Abstract
An efficient approach for the parallel solid phase synthesis of isoxazole and isoxazoline derivatives has been developed. The isoxazoles and isoxazolines were constructed through a 1,3-dipolar cycloaddition reaction of nitrile oxides, with resin-bound alkynes or alkenes. The cycloaddition reaction conditions performed on solid phase supports was optimized, and an array of resin bound carboxylic acid building blocks were utilized for distinct conversions. This methodology presents a new alternative to the diversity oriented synthesis of disubstituted isoxazoles and isoxazolines different from existing routes which are limited in structural diversity
Keywords: Isoxazoles; Isoxazolines; Heterocycles; 1,3-Dipolar cycloadditions; Solid-phase synthesis; Combinatorial chemistry
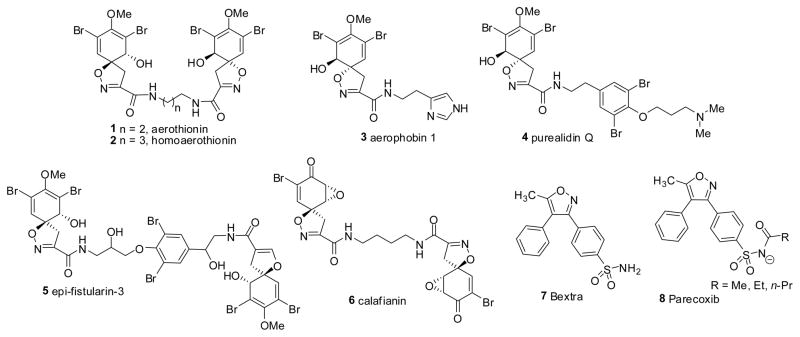
Heterocycles display an array of significant bioactive properties,1–3 and heterocyclic scaffolds are present in a wide variety of drugs as well as drug like molecules of pharmaceutical relevance.4,5 Among the family of heterocyclic compounds, isoxazoles and isoxazolines are an important class of heterocycles displaying a wide variety of biological properties including antiviral,6 antitubulin,7 as well as anti-inflammatory activity.8 The synthesis of this family of heterocycles continue to attract the attention of synthetic organic and medicinal chemists.9 The isoxazoline framework is a prevalent feature of several natural products 1–6,10,11 and the isoxazole structural motif is found in the COX II inhibitors, bextra 7 and parecoxib 8 (Fig.1).9a,12 A plethora of methodologies exist toward the synthesis of isoxazoles and isoxazolines,13,14 and most of them endeavor nitrile oxide cycloaddition (NOC) as a key step.15
Figure 1.
Naturally occurring isoxazolines and pharmaceutically relevant isoxazoles.
On the other hand, combinatorial chemistry and high-throughput screening have changed the scale on which drug discovery programs are carried out.4,5 The inherent potential of this technique aid to accelerate the drug discovery process through rapid synthesis and subsequent screening of much larger numbers of compounds than previously possible.16 The versatile and potential capability afforded by the tea-bag approach,17 has led to the isolation and recognition of an array of bioactive peptides, including antibacterials, antigenic peptides, opioid receptor agonists and antagonists, inhibitors and several heterocyclic compounds of biomedical importance.18 In continuation of our research investigation toward the design, and development of novel heterocycles, we developed a parallel solid-phase nitrile oxide cycloaddition (NOC) strategy to synthesize a variety of isoxazoles and isoxazolines utilizing 1,3-dipolar cycloaddition chemistry.9,15 The hydroximoyl chlorides 11 for the 1,3-dipolar cycloaddition are not commercially available and were conveniently synthesized in two steps utilizing solution phase chemistry.8,9a This methodology presents a new alternative to the diversity oriented synthesis of disubstituted isoxazoles and isoxazolines different from existing routes which, are limited in structural diversity.19,20
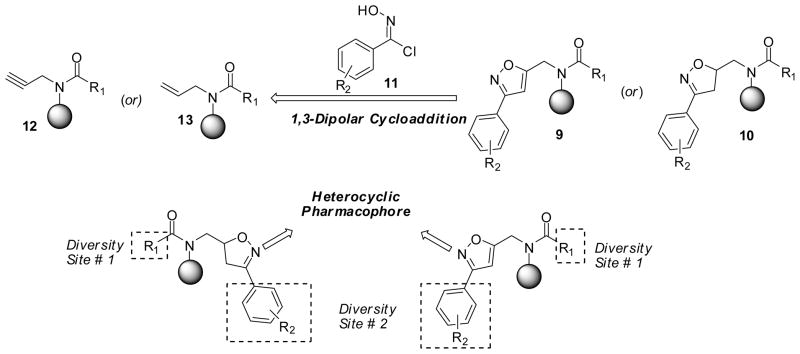
We envisioned the synthesis of isoxazole 9 and isoxazolines 10 via 1,3-dipolar cycloaddition of in situ generated nitrile oxides with alkyne 12 or alkenes 13. The required alkyne and alkene precursors are synthesized from the corresponding resin-bound carboxylic acid. The retrosynthetic rationale for the parallel solid-phase synthesis of isoxazoles and isoxazolines is illustrated in Scheme 1.
Scheme 1.
Retrosynthetic illustration of structurally diverse isoxazoles and isoxazolines.
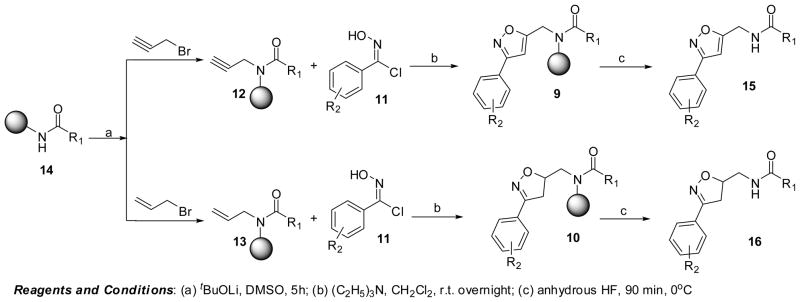
The parallel solid phase synthesis of all compounds were carried out utilizing Houghten’s tea-bag approach, wherein the resin is packed within sealed polypropylene mesh packets.17 The first position of diversity was introduced by coupling several carboxylic acids to p-methylbenzhydrylamine resin. The generated secondary amide 14 was then alkylated in the presence of lithium t-butoxide and an alkylating agent (allyl bromide or propargyl bromide).21 The parallel synthesis of the resin-bound isoxazoles 9 and isoxazolines 10 was initiated by the nitrile oxide cycloaddition of the resin-bound alkynes and alkene derivatives. Structurally diverse combinations of carboxylic acids (cyclopentanecarboxylic acid, 1-cyclopenteneacetic acid, benzoic acid, 2-nitrobenzoic acid, piperonylic acid, syringyl, 4-biphenylcarboxylic acid, 1-napthalenecarboxylic, diphenylacetic acid, 1-phenyl-1-cyclopropylcarboxylic acid, and 1-phenyl-1-cyclopentanecarboxylic acid) were selected.
To investigate the feasibility of nitrile oxide cycloadditions (NOC) on solid support, three different hydroximoyl chlorides were freshly prepared and were treated with triethylamine to generate the corresponding nitrile oxides9,13c,f,14i,5a,b Treatment of resin-bound dipolarophiles 12,13 with the generated nitrile oxides proceeded in a 1,3-dipolar fashion and furnished diversified isoxazoles and isoxazolines.22 In case of resin coupled with piperonylic acid, we also observed the formation of a diol (partially cleaved protected diol) in addition to the main product. All of the cycloadditions occurred in good yields and were isolated in high purities (Table 1 and 2). The protocol for the parallel synthesis of structurally diverse isoxazoles and isoxazolines is outlined in Scheme 2.
Table 1.
Isoxazoles from resin-bound alkynes
| Entry | R1 | R2 | Mass calcd./found | Yields (%)a |
|---|---|---|---|---|
| 15a | Piperonyl | H | 322.3/323.2 (MH+) | 87 |
| 15b | Cyclopentyl | H | 270.3/271.4 (MH+) | 66 |
| 15c | 1-Cyclopenteneacetyl | H | 282.3/283.2 (MH+) | 91 |
| 15d | 4-Biphenyl | H | 354.4/355.2 (MH+) | 63 |
| 15e | Syringyl | H | 354.4/356.0 (MH+) | 54 |
| 15f | 1-Phenyl-1-cyclopropyl | H | 318.3/319.6 (MH+) | 52 |
| 15g | Diphenylacetyl | H | 368.4/369.5 (MH+) | 75 |
| 15h | Phenyl | 4-Ph | 354.4/355.2 (MH+) | 90 |
| 15i | Piperonyl | 4-Ph | 398.4/399.3 (MH+) | 83 |
| 15j | Cyclopentyl | 4-Ph | 346.4/347.4 (MH+) | 93 |
| 15k | 1-Cyclopenteneacetyl | 4-Ph | 358.4/359.2 (MH+) | 94 |
| 15l | 2-Nitrophenyl | 4-Ph | 399.4/400.4 (MH+) | 55 |
| 15m | 4-Biphenyl | 4-Ph | 430.5/431.4 (MH+) | 63 |
| 15n | 1-Phenyl-1-cyclopropyl | 4-Ph | 394.5/395.3 (MH+) | 75 |
| 15o | Diphenylacetyl | 4-Ph | 444.5/445.3 (MH+) | 62 |
| 15p | Syringyl | 4-Ph | 430.4/453.2 (MH+) | 63 |
| 15q | 1-Napthyl | 4-Ph | 404.5/405.4 (MH+) | 73 |
| 15r | 2-Nitrophenyl | 2,6-diCl | 392.2/393.5 (MH+) | 70 |
| 15s | Piperonyl | 2,6-diCl | 391.2/392.4 (MH+) | 91 |
| 15t | Phenyl | 2,6-diCl | 347.4/349.3 (MH+) | 92 |
| 15u | Cyclopentyl | 2,6-diCl | 415.4/416.3 (MH+) | 79 |
| 15v | 1-Phenyl-1-cyclopentyl | 2,6-diCl | 415.3/438.2 (MNa+) | 75 |
| 15w | 4-Biphenyl | 2,6-diCl | 423.3/446.1 (MNa+) | 86 |
The products were run on a Vydac column, gradients 5 to 95% formic acid in Acetonitrile in 7 min.
The yields are based on the weight of purified products and are relatives to the initial loading of the resin. (The purity of the purified compounds is higher than 95% for all the compounds).
Table 2.
Isoxazolines from resin-bound alkenes
| Entry | R1 | R2 | Mass calcd./found | Yields (%)a |
|---|---|---|---|---|
| 16a | Cyclopentyl | H | 272.3/273.1 (MH+) | 96 |
| 16b | 1-Cyclopenteneacetyl | H | 284.1/285.1 (MH+) | 92 |
| 16c | 4-Biphenyl | H | 356.4/357.3 (MH+) | 67 |
| 16d | 1-Phenyl-1-cyclopropyl | H | 320.3/321.2 (MH+) | 87 |
| 16e | Syringyl | H | 356.3/357.2 (MH+) | 77 |
| 16f | Piperonyl | 4-Ph | 400.4/423.2 (MNa+) | 63 |
| 16g | Cyclopentyl | 4-Ph | 348.4/349.3 (MH+) | 93 |
| 16h | 1-Cyclopenteneacetyl | 4-Ph | 360.4/361.3 (MH+) | 88 |
| 16i | 2-Nitrophenyl | 4-Ph | 401.5/402.4 (MH+) | 84 |
| 16j | Phenyl | 4-Ph | 356.3/357.3 (MH+) | 84 |
| 16k | 1-Phenyl-1-cyclopropyl | 4-Ph | 396.5/397.4 (MH+) | 77 |
| 16l | Diphenylacetyl | 4-Ph | 446.4/447.4 (MH+) | 82 |
| 16m | Syringyl | 4-Ph | 432.4/433.2 (MH+) | 87 |
| 16n | 1-Napthyl | 4-Ph | 406.5/429.3 (MNa+) | 71 |
| 16o | 4-Biphenyl | 4-Ph | 432.5/455.5 (MNa+) | 61 |
| 16p | 1-Phenyl-1-cyclopentyl | 4-Ph | 424.5/447.3 (MNa+) | 72 |
| 16q | Phenyl | 2,6-diCl | 349.2/372.3 (MNa+) | 96 |
| 16r | 2-Nitrophenyl | 2,6-diCl | 394.2/417.1 (MNa+) | 95 |
| 16s | Piperonyl | 2,6-diCl | 393.2/417.2 (MNa+) | 96 |
| 16t | Cyclopentyl | 2,6-diCl | 417.3/440.2 (MNa+) | 87 |
| 16u | 1-Phenyl-1-cyclopentyl | 2,6-diCl | 417.3/440.1 (MNa+) | 77 |
| 16v | 4-Biphenyl | 2,6-diCl | 425.3/447.4 (MNa+) | 78 |
The products were run on a Vydac column, gradients 5 to 95% formic acid in Acetonitrile in 7 min.
The yields are based on the weight of purified products and are relatives to the initial loading of the resin (The purity of the purified compounds is higher than 95% for all the compounds).
Scheme 2.
Solid phase synthesis of structurally diverse isoxazoles and isoxazolines
In conclusion, we have developed an efficient parallel solid-phase methodology to construct diversity oriented isoxazoles and isoxazolines via 1,3-dipolar cycloaddition reaction.22 Coupling of carboxylic acids to the resin introduced the first position of diversity which, following N-alkylation with allyl bromide or propargyl bromide furnished several resin-bound alkenes and alkynes. 1,3-Dipolar cycloaddition of the resin bound alkenes or alkynes with nitrile oxides led to the formation of the corresponding disubstituted isoxazole and isoxazolines.
Supplementary Material
Acknowledgments
The authors would like to thank the State of Florida Funding, NIH (1R03DA025850-01A1, Nefzi; 5P41GM081261-03, Houghten; 3P41GM079590-03S1, Houghten) for providing generous financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. J Med Chem. 1997;40:1347. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 2.(a) Bhat GA, Montero JLG, Panzica RP, Wotring LL, Townsend LB. J Med Chem. 1981;24:1165. doi: 10.1021/jm00142a009. [DOI] [PubMed] [Google Scholar]; (b) Petrie CR, III, Cottam HB, McKernan PA, Robins RK, Revankar GR. J Med Chem. 1985;28:1010. doi: 10.1021/jm00146a007. [DOI] [PubMed] [Google Scholar]
- 3.(a) Elguero J. In: Comprehensive Heterocyclic Chemistry II. Katrizky AR, Rees CW, Scriven EFV, editors. Vol. 3. Pergamon; Oxford: 1996. p. 1. [Google Scholar]; (b) Patel JB, Malick JB, Salama AI, Goldberg ME. Pharmacol Biochem And Behavior. 1985;23:675. doi: 10.1016/0091-3057(85)90436-8. [DOI] [PubMed] [Google Scholar]
- 4.(a) Meng L, Lorsbach BA, Sparks TC, Fettinger JC, Kurth MJ. J Comb Chem. 2010;12:129. doi: 10.1021/cc900133k. [DOI] [PubMed] [Google Scholar]; (b) Nefzi A, Ostresh JM, Houghten RA. Chem Rev. 1997;97:449. doi: 10.1021/cr960010b. [DOI] [PubMed] [Google Scholar]; (c) Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 5.(a) Nefzi A, Ostresh JM, Yu J, Houghten RA. J Org Chem. 2004;69:3603. doi: 10.1021/jo040114j. [DOI] [PubMed] [Google Scholar]; (b) Gallop MA, Barret RW, Dower WJ, Fodor SPA, Gordon EM. J Med Chem. 1994;37:1385. doi: 10.1021/jm00036a001. [DOI] [PubMed] [Google Scholar]; (c) Thompson LA, Ellman JA. Chem Rev. 1996;96:555. doi: 10.1021/cr9402081. [DOI] [PubMed] [Google Scholar]; (d) Dadiboyena S, Nefzi A. Tetrahedron Lett. 2011;52:7030. doi: 10.1016/j.tetlet.2011.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Lee YS, Kim BH. Bioorg Med Chem Lett. 2002;12:1395. doi: 10.1016/s0960-894x(02)00182-8. [DOI] [PubMed] [Google Scholar]; (b) Srivastava S, Bajpai LK, Batra S, Bhaduri AP, Maikhuri JP, Gupta G, Dhar JD. Bioorg Med Chem. 1999;7:2607. doi: 10.1016/s0968-0896(99)00188-1. [DOI] [PubMed] [Google Scholar]
- 7.(a) Simoni D, Grisolia G, Giannini G, Roberti M, Rondanin R, Piccagli L, Baruchello R, Rossi M, Romagnoli R, Invidiata FP, Grimaudo S, Jung MK, Hamel E, Gebbia N, Crosta L, Abbadessa V, Di Cristina A, Dusonchet L, Meli M, Tolomeo M. J Med Chem. 2005;48:723. doi: 10.1021/jm049622b. [DOI] [PubMed] [Google Scholar]; (b) Kaffy J, Pontikis R, Carrez D, Croisy A, Monneret C, Florent JC. Bioorg Med Chem. 2006;14:4067. doi: 10.1016/j.bmc.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY, Zweifel BS, Seibert K. J Med Chem. 2000;43:775. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- 9.(a) Dadiboyena S, Xu J, Hamme AT., II Tetrahedron Lett. 2007;48:1295. doi: 10.1016/j.tetlet.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dadiboyena S, Nefzi A. Eur J Med Chem. 2010;45:4697. doi: 10.1016/j.ejmech.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Berquist PR, Wells RJ. Chemotaxonomy of the Porifera: The Development and Current Status of the Field. In: Scheuer PJ, editor. Marine Natural Products: Chemical and Biological Perspectives. Vol. 5. Academic Press; New York: 1993. pp. 1–50. [Google Scholar]; (b) Faulkner DJ. Nat Prod Rep. 1998:113. doi: 10.1039/a815113y. [DOI] [PubMed] [Google Scholar]; (c) Faulkner DJ. Nat Prod Rep. 1997:259. [Google Scholar]
- 11.(a) Faulkner J. Nat Prod Rep. 2001;18:1. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]; (b) Boehlow TR, Spilling CD. Nat Prod Lett. 1995;7:1. [Google Scholar]; (c) Faulkner JD. Nat Prod Rep. 2002;19:1. doi: 10.1039/b009029h. [DOI] [PubMed] [Google Scholar]; (d) Encarnacion RD, Sandoval E, Malmstrom J, Christophersen C. J Nat Prod. 2000;63:874. doi: 10.1021/np990489d. [DOI] [PubMed] [Google Scholar]; (e) Kernan MR, Canbie RC, Bergquist PR. J Nat Prod. 1990;53:615. [Google Scholar]; (f) Compagnone RS, Avila R, Suarez AI, Abrams OV, Rangel HR, Arvelo F, Pina IC, Merentes E. J Nat Prod. 1999;62:1443. doi: 10.1021/np9901938. [DOI] [PubMed] [Google Scholar]
- 12.(a) Talley JJ, Bertenshaw SR, Brown DL, Carter JS, Graneto MJ, Kellogg MS, Koboldt CM, Yuan J, Zhang YY, Seibert K. J Med Chem. 2000;43:1661. doi: 10.1021/jm000069h. [DOI] [PubMed] [Google Scholar]; (b) Dadiboyena S, Nefzi A. Eur J Med Chem. 2011;46:5258. doi: 10.1016/j.ejmech.2011.09.016. [DOI] [PubMed] [Google Scholar]; (c) Toyokuni T, Dileep Kumar JS, Walsh JC, Shapiro A, Talley JJ, Phelps ME, Herschman HR, Barrio JR, Satyamurthy N. Bioorg Med Chem Lett. 2005;15:4699. doi: 10.1016/j.bmcl.2005.07.065. [DOI] [PubMed] [Google Scholar]; (d) Sandanayaka VP, Youjun Y. Org Lett. 2000;2:3087. doi: 10.1021/ol006256r. [DOI] [PubMed] [Google Scholar]
- 13.For isoxazoles, see: Waldo JP, Larock RC. Org Lett. 2005;23:5203. doi: 10.1021/ol052027z.Bourbeau MP, Rider JT. Org Lett. 2006;8:3679. doi: 10.1021/ol061260+.Moore JE, Davies MW, Goodenough KM, Wybrow RAJ, York M, Johnson CN, Harrity JPA. Tetrahedron. 2005;61:6707.Cecchi L, De Sarlo F, Machetti F. Eur J Org Chem. 2006:4852.Grecian S, Fokin VV. Angew Chem, Int Ed. 2008;47:8285. doi: 10.1002/anie.200801920.Crossley JA, Browne DL. J Org Chem. 2010;75:5414. doi: 10.1021/jo1011174.Lee CC, Fitzmaurice RJ, Caddick S. Org Biomol Chem. 2009;7:4349. doi: 10.1039/b911098d.
- 14.For isoxazoline natural products, see: Goldenstein K, Fendert T, Proksch P, Winterfeldt E. Tetrahedron. 2000;56:4173.Harburn JJ, Rath NP, Spilling CD. J Org Chem. 2005;70:6398. doi: 10.1021/jo050846r.Adamo MFA, Donati D, Duffy EF, Sarti-Fantoni P. J Org Chem. 2005;70:8395. doi: 10.1021/jo051181w.Marsini MA, Huang Y, Van De Water R, Pettus TRR. Org Lett. 2007;9:3229. doi: 10.1021/ol0710257.Wasserman HH, Wang J. J Org Chem. 1998;63:5581.Boehlow TR, Harburn JJ, Spilling CD. J Org Chem. 2001;66:3111. doi: 10.1021/jo010015v.Masatoshi M, Yamada K, Hoshino O. Tetrahedron. 1996:14713.Ogamino T, Nishiyama S. Tetrahedron. 2003;59:9419.Bardhan S, Schmitt DC, Porco JA., Jr Org Lett. 2006;8:927. doi: 10.1021/ol053115m.
- 15.Huisgen R. Angew Chem Intl Ed. 1963;2:565.Huisgen R. Angew Chem Intl Ed. 1963;2:633.For more examples, see: Dadiboyena S, Valente EJ, Hamme AT., II Tetrahedron Lett. 2010;51:1341. doi: 10.1016/j.tetlet.2010.01.017.Dadiboyena S, Hamme AT., II Tetrahedron Lett. 2011;52:2536. doi: 10.1016/j.tetlet.2011.03.004.Dadiboyena S, Valente EJ, Hamme AT., II Tetrahedron Lett. 2009;50:291. doi: 10.1016/j.tetlet.2008.10.145.
- 16.Yu Y, Nefzi A, Ostresh JM, Houghten RA. In: Innnovations and Perspectives in Solid Phase Synthesis and Combinatorial Libraries. Epton Roger., editor. Mayflower Worldwide Ltd; London UK: 2004. [Google Scholar]
- 17.Houghten RA. Proc Natl Acad Sci USA. 1985;82:5131. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Houghten RA, Wilson DB, Pinilla C. Drug Discovery Today. 2000;5:276. doi: 10.1016/s1359-6446(00)01513-0. [DOI] [PubMed] [Google Scholar]; (b) Houghten RA, Pinilla C, Appel JR, Blondelle SE, Dooley CT, Eichler J, Nefzi A, Ostresh JM. J Med Chem. 1999;42:3743. doi: 10.1021/jm990174v. [DOI] [PubMed] [Google Scholar]
- 19.(a) Sammelson RE, Miler RB, Kurth MJ. J Org Chem. 2000;65:2225. doi: 10.1021/jo991551e. [DOI] [PubMed] [Google Scholar]; (b) Jeddeloh MR, Holden JB, Nouri DH, Kurth MJ. J Comb Chem. 2007;9:1041. doi: 10.1021/cc700117a. [DOI] [PubMed] [Google Scholar]
- 20.(a) Shankar BB, Yang DY, Ganguly AK. Tetrahedron Lett. 1998;39:2447. [Google Scholar]; (b) Curran DP, Choi S-M, Gothe SA, Lin F-T. J Org Chem. 1990;55:3710. [Google Scholar]
- 21.(a) Ostresh JM, Schoner CC, Hamashin VT, Nefzi A, Meyer JP, Houghten RA. J Org Chem. 1998;63:8622. [Google Scholar]; (b) Do Erner B, Husar GM, Ostresh JM, Houghten RA. Bioorg Med Chem. 1996;4:709. doi: 10.1016/0968-0896(96)00067-3. [DOI] [PubMed] [Google Scholar]; (c) Nefzi A, Ostresh JM, Houghten RA. Tetrahedron. 1999;55:335. [Google Scholar]
- 22.General procedure for the 1,3-Dipolar cycloaddition: Resin-bound carboxylic acid coupling and N-alkylation using allyl bromide or propargyl bromide were performed according to the literature precedents.21 Resin-bound alkyne (alkene) and the hydroximoyl chloride (10 equiv.) in 10 mL of dry dichloromethane (DCM) was treated with triethylamine (0.16 mL, 10 equiv) and the reaction mixture was stirred overnight. The excess solution was decanted, and the resin was washed with DCM (2 x 5 mL). The resin was cleaved with anhydrous HF (10 mL) for 90 min at 0 °C, and the desired isoxazole (isoxazoline) was obtained following extraction with 95% AcOH in H2O and lyophilization as a colorless powder. The isoxazole (isoxazoline) was purified by preparative reverse-phase HPLC; Cyclopentanecarboxylic acid (3-phenyl-isoxazol-5-ylmethyl)-amide (15b): 1H NMR (500 MHz; DMSO-d6): δ 1.50–1.52 (m, 2H), 1.61–1.67 (m, 4H), 1.77–1.79 (m, 2H), 2.61–2.64 (m, 1H), 4.44 (d, J = 6.0 Hz, 2H), 6.81 (s, 1H), 7.49–7.52 (m, 3H), 7.84–7.87 (m, 2H), 8.49 (t, J = 6.0 Hz, 1H); MS (ESI) m/z calcd for C16H18N2O2 [M+H+]: 270.3; found: 271.4; Benzo[1,3]dioxole-5-carboxylic acid (3-biphenyl-4-yl-isoxazol-5-ylmethyl)-amide (15i): 1H NMR (500 MHz; DMSO-d6): δ 1.23 (s, 2H), 4.64 (d, J = 5.5 Hz, 2H), 6.55 (s, 1H), 7.02 (d, J = 8.0 Hz, 1H), 7.41 (t, J = 7.0 Hz, 1H), 7.45 (s, 1H), 7.50 (q, J = 8.0 Hz, 3H), 7.74 (d, J = 8.5 Hz, 2H), 7.80 (d, J = 8.5 Hz, 2H), 7.97 (d, J = 8.0 Hz, 2H), 9.07 (t, J = 6.0 Hz, 1H); MS (ESI) m/z calcd for C24H18N2O4 [M+H+]: 398.4; found: 399.3; Cyclopentanecarboxylic acid (3-biphenyl-4-yl-isoxazol-5-ylmethyl)-amide (15j): 1H NMR (500 MHz; DMSO-d6): δ 1.23 (s, 4H), 1.50–1.52 (m, 1H), 1.63–1.68 (m, 2H), 1.77–1.80 (m, 1H), 2.62–2.65 (m, 1H), 4.46 (t, J = 5.8 Hz, 2H), 6.88 (s, 1H), 7.41 (t, J = 7.2 Hz, 1H), 7.50 (t, J = 8.0 Hz, 2H), 7.74 (t, J = 9.5 Hz, 2H), 7.81 (t, J = 8.1 Hz, 2H), 7.95 (t, J = 10.5 Hz, 2H), 8.49–8.51 (m, 1H); MS (ESI) m/z calcd for C22H22N2O2 [M+H+]: 346.4; found: 347.4; Benzo[1,3]dioxole-5-carboxylic acid [3-(2,6-dichloro-phenyl)-isoxazol-5-ylmethyl]-amide (15s): 1H NMR (500 MHz; DMSO-d6): δ 4.69 (d, J = 5.5 Hz, 2H), 6.10 (s, 2H), 6.59 (s, 1H), 7.01 (d, J = 8.5 Hz, 1H), 7.43 (s, 1H), 7.50 (d, J = 10.0 Hz, 1H), 7.54–7.63 (m, 1H), 7.64–7.65 (m, 2H), 9.1 (t, J = 5.5 Hz, 1H); MS (ESI) m/z calcd for C17H12Cl2N2O4 [M+H+]: 391.2; found: 393.2; Biphenyl-4-carboxylic acid [3-(2,6-dichloro-phenyl)-isoxazol-5-ylmethyl]-amide (15w): 1H NMR (500 MHz; DMSO-d6): δ 4.75 (d, J = 4.5 Hz, 2H), 6.62 (s, 1H), 7.41 (m, 1H), 7.50 (t, J = 7.0 Hz, 2H), 7.56 (t, J = 8.0 Hz, 1H), 7.64 (t, J = 8.5 Hz, 2H), 7.74 (d, J = 8.5 Hz, 2H), 7.80 (d, J = 8.5 Hz, 2H), 8.01 (d, J = 8.5 Hz, 2H), 9.31 (t, J = 6.0 Hz, 2H); MS (ESI) m/z calcd for C23H16Cl2N2O2 [M+Na+]: 423.2; found: 446.1; Cyclopentanecarboxylic acid (3-phenyl-4,5-dihydro-isoxazol-5-ylmethyl)-amide (16a): 1H NMR (500 MHz; DMSO-d6): δ 1.44–168 (m, 8H), 2.54 (t, J = 7.5 Hz, 1H), 3.13 (dd, J = 7.0 Hz, 17 Hz 1H), 3.22–3.24 (m, 1H), 3.44 (dd, J = 11.0 Hz, 17 Hz 1H), 4.72–4.78 (m, 1H), 7.43–7.46 (m, 3H), 7.62–7.65 (m, 2H), 8.04 (t, J = 6.0 Hz, 1H); MS (ESI) m/z calcd for C23H16Cl2N2O2 [M+H+]: 272.3; found: 273.1; Benzo[1,3]dioxole-5-carboxylic acid (3-biphenyl-4-yl-4,5-dihydro-isoxazol-5-ylmethyl)-amide (16f): 1H NMR (500 MHz; DMSO-d6): δ 3.40 (dd, J = 7 Hz, 17.5 Hz, 1H), 3.61–3.70 (m, 1H), 3.77–3.81 (m, 1H), 4.34 (dd, J = 5.5 Hz, 12 Hz, 1H), 4.43 (dd, J = 3.5 Hz, 12 Hz, 1H), 5.07–5.13 (m, 1H), 6.11 (s, 2H), 6.80–6.82 (m, 1H), 6.98–7.00 (m, 2H), 7.09–7.10 (m, 1H), 7.32 (s, 1H), 7.40–7.42 (m, 1H), 7.48–7.54 (m, 4H), 7.72–7.74 (m, 2H); MS (ESI) m/z calcd for C24H20N2O4 [M+Na+]: 400.4; found: 423.2; Cyclopentanecarboxylic acid (3-biphenyl-4-yl-4,5-dihydro-isoxazol-5-ylmethyl)-amide (16g): 1H NMR (500 MHz; DMSO-d6): δ 1.45–1.69 (m, 6H), 2.55–2.58 (m, 1H), 3.15–3.19 (m, 1H), 3.24–3.32 (m, 1H), 3.46–3.51 (m, 1H), 4.74–4.80 (m, 1H), 7.4 (t, J = 8.0 Hz, 1H), 7.49 (t, J = 7.5 Hz, 2H), 7.71–7.73 (m, 4H), 7.77–7.78 (m, 2H), 8.05 (t, J = 6.0 Hz, 1H); MS (ESI) m/z calcd for C22H24N2O2 [M+H+]: 348.4; found: 349.3; N-[3-(2,6-Dichloro-phenyl)-4,5-dihydro-isoxazol-5-ylmethyl]-2-nitro-benzamide (16r): 1H NMR (500 MHz; DMSO-d6): δ 3.14 (dd, J = 6.5 Hz, 17.5Hz, 1H), 3.41–3.55 (m, 3H), 4.96–5.00 (m, 1H), 7.52–7.55 (m, 1H), 7.61–7.64 (m, 3H), 7.8 (t, J = 7.5 Hz, 1H), 8.05 (d, J = 10 Hz, 1H), 9.06 (t, J = 6.0 Hz, 1H); MS (ESI) m/z calcd for C17H13Cl2N3O4 [M+Na+]: 394.20; found: 417.1; Benzo[1,3]dioxole-5-carboxylic acid [3-(2,6-dichloro-phenyl)-4,5-dihydro-isoxazol-5-ylmethyl-]-amide (16s): 1H NMR (500 MHz; DMSO-d6): δ 3.14 (dd, J = 6.5 Hz,17.5 Hz, 2H), 3.64–3.48 (m, 2H), 3.55–3.61 (m, 1H), 4.98–5.0 (m, 1H), 6.1 (s, 2H), 6.99 (d, J = 10 Hz, 2H), 7.43 (s, 1H), 7.48–7.58 (m, 2H), 7.60 (d, J = 5.0 Hz, 2H), 8.65 (t, J = 6.0 Hz, 1H); MS (ESI) m/z calcd for C18H14Cl2N2O4 [M+Na+]: 393.22; found: 417.2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.