Summary
Graft-versus-host disease (GVHD) is partly mediated by host antigen presenting cells (APCs) that activate donor T-cells. Extracorporeal photopheresis (ECP) can modulate APC function and benefit some patients with GVHD. We report the results of a study using ECP administered prior to a standard myeloablative preparative regimen intended to prevent GVHD. Grade II-IV aGVHD developed in 9 (30%) of 30 recipients of HLA-matched related transplants and 13 (42%) of 31 recipients of HLA-matched unrelated or HLA-mismatched related donor transplants. Actuarial estimates of overall survival (OS) at day 100 and 1 year post transplant were 89% (95% CI, 78%-94%) and 77% (95% CI, 64%-86%), respectively. There were no unexpected adverse effects of ECP. Historical controls receiving similar conditioning and GVHD prophylaxis regimens but no ECP were identified from the database of the Center for International Blood and Marrow Transplant Research and multivariate analysis indicated a lower risk of grade II-IV aGVHD in patients receiving ECP (p=0.04). Adjusted OS at one year was 83% in the ECP study group and 67% in the historical control group (relative risk 0.44, 95% CI, 0.24-0.80) (p= 0.007). These preliminary data may indicate a potential survival advantage with ECP for transplant recipients undergoing standard myeloablative hematopoietic cell transplantation.
Keywords: Photopheresis, Allogeneic, GVHD
Introduction
Acute graft-versus-host disease (aGVHD), and complications of its treatment, are the major causes of morbidity and mortality following allogeneic hematopoietic stem cell transplantation (HCT) [1-5]. The pathophysiology of aGVHD involves tissue damage after the preparative regimen, presentation of host antigen by host dendritic cells (DCs) to donor T cells, and the activation and release of inflammatory cytokines [6]. Using standard GVHD prophylaxis with a calcineurin inhibitor and methotrexate, 20-40% of recipients of HLA-matched related donor transplants and 50-80% of recipients of unrelated donor transplants develop grades II-IV aGVHD [2-5, 7].
Extracorporeal photopheresis (ECP) involves ex vivo incubation of leukapheresed peripheral blood mononuclear cells with 8-methoxypsoralen (8-MOP), exposing these cells to ultraviolet A light, and then re-infusing the treated cells into the patient. 8-MOP is a DNA-intercalating agent that when exposed to ultraviolet A light undergoes a conformational change leading to intra- and inter-strand photo-adducts, prevention of transcription, and apoptosis of affected cells. Significant clinical responses to ECP when used to treat both acute and chronic GVHD are described [8-18]. In patients with chronic GVHD, ECP is associated with the normalization of CD4/CD8 ratios, increased numbers of natural killer cells, decreased numbers of DCs, a shift from DC1 to DC2, and a shift from Th1 to Th2 immune responses [19-20].
The mechanism of action of ECP and its potential for preventing aGVHD is suggested by studies in murine models. Data from Shlomchik and colleagues demonstrate that host antigen-presenting cells are required for induction of GVHD [21], and it is thought that ECP may prevent GVHD by inactivation of these cells. Other data suggests that ECP-induced apoptosis may help regulate tolerance through several potential mechanisms including inhibition of pro-inflammatory cytokines [22, 23], anti-inflammatory cytokine production [23, 24], decreased stimulation of effector T cells [25], depletion of effector T cells [26], and stimulation of regulatory T cells [27-29]. A recent study of ECP in a murine model of experimental GVHD demonstrated reversal of established GVHD by increasing donor regulatory T cell populations and indirectly reducing donor effector T cells [30]. Foss and colleagues conducted a clinical study using ECP to decrease host DCs as part of a novel reduced intensity preparative regimen [31]. This study demonstrated a lower than expected incidence of aGVHD with no untoward effects on engraftment or disease relapse. In a separate analysis of these data, the investigators also reported strong correlation of acute and chronic GVHD with the persistence of host DCs at day 100 post-HCT [32].
We report the results of the first multi-institutional Phase II study adding pre-transplant ECP to cyclosporine (CSP) and methotrexate (MTX) in conjunction with a standard myeloablative preparative regimen to prevent aGVHD after allogeneic HCT. This manuscript describes the primary outcomes of the study patients and compares those to a historical control group registered with the Center for International Blood and Marrow Transplant Research (CIBMTR).
Subjects, Materials, and Methods
Subjects
The study and consent forms were approved by the institutional review boards, or equivalent body, of all participating sites. All subjects signed approved consent forms prior to beginning study treatment. Eligibility criteria included subjects 18 to 60 years of age with hematologic malignancies and organ function that did not preclude treatment with a myeloablative preparative regimen and allogeneic HCT. Subjects were eligible for the study if they had a diagnosis of a hematologic malignancy for which a treatment option would be an allogeneic bone marrow or peripheral blood stem cell (PBSC) transplantation. Subjects could be enrolled whether their disease was in remission or not, or after a first or second relapse of disease. Subjects were required to weigh at least 40 kg, have a platelet count above 20,000/cmm and no known sensitivity to psoralen or citrate products. Subjects had to have a related donor that was serologically or molecularly matched at all HLA-A, B and DR loci or mismatched at one HLA-A or -B locus but molecularly matched at HLA-DR loci, or have an unrelated donor that was molecularly matched at HLA-A, -B, and DR loci. Since enrollment occurred from October of 2002 through January of 2004, HLA-C matching was not routinely performed.
Preparative regimen and prophylaxis against GVHD
Subjects received CY (60 mg/kg per day for 2 consecutive days) and TBI (10-13.5 Gy delivered over 3 or 4 days in fractionated doses.). GVHD prophylaxis was CSP 3-5 mg/kg IV beginning D −1 and adjusted to keep trough levels between 200 and 600 ng/ml. The CSP was changed to oral administration when clinically tolerated and tapered no sooner than D 100, except in subjects who had relapse or intolerance to CSP. Subjects who received their HCT from a matched related donor received MTX 10 mg/m2 IV on day 1, and 5 mg/m2 on days, 3, 6, and 11, while subjects who had a mismatched related donor or a matched unrelated donor received MTX 15 mg/m2 on day 1, and 10 mg/m2 on days 3, 6, and 11. The dosing of MTX was based on institutional standards at participating centers and agreement among the investigators to provide uniform prophylaxis for aGVHD. Supportive care and prophylactic anti-microbial treatment were given per each study site’s institutional guidelines.
Extracorporeal photopheresis
ECP was performed using the UVAR XTS machine (Therakos, Exton, PA) as previously described [33]. In general, collection of at least 1500 ml of buffy coat blood was performed for each treatment prior to using methoxsalen solution (UVADEX® Therakos, Exton, PA) which was injected into the recirculation bag of the ECP circuit after collection of the buffy coat was complete, but prior to the photoactivation process. After completion of photoactivation, the treated buffy coat was reinfused into the subject. Patients received ECP on 2 consecutive days within 4 days prior to beginning the preparative regimen.
GVHD and adverse event grading
Grading of adverse events were performed according to established criteria [33]. The modified Seattle-Glucksberg criteria were used for staging of acute GVHD [34], and the diagnostic criteria of limited and extensive chronic GVHD were as described by Schulman [35]. In order to ensure uniformity in the diagnosis of acute and chronic GVHD, investigators underwent training on the diagnostic criteria prior to the initiation of the trial. Each investigator employed appropriate diagnostic methods at his/her study site to determine the presence of acute or chronic GVHD.
Statistics
Study Analysis
The primary analysis was the incidence rate of grade II-IV acute GVHD in the first 100 days after transplantation and was calculated using the cumulative incidence function to accommodate the competing risk of death without the development of acute GVHD. Similarly, the cumulative incidence method was used to compute the incidence rates for chronic GVHD, transplant related mortality, and relapse to accommodate competing risks.
The probabilities of overall survival (OS) and disease free survival (DFS) were described using Kaplan-Meier product limit estimates with 95% confidence intervals. Treatment failure (death or relapse) was the event used in the DFS assessment. Descriptive statistics, such as median time to event, were also calculated.
Comparison with historical controls
Historical controls were identified using the database maintained by the CIBMTR. The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR) at the Medical College of Wisconsin, and the National Marrow Donor Program (NMDP) that comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic stem cell transplants to a coordinating statistical center. Participating centers are required to report all transplants consecutively and compliance is monitored by on-site audits. All patients in the database are followed longitudinally, and yearly evaluations are included. Computerized checks for errors, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR during this time period were done with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
The CIBMTR collects data at two levels: registration and research. Registration data include disease type, age, sex, pre-transplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow- and/or blood-derived stem cells), high-dose conditioning regimen, post-transplant disease progression and survival, development of a new malignancy and cause of death. Requests for data on progression or death for registered patients are at six-month intervals. All participating CIBMTR sites contribute registration data. Research data are collected on subset of registered patients selected using a weighted randomization scheme to ensure representativeness and include detailed disease, pre- and post-transplant clinical information. Historical controls for this study were derived from the Research database.
ECP study subjects were transplanted between 2002 and 2004. CIBMTR controls were selected by applying the eligibility criteria for this study to subjects transplanted between 1997 and 2004. The longer time frame for the controls was used to ensure adequate numbers of subjects for adjusted comparisons with reasonable statistical power. Outcome data for both study and control subjects were censored at one year in order to adjust for the differences in length of follow up. Since study subjects and controls were transplanted in two different time periods, the year of transplant (1997 to 1999 versus 2000 to 2004) was examined for potential impact on outcomes of controls. There was no difference in one-year outcomes of control subjects transplanted in 1997 to 1999 versus those transplanted in 2000-2004.
Study subjects and controls were compared using multivariate Cox regression analyses. Tests for proportionality were performed by adding a time-dependent covariate. These tests indicated that the proportionality assumptions were valid. A stepwise backward method was used to identify significant covariates (other than use of ECP) associated with outcomes. The variables considered in model building were age, gender, race, donor relationship, HLA-matching, graft type, disease type, disease status at transplant, and cytomegalovirus (CMV) serologic status. Treatment effect (ECP) was included in every step of model building. Tests for potential interactions between ECP treatment and other significant covariates revealed no significant interactions. One-year adjusted probabilities of overall and disease free survival were estimated from the final Cox models, stratified on treatment received and weighted by the pooled sample proportion value for each prognostic factor. These adjusted probabilities estimate likelihood of outcomes in populations with similar prognostic factors.
Results
Subject and Donor Characteristics
Sixty-six subjects were enrolled in the ECP study. Nine study centers enrolled from 1 to 16 subjects each (mean of 7 subjects per study center). However, 3 subjects did not receive study treatment with ECP; one patient withdrew study consent, one patient had a delay in transplant, and one center had mechanical problems with the ECP instrument. One subject received ECP but was not transplanted because of rapid progression of disease. After the completion of the study sixty-two subjects were considered to be evaluable in the modified intent-to-treat population dataset. Two of these subjects received only one ECP treatment prior to starting the preparative regimen because of subsequent mechanical problem with the ECP instrument. Subject, disease, donor characteristics, disease status and GVHD prophylaxis regimen information are summarized in Table 1.
Table 1. Subject Characteristics.
| N (%) | |
|---|---|
| Modified intent to treat population | 62 |
| Median age, years (range) | 39 (20-60) |
| Sex M/F | 41/21 |
| Stem cell source | |
| bone marrow | 30 (48) |
| peripheral blood | 31 (52) |
| Donor | |
| 6/6 matched related donor | 30 (48) |
| 5/6 matched related donor | 1 (2) |
| 6/6 matched unrelated donor | 31 (50) |
| Diagnosis | |
| AML | 19 (31) |
| ALL | 14 (23) |
| CML | 15 (24) |
| NHL | 5 (8) |
| MDS | 3 (5) |
| CLL | 3 (5) |
| Other | 3 (5) |
| Disease Status at transplant | |
| CR 1 | 26 (42) |
| After 1st or 2nd Relapse | 29 (47) |
| Primary Refractory | 7 (11) |
AML indicates acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; NHL, non-hodgkins lymphoma; MDS, myelodysplastic syndrome; CLL, chronic lymphocytic leukemia.
Engraftment
One subject did not engraft and died of respiratory failure (cause unknown) at Day 28. All other subjects had satisfactory neutrophil and platelet recovery and there were no late graft failures. The median (range) times to achieve a neutrophil count > 500/ul were 21 (14-39) days for subjects receiving bone marrow grafts and 14 (13-28) days for those receiving peripheral blood grafts. Corresponding times to achieve platelets > 20,000/cmm were 14 (11-43) and 14 (6-56) days, respectively.
Graft-versus-Host Disease
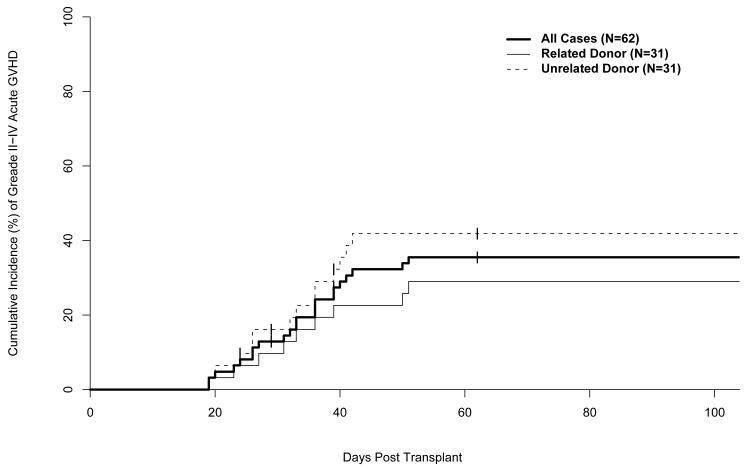
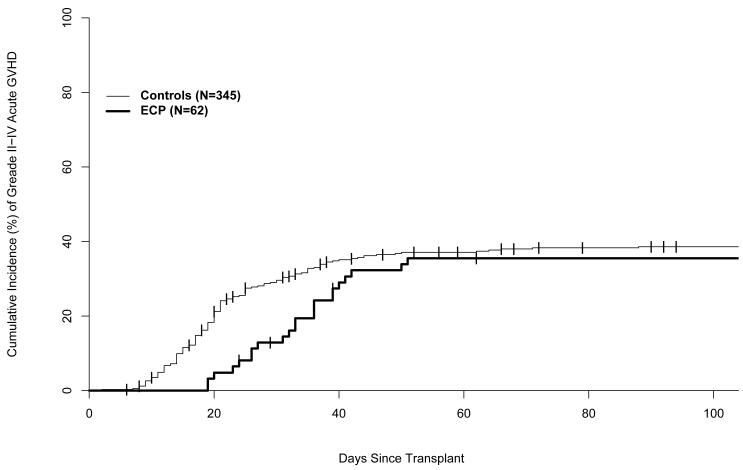
Grades II-IV aGVHD developed in 22 (36%) of the 62 subjects, including 9 (30%) of 30 related donor HCT recipients and 13 (42%) of 31 matched unrelated or one HLA antigen mismatched related donor HCT recipients (Table 2). The skin was the organ most frequently and most severely involved with 38% of subjects having stage 2 or higher skin involvement, while 16% and 13% of subjects had stage 2 or higher involvement of the gastrointestinal (GI) tract or liver, respectively (Table 2). The 100-day cumulative incidence of grades II-IV aGVHD was 35% (95% CI: 23%-48%) (Figure 1). Forty (66%) patients had biopsy proven acute GVHD. No patients were described as having early chronic GVHD before day 100 or late acute GVHD after day 100. The median time to first diagnosis and maximal grade of grade II-IV acute GVHD was 35 days for both, with a range of 18-52 days for first diagnosis and 22-96 days for maximal grade.
Table 2. Graft-Versus-Host Disease.
| Matched Related Donors |
Matched Unrelated Donor* |
|
|---|---|---|
| N (%) | 30 (%) | 32 (%) |
| Acute GVHD grade | ||
| 0 | 17 (56) | 10(32) |
| I | 4 (13) | 9 (28) |
| II | 6 (20) | 4 (12) |
| III | 2 (6) | 7 (22) |
| IV | 1 (4) | 2 (6) |
| Skin stage | ||
| 0 | 18 (60) | 10(32) |
| 1 | 3(10) | 7(22) |
| 2 | 5 (16) | 7(22) |
| 3 | 4(14) | 6(18) |
| 4 | 0 (0) | 2 (6) |
| GI stage | ||
| 0 | 25(84) | 22(68) |
| 1 | 3(10) | 2(6) |
| 2 | 2(6) | 1(4) |
| 3 | 0 (0) | 5(16) |
| 4 | 0(0) | 2(6) |
| Liver stage | ||
| 0 | 29(96) | 24(76) |
| 1 | 0(0) | 1(4) |
| 2 | 0(0) | 2(6) |
| 3 | 0 (0) | 2(6) |
| 4 | 1(4) | 3(10) |
Matched unrelated donor category includes one patient who was a one antigen mismatch related donor.
Figure 1.
Cumulative incidence of aGVHD. Unrelated donor analysis includes one patient who had a 5/6 HLA matched related donor.
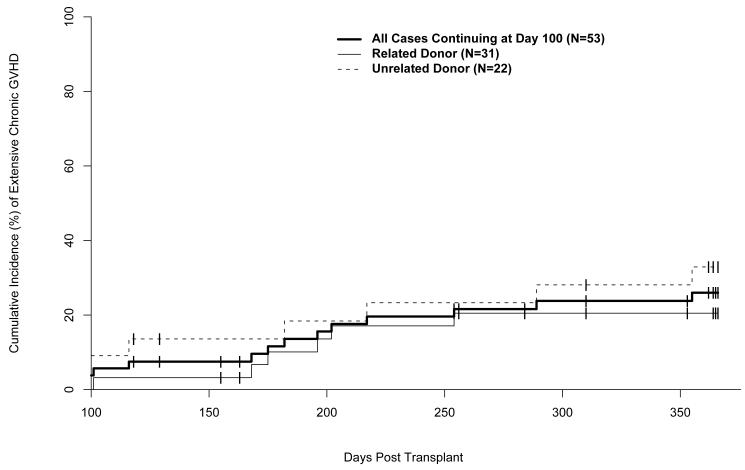
Fifty-three subjects were evaluable for chronic GVHD. Seven patients died prior to day 100 (acute GVHD 3, idiopathic pneumonia 1, multi organ system failure 3), and two patients had insufficient data collected to determine if chronic GVHD occurred. Chronic GVHD occurred in 21 (40%) of 53 subjects (limited: 8 (15%); extensive: 13 (25%)). The one-year cumulative incidence of limited and extensive chronic GVHD was 38% (95% CI: 21 - 47%) (Figure 2).
Figure 2.
Cumulative incidence of extensive chronic GVHD. BMT indicates bone marrow transplant; PBSC, peripheral blood stem cell transplant. Unrelated donor analysis includes one patient who had 5/6 HLA matched related donor.
Toxicity
The most frequent serious adverse events that occurred during the study were fever 8 (13%), febrile neutropenia 4 (7%), and multiple organ system failure 3 (5%). Adverse events directly attributable to the ECP included two subjects who experienced hypotension while undergoing ECP. CMV reactivation occurred in 17 (27%) subjects, with two subjects having CMV disease in lung tissue documented in biopsy specimens from bronchoscopy. Two (3%) subjects had systemic fungal infections while participating in the study, one occurring 19 days post transplant and the other 9 months post transplant.
Survival
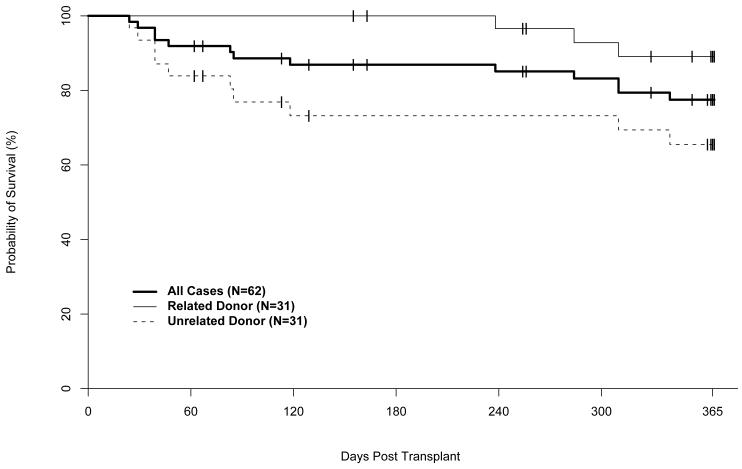
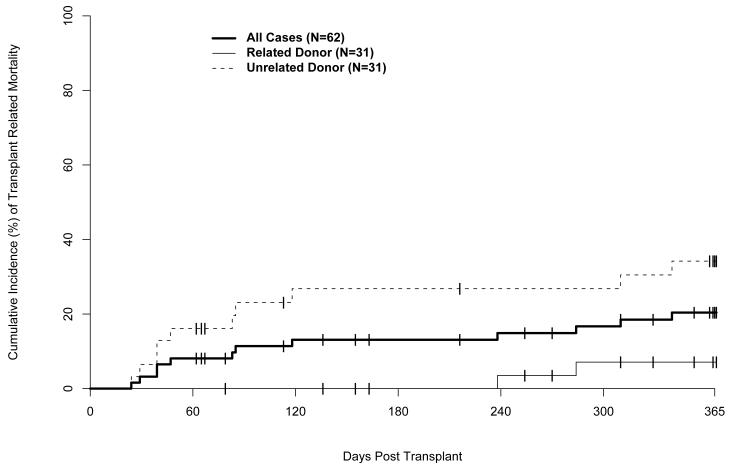
Median follow up of surviving patients is 371 days (range 366-643), with 48 (77%) of 62 subjects surviving. Kaplan Meier estimates of survival at day 100 and 1-year post transplant were 89% (95% CI: 78%-97%) and 77% (95% CI: 64%-86%), respectively. One-year probabilities of overall survival (OS) for related and unrelated donor HCT recipients were 89% (95% CI: 70%-96%) and 66% (95% CI: 46%-80%), respectively (Figure 3). One-year probabilities of disease-free survival (DFS) were 69% (95%CI: 64%-86%) for all patients, 79% (95%CI: 59%-90%) after related donor HCT and 60% (95%CI: 40%-75%) after unrelated donor HCT. Relapse occurred in 7 (11%) patients. Fourteen (23%) subjects died: 3 after related and 11 after unrelated donor transplants. Causes of death were relapse (1), acute GVHD (3), chronic GVHD (1), infection (4), multiple organ system failure (3), and idiopathic pneumonitis (2). The one-year cumulative incidence of transplant related mortality (TRM) was 21% (95% CI: 11%-31%); the cumulative incidences after related and unrelated donor transplants were 10% (95%CI: 1%-20%) and 31% (95%CI: 18%-50%), respectively (Figure 4).
Figure 3.
Overall survival. Unrelated donor includes one patient who had a 5/6 HLA matched related donor.
Figure 4.
Cumulative incidence of transplant related mortality. Unrelated donor includes one patient who had a 5/6 HLA matched related donor.
ECP Study Subjects Compared to CIBMTR Controls
The CIBMTR database was used to search for historical controls with characteristics similar to the study subjects. Control subjects were required to meet the eligibility criteria defined in Table 3. A total of 347 control subjects were identified. Their characteristics are compared with those of the study subjects in Table 4. ECP-treated subjects were more likely than controls to be older than 40 years, to be Caucasian, and to have an unrelated donor. The distribution of underlying diseases was also significantly different. The potential impact of these differences was considered in multivariate analyses.
Table 3. Selection Criteria for Historical Controls.
|
HLA-id sib indicates human leukocyte antigen identical sibling; 1-antigen mm, 1 antigen mismatch; TBI, total body irradiation; CY, cyclophosphamide; MTX, methotrexate; CSA, cyclosporine.
Table 4. Characteristics of Study and Historical Control Subjects.
| Study | CIBMTR | |
|---|---|---|
| N=62 | N=347 | |
| Age, median | 39 y | 36 y |
| > 40 y * | 48% | 34% |
| Caucasian * | 85% | 68% |
| HLA-identical sib donor * | 48% | 68% |
| Sex-match: F to M* | 51% | 23% |
| D/R CMV | ||
| −/− | 22% | 35% |
| −/+ | 18% | 18% |
| +/− | 13% | 10% |
| +/+ | 47% | 37% |
| Peripheral blood graft | 52% | 39% |
| Disease * | ||
| Acute leukemia | 53% | 49% |
| Chronic leukemia | 29% | 33% |
| MDS | 5% | 7% |
| NHL | 8% | 12% |
| Other | 5% | <1% |
| Advanced Disease | 48% | 44% |
p < 0.05
Sex match: F to M indicates female to male; D/R CMV, donor recipient cytomegalovirus status; advanced disease defined as beyond first complete remission or never obtaining remission.
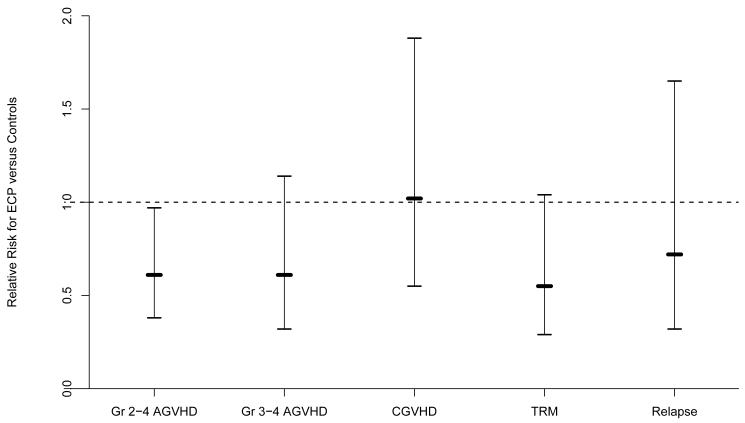
Multivariate analysis revealed a significantly lower rate of developing grade II-IV aGVHD (relative risk [RR] 0.61, 95% CI, 0.38-0.97) (p=0.04) in ECP-treated subjects compared to historical controls (Figure 5). This lower rate was do to a substantial delay in the onset of aGVHD in the ECP-treated subjects rather than an absolute decrease in the incidence (Figure 6). The cumulative incidence probability of aGVHD grades II-IV was 36% (95% CI: 25%-48%) in the study patients and 39% (95% CI: 33%-44%) in the historical control cohort (Figure 6). There was also less TRM in the ECP-treated subjects compared to the historical controls, but this was not statistically significant (RR 0.55, 95% CI, 0.29-1.04) (p=0.065). There was no difference in veno-occlusive disease or interstitial pneumonitis between the groups. However, opportunistic infections occurred in 85 (24%) of the control patients and 5 (8%) of the study patients (p=0.008).
Figure 5.
Multivariate analysis.
Figure 6.
Cumulative incidence probability of aGVHD grades II-IV.
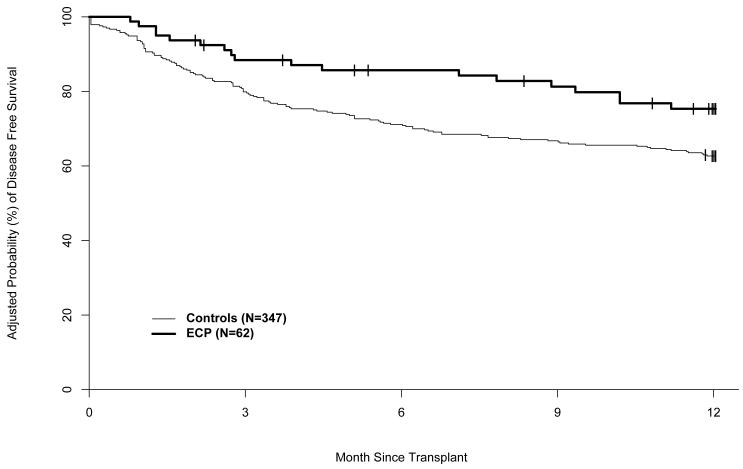
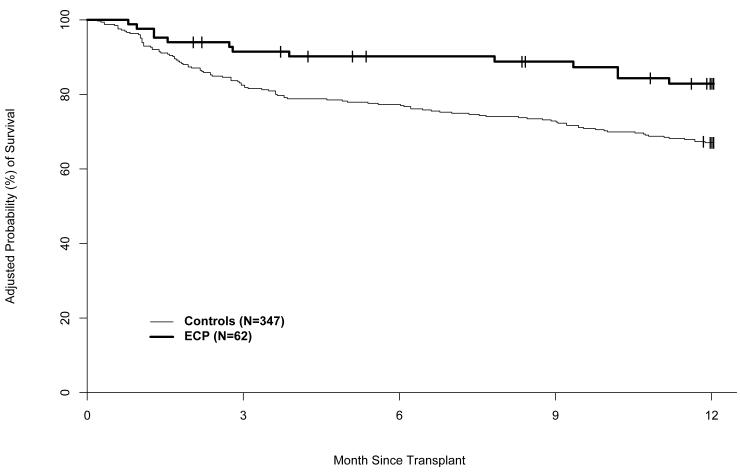
The adjusted probabilities of disease free and overall survival were significantly higher in the ECP-treated subjects than in the historical controls (Figures 7 and 8). The adjusted DFS rates at one year were 74% (95% CI: 62%-82%) in the ECP-treated subjects and 63% (95% CI: 58%-67%) in the historical control cohort (RR of treatment failure [relapse or death] 0.60, 95% CI: 0.36-0.99) (p=0.045). The adjusted OS rates at one year were 83% (95% CI: 72%-90%) in the ECP-treated subjects and 67% (95% CI: 62%-71%) in the historical controls (RR of mortality 0.44, 95% CI, 0.24–0.80) (p= 0.007).
Figure 7.
Adjusted probability of disease free survival.
Figure 8.
Adjusted probability of overall survival.
Discussion
Many factors contribute to the survival of patients after allogeneic HCT. Although control of the underlying disease is necessary, significant transplant-related toxicity can undermine this long-term goal. GVHD and its treatment, with the subsequent increased risks of infection, significantly contribute to TRM after allogeneic HCT [1-5]. Previous studies in patients receiving myeloablative preparative regimens have led to adoption of a calcineurin inhibitor and MTX for prevention of aGVHD as a standard regimen at many transplant centers [1, 36]. Efforts to further reduce the risk of aGVHD by increasing immune suppression or depleting donor T-cells have not been completely successful because increased risks of relapse or infection. Ultimately, the goal of separating GVHD from the graft-versus-malignancy effect has remained elusive.
The current study was undertaken to explore whether ECP could decrease the expected incidence of aGVHD in subjects who received a standard myleoablative preparative regimen and related or unrelated donor allogeneic HCT. The primary endpoint of the current study was the incidence of grades II-IV aGVHD. The overall rate was 30% with related donors and 40% with unrelated donors. These results fall in the range of reported incidences of aGVHD in other studies [1-7], and are higher than the incidence reported by Foss and colleagues using a reduced intensity conditioning regimen and ECP [31]. The more cytotoxic preparative regimen used in our current study may have contributed to greater tissue damage in subjects and more inflammatory cytokine release compared to the reduced intensity regimen used by Foss. It is also possible that the novel preparative regimen (rather than the ECP treatments) and/or the different patient population were responsible for the low incidence of aGVHD in the Foss study. A randomized phase II study is currently underway with or without ECP to address this question (NCT00402714; www.clinicaltrials.gov).
The schedule and dosing of MTX in this study was developed by agreement of investigators at participating centers. The MTX schedule for unrelated donors in this study has been well described and replicated [36-38]. Lower doses of MTX for matched related donor transplants have been used and resulted in adequate engraftment and aGVHD control, with less MTX associated toxicty [36, 39]. However, the total dose and number of doses of MTX may be less well established and reflect variations in institutional practice. In this multi-center international study the investigators agreed to use the lower doses of MTX used in this study for matched related donor transplants. This study failed to capture the total dose of MTX actually delivered to study patients, as well as information on trough levels and taper schedule of CSP, and specific therapy used for treatment of aGVHD. It is possible that variations in the dose of MTX and CSP delivered in this study impacted the incidence of aGVHD and comparison with controls. The lower dose MTX regimen for matched related donor study patients may have increased the risk for aGVHD and masked some of the effect of ECP on aGVHD prevention.
The ECP intervention in the current study did not appear to alter regimen-related toxicity or engraftment. Study-related adverse events were similar to those commonly seen with a standard myeloablative preparative regimen and allogeneic HCT. The two episodes of hypotension during the ECP procedure were readily reversible with intravenous fluids and the subjects were able to continue with treatment. Post-transplant recovery of neutrophils and platelets in the current study were consistent with expected rates after bone marrow and peripheral blood stem cell transplants. Transplant-related mortality was low, with no related donor HCT recipients and only 7 unrelated donor HCT recipients dying of transplant-related causes in the first 100 days. Most TRM was due to GVHD or infection in patients under treatment for GVHD. No TRM could be attributed to toxicities related to the ECP treatment.
Collaboration with the CIBMTR was undertaken at the completion of the study to identify suitable historical controls for comparison with ECP study subjects to put the results of this single Phase II study in perspective. Controls were selected using the eligibility criteria for the patients in the current study. However, there were some differences in the distribution of characteristics between the groups as described in Table 5, with ECP study subjects more likely to be older and to receive an unrelated donor graft, both associated with increased risk of GVHD. Despite the presence of these key demographic differences favoring the historical control group, acute GVHD occurred more slowly in the ECP-treated cohort.
Multivariate analysis, adjusting for the differences in prognostic factors, revealed a significant difference in rate of aGVHD (grade II-IV) between the groups (Figure 6), with a significant delay in the time to onset of aGVHD, suggesting that ECP may have slowed but not completely eliminated the presentation of host antigen to donor effector cells. This delay (rather than prevention) of aGVHD may suggest that there is a dose response of ECP on antigen presentation in the setting of a large amount of antigen and inflammatory cytokine release from cytotoxic insults occurring during the preparatory regimen. Prior clinical and pre-clinical studies of ECP have not determined an optimal frequency or schedule of ECP, and further investigation is needed to address whether there is a dose response of ECP on the ability to reduce DCs and/or inflammatory cytokines and correlate this with antigen presentation and the clinical presentation of GVHD. It is possible that more ECP would be needed to reduce DCs and/or inflammatory cytokines prior to a large cytotoxic insult than to a smaller one. Gatza and colleagues demonstrated in a murine model that ECP reverses experimental GVHD through regulatory T cells [30] and future studies on ECP for GVHD prevention and treatment in humans will need to examine this effect.
Though the absolute incidence of aGVHD was not lower with ECP, multivariate analysis revealed a trend to less TRM, significantly less treatment failure (relapse and TRM), and higher overall and disease free survival in the ECP study cohort compared to the historical control cohort. Regimen related toxicities were similar between the groups, except for significantly less opportunistic infections in the ECP treated patients compared to historical controls. Importantly, ECP did not appear to increase the risk for infections or relapse rates that have been observed in other attempts to prevent GVHD [36]. Later onset of aGVHD may itself be beneficial, since it may permit more recovery from the preparative regimen and transplant procedure, and a higher degree of immune reconstitution, allowing these patients to better tolerate the side effects of corticosteroids, have less end organ damage, and to overcome infections. Unfortunately, insufficient data were collected in this study to assess type and dose of immunosuppressive therapy used to treat aGVHD and any association with adverse events or clinical outcomes. ECP did not appear to suppress the allo-immune mediated graft versus malignancy effect as relapse rates were not significantly different between the two groups, though the follow-up time for the evaluation of relapse was short.
Caveats to the interpretation of this study include: (1) This was a large multi-institutional study, but was primarily conducted in large transplant centers and there may be some center effect compared to the many different types of transplant centers that report to the CIBMTR. (2) As is common to all non-randomized studies, some supportive care measures or unknown prognostic factors may have been different in the study patients and controls which may have contributed to the differences in survival. (3) Study centers were allowed to use institutional standards for aGVHD treatment and supportive care which may have differed from treatments given to historical controls.
In conclusion, the use of ECP prior to a standard myeloablative preparative regimen appears safe with no unexpected serious adverse events related to the ECP, and no apparent untoward effects on either engraftment or relapse rates. Comparison with historical controls suggests that this approach may improve overall and disease-free survival, though longer follow-up, larger sample sizes, and randomized comparisons to standard approaches are necessary.
Supplementary Material
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); and, two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research. Therakos, Inc. sponsored the study and provided data collection and monitoring of study patients. Dennis Parenti, Jose Gallo and Jon Rogers are employees of Therakos. Paul Shaughnessy, Koen van Besien, Francine Foss, and Sunil Abhyankar have served on advisory boards, received honoraria, or received research funding from Therakos.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Martin PJ, Schoch G, Fishter L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: Initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 3.Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–1030. [PubMed] [Google Scholar]
- 4.Martin PJ, McDonald GB, Sanders EJ, et al. Increasing frequent diagnosis of acute gastrointestinal graft versus host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320–327. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 5.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft versus host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara JL, Antin JH. The pathophysiology of graft versus host disease. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic cell Transplantation. Blackwell Science, Inc; Malden, MA: 1999. pp. 19–27. [Google Scholar]
- 7.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 8.Greinix HT, Volc-Platzer B, Kalhs P, Fisher G, Rosenmayr A, Keil F, et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood. 2000;96:2426–2431. [PubMed] [Google Scholar]
- 9.Greinix HT, Volc-Platzer B, Rabitsch W, Gmeinhart B, Guevara-Pineda C, Kalhs P, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92:3098–3104. [PubMed] [Google Scholar]
- 10.Seaton ED, Szydio RM, Kanfer E, Apperley JF, Russell-Jones R. Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood. 2003;102:1217–1223. doi: 10.1182/blood-2002-11-3351. [DOI] [PubMed] [Google Scholar]
- 11.Greinix HT, Volc-Platzer B, Knobler RM. Extracorporeal photochemotherapy in the treatment of severe graft-versus-host disease. Leukemia and Lymphoma. 2000;36:425–434. doi: 10.3109/10428190009148389. [DOI] [PubMed] [Google Scholar]
- 12.Foss FM, Divenuti GM, Chin K, Sprague K, Grodman H, Klein A, et al. Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005;35:1187–1193. doi: 10.1038/sj.bmt.1704984. [DOI] [PubMed] [Google Scholar]
- 13.Apisarnthanarax N, Donato M, Korbling M, Couriel D, Gajewski J, Giralt S, et al. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: feasibility and results. Bone Marrow Transplant. 2003;31:459–465. doi: 10.1038/sj.bmt.1703871. [DOI] [PubMed] [Google Scholar]
- 14.Dall’Amico R, Messina C. Extracorporeal photochemotherapy for the treatment of graft-versus-host disease. Therapeutic Apheresis. 2002;6:296–304. doi: 10.1046/j.1526-0968.2002.00448.x. [DOI] [PubMed] [Google Scholar]
- 15.Owsianowski M, Gollnick H, Siegert W, Schwerdtfeger R, Orfanos CE. Successful treatment of chronic graft-versus-host disease with extracorporeal photopheresis. Bone Marrow Transplant. 1994;14:845–848. [PubMed] [Google Scholar]
- 16.Rossetti F, Zulian F, Dall’Amico R, Messina C, Montini G, Zacchello F. Extracorporeal photochemotherapy as single therapy for extensive, cutaneous, chronic graft-versus-host disease. Transplantation. 1995;15:149–151. doi: 10.1097/00007890-199501150-00029. [DOI] [PubMed] [Google Scholar]
- 17.Couriel DR, Hosing C, Saliba R, Shpall EJ, Anderlini P, Rhodes B, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 18.Flowers ME, Apperley JF, van Besien K, Elmaagacil A, Grigg A, Reddy V, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for the treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–2674. doi: 10.1182/blood-2008-03-141481. [DOI] [PubMed] [Google Scholar]
- 19.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood. 2002;100:941–947. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- 20.Alcindor T, Gorgun G, Miller KB, Roberts TF, Sprague K, Schenkein DP, et al. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001;98:1622–1625. doi: 10.1182/blood.v98.5.1622. [DOI] [PubMed] [Google Scholar]
- 21.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert NE, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 22.Lucas M, Stuart LM, Savill J, Lacy-Hulber A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–2615. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 23.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-geta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-geta 1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker RN, Erwig L, Pearce WP, Devine A, Rees AJ. Differential effects of necrotic or apoptotic cell uptake on antigen presentation by macrophages. Pathobiology. 1999;67:302–305. doi: 10.1159/000028085. [DOI] [PubMed] [Google Scholar]
- 26.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda A, Schwarz A, Kernebeck K, Gross N, Aragane Y, Peritt D, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen specific regulatory T cells. J Immunol. 2005;174:5968–5976. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 28.Lamioni A, Parisi F, Isacchi G, Giorda E, Di Cesare S, Landolfo A, et al. The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79:846–850. doi: 10.1097/01.tp.0000157278.02848.c7. [DOI] [PubMed] [Google Scholar]
- 29.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Immunology. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 30.Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller KB, Roberts TF, Chan G, Schenkein DP, Lawrence D, Sprague K, et al. A novel reduced intensity regimen for allogeneic hematopoietic stem cell transplantation associated with a reduced incidence of graft-versus-host disease. Bone Marrow Transplant. 2004;33:881–889. doi: 10.1038/sj.bmt.1704454. [DOI] [PubMed] [Google Scholar]
- 32.Chan GW, Gorgun G, Miller KB, Foss FM. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Bio Blood and Marrow Transplant. 2003;9:170–176. doi: 10.1053/bbmt.2003.50006. [DOI] [PubMed] [Google Scholar]
- 33.Common Terminology Criteria for Adverse Events. Version 3.0. 2003 Dec 12; [Google Scholar]
- 34.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplant. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 36.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Semin Hemato. 2006;43:32–41. doi: 10.1053/j.seminhematol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: Long term follow up of a controlled trial. Blood. 1989;73:1729–1734. [PubMed] [Google Scholar]
- 38.Nash R, Pepe MS, Storb R, Longton G, Pettinger M, Anasetti C, et al. Acute graft-versus host disease: Analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood. 1992;80:1838–1845. [PubMed] [Google Scholar]
- 39.Deeg HJ, Spitzer TR, Cottler-Fox M, Cahill R, Pickle LW. Conditioning-related toxicity and acute graft-versus-host disease in patients given methotexate/cyclosporine prophylaxis. Bone Marrow Transplant. 1991;7:193–198. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.