Abstract
The skeleton is a potential metastatic target of many malignant tumors. Up to 85% of prostate and breast cancer patients may develop bone metastases causing severe pain syndromes in many of them. In patients suffering from multilocular, mainly osteoblastic lesions and pain syndrome, radionuclide therapy is recommended for pain palliation. Low-energy beta-emitting radionuclides (153samarium-ethylenediaminetetrameth-ylenephosphonate (EDTMP) and 89strontium) deliver high radiation doses to bone metastases and micrometastases in the bone marrow, but only negligible doses to the hematopoietic marrow. The response rate regarding pain syndrome is about 75%; about 25% of the patients may even become pain free. The therapy is repeatable, depending on cell counts. Concomitant treatment with modern bisphosphonates does not interfere with the treatment effects. Clinical trials using a new, not yet approved nuclide (223Radium) and/or combinations of chemotherapy and radionuclides are aiming at a more curative approach.
Keywords: Bone metastases, Pain palliation, Radionuclides, 153Sm-EDTMP, 89Sr, 223Ra
Abstract
Zahlreiche maligne Tumoren metastasieren in das Skelett. Bei bis zu 85% der Patienten mit einem Prostataoder Mammakarzinom finden sich Knochenmetastasen, die bei vielen zu einer ausgeprägten Schmerzsymptomatik führen. Bei Patienten, die an einer multilokulären Knochenmetastasierung und Schmerzen leiden, kann eine palliative Schmerztherapie mit Radionukliden durchgeführt werden. Niedrigenergetische Beta-Strahler (153Samarium-Ethylendiamintetra(methylenphosphon-säure) (EDTMP) und 89Strontium) bewirken eine hohe Strahlendosis auf die Knochenmetastasen und die Mikrometastasen im Knochenmark bei nur geringer Dosis auf das Mark selbst. Die Ansprechrate auf die Therapie beträgt 70–80%, etwa 25% der Patienten werden schmerzfrei. Die Therapie kann in Abhängigkeit vom Blutbild wiederholt werden. Eine gleichzeitige Therapie mit modernen Bisphosphonaten führt nicht zu einer Wirkungsänderung. Klinische Studien mit einem neuen, noch nicht zugelassenen Nuklid (223Radium) oder Kombinationstherapien mit Chemotherapeutika und Radionukliden zielen auf einen mehr kurativen Effekt und zeigen vielversprechende Ergebnisse.
Introduction
The incidence rate of many common primary tumors is still rising and, due to progression in efficacious treatment, many patients survive for a longer time.
Because the skeleton is a potential metastatic target for the majority of malignant extracranial tumors [1], an increasing number of patients will suffer from painful bone metastases, which can significantly impair the patient's health. Postmortem studies indicate that approximately 80% of all patients with prostate cancer and 75% of breast carcinoma patients develop bone metastases, which together account for approximately 80% of all skeletal metastases [2]. By comparison, bone metastases occur in approximately 20–40% of patients with lung or renal cancer [3] (table 1). World Health Organization (WHO) data suggest that approximately 4 million people worldwide experience daily pain due to malignant disease; in half of these people, metastatic bone discomfort is the dominant source of symptoms [4]. The majority of patients with bone metastases develop severe pain as their disease progresses, resulting in a considerable reduction in their quality of life. A multidisciplinary approach to symptom palliation is recommended, tailoring treatment to individual need, with the aim of individualized treatment being ‘to add life to the years, not years to the life’.
Table 1.
Incidence of bone metastases reported in postmortem studies [54]
| Tumor | Mean frequency, % | Range, % |
|---|---|---|
| Breast | 73 | 47–85 |
| Prostate | 68 | 33–85 |
| Thyroid | 42 | 28–85 |
| Kidney | 35 | 33–40 |
| Lung | 36 | 30–55 |
| Esophagus | 6 | 5–7 |
| Gastrointestinal | 5 | 3–11 |
| Rectum | 11 | 8–13 |
The uptake of bone-seeking radiotracers used for radionuclide therapy of bone metastases depends on the osteoblastic activity and the calcification of the tumor tissue. In the past, the morphology of bone metastases arising from primary prostate cancer was typically characterized as mainly osteoblastic, whereas plasmocytoma and renal cell carcinoma have been associated with predominantly osteolytic bone lesions. Mixed patterns of osteoblastic and osteolytic metastases are more common in breast, lung, colorectal and pancreatic malignancies [5, 6]. More recently, when comparing the morphology of breast cancer metastases by computed tomography in the time period 1996–2000 versus 2001–2005, a higher prevalence of osteosclerosis was observed in the later period (1996–2000: osteolytic 53.7%, osteosclerotic 32.1%, mixed type 14.3%; 2001–2005: osteolytic 9.4%, osteosclerotic 71.9%, mixed type 18.7%). This may be due to the application of systematic adjuvant bisphosphonate treatment [7].
Approximately 75% of patients with bone metastases complain of pain as their main symptom and the dominant reason for a decreased quality of life [8]. Appropriate pain management may be difficult, particularly in case of poorly localized discomfort [9]. The prognosis of patients with metastases confined to the skeleton is usually superior to that of patients with soft-tissue metastases, in lungs, liver or lymph nodes, for example [10], and therefore merits careful consideration.
In addition to analgesic drugs (prescribed according to the WHO scheme) [4], local external-beam radiation therapy, and surgical interventions, especially in locally restricted disease, several radiopharmaceuticals have been developed for the systemic palliation of bone pain with more multilocular skeletal involvement.
Radionuclide Therapy
The first application of the bone-seeking radiopharmaceutical strontium-89 [89Sr]-chloride was described by Pecher in 1940/41 [11], followed by the first report of pain palliation in a patient with bone metastases from breast carcinoma using phosphorous-32 [32P] by Friedell [12].
In Europe, strontium [89Sr]-chloride is approved for bone pain palliation in patients with bone metastases of prostate cancer, whereas samarium [153Sm]-ethylenediaminetetrameth-ylenephosphonate (EDTMP) is approved for the treatment of pain from all osteoblastic bone metastases. Phosphor [32P]-orthophosphate is used in several other countries. 89Sr is a calcium analog and is incorporated into the newly formed hydroxyapatite of the bone matrix. 153Sm is radiolabeled to a bisphosphonate (EDTMP) and adsorbed onto the hydroxyapatite surface of metabolically active bone by the same mechanism as technetium [99mTc]-labeled bisphosphonates used for diagnostic bone scintigraphy.
Selective uptake depends on the degree of the metabolic (i.e. osteoblastic) response elicited in normal bone by the presence of metastatic tissue. Increased bone turnover leads to enhanced incorporation of bone-seeking radiopharmaceuticals at metastatic sites, by comparison with normal bone, and can therefore deliver a high, targeted local radiation dose. Skeletal uptake of the radiolabeled bisphosphonate 153Sm-EDTMP is in the order of 48% of the administered activity [13]. The effective half life of 89Sr in bone metastases is greater than 50 days, compared with 14 days in normal bone [14] (table 2).
Table 2.
Physical characteristics of radiopharmaceuticals used for bone pain palliation
| Radionuclide | Carrier | Physical half-life, days | βmax, MeV | βmean, MeV | Mean rangec in tissue, mm | γ-Energy, keV (%) |
|---|---|---|---|---|---|---|
| 89Sr | chloride | 50.5 | 1.46 | 0.583 | 6.7 | – |
| 153Sm | EDTMP | 1.95 | 0.8 | 0.224 | 3.4 | 103 (28) |
| 32Pa | phosphate | 14.28 | 1.71 | 0.695 | 7.9 | – |
| 188Reb | HEDP | 0.71 | 2.12 | 0.76 | 11.0 | 155 (1) |
| 117mSnb | DTPA | 13.6 | no beta-emission | 0.3 | CE 159 | |
| 33Pb | phosphate | 25.34 | 0.249 | 0.85 | 0.05 | |
| 223Rab | chloride | 11.4 | alpha-emitter (eff. energy 26.4 MeV) | < 100 µm |
Not approved in Germany.
Clinical trial only.
Mean range in periosseous soft tissue.
EDTMP = Ethylenediaminotetramethylenephosphonate, HEDP = hydroxyethylenediphosphonate, DTPA = diethylenetriaminopentaacetate, CE = conversion electrons.
32P as sodium phosphate is no longer approved in many countries because of documented myelotoxocity associated with therapeutic administration. More recently, a clinical trial comparing 89Sr and 32P in patients suffering from bone metastases reported slightly higher toxicity in the 32P group but comparable efficacy in terms of pain palliation [15]. Further research will be necessary to confirm these results particularly in heavily pretreated patients who may have limited bone marrow reserves.
Clinical trials are in progress evaluating the therapeutic potential of other radionuclides for bone pain palliation. These include: tin [117mSn]-diethylenetriaminopentaacetate (DTPA), sodium [33P]-phosphate, rhenium [188Re]-hydroxye-thylidenediphosphonate (HEDP), lutetium [177Lu]-EDTMP, and radium [223Ra]-chloride.
In addition to clinical variables such as skeletal metastatic burden, disease distribution, and prior treatment, myelosuppression resulting from systemic bone-seeking radiopharmaceutical therapy reflects the effective half-life, particle energy and particle range of the radionuclide used. The use of low-energy beta-emitting radionuclides would be expected to deliver a high absorbed dose to the bone surface, but a negligible dose to the hematopoietic bone marrow [16]. Theoretical dose calculations predict a 3–6-fold advantage in terms of myelotoxicity risk if 33P were substituted for 32P, for example [17]. The same is true for conversion electons of 117mSn or alpha-emitters like 223Ra.
To reduce the myelotoxicity, the therapeutic potential of conversion electron-emitting radiolabels such as 117mSn-DTPA has been reported. Conversion electrons ejected during the decay of this nuclide have a 1.7–5.5 times lower energy than beta-particles conventionally used for systemic treatment for pain palliation [18]. But the limiting factor of this compound used in a phase I/II clinical study was not the radiation dose to the marrow but the high amount of DTPA in the current formulation, in a 20-fold molar excess over tin [19]. More recently, a new 1:1 chelate was synthesized [20].
Estimates of the absorbed radiation dose delivered to osteoblastic bone metastases vary widely, ranging from 6–61 cGy/MBq for 89Sr, 1000–14,000 cGy from a standard treatment activity of 1295 MBq 186Re-HEDP (this radiopharmaceutical was recently withdrawn from the market), and a mean dose of 87 Gy from 2590 MBq 153Sm-EDTMP. A dose of 54 mGy/MBq was reported using 117mSn-DTPA, with the bone uptake ranging from 34 to 83% of the injected activity [21].
The bone-seeking alpha-particle emitter radium-223 is predicted to deliver a high absorbed radiation dose to the bone surface, with sparing of the bone marrow compartment. From data of animal experiments, a total skeletal dose of 553–790 Gy was calculated after administration of 3750 kBq 223Ra per kilogram bodyweight [22]. Following intravenous administration, skeletal uptake peaks within 1 h of injection, with no subsequent redistribution. Phase I and II studies confirm low temporary myelosuppression approximately 4 weeks post treatment, but this rarely exceeds WHO grade I/II even at high activities (200 kBq/kg) in heavily pretreated patients [23]. Less than 1% of 292 patients developed grade IV hematological toxicity; grade III toxicity for hemoglobin was experienced by 4.8%, and for platelets, neutrophiles and white blood cells by < 3%. The preliminary results of a double-blind, randomized, placebo-controlled phase III trial (ALSYMPCA) with its primary endpoint of survival show low toxicity and a mean survival of 14 months for the radium-223 group compared to 11.2 months for the placebo group. The median time of new skeletal events was 13.6 versus 8.4 months.
The mechanism and radiobiology of pain reduction using unsealed source therapy is not yet fully understood. A direct radiation effect on neuronal tissue seems unlikely due to the well-known high radiation resistance of peripheral neurons. It is more conceivable that radiation to cells and tissues surrounding the metastasis promotes cell signaling changes, resulting in modulation of both pain reception and transmission. Possible target cells are likely to include macrophages, mast cells, thrombocytes, lymphocytes, and endothelial cells, which influence secretion of pain mediators such as ATP, histamine, prostaglandin E (PGE), interleukin (IL)−1 and −2, leukotrienes, and substance P. Animal experiments [24] have shown that 223Ra inhibits the differentiation of osteoclasts, and probably thereby also the progression of mainly osteolytic breast cancer bone metastases.
Indications, Contraindications and Procedure of Pain Palliation Treatment
Surgical stabilization and/or external-beam radiation are the treatments of choice for the management of solitary, painful bone metastases, bones at high risk of pathological fracture, and in patients with impending spinal cord compression.
Systemic radionuclide therapy is indicated to manage multifocal metastatic bone pain following failure of conventional analgesics and to palliate recurrent pain within a previously irradiated site. It is likewise indicated if the side effects of high-dose analgesics become intolerable and significantly compromise the quality of life, even if pain control is adequate.
Strontium [89Sr]-chloride is approved for pain palliation in patients with bone metastases from prostate cancer; samarium [153Sm]-EDTMP may also be used in patients suffering from osteoblastic metastases of other tumor types. The activity of 153Sm-EDTMP is adjusted for the patient's body weight (37 MBq/kg), whereas 89Sr-chloride is prescribed as standardized activity (150 MBq).
A prerequisite for radionuclide treatment of metastatic bone pain is the demonstration of multifocal abnormal skeletal uptake on conventional 99mTc phosphate bone scintigraphy, corresponding to known pain sites [58]. Patients should have reasonable bone marrow reserves, as evidenced by (near) normal blood counts. The gamma-emission of Sm-153 is useful for early post-therapy imaging to confirm selective tracer uptake and appropriate targeting.
Due to the delay between treatment administration and onset of pain relief, which may take 1 week in case of [153Sm]-EDTMP and up to 4 weeks using 89Sr, patients should have a life expectancy of at least 3 months. Absolute contraindications to radionuclide therapy include pregnancy, breast-feeding and severe bone marrow depression, for beta-emitters indicated by platelets < 60,000/µl or leucopenia < 2400/µl [25]. Acute spinal cord compression, disseminated intravascular coagulation, and impaired renal function (urea > 12 mmol/l or creatinine > 150 mmol/l) are regarded as additional contraindications in German, European and American guidelines for pain palliation treatment using Sm-153-EDTMP or Sr-89.
Patients with urinary incontinence should be catheterized prior to treatment, to mitigate the risk of radioactive urine contamination. Specialist referral is advised where bones are considered at risk of pathological fracture. To allow time for bone marrow recovery and avoid unpredictable cumulative toxicity, unsealed source treatment should be delayed for 6–8 weeks after completion of chemotherapy. It is recommended that further chemotherapy be deferred for at least 8–12 weeks, depending on the radiopharmaceutical used. A 2–3-month delay is recommended after large-field radiation therapy.
Concomitant treatment with modern bisphosphonates, which are characterized by very low effective levels, does not interfere with the uptake of bone-seeking radionuclides [23, 26, 59]. This is in contrast to former concerns regarding the classical drugs clodronate or etidronate. Focal abnormal uptake should, however, be confirmed in every patient by pre-therapeutic bone imaging and correlated with the localization of the pain.
Following appropriate oral hydration, the bone-seeking radiopharmaceutical is administered intravenously via a peripheral cannula, usually in an outpatient setting, depending on local legislation. Prior to discharge, the uptake and distribution of the activity of 153Sm-EDTMP can be documented by whole-body scanning 5–24 h post injection. Renal excretion of the unbound fraction of the radionuclide is very rapid, i.e. 71% within 3 days [27] compared with 53% of the unbound 153Sm-EDTMP excreted via the kidneys within 6–8 h after injection [28].
Blood counts, especially thrombocytes and white blood cells, must be monitored weekly to track expected, temporary bone marrow suppression. Marrow recovery is usual within 8 weeks of 153Sm-EDTMP administration and within 12 weeks of 89Sr treatment, with the speed and completeness of hematopoietic regeneration being determined by the underlying bone marrow reserves. With appropriate patient selection and careful monitoring, clinically significant or protracted bone marrow suppression requiring red cell or platelet transfusion is rare. Palliative pain therapy may be repeated to treat recurrent symptoms, a minimum of 8–12 weeks after previous 153Sm-EDTMP administration or 3–4 months after therapy with 89Sr. Hematotoxicity caused by 223Ra is much less because of the rapid uptake in bone (> 75 of the administered activity is cleared from the blood and plasma within 15 min after injection) and the very short range of alpha-particles [29].
Clinical Results
Of the patients with metastatic prostate or breast cancer, 70–80% report symptom benefit following treatment with bone-seeking radiopharmaceuticals (figs. 1 and 2). Pain relief typically occurs within 1 week of intravenous 153Sm-EDTMP administration and usually lasts for about 8–12 weeks, although prolonged responses of up to 12 months have been reported [23]. The advantage of 89Sr is a longer mean response duration of approximately 4 to 6 months, but this benefit must be weighed against the delayed onset of symptom palliation of 14–28 days after radiopharmaceutical administration [30] and the increased risk of myelosuppression.
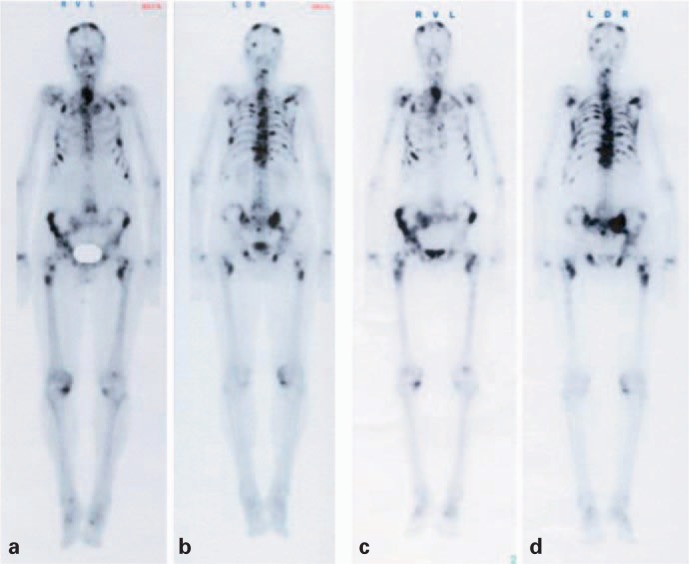
Fig. 1.
A 76-year-old male patient with prostate cancer. Whole-body bone scan 2 h after intravenous injection of 698 MBq 99mTc-DPD Multiple osteoblastic metastases are seen from both the anterior (a) and posterior (b) projection. Post-therapy whole-body scan (c, d) 24 h after application of the second treatment with 153Sm-EDTMP. The cumulative activity of both treatments was 6.7 GBq. The scan shows mild progression of the bony lesions. The PSA level increased up to 1,210 ng/ml, from the staging scan to 5 months after the second treatment. The patient is pain free since the first therapy.
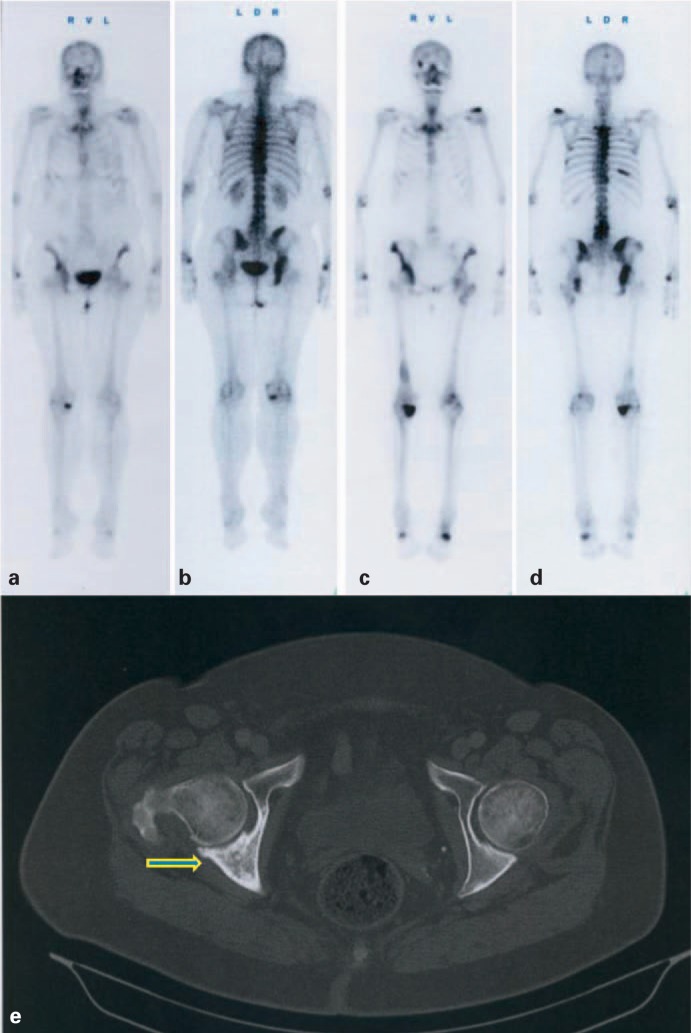
Fig. 2.
A 68-year-old female patient with breast cancer. Whole-body bone scan 3 h after intravenous injection of 656 MBq 99mTc-DPD, in anterior (a) and posterior view (b). Last post-therapy whole-body scan (c, d) 23 h after intravenous injection of 3.2 GBq 153Sm-EDTMP. Mild progressive disease after a cumulative activity of 17.0 GBq 153Sm-EDTMP; cancer antigen (Ca) 15–3 was increased from 65.5 U/ml (staging scan) to 175 U/ml 15 months later. (e) Computed tomography of the pelvis after the third 153Sm-EDTMP and continuous bisphosphonate therapy, showing calcification of a large, mainly lytic lesion of the pelvis (arrow).
No reliable response predictors have been established [31, 32]. Prostate-specific antigen (PSA) decline in prostate cancer patients treated using radionuclide therapy does not correlate with pain palliation. In a small study of 50 patients treated with 89Sr for metastatic castration-resistant prostate cancer (CRPC), a decrease or stabilization of the PSA levels after treatment (28% of patients, n = 14) was associated with a significant mean survival improvement from 275 to 641 days and prolonged time to pain progression (67 to 142 days) [33]. In a review of data published in evidence-based trials, Serafini [34] summarized reported response rates, in terms of pain palliation, of different radionuclides (table 3). These results were confirmed or completed by other groups [35, 36].
Table 3.
Clinical response rate on systemic radionuclide therapy
| Nuclide | Primary tumor | Response, % | Reference |
|---|---|---|---|
| 89Sr | n.r. | 70–90 | [34] |
| 153Sm | n.r. | 70–80 | [55] |
| 186Re | breast cancer | 50 | [56] |
| 186Re | breast cancer | 92 | [34] |
| 89Sr | breast cancer | 36 | [57] |
| 186Re | prostate cancer | 83 | [56] |
n.r. = Not reported.
Perspectives
There is growing interest in extending the role of bone-seeking radiopharmaceuticals beyond pain palliation towards treatment delivered with tumoricidal intent. The potential advantage of early treatment in patients with asymptomatic metastases to achieve durable disease control is well recognized [37]. Response duration is longer in patients treated early in the natural history of their disease than in subjects with advanced metastases [32]. This observation may be attributable to the effect of long-range beta-radiation on bone marrow micrometastases. Such micrometastases were detected by polymerase chain reaction (PCR) in the bone marrow of patients with prostate cancer who were staged N0 by clinical investigation and imaging procedures [38]. Other tumoricidal options include activity escalation, repeated radionuclide administration and multi-modality regimens designed to exploit potential synergies between radionuclide treatment and external-beam radiotherapy or chemotherapy. Preliminary activity-ranging studies using 153Sm-EDTMP suggested improved response rates, superior response quality and prolonged survival in patients treated using high administered activities [39, 40]. The disadvantage of further activity escalation was dose-limiting myelosuppression. A subsequent phase I study demonstrated PSA reduction in CRPC patients treated with high-activity 186Re-HEDP and peripheral stem cell support [41].
The efficacy of repeated radionuclide therapy was reported in a phase II trial comparing the response rate in CRPC patients with bone metastases after 1 or 2 administrations of 188Re-HEDP within 8 weeks (table 4). Pain palliation was significantly higher and associated with > 50% PSA reduction in 39% of the patients in the double-dose group compared with 7% in the single-dose group. The mean survival increased from 7 to 13 months [42] in the double-dose cohort. A more recently published retrospective analysis of these data of the same group showed, in a total number of 60 patients suffering from bone metastases of hormone-refractory prostate cancer, an improvement of the mean survival from 4.5 to 15.66 months, in the subgroup with multiple (3 and more) successive administrations of 188Re-HEDP [43]. Similar results were published by Turner and Claringbold [44] administering, in a phase II trial, either a single or repeated activity of 153Sm-EDTMP. The mean survival in the repeated-therapy group was 9 months versus 4 months in the single-activity group.
Table 4.
Studies with evidence of improved survival after radionuclide therapy
| Study design | Cancer type | Median survival, weeks | Δ Survival, weeks | Ref. |
|---|---|---|---|---|
| 223Ra versus placebo | HRPC | 92.9/49.4 | + 43.5 | [52] |
| 153Sm-EDTMP single versus repeated | HRPC, BC, others | 16/54 | + 38 | [44] |
| 188Re-HEDP single versus repeated | HRPC | 18/64 | + 46 | [43] |
HRPc = Hormone-refractory prostate cancer, BC = breast carcinoma.
Also pain control was significantly better in the repeated-therapy group (24 versus 8 weeks). These data were confirmed by other publications [45]. Interestingly, the response of metastases already existing prior to the first therapy was significantly better than the response of those appearing during repeated therapy [45]. In a separate study, 6 of 40 patients with metastatic breast cancer treated with 89Sr for painful skeletal metastases received repeated 89Sr administrations. Higher overall response rates (83% versus 60%) were recorded in the re-treatment subset by comparison with patients who had received a single treatment [35]. Prolonged response duration (3.08 ± 0.48 versus 5.33 ± 2.36 months) was reported in breast cancer patients receiving multiple 89Sr administrations compared with patients who had received a single treatment [6]. These data have not been confirmed in larger randomized studies. Following palliative external-beam radiotherapy to a dominant pain site, the administration of systemic radionuclide as consolidation treatment was shown to delay the development of new bone pain in patients with metastatic CRPC [46, 47].
There is growing evidence to support the addition of cytotoxic chemotherapy to radionuclide treatment in patients with predominantly osteoblastic bone metastases (‘chemosensitization’). In cell cultures, the co-incubation of radionuclides with cisplatin showed a synergistic effect with strong correlation between radiation dose and cisplatin concentration [48]. These results were confirmed by two randomized clinical trials in men with CRPC. Significant prolongation of mean survival, improved quality and duration of pain reduction, and delayed pain in clinically silent metastases were observed in patients treated using 89Sr/cisplatin compared with 89Sr/placebo. There was no significant difference in hematological toxicity between the two groups [49, 50]. Tu et al. randomized CRPC patients pretreated with induction doxorubicin and vinblastine to receive further doxorubicin as monotherapy or doxorubicin with 89Sr. A greater than 80% PSA reduction was observed in 72% of the subjects who had received doxorubicin with 89Sr compared with 36% of those who had received doxorubicin alone. The mean survival increased from 17 months in the monotherapy arm to 28 months in the combined treatment group [51]. Early results indicate that high-linear energy transfer (LET) therapeutic alpha-particle-emitting radionuclides exert a tumoricidal effect in skeletal metastases. A randomized, placebo-controlled phase II study using fractionated 223Ra in metastatic CRPC patients demonstrated significant reductions in bone alkaline phosphatase, delayed time to PSA progression (26 versus 8 weeks), and prolonged median overall survival (65.3 versus 46.4 weeks) in the active treatment arm by comparison with the control group. The hematological toxicity was similar in both groups [52].
Summary and Future Aspects
Radionuclide treatment for metastatic bone pain palliation is a safe and effective option for patients with multifocal osteoblastic metastases. Symptom benefit is reported in 70–80% of patients with metastatic breast and prostate cancer, although lower response rates are observed in patients with other primary tumors [1, 35]. Approximately 20% of patients become pain free after radionuclide therapy. The majority of patients are able to reduce or withdraw opioid analgesics, but most continue on non-steroidal anti-inflammatory medication. Economic analyses demonstrate that targeted radionuclide therapy is a cost-effective alternative to repeated external-beam irradiation in patients with multifocal skeletal metastases. For beta- as well as for alpha-emitters there are convincing data that, besides the pain palliation effect, even a prolongation of the mean survival is obvious. The therapeutic potential of new radiopharmaceuticals for bone pain palliation is under investigation. The high absorbed dose delivered by alpha-emitting radionuclides, for example, is predicted to achieve a direct antitumor effect in bone. Limited hematological toxicity resulting from the short particle range of both alpha and conversion electron emitters may allow easier integration with other treatments without the penalty of cumulative myelotoxicity.
Several multi-modality bisphosphonate, chemotherapy and radiopharmaceutical regimens have been investigated. The results consistently suggest superior symptom control and prolonged survival using combined treatment rather than either chemotherapy or radiopharmaceuticals alone. Phase II studies combining either 223Ra or 153Sm with docetaxel in CRPC are in progress. In the long term, these radiopharmaceuticals might also offer opportunities for fractionated therapy to achieve both sustained symptom benefit and sustained skeletal disease control.
A totally different approach is the use of radiolabeled monoclonal antibodies as presented recently for diagnostic imaging of prostate cancer metastases: (S)-2-(3-((S)-1-carboxy-5-((4-123I-iodobenzyl)amino)pentyl)ureido)pentanedioic acid (123I-MIP-1072). This small-molecule glutamate urea heterodimer inhibits the N-acetylated α-linked acidic dipeptidase enzymatic activity of the prostate-specific membrane antigen (PSMA). In patients, a high specificity of this tracer for prostate cancer tumor cells was observed. A trial is in progress treating patients with metastases from prostate cancer using 131I-MIP-1072 [53].
Disclosure Statement
M.F. is advisor to CISbio Germany, member of the IBA group. There is no conflict of interest for W.U.K.
References
- 1.Elgazzar AH, Maxon HR. Radioisotope therapy of cancer related bone pain. In: Limouris GS, Shukla SK, editors. Radionuclides for therapy. Athens: Mediterra Publishers; 1993. pp. 111–116. [Google Scholar]
- 2.Roodman GD. Mechanisms of bone lesions in multiple myeloma and lymphoma. Cancer. 1997;80:1557–1514. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1557::aid-cncr5>3.3.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation Cancer pain relief and palliative care. World Health Organ Tech Rep Ser. 1990;804:7–73. [PubMed] [Google Scholar]
- 5.Galasko CSB. Mechanisms of lytic and blastic metastatic disease of bone. Clin Orthop. 1982;169:20–27. [PubMed] [Google Scholar]
- 6.Kasalický J, Kraská V. The effect of repeated strontium-89 chloride therapy in bone pain palliation in patients with skeletal cancer metastases. Eur J Nucl Med. 1998;25:1362–1367. doi: 10.1007/s002590050309. [DOI] [PubMed] [Google Scholar]
- 7.Quattrocchio CC, Piicucchi S, Sammarra M, Santini D, Vincenzi B, Tonini G, Grasso RF, Zobel BB. Bone metastases in breast cancer: higher prevalence of osteoblastic lesions. Radiol Med. 2007;112:1049–1059. doi: 10.1007/s11547-007-0205-x. [DOI] [PubMed] [Google Scholar]
- 8.Tannock I, Gospodarowicz M, Meakin W, Panzarella T, Stewart L, Rider W. Treatment of metastatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol. 1989;7:590–597. doi: 10.1200/JCO.1989.7.5.590. [DOI] [PubMed] [Google Scholar]
- 9.Wagner G. Frequency of pain in patients with cancer. Recent Results Cancer Res. 1984;89:64–71. doi: 10.1007/978-3-642-82028-1_7. [DOI] [PubMed] [Google Scholar]
- 10.Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336–340. doi: 10.1038/bjc.1998.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pecher C. Biological investigations with radioactive calcium and strontium: preliminary report on the use of radioactive strontium in the treatment of metastatic bone cancer. Univ Calif Pub Pharmacol. 1942;2:117–149. [Google Scholar]
- 12.Brucer M. A chronology of nuclear medicine. St. Louis: Heritage Publications; 1990. [Google Scholar]
- 13.Brenner W, Kampen WU, Kampen AM, Henze E. Skeletal uptake and soft tissue retention of 186-Re-HEDP and 153-Sm-EDTMP in patients with metastatic bone disease. J Nucl Med. 2001;42:231–236. [PubMed] [Google Scholar]
- 14.Blake GM, Zivanovic MA, McEwan AJB, Ackery DM. Sr-89 therapy: strontium kinetics in disseminated carcinoma of the prostate. Eur J Nucl Med. 1986;12:447–454. doi: 10.1007/BF00254749. [DOI] [PubMed] [Google Scholar]
- 15.Fettich J, Padhy A, Nair N, et al. Comparative clinical efficacy and safety of phosphorus-32 and strontium-89 in the palliative treatment of metastatic bone pain: Results of an IAEA coordinated research project. World J Nucl Med. 2003;2:226–231. [Google Scholar]
- 16.Bouchet LG, Bolch WE, Goddu SM, et al. Considerations in the selection of radiopharmaceuticals for palliation of bone pain from metastatic osseous lesions. J Nucl Med. 2000;41:682–687. [PubMed] [Google Scholar]
- 17.Goddu SM, Bbishayee A, Bouchet LG, et al. Marrow toxicity of 33-P versus 32-P-orthophosphate: implication for therapy of bone pain and bone metastases. J Nucl Med. 2000;41:941–951. [PubMed] [Google Scholar]
- 18.Hillegonds DJ, Franklin S, Shelton DK, et al. The management of painful bone metastases with an emphasis on radionuclide therapy. J Natl Med Assoc. 2007;99:785–794. [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava SC. Treatment of bone and joint pain with electron emitting radiopharmaceuticals. Int J Nucl Med Biol. 2004;19:89–97. [Google Scholar]
- 20.Srivastava SC, Meinken GE, Li Z, et al. A novel sythetic method for preparing Sn-117m stannic DTPA for therapeutic use. J Nucl Med. 2002;43:372. [Google Scholar]
- 21.Atkins HL, Mausner LF, Srivastava SC, Meinken GE, et al. Tin-117m(4+)-DTPA for palliation of pain from osseous metstases: a pilot study. J Nucl Med. 1995;36:725–729. [PubMed] [Google Scholar]
- 22.Larsen RH, Saxtorph H, Skydsgaard M, Borrebaek J, Jonasdottier TJ, et al. Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: histology, clinical chemistry and haematology. In vivo. 2006;20:325–332. [PubMed] [Google Scholar]
- 23.McEwan AJB. Palliation of bone pain. In: Murray IPC, Ell PJ, editors. Nuclear Medicine in Clinical Diagnosis and Treatment. Edinburgh: Churchill Livingston; 1994. pp. 877–892. [Google Scholar]
- 24.Suomine MI, Rissanen JK, Käkönen R, Mumberg D, Ziegelbauer K, Halleen J, Scholz A.Alpharadin inhibits osteoclast differentiation in vitro and progression of established breast cancer bone metastases in vivo. American Association for Cancer Research Annual Meeting 2011;abstr 2664.
- 25.Fischer M. Leitlinie für die Radionuklidtherapie bei schmerzhaften Knochenmetastasen. Nuklearmedizin. 1999;38:270–272. [PubMed] [Google Scholar]
- 26.Lau WF, Hicks R, Binns D. Differential effects of bisphosphonate on Paget's disease and metastatic prostatic carcinoma bone scan findings. Clin Nucl Med. 2001;26:347–348. doi: 10.1097/00003072-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Maxon HR, Deutsch EA, Thomas SR, Libson K. Re-186 (Sn) HEDP for treatment of multiple metastatic foci in bone: human biodistribution and dosimetric studies. Radiology. 1988;166:501–507. doi: 10.1148/radiology.166.2.3122267. [DOI] [PubMed] [Google Scholar]
- 28.Singh A, Holmes RA, Farhangi M, Volkert WA. Human pharmacokinetics of samarium-153 EDTMP in metastatic cancer. J Nucl Med. 1989;30:1814–1818. [PubMed] [Google Scholar]
- 29.Lewington V, Parker C, Hindorf C, Flux G, Chittenden S, et al. Alpharadin, a novel targeted approach for treatment of bone metastases from CRPC – calculated alpha-particle dosimetry compared to a favourable clinical safety profile. American Society of Clinical Oncology Annual Meeting 2010; abstr no 216.
- 30.Pons F, Herranz R, Garcia A, Vidal-Sicart S, Conill C, Grau JJ, Alcover J, Fuster D, Setoain J. Strontium-89 for palliation of pain from bone metastases in patients with prostate and breast cancer. Eur J Nucl Med. 1997;24:1210–1214. doi: 10.1007/s002590050143. [DOI] [PubMed] [Google Scholar]
- 31.McCready VR, O'Sullivan J, Dearnaly D, Cook G. Prediction of response of skeletal metastases from cancer of the prostate to high activity 186-Re HEDP therapy. J Nucl Med. 2002;43(suppl) abstr 316. [Google Scholar]
- 32.Sciuto R, Tofani A, Festa A, et al. Short- and long-term effects of 186-Re-1,1-hydroxyethylidene diphosphonate in the treatment of painful bone metastases. J Nucl Med. 2000;41:647–654. [PubMed] [Google Scholar]
- 33.Zyskowski A, Lamb D, Morum P, et al. Strontium-89 treatment for prostate cancer bone metastases: does a prostate specific antigen response predict for improved survival? Australian Radiol. 2001;45:39–42. doi: 10.1046/j.1440-1673.2001.00871.x. [DOI] [PubMed] [Google Scholar]
- 34.Serafini AN. Therapy of metstatic bone pain. J Nucl Med. 2001;42:895–906. [PubMed] [Google Scholar]
- 35.Fuster D, Herranz R, Vidal-Sicart S, et al. Usefulness of strontium-89 for bone pain palliation in metastatic breast cancer patients. Nucl Med Commun. 2000;21:623–626. doi: 10.1097/00006231-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Liepe K, Franke W-G, Koch R, et al. Comparison of rhenium-188, rhenium-186 and strontium in palliation of painful bone metastases. Nuklearmedizin. 2000;39:146–151. [PubMed] [Google Scholar]
- 37.Krishnamurthy GT, Krishnamurthy S. Invited commentary: Radionuclides for metastatic bone pain palliation: a need for rational re-evaluation in the new millenium. J Nucl Med. 2000;41:688–691. [PubMed] [Google Scholar]
- 38.Deguchi T, Yang M, Ehara H, et al. Detection of micrometastatic prostate cancer cells in the bone marrow of patients with prostate cancer. Br J Cancer. 1997;75:634–638. doi: 10.1038/bjc.1997.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins C, Eary JF, Donaldson G, et al. Samarium-153 EDTMP in bone metastases of hormone refractory prostate carcinoma: a phase I/II trial. J Nucl Med. 1993;34:1839–1844. [PubMed] [Google Scholar]
- 40.Resche I, Chatal JF, Pecking, et al. A dose controlled study of Sm-153 ethylenediaminetetra-methylenephosphonate in the treatment of patients with painful bone metastases. Eur J Cancer. 1997;33:1583–1591. doi: 10.1016/s0959-8049(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 41.O'Sullivan JM, McCready VR, Flux G, et al. High activity rhenium-186 HEDP with autologous blood stem cells rescue: a phase I study in progressive hormone refractory prostate cancer metastatic to bone. Br J Cancer. 2002;86:1715–1720. doi: 10.1038/sj.bjc.6600348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmedo H, Manka-Waluch A, Albers P, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: randomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol. 2003;21:2869–2875. doi: 10.1200/JCO.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 43.Biersack HJ, Palmedo H, Andris A, Rogenhofer S, et al. Palliation and survival after repeated 188Re-HEDP therapy of hormone-refractory bone metastases of prostate cancer: a retrospective analysis. J Nucl Med. 2011;52:1721–1726. doi: 10.2967/jnumed.111.093674. [DOI] [PubMed] [Google Scholar]
- 44.Turner JH, Claringbold PG. A phase II study of treatment of painful multifocal skeletal metastases with single and repeated dose samarium-153 ethylenediaminetetramethylenephosphonate. Eur J Cancer. 1991;27:1084–1086. doi: 10.1016/0277-5379(91)90297-q. [DOI] [PubMed] [Google Scholar]
- 45.Sinzinger H, Palumbo B, Özker K. The Vienna protocol and perspectives in radionuclide therapy. Q J Nucl Med Mol Imaging. 2011;55:420–430. [PubMed] [Google Scholar]
- 46.Bolger JJ, Dearnaley DP, Kirk D, Lewington VJ, et al. Strontium-89 (Metastron) versus external beam radiotherapy in patients with painful bone metastases secondary to prostate cancer: preliminary report of a multicenter trial. Semin Oncol. 1993;20(suppl 2):32–33. [PubMed] [Google Scholar]
- 47.Porter AT, McEwan AJB, Powe JE, Reid R, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Rad Oncol Biol Phys. 1993;25:805–813. doi: 10.1016/0360-3016(93)90309-j. [DOI] [PubMed] [Google Scholar]
- 48.Geldof AA, de Rooij L, Versteegh RT, et al. Combination 186-Re-HEDP and cisplatin supraadditive treatment effects in prostate cancer cells. J Nucl Med. 1999;40:667–671. [PubMed] [Google Scholar]
- 49.Sciuto R, Festa A, Rea S, et al. Effects of low-dose cisplatin on 89-Sr therapy for painful bone metastases from prostate cancer: a randomized clinical trial. J Nucl Med. 2002;43:79–86. [PubMed] [Google Scholar]
- 50.Tu SM, Delpass ES, Jones D, et al. Strontium-89 combined with doxorubicin in the treatment of patients with androgen independent prostate cancer. Urol Oncol. 1997;2:191–197. doi: 10.1016/s1078-1439(97)00013-6. [DOI] [PubMed] [Google Scholar]
- 51.Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:336–341. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson S, Franzen L, Parker C, et al. Bone targeted radium-223 in symptomatic, hormone refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 53.Hiller SM, Kern AM, Maresca KP, Marquis JC, Eckelman WC, Joyal JL, Babich JW. 123I-MIP-1072, a small-molecule inhibitor of prostate-specific membrane antigen is effective at monitoring tumor response to taxane therapy. J Nucl Med. 2011;52:1087–1093. doi: 10.2967/jnumed.110.086751. [DOI] [PubMed] [Google Scholar]
- 54.Paulus P. Re-186-HEDP in routine use: correlation between dose rate measurements and clinical efficacy. The Update. 1995;1:17–20. [Google Scholar]
- 55.Serafini AN. Samarium Sm-153 lexidronam for the palliation of bone pain associated with metastases. Cancer. 2000;88(suppl):2034–2039. doi: 10.1002/1097-0142(20000615)88:12+<2934::aid-cncr9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 56.Kalesnikov-Gauthier H, Carpenier P, Depreux P, et al. Evaluation of toxicity and efficacy of 186-Rehydroxyethylidene diphosphonate in patients with painful bone metastases of prostate or breast cancer. J Nucl Med. 2000;41:1689–1694. [PubMed] [Google Scholar]
- 57.Baziotis N, Yakoumakis E, Zissimopoulos, et al. Strontium-89 chloride in the treatment of bone metastases from breast cancer. Oncology. 1998;55:377–381. doi: 10.1159/000011881. [DOI] [PubMed] [Google Scholar]
- 58.Fischer M, Böhme K. Nuklearmedizinische Schmerztherapie bei metastasiertem Prostatakarzinom. Der Nuklearmediziner. 1996;5:339–344. [Google Scholar]
- 59.Marcus CS, Saeed S, Mlikotic A, Mishkin F, Pham HL, Javellana T, Diestelhorst S, Minami C. Lack of effect of a bisphosphonate (pamidronate disodium) infusion on subsequent skeletal uptake of Sm-153-EDTMP. Clin Nucl Med. 2002;27:427–430. doi: 10.1097/00003072-200206000-00008. [DOI] [PubMed] [Google Scholar]