Abstract
While allogeneic hematopoietic cell transplantation (HCT) has proven curative potential for myelodysplastic syndrome (MDS), relapse after HCT remains a problem. Pre-transplant cytoreduction with induction chemotherapy (IC) has been utilized to reduce relapse rates, but is associated with significant toxicity and mortality. Hypomethylating agents may achieve cytoreduction with limited toxicity; however, data on the effect of pre-HCT hypomethylation on post-HCT outcomes are limited. We retrospectively reviewed results in 68 patients who underwent allogeneic HCT for MDS or acute myeloid leukemia (AML) transformed from MDS. Thirty-five patients had received cytoreduction with azacitidine prior to HCT with either a high-dose (40%) or a reduced-intensity (60%) conditioning regimen, and 33 had undergone IC prior to HCT with high-dose conditioning. The estimated one-year overall survival was 57% in the azacitidine group and 36% in the IC group. The risk of post-HCT mortality (HR 0.68, 95% CI 0.35–1.30), non-relapse mortality (HR 0.99, 95% CI 0.41–2.34), and relapse (HR 0.34, 95% CI 0.41–2.34) were lower in the azacitidine group compared to the IC group, but only the hazard for relapse was significantly lower. After adjustment for cytogenetic risk, IPSS, and donor, the rates of post-HCT relapse for the two cohorts were similar. While the current study was retrospective and non-randomized and needs to be interpreted in this context, the results add to the growing evidence that pre-HCT therapy with azacitidine is associated with less toxicity than IC, and may allow for similar post-HCT outcomes.
Keywords: azacitidine, MDS, induction chemotherapy, hypomethylation, reduced-intensity conditioning
INTRODUCTION
Myelodysplastic syndrome (MDS) represents a group of clonal myeloid stem cell disorders with a heterogeneous spectrum of presentation, ranging from an indolent course over several years to rapid progression to acute myeloid leukemia (AML). The natural history of patients with MDS is varied with a median survival ranging from 5.7 years for patients with low-risk IPSS (International Prognostic Scoring System) scores, to 0.4 years for those with high-risk scores at the time of diagnosis. The only known curative treatment modality for MDS is allogeneic hematopoietic cell transplantation (HCT). Yet, despite the demonstrated benefit of HCT, patients with advanced MDS are at a substantial risk of relapse after HCT [1–3]. A central question, therefore, is whether patients should receive pre-HCT “debulking” therapy to reduce the risk of post-HCT relapse, and if so, what is the optimum modality to achieve that objective? As MDS is primarily a disease of older age, often complicated by medical comorbidities, a major concern is toxicity and mortality related to intensive treatment. Several retrospective studies have explored efficacy of induction chemotherapy (IC) before HCT aimed at decreasing the rate of relapse and improving post-transplant outcomes [4–6]. Pre-transplant IC may reduce the incidence of post-HCT relapse, but is associated with considerable morbidity and mortality, and patients may not come to HCT. In fact, a prospective study of 259 patients with high-risk MDS or AML over the age of 50 years found that of the 99 patients who achieved complete remissions after IC, only 53 had a consultation considering HCT, and just 14 patients ultimately received an HCT [7]. The remaining patients were not considered well enough for HCT for various reasons, including their clinical status before IC.
Administration of the azanucleosides, azacitidine and decitabine, is associated with only mild toxicity and has been shown to delay progression to AML and, in the case of azacitidine, to extend survival by 9.5 months as compared to conventional care [8–10]. Given their activity against MDS and the low toxicity profile, azanucleosides represent an attractive alternative to traditional IC as a pre-HCT cytoreductive modality.
The ideal strategy to discern the post-HCT benefit of pre-HCT cytoreductive therapy, and to identify the optimal agent, would be in the form of a randomized prospective study. Patients enrolled would be randomized to one of three arms, i.e. either proceed to immediate HCT, or receive pre-HCT cytoreduction with IC or with azacitidine. Such a study has not been conducted and may not be feasible or practical. In fact, several studies designed and launched to answer similar questions have been closed due to poor accrual. With the lack of randomized prospective data, and despite the inherent limitations, retrospective analyses provide the only means to generate evidence on which to base clinical practice. Thus, to compare the usefulness of pre-HCT azacitidine and IC for post-HCT outcome, we conducted a retrospective analysis of post-HCT results in patients with advanced MDS who underwent pre-HCT cytoreduction at our institution.
PATIENTS AND METHODS
Patients
Consecutive patients with advanced MDS or AML developing from antecedent MDS (tAML) who received an allogeneic HCT between August 2004 and May 2010 were screened for treatment with azacitidine prior to HCT. Included were patients who received other chemotherapeutic interventions prior to azacitidine. However, patients who received other chemotherapy after azacitidine and before HCT were excluded. A historical cohort comprised of patients with advanced MDS or tAML, who received IC prior to HCT at our center from December 1992 to October 2002, was included in the analysis as a comparator group [5].
Patients who received azacitidine were older than IC-treated patients (median 60 versus 47 years). The majority of patients in both groups had de novo disease. The median time from diagnosis to HCT was similar for the two groups. Patients for whom bone marrow biopsies were available to evaluate cellularity before cytoreductive therapy, all showed hypercellular marrows with a cellularity >60%. There were more patients with advanced MDS/tAML in the IC-treated group. However, there were no significant differences between the groups with regards to marrow myeloblast count at the time of HCT (Table 1).
Table 1.
Patient and Disease Characteristics
| Characteristic | Pre-transplant therapy | |
|---|---|---|
| AZA | IC | |
| No. of patients | 35 | 33 |
| Age (y), range (median) | 4–74 (60) | 2–64 (47) |
| Gender, M/F (No. of patients) | 22/13 | 17/16 |
| Etiology (No. of patients [%]) | ||
| De Novo | 29 (83%) | 28 (85%) |
| Secondary | 6 (17%) | 5 (15%) |
| Disease duration in months (range [median]) | 4–112 (9) | 0–62 (7) |
| WHO Classification (No. of patients [%]) | ||
| RARS | 2 (6%) | 2 (6%) |
| RCMD | 9 (26%) | 3 (9%) |
| RAEB-1 | 3 (9%) | 7 (21%) |
| RAEB-2 | 12 (34%) | 10 (30%) |
| tAML | 2 (6%) | 11 (33%) |
| CMML | 3 (9%) | 0 (0%) |
| JMML | 1 (3%) | 0 (0%) |
| MDS-U | 3 (9%) | 0 (0%) |
| IPSS risk group (No. of patients [%]) | ||
| Low | 6 (17%) | 0 (0%) |
| Intermediate-1 | 11 (31%) | 10 (30%) |
| Intermediate-2 | 9 (26%) | 8 (24%) |
| High | 9 (26%) | 15 (45%) |
| Cytomegalovirus serology (No. of patients [%]) | ||
| Positive | 27 (77%) | 20 (61%) |
| Negative | 8 (23%) | 13 (39%) |
| Myeloblast at transplant (No. of patients [%]) | ||
| <5% | 18 (51%) | 19 (58%) |
| 5–9% | 8 (23%) | 5 (15%) |
| 10–19% | 7 (20%) | 5 (15%) |
| ≥20% | 2 (6%) | 4 (12%) |
| Graft-versus-host-disease prophylaxis (No. of patients [%]) | ||
| MTX, cyclosporine | 1 (3%) | 27 (82%) |
| MTX, tacrolimus | 15 (43%) | 0 (0%) |
| MTX, cyclosporine, tacrolimus | 0 (0%) | 1 (3%) |
| MTX, tacrolimus, rapamycin | 1 (3%) | 0 (0%) |
| Cyclosporine, MMF | 15 (43%) | 4 (12%) |
| Tacrolimus and MMF | 2 (6%) | 0 (0%) |
| Tacrolimus, MMF, Cy | 1 (3%) | 0 (0%) |
| T-cell depletion | 0 (0%) | 1 (3%) |
| Donor (No. of patients [%]) | ||
| HLA-identical sibling | 13 (37%) | 16 (48%) |
| HLA-identical unrelated | 13 (37%) | 17 (52%) |
| Alternative related donor | 1 (3%) | 0 (0%) |
| HLA-mismatched unrelated | 5 (14%) | 0 (0%) |
| Umbilical Cord Blood | 3 (9%) | 0 (0%) |
| Conditioning regimen (No. of patients [%]) | ||
| tBuCy | 12 (34%) | 21 (64%) |
| BuTBI | 0 (0%) | 12 (36%) |
| CyTBI | 1 (3%) | 0 (0%) |
| FluBu | 1 (3%) | 0 (0%) |
| FluTBI(200) | 11 (31%) | 0 (0%) |
| TreoFluTBI(200) | 6 (17%) | 0 (0%) |
| FluTBI(200) + I-131 | 2 (6%) | 0 (0%) |
| FluCyTBI(200) + I-131 | 1 (3%) | 0 (0%) |
| FluCyTBI(200) + ATG | 1 (3%) | 0 (0%) |
| Conditioning intensity (No. of patients [%]) | ||
| High intensity | 14 (40%) | 33 (100%) |
| Reduced intensity | 21 (60%) | 0 (0%) |
ATG = antithymocyte globulin; AZA = Azacitidine; Bu = busulfan; CMML = chronic myelomonocytic leukemia; Cy = cyclophosphamide; I-131 = radiolabeled anti-CD45 antibody; IC= induction chemotherapy; IPSS = International Prognostic Scoring System; JMML = juvenile myelomonocytic leukemia; MDS-U = myelodysplastic syndrome unclassified; MMF = mycophenolate mofetil; MTX = methotrexate; RAEB = refractory anemia with excess blasts; RARS = refractory anemia with ring sideroblasts; RCMD = refractory cytopenias with multilineage dysplasia; tAML = acute myeloid leukemia transformed from myelodysplastic syndrome; TBI = total body irradiation; Treo = treosulfan.
Pre-transplant Therapy
The initiation and duration of treatment with azacitidine or IC was at the discretion of the treating physician. Azacitidine was administered at doses of 75 mg/m2/day on days 1 through 7 of a 28-day cycle [8]. A minimum of one cycle of azacitidine was administered prior to proceeding to HCT. The median cycles of azacitidine administered was three (range 1–11). Among the patients who received IC, most (61%) were given cytarabine for 7 days and an anthracycline for 3 days (7+3). Other patients received topotecan and cytarabine (21%), DCTER (dexamethasone, cytarabine, thioguanine, etoposide, daunorubicin, 12%), FLAG (fludarabine, cytarabine, filgrastim, 3%), and high-dose cytarabine (3%). Patients received IC 1 to 6 (median 2) months before transplantation.
Transplant Conditioning Regimens
All patients in the IC group were conditioned with high-dose regimens, whereas in the azacitidine group, 14 patients (40%) underwent conditioning with high-dose regimens, and 21 (60%) received reduced-intensity conditioning (RIC) as previously defined (p < 0.001) [11]. Overall, 33 patients were conditioned with a previously described regimen comprising targeted busulfan and cyclophosphamide (tBuCy) [12].
Twelve patients, all in the IC group, were conditioned with Bu and total body irradiation (TBI) (BuTBI) [13]. TBI and cyclophosphamide (Cy) was employed for one patient in the azacitidine group [14]. Fludarabine and targeted Bu was used to condition one patient in the azacitidine group (FluBu) [15]. Eleven patients in the azacitidine group were conditioned with Flu and low-dose TBI (FluTBI) [16], while six received treosulfan, Flu, and low-dose TBI (TreoFluTBI) (3 × 14 g/m2 of Treo, 5 × 30 mg/m2 of IV Flu, and 200 cGy TBI).
Two patients were conditioned with radiolabeled anti-CD45 antibody plus Flu and low-dose TBI (FluTBI + I-131) [17]. One patient in the azacitidine group received radiolabeled anti-CD45 antibody with fludarabine, low-dose TBI, and cyclophosphamide before and after stem cell infusion (FluCyTBI + I-131; 0.5 mg/kg of 131I-anti-CD45 antibody, 2 × 14.5 mg/kg of Cy, 5 × 30 mg/m2 of IV Flu, 200 cGy TBI, and 50 mg/kg Cy post-HCT), and one was conditioned with a regimen comprising Flu, Cy, low-dose TBI and antithymocyte globulin (FluCyTBI + ATG; 50 mg/kg Cy, 3 × 30mg/kg of ATG, 5 × 40 mg/m2 Flu, and 200 cGy TBI).
Donors and Source of Hematopoietic Cells
Twenty-nine patients received hematopoietic cells from HLA-matched siblings, and 30 from HLA-matched unrelated donors. Patients in the azacitidine group were more likely to have received stem cells from alternative donors (p = 0.002). Five patients received stem cells from HLA-mismatched unrelated donors, three received umbilical cord blood (two units), and one received stem cells from an HLA haploidentical related donor.
Graft-Versus-Host Disease Prophylaxis and Treatment
Graft-versus-host disease (GVHD) prophylaxis consisted of intravenous methotrexate and cyclosporine in 28 patients [18], methotrexate and tacrolimus in 15 [19], cyclosporine and mycophenolate mofetil in 19 [20], and miscellaneous regimens in 6 patients (Table 1). First-line treatment of acute GVHD consisted of methylprednisolone at doses of 1 or 2 mg/kg/day depending on the severity of presentation. The treatment of steroid-refractory GVHD included a variety of approaches [21].
Supportive Care
Antimicrobial prophylaxis consisted of acyclovir, a fluoroquinolone, and either fluconazole or voriconazole. Granulocyte colony stimulating factor was not routinely used after HCT as part of supportive care. Trimethoprim/sulfamethoxasole or dapsone was given for prophylaxis of Pneumocystis jiroveci. All patients underwent surveillance for cytomegalovirus reactivation and were treated with preemptive ganciclovir, valganciclovir, or foscarnet as appropriate.
Definition of Endpoints
The primary endpoint in this analysis was overall survival (OS). Secondary endpoints were relapse, non-relapse mortality (NRM), relapse-free survival (RFS), and GVHD. Relapse was defined as the presence of myeloid cells with immunophenotypic abnormalities identified by flow cytometry consistent with those prior to HCT, morphologic evidence of dysplasia, or the presence of cytogenetic abnormalities identified prior to HCT. GVHD was diagnosed and graded according to consensus criteria [22]. Relapse was considered the primary cause of failure in patients with evidence of relapse regardless of the proximate cause of death [23].
Statistical Analysis
Data were analyzed as of March 24, 2011. OS and RFS were estimated using the Kaplan-Meier method [24]. Relapse and NRM were summarized using cumulative incidence estimates, with NRM a competing risk for relapse and relapse a competing risk for NRM. Cox regression was used to compare the hazards of failure between the azacitidine and IC groups for the endpoints OS, NRM, RFS, and relapse, and logistic regression was used for acute GVHD.
RESULTS
Overall Survival and NRM
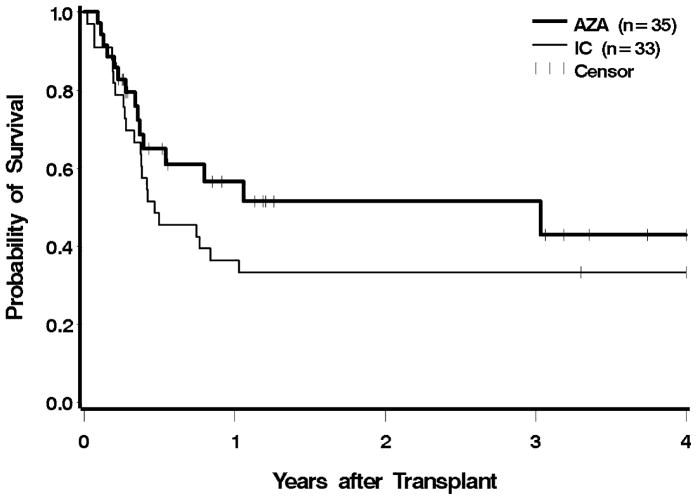
The median post-HCT follow-up among survivors was 10.6 (range 2.6–59.5) months for the azacitidine group, and 95.9 (range 78.6–180.3) months for the IC group. At the time of analysis, 20 and 9 patients were alive in the azacitidine and the IC groups, respectively. There were no engraftment failures in either group. The 100-day mortality for the azacitidine group was 29%, versus 27% in the IC group (Figure 1). The estimated one-year overall survival was 57% in the azacitidine group and 36% in the IC group. The risk of post-HCT mortality was 32% lower in the azacitidine group compared to the IC group (HR 0.68, 95% CI 0.35–1.30); after adjusting for cytogenetic risk, IPSS, and type of donor, the reduction in mortality hazard was lessened to 13%; neither of these reductions was statistically significant. We performed the same analysis excluding patients in whom MDS had progressed to AML, and the findings were similar (unadjusted HR 0.72; adjusted HR 0.9) (Table 2).
Figure 1.
Overall survival following HCT according to pre-transplant therapy: azacitidine (AZA) vs. induction chemotherapy (IC). Patients were censored at the last follow up. The difference between AZA and IC was not significant at p = 0.24.
Table 2.
Regression Analysis of HCT Outcomes
| Univariate analysis | Excluding AML Patients | |||
|---|---|---|---|---|
| Outcome | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Overall survival | 0.68 (0.35–1.30) | 0.24 | 0.73 (0.35–1.51) | 0.39 |
| Relapse | 0.34 (0.12–0.94) | 0.04 | 0.35 (0.12–1.01) | 0.05 |
| Non-relapse mortality | 0.99 (0.41–2.34) | 0.98 | 1.2 (0.43–3.31) | 0.73 |
| Relapse-free survival | 0.62 (0.33–1.16) | 0.14 | 0.66 (0.33–1.32) | 0.23 |
| Adjusted for cyto risk, IPSS, and donor | ||||
| Outcome | HR (95% CI) | p-value | HR (95% CI) | p-value |
|
| ||||
| Overall survival | 0.87 (0.44–1.69) | 0.68 | 0.9 (0.43–1.9) | 0.79 |
| Relapse | 043 (0.15–1.20) | 0.1 | 0.41 (0.14–1.2) | 0.11 |
| Non-relapse mortality | 1.06 (0.45–2.54) | 0.88 | 1.37 (0.49–3.82) | 0.55 |
| Relapse-free survival | 0.72 (0.38–1.38) | 0.32 | 0.78 (0.38–1.57) | 0.48 |
Hazard Ratio (HR) reflects hazard of failure in group that received azacitidine relative to the hazard of failure in the group that received IC.
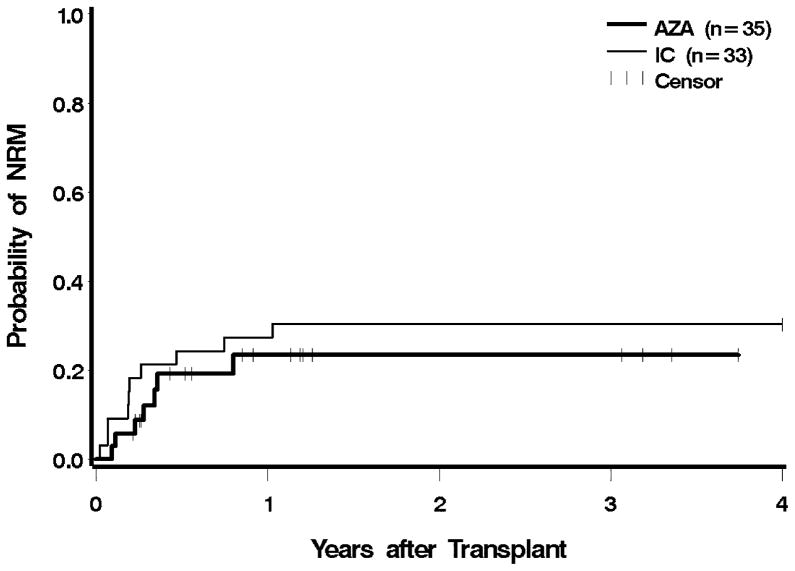
Seven patients in the azacitidine cohort died while in remission. Causes of death included infection and multisystem organ failure (four patients) with or without GVHD (two patients). One patient committed suicide. In the IC cohort, nine patients died without evidence of MDS or AML. Five of these patients died with GVHD associated with infection or organ failure, and four patients died as a result of infections. Eight patients (23%) in the azacitidine group, and 15 (45%) in the IC group died from relapse. Post-HCT NRM was similar for the azacitidine group relative to the IC group both before and after adjustment for cytogenetic risk, IPSS, and type of donor (Figure 2). An analysis excluding patients with transformation to AML showed that the azacitidine cohort had a slightly higher hazard for NRM, which, however, was not statistically significant (Table 2).
Figure 2.
Non-relapse mortality following HCT according to pre-transplant therapy: azacitidine (AZA) vs. induction chemotherapy (IC). Patients were censored at the last follow up. The difference between AZA and IC was not significant at p = 0.98.
Relapse and RFS
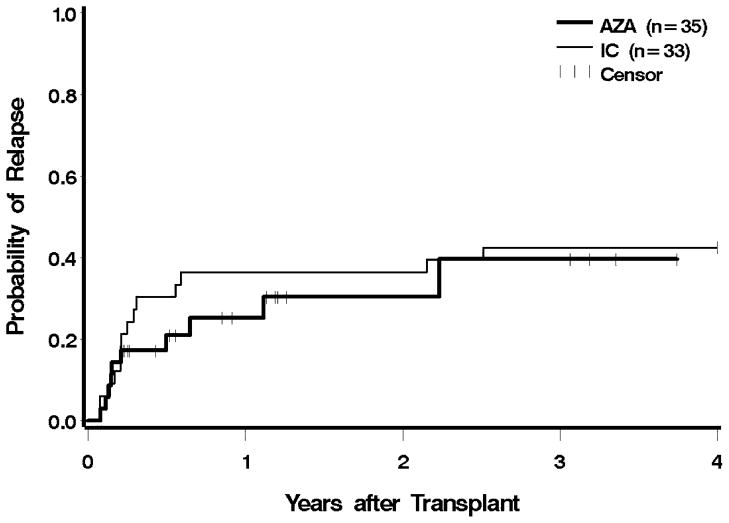
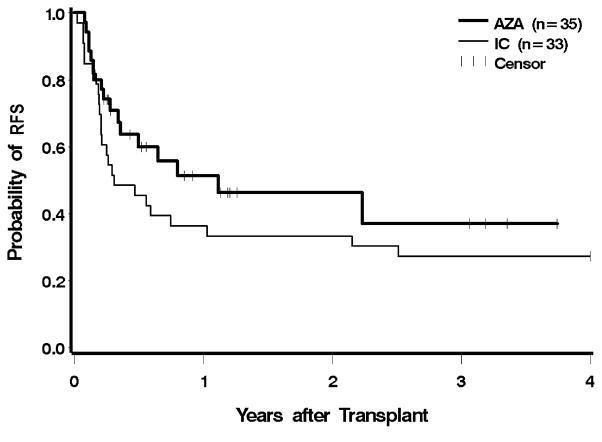
Ten patients (29%) in the azacitidine group, and 13 (39%) in the IC group, had evidence for recurrent disease after transplantation; two patients in the azacitidine group were alive with post-HCT relapse at the time of analysis. One patient in each group relapsed within 30 days of HCT, and two in each group relapsed more than one year after HCT (Figure 3). Pre-HCT exposure to azacitidine led to a 66% lower hazard of relapse compared to IC, which was significantly different (CI 0.12–0.94, p = 0.04). After adjustment for cytogenetic risk, IPSS, and donor type, the hazard of relapse was still 58% lower with azacitidine, but following adjustment, the findings were no longer statistically significant (p = 0.1). The hazard for relapse remained lower following the exclusion of AML patients, but these differences were no longer statistically significant (unadjusted p = 0.05, adjusted for cytogenetic risk, IPSS, and donor type p = 0.11). The risk of relapse or death was reduced by 38% in the azacitidine group before adjustment, and by 27% after adjustment; neither difference was statistically significant (Figure 4 and Table 2).
Figure 3.
Relapse following HCT according to pre-transplant therapy: azacitidine (AZA) vs. induction chemotherapy (IC). Patients were censored at the last follow up. The difference between AZA and IC was significant at p = 0.04.
Figure 4.
Relapse-free survival following HCT according to pre-transplant therapy: azacitidine (AZA) vs. induction chemotherapy (IC). Patients were censored at the last follow up. The difference between AZA and IC was not significant at p = 0.14.
GVHD
Twenty-four of 34 evaluable patients (71%) in the azacitidine group, and 24 of 31 (77%) in the IC group developed grades II–IV acute GVHD. The odds of grades II–IV acute GVHD were reduced by 33% in the azacitidine group before adjustment, and by 40% after adjustment, but neither difference was statistically significant. Only 2 patients (6%) in the azacitidine group developed grades III–IV acute GVHD as compared to 13 (42%) in the IC group. The difference was statistically significant (OR 0.10, 95% CI 0.02–0.48, p = 0.004) after adjustment (Table 3). Chronic GVHD occurred in 11 patients (32%) in the azacitidine group and in 13 patients (42%) who received IC.
Table 3.
Graft-Versus-Host-Disease
| Univariate analysis | ||
|---|---|---|
| Outcome | OR (95% CI) | p-value |
| Grades 2–4 aGVHD | 0.67 (0.21–2.16) | 0.5 |
| Grades 3–4 aGVHD | 0.10 (0.02–0.48) | 0.0044 |
| Adjusted for donor | ||
| Outcome | OR (95% CI) | p-value |
|
| ||
| Grade 2–4 aGVHD | 0.60 (0.17–2.08) | 0.42 |
| Grade 3–4 aGVHD | 0.09 (0.02–0.45) | 0.05 |
aGVHD = acute graft-versus-host-disease
Odds Ratio (OR) reflects hazard of failure in the group that received azacitidine relative to the hazard of failure in the group that received IC.
DISCUSSION
Although advances have been made in recent years in the non-transplant treatment of MDS, allogeneic HCT remains the only modality with known curative potential [25]. MDS is currently the third most common indication for allogeneic HCT as reported to the Center for International Blood and Marrow Transplantation Research [26]. Due to the high frequency of advanced disease state at HCT, post-transplant relapse remains a major cause of failure of HCT for MDS [1–3]. Pre-transplant chemotherapy, similar to that used as IC for AML, has been utilized in this setting [4–6]. More recently, a form of low-intensity therapy with hypomethylating azanucleosides has been shown in randomized phase III trials in the non-transplant setting to decrease the risk of or delay leukemic transformation and prolong survival by approximately 9 months [8–10]. These agents offer attractive therapy options for MDS, as the largest barriers to effective treatment are patient age and comorbidities and the associated decreased ability to tolerate high-dose therapy.
Azanucleosides are now commonly administered as pre-HCT cytoreductive therapy for patients with MDS. However, data are lacking in regards to their effect on HCT outcomes, particularly in comparison to traditional IC. The American Society for Blood and Marrow Transplantation has, therefore, identified pre-HCT cytoreductive therapy as a critical area for further investigation [27].
The current study presents results in two cohorts of patients with high-risk MDS, one cohort treated pre-HSCT with conventional IC, the other with azacitidine. The cohorts were non-concurrent and non-randomized, and therefore results must be interpreted with caution. As advances have been made in the treatment of AML, the overall survival has improved [28], and it is possible that a concurrent comparative group would yield different results. However, few patients concurrently treated with IC were available, underscoring the preference of clinicians for hypomethylation, even though randomized trials are lacking. Additionally, there is an imbalance in diagnosis, with more patients with transformation to AML treated with induction chemotherapy as opposed to MDS. Nevertheless, the results are consistent with reports by others, and show that pre-HCT therapy with azacitidine was well tolerated and resulted in post-HCT outcomes that did not show any evidence of being worse than those observed with IC, even after adjusting for various risk factors. The present results also indicate that despite the significantly older age of patients in the azacitidine cohort, NRM was not higher than observed in younger patients of the IC cohort. Of course, comparability is limited as younger patients typically received IC pre-HCT and were conditioned with high-intensity transplant conditioning regimens. The ideal method to address these issues would be a prospective randomized trial; however, prior attempts to conduct randomized trials evaluating pre-transplant treatment in MDS have failed due to lack of accrual. We calculated that if the true reduction in mortality was 32% with azacitidine as suggested in our data, then 220 patients would need to be randomized between IC and azacitidine in order to have 80% power to detect this degree of difference.
There was a trend towards lower rates of acute GVHD in patients pre-treated with azacitidine, which was significant for grades III-IV disease. This outcome may be related to lessened tissue injury by azacitidine (compared to IC) [14,29,30], or a direct anti-GVHD effect of azacitidine, as also suggested by others [31,32].
Several transplant teams have reported data on the pre-HCT use of hypomethylating agents. Field, et al., analyzed results in a cohort of 54 consecutive patients with MDS who underwent allogeneic HCT, divided into two groups based on treatment with azacitidine prior to HCT [33]. Thirty patients received azacitidine, and 24 did not. Six of the 30 patients in the azacitidine group and 10 of the 24 in the comparator group progressed to AML and were treated with IC before HCT. All patients were conditioned for HCT with a high-dose regimen of Flu and Bu. The cumulative incidence of NRM was similar for the two groups. The 1-year estimates of OS, RFS, and cumulative incidence of relapse were 47%, 41%, and 20% for patients who had received azacitidine, versus 60%, 51%, and 32% for those who had not. While azacitidine treatment did not appear to result in a net gain, the authors suggested that azacitidine could be a useful bridge to HCT.
Recently, Kim, et al., reported the outcomes in 19 patients who were treated with azacitidine or decitabine [34]. Three patients developed progressive disease prior to HCT and were given IC with anthracycline and cytarabine. Eighteen of the patients underwent HCT following RIC. NRM was 11%. The overall survival rates at 1 and 2 years were 95% and 68%, respectively. At the most recent follow-up, 13 patients were alive and 4 had died due to relapse and progression to AML. The reported 1-year OS in this study was higher than in the azacitidine-treated patients in the present study. However, the studies are not strictly comparable as patient and disease-risk parameters differed considerably.
Similar results have been reported in smaller patient cohorts by other investigators [35–37]. While azacitidine and decitabine differ in their mechanism of action, results with both agents appeared to be comparable. The results of these previously reported studies are comparable to the outcomes of the azacitidine cohort in the present study. The small number of patients included in the single-arm studies limited the power of analysis. Also, 27% of the patients in one study (Lübbert and colleagues) [37], as well as 25% (Cogle, et al.) [35] and 30% (Field, et al.) [33] in others, received second-line cytoreduction with either low-dose melphalan or IC (similarly treated patients were excluded from the present study), confounding the effect of hypomethylating agents on post-HCT outcomes.
McCarty, et al., investigated the role of azacitidine versus IC in the pre-HCT setting in 25 patients with high-risk MDS or tAML [38]. Patients received four cycles of azacitidine, then, based on myeloblast count, they either received more azacitidine or, if there was no evidence of response, crossed over to IC with FLAG. Twenty-one of the patients went on to receive HCT following a high-dose conditioning regimen. With one year follow-up, both NRM and relapse rates were higher among patients who received IC, not a surprising outcome, as the study was designed such that only patients who did not respond to azacitidine received IC before transplant.
The retrospective nature of the current study and the sequential treatment intervals of the two cohorts limit the conclusions that can be drawn as recent reports note improved HCT outcomes and AML treatment results have improved over time [28,39]. However, we chose a comparator group that predated the widespread use of azacitidine as cytoreductive or bridging therapy before HCT, thereby reducing the potentially confounding effect of a bias in treatment selection. Treatment selection bias may exert an influence on post-HCT outcomes as more practitioners and patients may opt for therapy with low toxicity as a pre-HCT regimen. It is not unlikely that patients, who previously would have received IC, now are treated with a hypomethylating agent instead.
An important concept materializing concurrently with the advent of hypomethylating agents is the development of RIC regimens and the relationship between the selection of pre-HCT therapy and the subsequent HCT conditioning regimen. It is reasonable to view the selection of a pre-HCT cytoreductive or bridging regimen and the proposed conditioning approach as a “package.” In addition to relapse, the major cause of failure in patients transplanted for MDS is NRM. The most powerful predictors of NRM are patient comorbidities and conditioning-regimen intensity. Patient and disease characteristics are a given; however, the regimen can be selected, and that selection should probably take into consideration the pre-HCT therapy the patient received [40,41].
In addition to cytoreduction as achieved with conventional IC, hypomethylating agents may offer benefits in regards to relapse reduction via epigenetic modulation. Several studies have described the methylation of promoter regions involved with the tumor suppressor genes CDH1 (E-cadherin) and CTNNA1 (catenin), as well as genes regulating the cell cycle such as CDKN2A (p16INK4a) and CDKN2B (p15INK4b) in MDS patients [42,43]. Furthermore, enhanced expression of cancer-testis antigens following hypomethylation may allow for a greater graft-versus-leukemia effect mediated by donor cells after HCT [44].
In summary, the results of our analysis support the concept that hypomethylating drugs are suitable for pre-HCT therapy and may offer advantages over conventional IC. However, many questions remain in regards to disease stage most amenable to this therapy, timing, number of cycles, and possibly the optimum transplant conditioning regimens to be used in combination with azacitidine pre-HCT.
Acknowledgments
This work was supported in part by National Institutes of Health grants K23HL084054 (B.S.), P01HL036444 (R. Storb), P01CA018029 (R. Storb), P30CA015704, and K99/R00HL088021 (M. Sorror). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
We thank the transplant teams at the Fred Hutchinson Cancer Research Center and the Seattle VA Puget Sound Health Care System for their contributions, all patients for participation in clinical trials, Wendy Wilson and Carlos Breton for maintaining the database, and Bonnie Larson and Helen Crawford for their help with manuscript preparation
Footnotes
Authors’ Disclosures:
AT Gerds: No disclosures to make.
TA Gooley: No disclosures to make.
HJ Deeg: No disclosures to make.
EH Estey: No disclosures to make
FR Appelbaum: No disclosures to make
BL Scott: Served as consultant to Celgene.
Authors Contributions:
AT Gerds: Conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing.
TA Gooley: Conception and design, data analysis and interpretation, and critiqued manuscript.
HJ Deeg: Conception and design, provided data, critiqued and approved the manuscript.
EH Estey: Provided data and critiqued manuscript.
FR Appelbaum: Provided data and critiqued manuscript.
BL Scott: Concept and design, provided data, critiqued the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Witte T, Hermans J, Vossen J, et al. Haematopoietic stem cell transplantation for patients with myelo-dysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2000;110:620–630. doi: 10.1046/j.1365-2141.2000.02200.x. [DOI] [PubMed] [Google Scholar]
- 2.Sierra J, Pérez WS, Rozman C, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100:1997–2004. [PubMed] [Google Scholar]
- 3.Deeg HJ, Storer B, Slattery JT, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201–1207. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 4.Yakoub-Agha I, de La Salmonière P, Ribaud P, et al. Allogeneic bone marrow transplantation for therapy-related myelodsyplastic syndrome and acute myeloid leukemia: a long-term study of 70 patients-report of the French Society of bone marrow transplantation. J Clin Oncol. 2000;18:963–971. doi: 10.1200/JCO.2000.18.5.963. [DOI] [PubMed] [Google Scholar]
- 5.Scott BL, Storer B, Loken M, Storb R, Appelbaum FR, Deeg HJ. Pretransplantation induction chemotherapy and posttransplantation relapse in patients with advanced myelodysplastic syndrome. Biol Blood Marrow Transplant. 2005;11:65–73. doi: 10.1016/j.bbmt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Nakai K, Kanda Y, Fukuhara S, et al. Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia. 2005;19:396–401. doi: 10.1038/sj.leu.2403640. [DOI] [PubMed] [Google Scholar]
- 7.Estey E, de Lima M, Tibes R, et al. Prospective feasibility analysis of reduced-intensity conditioning (RIC) regimens for hematopoietic stem cell transplantation (HSCT) in elderly patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) Blood. 2007;109:1395–1400. doi: 10.1182/blood-2006-05-021907. [DOI] [PubMed] [Google Scholar]
- 8.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 9.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 11.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCune JS, Batchelder A, Deeg HJ, et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant. 2007;13:853–862. doi: 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Jurado M, Deeg HJ, Storer B, et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome after conditioning with busulfan and fractionated total body irradiation is associated with low relapse rate but considerable nonrelapse mortality. Biol Blood Marrow Transplant. 2002;8:161–169. doi: 10.1053/bbmt.2002.v8.pm11939606. [DOI] [PubMed] [Google Scholar]
- 14.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: A randomized trial of two irradiation regimens. Blood. 1990;76:1867–1871. [PubMed] [Google Scholar]
- 15.Bornhäuser M, Storer B, Slattery JT, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102:820–826. doi: 10.1182/blood-2002-11-3567. [DOI] [PubMed] [Google Scholar]
- 16.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 17.Pagel JM, Gooley TA, Rajendran J, et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114:5444–5453. doi: 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storb R, Deeg HJ, Pepe M, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: Long-term follow-up of a controlled trial. Blood. 1989;73:1729–1734. [PubMed] [Google Scholar]
- 19.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 20.Bolwell B, Sobecks R, Pohlman B, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34:621–625. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 21.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109:4119–4126. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13:1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Greenberg PL, Attar E, Bennett JM, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™): Myelodysplastic Syndromes Version 1.2011. Journal of the National Comprehensive Cancer Network. 2011;9:30–56. doi: 10.6004/jnccn.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2010. 2010 Available at: http://wwwcibmtrorg.
- 27.Oliansky DM, Antin JH, Bennett JM, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of myelodysplastic syndromes: an evidence-based review. Biol Blood Marrow Transplant. 2009;15:137–172. doi: 10.1016/j.bbmt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–494. doi: 10.1200/JCO.2010.30.1820. (Review) [Erratum appears in J Clin Oncol. 2011 Jun 1;29(16):2293] [DOI] [PubMed] [Google Scholar]
- 29.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 30.Deeg HJ, Spitzer TR, Cottler-Fox M, Cahill R, Pickle LW. Conditioning-related toxicity and acute graft-versus-host disease in patients given methotrexate/cyclosporine prophylaxis. Bone Marrow Transplant. 1991;7:193–198. [PubMed] [Google Scholar]
- 31.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116:129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Field T, Perkins J, Huang Y, et al. 5-azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010;45:255–260. doi: 10.1038/bmt.2009.134. [DOI] [PubMed] [Google Scholar]
- 34.Kim D-Y, Lee J-H, Park Y-H, et al. Feasibility of hypomethalating agents followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome. Bone Marrow Transplant. doi: 10.1038/bmt.2011.86. prepublished online April 11, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Cogle CR, Imanirad I, Wiggins LE, et al. Hypomethylating agent induction therapy followed by hematopoietic cell transplantation is feasible in patients with myelodysplastic syndromes. Clinical Advances in Hematology & Oncology. 2010;8:40–46. [PubMed] [Google Scholar]
- 36.De Padua SL, de Lima M, Kantarjian H, et al. Feasibility of allo-SCT after hypomethylating therapy with decitabine for myelodysplastic syndrome. Bone Marrow Transplant. 2009;43:839–843. doi: 10.1038/bmt.2008.400. [DOI] [PubMed] [Google Scholar]
- 37.Lübbert M, Bertz H, Rüter B, et al. Non-intensive treatment with low-dose 5-aza-2′-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone Marrow Transplant. 2009;44:585–588. doi: 10.1038/bmt.2009.64. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy JM, Shickle L, Roberts CH, Candler KS, Chung HM. 5Azacytidine prior to allogeneic transplantation effectively reduces relapse, TRM and overall mortality in high-risk myelodysplastic and secondary AML. Bone Marrow Transplant. 2008;41:S212–S213. #P746 [abstr.] [Google Scholar]
- 39.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartenstein M, Deeg HJ. Hematopoietic stem cell transplantation for MDS. Hematol Oncol Clin North Am. 2010;24:407–422. doi: 10.1016/j.hoc.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martino R, Caballero MD, Simón JA, et al. Evidence for a graft-versus-leukemia effect after allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning in acute myelogenous leukemia and myelodysplastic syndromes. Blood. 2002;100:2243–2245. doi: 10.1182/blood-2002-02-0400. [DOI] [PubMed] [Google Scholar]
- 42.Aggerholm A, Holm MS, Guldberg P, Olesen LH, Hokland P. Promoter hypermethylation of p15INK4B, HIC1, CDH1, and ER is frequent in myelodysplastic syndrome and predicts poor prognosis in early-stage patients. Eur J Haematol. 2006;76:23–32. doi: 10.1111/j.1600-0609.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- 43.Ye Y, McDevitt MA, Guo M, et al. Progressive chromatin repression and promoter methylation of CTNNA1 associated with advanced myeloid malignancies. Cancer Res. 2009;69:8482–8490. doi: 10.1158/0008-5472.CAN-09-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodyear O, Agathanggelou A, Novitzky-Basso I, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116:1908–1918. doi: 10.1182/blood-2009-11-249474. [DOI] [PubMed] [Google Scholar]