Summary
Over the past decade, the Hippo tumor suppressor pathway has emerged as a central regulator of growth in epithelial tissues. Research in Drosophila and in mammals has shown that this kinase signaling cascade regulates the activity of the transcriptional coactivator and oncoprotein Yorkie/Yap. In this review, we discuss recent findings that emphasize the cell cortex - specifically the actin cytoskeleton, intercellular junctions and the protein complexes determine cell polarity - as a key site for Hippo pathway regulation. We also highlight where additional research is needed to integrate known functional interactions between Hippo pathway components.
Introduction
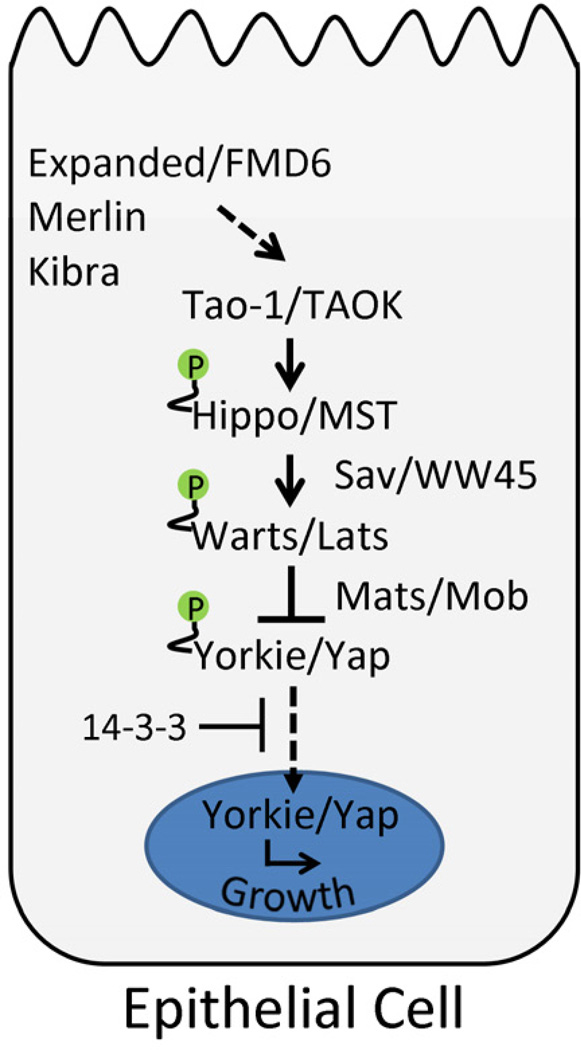
The Hippo (Hpo) tumor suppressor pathway is a conserved signaling pathway that regulates levels of cell proliferation and apoptosis in growing epithelial tissues. First identified in Drosophila, the Hpo pathway has since been implicated in mammalian organ size control and several types of human cancer (Pan, 2010; Chan et al., 2011a). The founding member of the pathway, Warts (Wts), was discovered in genetic mosaic screens designed to identify genes involved in cell growth and proliferation (Justice et al., 1995; Xu et al., 1995). Drosophila wts encodes a Ser/Thr kinase that bears significant homology to Cbk1 and the cell cycle regulators Dbf20 and Dbf2 in budding yeast. In mosaic animals, wts mutant cells overproliferate compared to their wild-type neighbors and form cuticular outgrowths from highly folded epithelial tissue. Subsequent genetic screens have revealed other genes, including salvador (sav), hippo (hpo) and mob as tumor suppressor (mats) that give strikingly similar phenotypes when clonally deleted in imaginal discs (Kango-Singh et al., 2002; Tapon et al., 2002; Harvey et al., 2003; Pantalacci et al., 2003; Udan et al., 2003; Wu et al., 2003; Lai et al., 2005). Studies in Drosophila and in mammals have shown that Hpo, Sav and Wts (and their mammalian orthologues MST1/2, WW45 and Lats1/2, respectively) form a protein complex that negatively regulates the oncoprotein and transcriptional co-activator Yorkie (Yki) or its mammalian orthologues Yap and Taz (Figure 1; Tapon et al., 2002; Harvey et al., 2003; Pantalacci et al., 2003; Udan et al., 2003; Wu et al., 2003; Huang et al., 2005; Lai et al., 2005; Callus et al., 2006). Biochemical experiments have shown that Hpo/MST can phosphorylate and activate Wts/Lats, which in turn phosphorylate and inactivate Yki/Yap (Wu et al., 2003; Chan et al., 2005; Huang et al., 2005; Dong et al., 2007; Wei et al., 2007; Zhao et al., 2007; Oh and Irvine, 2008). The phosphorylation of Yki/Yap at several key serine residues promotes cytoplasmic retention by 14-3-3 and inhibits the transcription of genes that promote tissue growth (Huang et al., 2005; Zhao et al., 2007; Oh and Irvine, 2008; Oh and Irvine, 2009). Sav/WW45 and Mats are thought to function primarily as scaffolding proteins to promote Hpo/MST and Wts/Lats activation, respectively. In the absence of pathway function, Yki/Yap accumulates in the nucleus where it binds to Scalloped/TEAD to form an active transcription factor complex that drives expression of growth promoting genes (Wu et al., 2008; Zhang et al., 2008; Zhao et al., 2008).
Figure 1. Functionally conserved components of the Hippo tumor suppressor pathway.
The FERM domain proteins Merlin and Expanded/FMD6 have been proposed to function upstream of the core kinase signaling cassette together with the WW domain protein Kibra. The sterile 20 kinase Tao-1 directly phosphorylates Hippo and MST kinases in vitro and promotes pathway activation in vivo. The scaffolding proteins Salvador/WW45 and Mats/Mob promote Hippo/MST and Warts/Lats kinase activity, respectively. Hippo/MST phosphorylates Warts/Lats, which in turn phosphorylates Yorkie/Yap to promote 14-3-3 binding. 14-3-3 sequesters Yorkie/Yap in the cytoplasm, preventing the transcription of target genes that promote tissue growth. In addition, Fat signaling (not shown) has been shown to regulate Warts stability and Yorkie activation in parallel to Hippo.
While the intracellular signaling events downstream of Hpo/MST that lead to Yki/Yap inactivation are largely defined, it remains less clear how the pathway is regulated upstream of Hpo/MST. Moreover, it is unknown to what extent extracellular cues play a role in activating the pathway. Over the past year or two, significant progress has been made in identifying new Hpo pathway components and establishing links between Hpo signaling and the actin cytoskeleton, cell junctions and cell polarity. In this review, we discuss recent advances in our understanding of upstream regulation of the Hpo pathway and how basic cellular processes such as actin polymerization and cell junctions influence Hpo signaling.
Bridging Cell Junctions and Hpo Signaling
Intercellular junctions, once primarily thought of as structural components, have been shown to play critical roles in cell signaling events – perhaps the best example being the dual functions of β-catenin as an adherens junction (AJ) protein and transcriptional coactivator for Wnt signaling (Jeanes et al., 2008). By functioning at the site of cell-cell contacts, junctional proteins are ideally positioned to monitor the extracellular environment and transmit growth inhibitory signals when cells reach high cell density. A central tenant of this model is that intercellular junctions trigger intracellular signaling events that inhibit further cell division and growth. As a critical regulator of epithelial tissue growth, the Hpo pathway is a good candidate for receiving growth inhibitory signals from cell junctions in polarized epithelial cells. Evidence that junctional complexes might signal through the Hpo pathway to inhibit cell proliferation first came from studies revealing that Yap nuclear localization and activity are inversely correlated with cell density (Zhao et al., 2007; Ota and Sasaki, 2008). At low cell density Yap is enriched within the nucleus and weakly phosphorylated, whereas at high cell density Yap is predominantly cytoplasmic and strongly phosphorylated. The latter observation suggests that MST and Lats kinase activity upstream of Yap are also regulated by cell density (Zhao et al., 2007), though this has not been directly demonstrated.
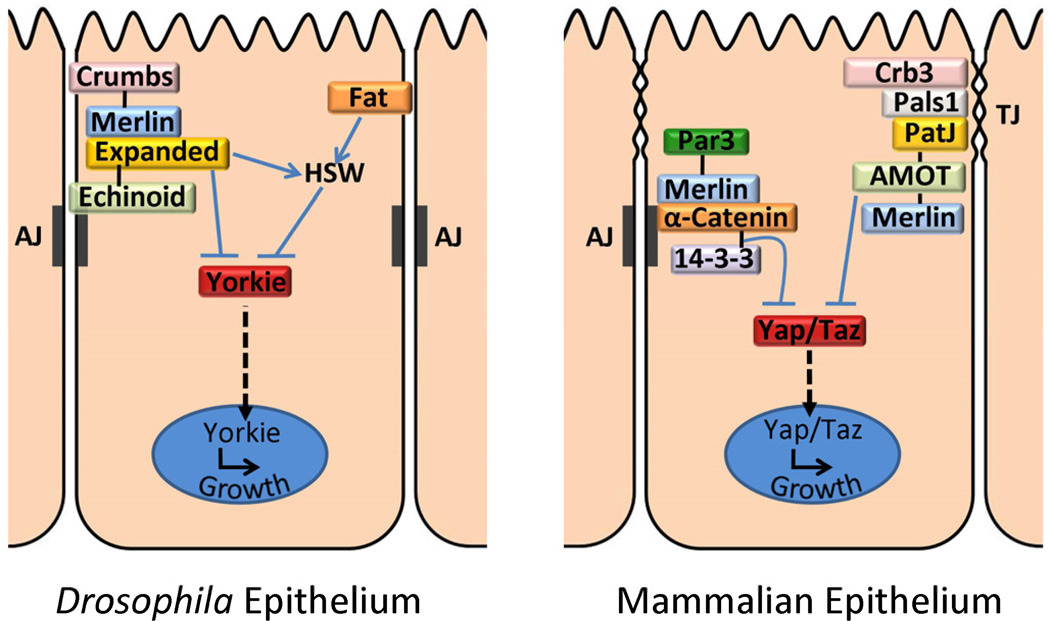
One potential mechanism for junctional regulation of Hpo pathway signaling is to sequester pathway components, thereby preventing their participation in signaling. This sort of mechanism has been proposed for the AJ in sequestering β-catenin and thus preventing it from participating in Wnt-pathway signaling (Jeanes et al., 2008). The first evidence for this type of mechanism in the Hpo pathway came from studies in Drosophila demonstrating that Yki binds directly to Expanded (Ex), a FERM domain protein (Figure 2; Badouel et al., 2009) and forms a complex with Hpo and Wts (Oh et al., 2009). These papers represented a fundamental shift in the Drosophila Hpo signaling paradigm because the results clearly demonstrated that Yki activity can be regulated independently of its phosphorylation state, thus blurring the lines between ‘upstream’ and ‘downstream’ Hpo pathway components. Prior to this study, the prevailing model was that Ex and Merlin function upstream of the core Hpo signaling cassette to regulate pathway activation (Hamaratoglu et al., 2006). Interestingly, like Merlin Ex localizes closely to AJs in the fly epithelium and has been implicated in regulating the endocytosis of transmembrane proteins such as Notch, E-cadherin, Fat and EGFR (McCartney et al., 2000; Maitra et al., 2006). Studies in Drosophila have shown that both the proto-cadherin Fat and the apical polarity protein Crumbs (Crb) regulate Ex subcellular localization, suggesting that Ex might be part of a larger junctional complex that functions to inhibit Yki transcriptional activity (Bennett and Harvey, 2006; Cho et al., 2006; Silva et al., 2006; Willecke et al., 2006; Chen et al., 2010; Grzeschik et al., 2010; Ling et al., 2010; Robinson et al., 2010). In support of this notion, the adhesion molecule Echinoid (Ed) and the WW domain protein Kibra were recently identified as Hpo pathway members that interact with both Ex and Yki (Figure 2; Baumgartner et al., 2010; Genevet et al., 2010; Yu et al., 2010; Yue et al., 2012). Interestingly, loss of Ed in the wing epithelium results in the mislocalization of Sav from the subapical cell membrane, but does not have an effect on the localization of Ex and Merlin.
Figure 2. Yorkie/Yap activity is regulated by apical junctions and polarity proteins.
In Drosophila, the FERM domain protein Expanded has been shown to physically interact with Yorkie at the cell membrane and prevent its translocation to the nucleus. The adhesion molecule Echinoid co-immunoprecipitates with several Hippo pathway components in S2 cells including Yorkie and promotes pathway activation. Fat and Crumbs are both large transmembrane proteins that localize to the subapical membrane and adherens junctions, respectively, and regulate the subcellular localization of Expanded. In addition, Fat is believed to regulate the Hippo/Salvador/Warts (HSW) kinase cascade independently of Expanded. In mammals, the adherens junction (AJ) protein α-catenin inhibits Yap activity by recruiting it to the cell membrane. In a similar fashion, the TJ protein AMOT and the subapical Crumbs complex (Crb3, Pals1 and PatJ) were recently shown to sequester Yap/Taz to the TJ, thereby inhibiting its oncogenic activity. For clarity, interactions with the HSW cascade have been omitted from the mammalian cell.
Recently, several studies have built on these observations by linking Yap to both AJ and tight junction (TJ) proteins and revealing additional mechanisms of Yap inactivation (Figure 2). Two groups recently showed that Yap interacts with the AJ protein α-catenin in primary mouse keratinocytes (Schlegelmilch et al., 2011; Silvis et al., 2011). Overexpression of activated Yap in keratinocytes results in a skin carcinoma-like phenotype that is accompanied by expansion of the basal stem cell population. Loss of α-catenin or disruption of AJs with EGTA in confluent keratinocytes results in increased nuclear Yap, suggesting that the AJ is a key mediator Yap subcellular localization. Moreover, knockdown of α-catenin increases Yap transcriptional activity in epithelial-derived cell lines. Surprisingly, loss of MST1/2 had no effect on skin proliferation in vivo, suggesting that Yap activity is primarily regulated by α-catenin in this context (Schlegelmilch et al., 2011).Silvis et al. (2011) also found that Yap phosphorylation state remained constant in α-catenin −/− cells when normalized to total Yap levels, as did Lats phosphorylation. Taken together, these results suggest that Yap is regulated independently of canonical Hpo signaling in the epidermis; however, it is important to note that 14-3-3, which specifically binds phosphorylated Yap, was also identified as an α-catenin binding protein and found to be necessary for α-catenin and Yap binding in vitro (Schlegelmilch et al., 2011). Thus, it seems possible that other kinases take the place of MST and Lats in regulating the interaction between Yap and α-catenin in the epidermis.
Potentially a key player in mediating density-dependent regulation of Yki/Yap function is Merlin, the product of the Neurofibromatosis 2 tumor-suppressor gene. Merlin is a member of the FERM domain superfamily, a group of proteins that have the ability to interact with the cytoplasmic tail of select transmembrane proteins as well as other cortical cytoplasmic proteins. As such, Merlin and other FERM domain proteins can coordinate protein interactions and intercellular signaling while functioning at the cytoplasmic face of the plasma membrane (McClatchey and Fehon, 2009). In Drosophila, immunolocalization studies place Merlin in the apical junctional zone, in close proximity to both the AJ and the apical polarity proteins (McCartney and Fehon, 1996). In mammalian cells, Merlin has been shown to mediate contact inhibition that suppresses cell division in cultured cells (Lallemand et al., 2003), and its phosphorylation state is cell density dependent (Shaw et al., 1998). In addition, a recent study has shown that Merlin physically interacts with α-catenin and Par3 in keratinocytes, suggesting that Merlin plays a role in linking cell polarity proteins to the AJ complex (Gladden et al., 2010). In Drosophila, Merlin has been shown to be an upstream regulator of Hpo pathway function (Hamaratoglu et al., 2006). Similarly, a recent study in the liver has found that mammalian Merlin functions antagonistically to Yap in liver homeostasis (Zhang et al., 2010), though other studies have suggested that Merlin functions independently of Yap in both the skin and liver (Benhamouche et al., 2010; Gladden et al., 2010). These studies raise a number of interesting but as yet unanswered questions, including whether Merlin, Yap and α-catenin form a complex at the AJ to restrict cell proliferation, how cell density regulates Merlin phosphorylation and activity, and whether Merlin has growth regulatory functions that are independent of its function in Hpo signaling? Regardless, Merlin seems an ideal candidate to mediate density-dependent signals that regulate growth.
In addition to interacting with α-catenin, Merlin was recently shown to bind angiomotin (AMOT) and localize to TJs (Figure 2; Yi et al., 2011). In this study, Merlin was found to compete with Rich1 for AMOT binding, thereby activating Rich1 and inhibiting Ras-MAPK signaling. Several recent papers have also shown that Yap and its close relative Taz can directly bind to AMOT family proteins (Chan et al., 2011b; Wang et al., 2011; Zhao et al., 2011). As with α-catenin, this interaction occurs independently of canonical Hpo signaling because mutating critical Lats phosphorylation residues does not prevent the association and inhibition of Yap by AMOT proteins (Zhao et al., 2011). Moreover, Yap requires its WW1 domain to bind AMOT, which is the same domain used to interact with TEAD-family transcription factors, suggesting that AMOT might restrict Yap transcriptional activity by competing for these binding sites. AMOT was first identified as a binding partner of angiostatin in yeast two hybrid screens, but has since been shown to function in maintaining TJs through association with an apical polarity complex consisting of PatJ, Par3 and Rich1 (Troyanovsky et al., 2001; Wells et al., 2006). Knockdown of AMOTL2 in MDCK cells results in an EMT-like phenotype characterized by increased cell migration, decreased E-cadherin and loss of cell-cell junctions, indicating that AMOT proteins are required to maintain epithelial identity (Wang et al., 2011). In primary keratinocytes, however, knockdown of AMOT family proteins does not cause any abnormalities, suggesting that in this context Yap regulation by AMOT might not be physiologically relevant (Silvis et al., 2011). Nonetheless, the finding that Merlin and Yap both interact with AMOT is intriguing given that Merlin and Yap also interact with α-catenin in keratinocytes. Although there is no obvious AMOT homologue in Drosophila, Merlin physically interacts with the adhesion protein Ed and the WW domain proteins Sav and Kibra in Drosophila S2 cells (Yu et al., 2010; Yue et al., 2012).
Taken together, these studies raise the intriguing possibility that intercellular junctions negatively regulate tissue growth by sequestering Yki/Yap to the cell cortex. However, it is important to note that a key prediction of this model, that Yki/Yap should localize to the junctional complex, has not been universally observed. While in some studies recruitment of Yki/Yap to the junctional region has been clearly demonstrated (Zhang et al., 2009; Zhao et al., 2011), in others Yki/Yap seems more uniformly cytoplasmic in distribution (Silvis et al., 2011). Additional studies will be required to determine what role Merlin and other junctionally localized proteins play in sequestering Yki/Yap and other Hpo pathway components to junctional protein complexes.
The Cytoskeleton and Hpo Pathway Activation
For some time now, it has been recognized that the actin cytoskeleton is required for the morphological and cell cycle changes that accompany cell-cell adhesion and extracellular matrix (ECM) binding (Mammoto and Ingber, 2009). Studies surrounding the actin cytoskeleton and cell growth have focused primarily on the Rho family of small GTPases because of their involvement in promoting actin polymerization and cytoskeletal tension. For instance, RhoA promotes stress fiber formation and ERK-dependent cell cycle progression in response to cell tension (Assoian and Klein, 2008). The related small GTPase Rac promotes cell cycle progression in response to integrin receptor binding at the ECM (Fournier et al., 2008). More recently, the actin cytoskeleton has been shown to regulate cell proliferation through the Hpo pathway in both flies and mammals. In mammalian tissue culture, Yap activity and subcellular localization is influenced by changes in cell morphology and the actin cytoskeleton, although there is currently some debate whether core Hpo signaling components are involved (Dupont et al., 2011; Wada et al., 2011; Zhao et al., 2012). For example,Wada et al. (2011) and Zhao et al. (2012) showed that disrupting the actin cytoskeleton regulates Yap activity in a Lats-dependent fashion. In addition, Lats kinase activity and its ability to phosphorylate Yap in vitro are modulated by pharmacological inhibition of microtubules (MTs) and F-actin (Zhao et al., 2012). An earlier study came to a different conclusion because disrupting F-actin did not affect Yap phosphorylation levels and mutating critical Lats phosphorylation residues did not prevent Yap inactivation by mechanical stress (Dupont et al., 2011). This discrepancy could be due to differences in methodology as well as the fact that different cell lines were used. Interestingly, inhibition or overexpression of RhoA also influenced Yap activation, suggesting that the Rho pathway impinges on Hpo signaling (Zhao et al., 2012). This effect is likely due to RhoA’s known ability to promote actin polymerization, though it could also relate to increased cortical contractility.
In agreement with the above studies in mammalian cells, recent work in Drosophila has revealed that several actin-modulating proteins influence Hpo pathway activation. Knocking down capping protein A or expressing a constitutively active form of the formin Diaphanous (Dia) results in increased Yki activity and tissue overgrowth, suggesting that increased F-actin accumulation promotes growth (Fernández et al., 2011; Sansores-Garcia et al., 2011). However, increasing cortical F-actin by other means, for example by expressing an activated form of the actin-binding protein Moesin or mutating the cofilin protein Twinstar, does not result in tissue overgrowth (Speck et al., 2003; Neisch and Fehon, unpublished; Fernández et al., 2011), indicating that the relationship between F-actin and growth is complicated. In addition,Fernandez et al. (2011) found that loss of hpo, sav, wts, ex or mats resulted in increased levels of F-actin, suggesting that Hpo signaling negatively regulates actin polymerization or stability. It remains to be resolved how F-actin is regulated by Hpo signaling and it is worth noting that there are conflicting reports on whether Yki expression promotes F-actin accumulation in vivo (Fang and Adler, 2010; Fernández et al., 2011), leaving open the possibility that actin accumulation is not downstream of Yki’s transcriptional role. Interestingly, phospho-Moesin staining is also increased in Hpo pathway mutants (Polesello and Tapon, 2007; Vanderzalm and Fehon, unpublished), suggesting that Moesin might be responsible for the observed F-actin accumulation.
It remains unclear how the actin cytoskeleton regulates Hpo signaling to elicit growth responses. One possibility is that Zyxin, which was recently identified as a negative regulator of Hpo signaling, integrates information about mechanical tension from the actin cytoskeleton with Hpo signaling (Rauskolb et al., 2011). Zyxin has been proposed to function within the Fat signaling branch of the Hpo pathway to regulate Wts protein stability in conjunction with the atypical myosin Dachs. Zyxin localizes to points of cell-cell contact in the wing epithelium and to focal adhesions in mammalian fibroblasts (Beckerle, 1997; Hirata et al., 2008; Rauskolb et al., 2011), making it an ideal candidate for transmitting signals about mechanical tension to the Hpo pathway.
It will also be important to determine what contribution, if any, MTs make to Hpo pathway regulation. The recent demonstration that Tao-1 acts as a Hpo pathway component and directly phosphorylates Hpo (Boggiano et al., 2011; Poon et al., 2011) provides a possible mechanistic link between MTs, the actin cytoskeleton and Hpo signaling. Tao-1 is known to negatively regulate MT stability in both mammals and Drosophila (Mitsopoulos et al., 2003; Timm et al., 2003; Liu et al., 2010; King et al., 2011). In Drosophila S2 cells, knocking down Tao-1 results in MT protrusions that resemble the effects of disrupting cortical actin, suggesting that Tao-1 might destabilize MTs at the cell cortex in response to signals from the actin cytoskeleton (Liu et al., 2010). In mammalian cells, Tao-1 functions through Par-1 and Tau to regulate MT stability and physically interacts with the actin-modulating proteins Tesk1 and Spred1 (King and Heberlein, 2011). Thus, in addition to regulating MT stability, Tao-1 appears to function at the interface between the actin and MT cytoskeletons. An interesting, though still speculative, possibility is that in addition to regulating MT stability, Tao-1 activity itself might be regulated by interaction with the MT and/or actin cytoskeletons. Indeed, a recent study suggests that Tao-1 activity is enhanced at the cell cortex (Liu et al., 2010). Although more studies will be needed to fully elucidate the role of Tao-1 in regulating cytoskeletal dynamics, it is tempting to propose that Tao-1 might integrate signals from both the actin and MT cytoskeletons to regulate Hpo signaling.
Cell Polarity and the Hpo Pathway
Polarized epithelial cells consist of an apical and basolateral membrane domain that is separated by cell-cell junctions (St Johnston and Ahringer, 2010). The membrane domains and the position of the junctions are defined by mutual antagonism between apical and basolateral protein complexes. Studies in Drosophila have revealed that many of the proteins that regulate cell polarity also function to control growth in epithelial tissues (Hariharan and Bilder, 2006). These include the apical polarity protein Crumbs (Crb) and the basolateral polarity proteins Lethal giant larvae (Lgl), Discs large (Dlg) and Scribble (Scrib). Loss of lgl gives rise to the “giant larvae” phenotype for which the gene was named more than 40 years ago (Schneiderman and Gateff, 1967). In addition, it has long been recognized that loss of cell polarity is one of the changes that accompanies EMT in advanced stage cancers (Wodarz and Nathke, 2007). An interesting, but perplexing question is how the loss of cell polarity contributes to tumorigenesis in epithelial tissues. One possibility, supported by evidence that E-cadherin is also downregulated in many human cancers, is that loss of cell polarity results in defective cell adhesion and contact inhibition. Indeed, loss of apical and basolateral polarity proteins in Drosophila has been shown to disrupt adherens junctions during embryogenesis (Tepass et al., 2001; Harris and Peifer, 2004). Another possibility, as alluded to earlier, is that cell polarity proteins directly regulate the signaling pathways that control tissue growth. Shedding some light on this, recent studies have linked both apical and basolateral polarity proteins to the Hpo pathway (Chen et al., 2010; Grzeschik et al., 2010; Ling et al., 2010; Robinson et al., 2010; Varelas et al., 2010).
The apical polarity protein Crb was recently identified as a component of the Hpo pathway in Drosophila and found to regulate the subcellular localization of Ex (Chen et al., 2010; Grzeschik et al., 2010; Ling et al., 2010; Robinson et al., 2010). Interestingly, both loss and overexpression of Crb in the wing epithelium causes overgrowth phenotypes. Crb contains two functionally important subdomains within its cytoplasmic tail, a PDZ binding motif (PBM), which physically interacts with other apical polarity proteins, and a FERM binding motif (FBM) that physically interacts with Ex and another FERM domain protein called Yurt (Ling et al., 2010; St Johnston and Ahringer, 2010){Robinson, 2010 #30}. To determine which of these domains mediates the growth regulatory activity of Crb, Robinson et al. (2010) performed in vivo experiments using transgenic animals carrying different pieces of the Crb intracellular domain. They found that overexpression of the FBM was sufficient to drive overgrowth in the absence of the PBM and that overexpression of the PBM did not cause overproliferation, but instead altered cell polarity. In addition, another study found that Ex protein was mislocalized in a crb allele lacking the FBM (crbΔFBM), but that Ex was normally localized in an allele lacking the PBM (crbΔPBM; Ling et al., 2010). Adult crbΔFBM wings were also significantly overgrown when compared to wild-type controls, suggesting that Crb regulates Hpo signaling through its FBM. Thus, recent work on Crb would argue that cell polarity and cell proliferation are regulated through distinct protein-protein interactions.
The mammalian Crb complex was also found to modulate Hpo signaling by interacting with Yap/Taz in Eph4 mammary epithelial cells (Varelas et al., 2010). Similar to regulation of Yap/Taz by a-catenin and AMOT, the Crb complex sequesters and inactivates Yap/Taz at high cell density in Eph4 cells. Disrupting the complex by knocking down either Crb3 or Pals1 relocalizes Yap/Taz to the nucleus and promotes its dephosphorylation and activation. It seems likely that the function of AMOT and the Crb complex in Yap/Tz regulation are interrelated, given that AMOT has been shown to bind PatJ, which is also a component of the mammalian Crb complex (Wells et al., 2006). Another interesting finding from theVarelas et al. (2010) study is that loss of Crb3 enhances TGF-β signaling and promotes EMT, suggesting a link between Crb, Yap/Taz and TGF-β in tumor progression.
Loss of the basolateral polarity protein Lgl was also recently shown to modulate Hpo signaling in the Drosophila eye, but, unlike crb mutants, Ex protein was not mislocalized in lgl mutant tissue (Grzeschik et al., 2010). Instead, Hpo and its negative regulator Ras associated family protein (Rassf) are mislocalized, suggesting that Lgl acts at the level of Hpo to regulate the pathway. Mechanistically it is unclear how Lgl regulates the Hpo pathway, but this effect might be mediated through aPKC or other apical polarity proteins because depletion of aPKC suppressed overgrowth in lgl mutants (Grzeschik et al., 2010). It is worth noting that Lgl1 knockout mice exhibit brain dysplasia and loss of cell polarity in neuroepithelial cells, suggesting that role of Lgl1 as a tumor suppressor has been evolutionarily conserved (Klezovitch et al., 2004). Thus, it would be interesting to determine if Lgl1 or other mammalian orthologs of Drosophila basolateral proteins similarly regulate Hpo signaling. Studies in flies, zebrafish and human cells have linked another basolateral polarity protein, Scribble, to the Hpo pathway as well (Skouloudaki et al., 2009; Cordenonsi et al., 2011; Chen et al., 2012). Together these studies suggest that loss of cell polarity, which has long been recognized as a hallmark of human cancer, has a causal relationship at least in part via its effects on the Hpo pathway.
Concluding Remarks
Recent studies in Drosophila and mammals demonstrate the importance of basic cellular processes such as actin polymerization, cell polarity and cell adhesion in Hpo pathway regulation. Moreover, they reveal that Yki/Yap activity can be inhibited through two distinct mechanisms: 1) The phosphorylation of Yki/Yap by the canonical Hpo pathway, which in turn promotes Yki/Yap degradation and cytoplasmic retention by 14-3-3 and 2) the recruitment of Yki/Yap to the cell cortex by direct protein-protein interactions. While these mechanisms are not mutually exclusive, it is becoming clear that in certain contexts canonical Hpo signaling is not required to sequester Yki/Yap in the cytoplasm and restrict its oncogenic activity. For example, in the Drosophila wing Ex, Hpo and Wts regulate Yki’s subcellular localization independently of its phosphorylation state and in mouse keratinocytes α-catenin recruits Yap to AJs independently of MST and Lats kinase activity (Badouel et al., 2009; Oh et al., 2009; Schlegelmilch et al., 2011; Silvis et al., 2011). In addition, core pathway components such as Mats/MOB1 become activated at the cell membrane when myristoylated (Hergovich et al., 2006; Ho et al., 2010), further highlighting the importance of the cell cortex as an organizing center for Hpo pathway activity. In regard to this, it is interesting to note that some Yki/Yap normally localizes to the cell cortex in both Drosophila and mammalian cells (Zhang et al., 2009; Zhao et al., 2011). An interesting future question will be to determine if Yki/Yap or other Hpo pathway components have functions at the cell membrane that are independent of their roles in transcription.
Given that the actin cytoskeleton and intercellular junctions both regulate Hpo signaling, it seems likely that these two modes of regulation are interrelated. An intriguing possibility is that the cortical cytoskeleton and junctional proteins cooperate to relay information about cell density and mechanical tension to the Hpo pathway. Indeed, this notion is supported by recent evidence in mammalian cells that mechanical tension can influence Yap activation (Dupont et al., 2011; Wada et al., 2011; Zhao et al., 2012). The addition of actin-modulating and junctional proteins to the Hpo signaling milieu makes the idea of mechanosensory regulation of tissue growth even more appealing. A challenge for future investigators will be to develop a cohesive Hpo signaling model that takes into account all of the known functional interactions between cell polarity, intercellular junctions and the actin cytoskeleton.
Acknowledgements
The authors would like to thank present and past members of our laboratory and colleagues in other laboratories who have contributed comments and ideas. JCB was supported by National Institutes of Health (NIH) training grants (T32 HD055164 and T32 GMO7197). RGF is supported by NIH grants NS034783 and GM087558.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18:347–352. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badouel C, Gardano L, Amin N, Garg A, Rosenfeld R, Le Bihan T, Mcneill H. The FERM-Domain Protein Expanded Regulates Hippo Pathway Activity via Direct Interactions with the Transcriptional Activator Yorkie. Dev. Cell. 2009;16:411–420. doi: 10.1016/j.devcel.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev. Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Beckerle MC. Zyxin: zinc fingers at sites of cell adhesion. Bioessays. 1997;19:949–957. doi: 10.1002/bies.950191104. [DOI] [PubMed] [Google Scholar]
- Benhamouche S, Curto M, Saotome I, Gladden AB, Liu C-H, Giovannini M, McClatchey AI. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr. Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 Phosphorylates Hippo/MST Kinases to Regulate the Hippo-Salvador-Warts Tumor Suppressor Pathway. Dev. Cell. 2011;21:888–895. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- Chan EHY, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HHW. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chen L, Chong YF, Huang C, Song H, Hong W. The Hippo pathway in biological control and cancer development. J. Cell Physiol. 2011a;226:928–939. doi: 10.1002/jcp.22435. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo Pathway-independent Restriction of TAZ and YAP by Angiomotin. J. Biol. Chem. 2011b;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-L, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl. Acad. Sci. USA. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc Natl Acad Sci U S A. 2012;109:484–489. doi: 10.1073/pnas.1113882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat. Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed Mariana F, Anders RA, Maitra A, Pan D. Elucidation of a Universal Size-Control Mechanism in Drosophila and Mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Fang X, Adler PN. Regulation of cell shape, wing hair initiation and the actin cytoskeleton by Trc/Fry and Wts/Mats complexes. Dev. Biol. 2010;341:360–374. doi: 10.1016/j.ydbio.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández BG, Gaspar P, Brás-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- Fournier AK, Campbell LE, Castagnino P, Liu WF, Chung BM, Weaver VM, Chen CS, Assoian RK. Rac-dependent cyclin D1 gene expression regulated by cadherin- and integrin-mediated adhesion. J. Cell Sci. 2008;121:226–233. doi: 10.1242/jcs.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the salvador/warts/hippo signaling network. Dev. Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev. Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 2010;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Hariharan IK, Bilder D. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu. Rev. Genet. 2006;40:335–361. doi: 10.1146/annurev.genet.39.073003.100738. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 2004;167:135–147. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- Hergovich A, Schmitz D, Hemmings BA. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem. Biophys. Res. Commun. 2006;345:50–58. doi: 10.1016/j.bbrc.2006.03.244. [DOI] [PubMed] [Google Scholar]
- Hirata H, Tatsumi H, Sokabe M. Zyxin emerges as a key player in the mechanotransduction at cell adhesive structures. Commun. Integr. Biol. 2008;1:192–195. doi: 10.4161/cib.1.2.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LL, Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated at the cell membrane to control tissue growth and organ size in Drosophila. Dev. Biol. 2010;337:274–283. doi: 10.1016/j.ydbio.2009.10.042. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, Bellen HJ, Halder G. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129:5719–5730. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- King I, Heberlein U. Tao kinases as coordinators of actin and microtubule dynamics in developing neurons. Commun. Integr. Biol. 2011;4:554–556. doi: 10.4161/cib.4.5.16051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I, Tsai LT, Pflanz R, Voigt A, Lee S, Jackle H, Lu B, Heberlein U. Drosophila tao controls mushroom body development and ethanol-stimulated behavior through par-1. J. Neurosci. 2011;31:1139–1148. doi: 10.1523/JNEUROSCI.4416-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z-C, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho L-L, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl. Acad. Sci. USA. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Rohn JL, Picone R, Kunda P, Baum B. Tao-1 is a negative regulator of microtubule plus-end growth. J. Cell Sci. 2010;123:2708–2716. doi: 10.1242/jcs.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr. Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr. Opin. Cell Biol. 2009;21:864–870. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- McCartney BM, Fehon RG. Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of Moesin and the neurofibromatosis 2 tumor suppressor, Merlin. J. Cell Biol. 1996;133:843–852. doi: 10.1083/jcb.133.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The neurofibromatosis-2 homologue, Merlin and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Fehon RG. Merlin and the ERM proteins--regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19:198–206. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsopoulos C, Zihni C, Garg R, Ridley AJ, Morris JD. The prostate-derived sterile 20-like kinase (PSK) regulates microtubule organization and stability. J. Biol. Chem. 2003;278:18085–18091. doi: 10.1074/jbc.M213064200. [DOI] [PubMed] [Google Scholar]
- Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD. In vivo analysis of Yorkie phosphorylation sites. Oncogene. 2009;28:1916–1927. doi: 10.1038/onc.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Reddy BV, Irvine KD. Phosphorylation-independent repression of Yorkie in Fat-Hippo signaling. Dev. Biol. 2009;335:188–197. doi: 10.1016/j.ydbio.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev. Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Léopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- Polesello C, Tapon N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 2007;17:1864–1870. doi: 10.1016/j.cub.2007.09.049. [DOI] [PubMed] [Google Scholar]
- Poon CLC, Lin JI, Zhang X, Harvey KF. The Sterile 20-like Kinase Tao-1 Controls Tissue Growth by Regulating the Salvador-Warts-Hippo Pathway. Dev. Cell. 2011;21:896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Pan G, Reddy BVVG, Oh H, Irvine KD. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 2011;9:e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein expanded. Curr. Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman HA, Gateff E. Control systems in insect development. Science. 1967;158:534–535. doi: 10.1126/science.158.3800.534-c. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, McClatchey AI, Jacks T. Regulation of the neurofibromatosis type 2 tumor suppressor protein, merlin, by adhesion and growth arrest stimuli. J. Biol. Chem. 1998;273:7757–7764. doi: 10.1074/jbc.273.13.7757. [DOI] [PubMed] [Google Scholar]
- Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The Tumor-Suppressor Gene fat Controls Tissue Growth Upstream of Expanded in the Hippo Signaling Pathway. Curr. Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien W-H, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci. Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouloudaki K, Puetz M, Simons M, Courbard JR, Boehlke C, Hartleben B, Engel C, Moeller MJ, Englert C, Bollig F, et al. Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proc Natl Acad Sci U S A. 2009;106:8579–8584. doi: 10.1073/pnas.0811691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O, Hughes SC, Noren NK, Kulikauskas RM, Fehon RG. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–87. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DCR, Schiripo TA, Haber DA, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- Timm T, Li X-Y, Biernat J, Jiao J, Mandelkow E, Vandekerckhove J, Mandelkow E-M. MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. EMBO J. 2003;22:5090–5101. doi: 10.1093/emboj/cdg447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky B, Levchenko T, Mansson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J. Cell Biol. 2001;152:1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Wada K-I, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J. Biol. Chem. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Shimizu T, Lai Z-C. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 2007;26:1772–1781. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, Lim C, et al. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125:535–548. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen C-L, Tao C, Zhang X, Halder G. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr. Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Näthke I. Cell polarity in development and cancer. Nat. Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, Persson NL, Shimono A, Speicher DW, Marmorstein R, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19:527–540. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng W-M, Pan D. Kibra functions as a tumor suppressor protein that regulates hippo signaling in conjunction with Merlin and expanded. Dev. Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T, Tian A, Jiang J. The cell adhesion molecule echinoid functions as a tumor suppressor and upstream regulator of the hippo signaling pathway. Dev. Cell. 2012;22:255–267. doi: 10.1016/j.devcel.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Milton CC, Humbert PO, Harvey KF. Transcriptional output of the Salvador/warts/hippo pathway is controlled in distinct fashions in Drosophila melanogaster and mammalian cell lines. Cancer Res. 2009;69:6033–6041. doi: 10.1158/0008-5472.CAN-08-4592. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Lu Q, Wang LH, Liu C-Y, Lei Q, Guan K-L. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang C-Y, Yu J, Guan K-L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]