical assistance with software design. JTR, JOP and the University of Washington hold intellectual property rights to the device being studied.
References
- 1.Gates GA, Miyamoto RT. Cochlear implants. N Engl J Med. 2003;349(5):421–3. doi: 10.1056/NEJMp038107. [DOI] [PubMed] [Google Scholar]
- 2.Francis HW, Niparko JK. Cochlear implantation update. Ped Clin North Am. 2003;50(2):341–61. doi: 10.1016/s0031-3955(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 3.Rubinstein JT. How cochlear implants encode speech. Curr Opin Otolaryngol Head Neck Surg. 2004 Oct;12(5):444–8. doi: 10.1097/01.moo.0000134452.24819.c0. [DOI] [PubMed] [Google Scholar]
- 4.Della Santina C, Migliaccio A, Patel A. Electrical stimulation to restore vestibular function development of a 3-d vestibular prosthesis. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7380–5. doi: 10.1109/IEMBS.2005.1616217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Della Santina CC, Migliaccio AA, Patel AH. A multichannel semicircular canal neural prosthesis using electrical stimulation to restore 3-d vestibular sensation. IEEE Trans Biomed Eng. 2007 Jun;54(6 Pt 1):1016–30. doi: 10.1109/TBME.2007.894629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong W, Merfeld DM. System design and performance of a unilateral horizontal semicircular canal prosthesis. IEEE Trans Biomed Eng. 2002 Feb;49(2):175–81. doi: 10.1109/10.979358. [DOI] [PubMed] [Google Scholar]
- 7.Lewis RF, Gong W, Ramsey M, Minor L, Boyle R, Merfeld DM. Vestibular adaptation studied with a prosthetic semicircular canal. J Vestib Res. 2003;12(2–3):87–94. [PubMed] [Google Scholar]
- 8.Merfeld DM, Gong W, Morrissey J, Saginaw M, Haburcakova C, Lewis RF. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng. 2006 Nov;53(11):2362–72. doi: 10.1109/TBME.2006.883645. [DOI] [PubMed] [Google Scholar]
- 9.Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng. 2007 Jun;54(6 Pt 1):1005–15. doi: 10.1109/TBME.2007.891943. [DOI] [PubMed] [Google Scholar]
- 10.Rubinstein JT, Della Santina CC. Development of a biophysical model for vestibular prosthesis research. J Vestib Res. 2003;12(2–3):69–76. [PubMed] [Google Scholar]
- 11.Shkel AM, Zeng FG. An electronic prosthesis mimicking the dynamic vestibular function. Audiol Neurootol. 2006;11(2):113–22. doi: 10.1159/000090684. [DOI] [PubMed] [Google Scholar]
- 12.Wall C, 3rd, Merfeld DM, Rauch SD, Black FO. Vestibular prostheses: the engineering and biomedical issues. J Vestib Res. 2003;12(2–3):95–113. [PubMed] [Google Scholar]
- 13.Wall C, 3rd, Kos MI, Guyot JP. Eye movements in response to electric stimulation of the human posterior ampullary nerve. Ann Otol Rhinol Laryngol. 2007 May;116(5):369–74. doi: 10.1177/000348940711600509. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki & Cohen
- 15.Schuknecht HF. Pathology of the ear. 2. Lea & Febiger; Pennsylvania: 1993. [Google Scholar]
- 16.Aschan G, Stahle J. Nystagmus in Menière’s Disease During Attacks: A Nystagmographical Study. Acta Oto-Laryngologica. 1957;47:2, 189–201. doi: 10.3109/00016485709130333. [DOI] [PubMed] [Google Scholar]
- 17.Blanks RH, Curthoys IS, Bennett ML, Markham CH. Planar relationships of the semicircular canals in rhesus and squirrel monkeys. Brain Res. 1985 Aug 12;340(2):315–24. doi: 10.1016/0006-8993(85)90928-x. [DOI] [PubMed] [Google Scholar]
- 18.Gantz BJ, Turner C, Gfeller KE. Acoustic plus electric speech processing: preliminary results of a multicenter clinical trial of the Iowa/Nucleus Hybrid implant. Audiol Neurootol. 2006;11( Suppl 1):63–8. doi: 10.1159/000095616. [DOI] [PubMed] [Google Scholar]
- 19.Turner CW, Reiss LA, Gantz BJ. Combined acoustic and electric hearing: Preserving residual acoustic hearing. Hear Res. 2007 Nov 29; doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limb CJ, Carey JP, Srireddy S, Minor LB. Auditory function in patients with surgically treated superior semicircular canal dehiscence. Otol Neurotol. 2006 Oct;27(7):969–80. doi: 10.1097/01.mao.0000235376.70492.8e. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal SK, Parnes LS. Human experience with canal plugging. Ann N Y Acad Sci. 2001 Oct;942:300–5. doi: 10.1111/j.1749-6632.2001.tb03754.x. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal SK, Parnes LS. Transmastoid superior semicircular canal occlusion. Otol Neurotol. 2008 Apr;29(3):363–7. doi: 10.1097/mao.0b013e3181616c9d. [DOI] [PubMed] [Google Scholar]
- 23.Nie K, Bierer SM, Ling L, Oxford T, Rubinstein JT, Phillips JO. Characterization of the electrically-evoked compound action potential of the vestibular nerve. Otology & Neurotology. 2011;32:88–97. doi: 10.1097/mao.0b013e3181f6ca45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 25.Phillips JO, Ling L, Fuchs AF, Siebold C, Plorde JJ. Rapid horizontal gaze movement in the monkey. J Neurophysiol. 1995 Apr;73(4):1632–52. doi: 10.1152/jn.1995.73.4.1632. [DOI] [PubMed] [Google Scholar]
- 26.Phillips JO, Bierer S, Fuchs AF, Kaneko CRS, Ling L, Nie K, Oxford T, Rubinstein JT. A multichannel vestibular prosthesis based on cochlear implant technology. Society for Neurosciences. 2008:1810. [Google Scholar]