Abstract
Tumor cell invadopodia mediate degradation of matrix barriers. A new study now demonstrates that a ring of active RhoC focuses invadopodial protrusion and degradation by regulating cofilin activity.
Complications from metastasis are the primary cause of breast cancer mortality, making the pathways that regulate this process attractive targets for therapeutic intervention. To penetrate surrounding tissues, cancer cells must invade the basement membrane, a network of extracellular matrix proteins that supports the overlying epithelium. Once they have escaped the tumor, metastasizing cells must migrate through the stroma and degrade the vascular subendothelial basement membrane to gain entry to the bloodstream. In culture, invasive cancer cells cross similarmatrix barriers by forming F-actin-rich protrusions called invadopodia [1], which provide localized delivery of matrix metalloproteinases to degrade these barriers. The formation of invadopodia correlates with cell invasiveness.
Invadopodia proceed through several different stages to mature into functional, matrix-degrading structures [2]. First, small clusters of branched F-actin, the actin-nucleation-promoting factors cortactin and N-WASp, cofilin, and the actin-related protein (Arp) 2/3 complex form invadopodia cores. These clusters have two fates: they can either dissociate or become stable invadopodia. Chemotactic stimuli within the tumor stroma, such as epidermal growth factor (EGF), promote new actin synthesis within invadopodia, leading to their stabilization, protrusion and, finally, degradation of the surrounding matrix [2–5]. Once they have emerged, invadopodia elongate through convergent extension of a central bundle of actin filaments. The initial invadopodial protrusion can enlarge to create a larger breach that ultimately allows the cell to penetrate the membrane and invade the surrounding tissue [6].
Tightly focused invadopodial penetration of the basement membrane appears to be a critical first step in invasion. But why the tight focus? Basement membranes are likely the most difficult barriers to breach. Focusing of invadopodia may concentrate matrix metalloproteinase activity. Moreover, the convergent elongation of actin filaments within a concentrated site of protrusion would be expected to produce the maximal unit force for basement membrane penetration. Invadopodia may also act as microsensors, testing the matrix environment to seek out favorable routes of invasion [7]. These factors likely explain why invadopodia must be so narrowly focused.
How is this tight focus maintained? The Ena/VASP family protein Mena localizes to invadopodia and promotes the formation and maturation of these protrusions [8]. By virtue of its ability to promote actin filament elongation, Mena likely supports convergent extension of actin filaments within the invadopodial core. In addition, actin-bundling proteins, such as fascin and T-fimbrin [9], stabilize F-actin bundles within the invadopodial core. Nevertheless, both of these mechanisms likely require a tight initial grouping of nascent elongating actin filaments. The major unresolved question is what corrals the nascent invadopodial protrusion. Now, in a paper in this issue of Current Biology, Bravo-Cordero et al. [10] reveal a novel mechanism by which the RhoC GTPase focuses actin polymerization within the assembling invadopodium. In so doing, the authors may have solved an important mystery as to the function of the RhoC GTPase in cancer metastasis.
Bravo-Cordero et al. [10] initially found that knockdown of the RhoC GTPase in highly metastatic MTLn3 rat breast carcinoma cells reduced their migration through matrix barriers. This finding is consistent with previous work demonstrating that RhoC is upregulated in invasive cancers and that RhoC overexpression can drive melanoma cell metastasis [11]. In contrast to its more famous relatives, RhoA, Rac1, and Cdc42, RhoC is comparatively understudied. Thus, the molecular mechanisms by which RhoC regulates tumor cell invasion and metastasis were unclear.
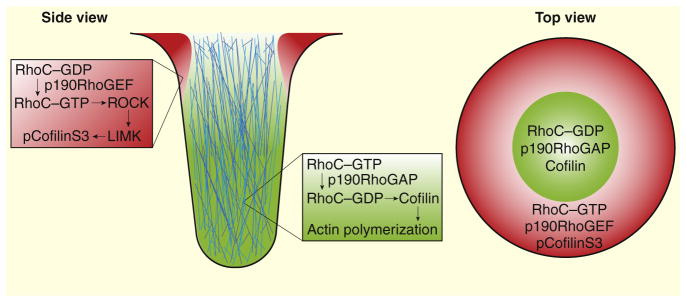
A major clue to RhoC function came from the analysis of invadopodial structure in RhoC knockdown cells. Rather than forming straight, narrow and focused invadopodia, RhoC knockdown cells have invadopodia that are wider, shorter, and often branched. When cells are plated on thin surfaces of fluorescently labeled matrix, the altered invadopodia of RhoC knockdown cells showed increased matrix degradation. Surprisingly, despite increased degradation, knockdown cells were unable to invade efficiently. The fact that these cells are deficient in invasive migration is consistent with the model that invadopodia must be tightly focused for proper invasive migration. The secret of RhoC control of invadopodial focus was revealed by localization studies using a RhoC fluorescence resonance energy transfer (FRET) biosensor to spatiotemporally visualize its activity. By simultaneously imaging the invadopodia-resident protein cortactin and the RhoC biosensor, the authors demonstrate that RhoC activity occurs in a tight ring surrounding the invadopodia core. This RhoC ring suggests a corral-like mechanism that confines molecules required for maturation and activity within the invadopodia core, restricting the size and directionality of degradation and invasion.
One intriguing potential target of RhoC activity is cofilin, an actin-severing protein that drives invadopodium formation through its ability to generate free actin barbed ends that serve as sites for new actin polymerization [12]. Bravo-Cordero et al. [10] initially sought to identify the mechanism that controls local cofilin activation in the invadopodia of invasive breast cancer cells. Serine phosphorylation of cofilin by LIM kinase (LIMK) leads to its inactivation by inhibiting its ability to bind actin. LIMK is phosphorylated and activated by ROCK, which is a downstream effector of the Rho subfamily of GTPases [13,14]. The upregulation of RhoC in invasive cells makes it a prime candidate for the regulation of cofilin in invadopodia [11].
Within the invadopodial core, cofilin is enriched and nonphosphorylated. In contrast, inactive phosphorylated cofilin is abundant just outside of the core. Results from Bravo-Cordero et al. [10] indicate that RhoC is a critical determinant of this sharp cofilin activity boundary (Figure 1). RhoC knockdown leads to an overall decrease in inactive phosphorylated cofilin due to the reduced action of ROCK and LIMK. As a result, inactivation of the RhoC–ROCK–LIMK pathway leads to increased generation of free actin barbed ends within invadopodial precursors, but the resulting invadopodia are less well focused and less efficient at supporting invasive migration. Interestingly, RhoA knockdown cells do not show a drop in phosphorylated cofilin, suggesting that RhoC, but not RhoA, regulates cofilin activity, adding to a growing list of evidence for non-redundant roles of Rho-family GTPases in cells.
Figure 1. RhoC regulates cofilin activity in invadopodia.
RhoC is activated by p190RhoGEF and these two proteins form a ring around the periphery of invadopodial cores, inactivating cofilin through the ROCK–LIMK pathway. RhoC is inactivated by p190RhoGAP within invadopodial cores, allowing active cofilin to generate actin barbed ends and promote polymerization.
Mounting evidence suggests that GTPases function in discrete zones of activity, which have been observed in numerous processes, including cytokinesis, wound healing, and locomotion [15]. These phenomena all require spatially constrained GTPase activity, similar to the RhoC activity zone observed surrounding invadopodia. GTPases are primarily activated by guanine nucleotide exchange factors (GEFs) and inhibited by GTPase-activating proteins (GAPs). One proposed method of zonal regulation is through the selective localization of these regulatory proteins [15]. In support of this, Bravo-Cordero et al. [10] showed that the RhoC-inactivating protein p190RhoGAP localizes to invadopodia to block RhoC activation within the core, while the activating p190RhoGEF is excluded from the core and enriched in areas where RhoC is active. These data offer yet another example of Rho GTPase activity zones used in the regulation of cytoskeletal dynamics.
Bravo-Cordero et al.’s [10] results have advanced the field by demonstrating an important, novel role for RhoC in the regulation of matrix degradation and basement membrane invasion by tumor cells. Although cofilin has been previously identified as an important regulator of actin polymerization, this work characterized the upstream regulatory pathway responsible for focusing cofilin activity within the invadopodial core. Finally, the ring of RhoC activity localized around invadopodia cores and the importance of this ring to the regulation of cofilin activity provides a further example of zonal regulation of Rho family GTPases. There are several questions that remain unanswered, and future work will undoubtedly focus on how GEFs and GAPs are physically constrained in their regulation of RhoC as well as how cofilin might be dephosphorylated by upstream signaling pathways.
Contributor Information
Stacey M. MacGrath, Email: stacey.macgrath@yale.edu.
Anthony J. Koleske, Email: anthony.koleske@yale.edu.
References
- 1.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 4.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldassarre M, Ayala I, Beznoussenko G, Giacchetti G, Machesky LM, Luini A, Buccione R. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006;85:1217–1231. doi: 10.1016/j.ejcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Schoumacher M, Louvard D, Vignjevic D. Cytoskeleton networks in basement membrane transmigration. Eur J Cell Biol. 2010;90:93–99. doi: 10.1016/j.ejcb.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122:3037–3049. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–828. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, Konig I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–644. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 12.Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip SC, Ghosh M, Eddy R, Backer JM, Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Cell Biol. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–667. doi: 10.1016/j.ejcb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 15.Bement WM, Miller AL, von Dassow G. Rho GTPase activity zones and transient contractile arrays. Bioessays. 2006;28:983–993. doi: 10.1002/bies.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]