Abstract
Several studies have suggested that interleukin (IL)-15 is a promising adjuvant that promotes cellular immunity when administered with human immunodeficiency virus (HIV) vaccine. Here we evaluated the effect of IL-15 plasmid on HIV-specific immune responses, especially cellular immunity, in eight rhesus monkeys. These monkeys were immunized three times with HIV DNA vaccine with or without IL-15 plasmid and boosted with recombinant Tiantan strain vaccinia virus-based HIV vaccine (rTV) 22 weeks after the first immunization. Although we did not detect any significant differences in the HIV-specific CD8+ T-cell response between monkeys with IL-15 coimmunization and monkeys with HIV vaccine alone, our results showed that the frequency of effector CD8+ memory T cells in the peripheral blood was significantly higher in monkeys with IL-15 coimmunization than those with HIV vaccine alone at almost all of the time points examined. Furthermore, the titers of anti-HIV antibodies were higher in Group T than those in Group C after rTV boosting. These findings in rhesus monkeys suggest that IL-15 may be useful as a cytokine adjuvant for HIV vaccine.
Keywords: adjuvant, effector memory CD8+ T cell, HIV vaccine, IL-15
Introduction
Since human immunodeficiency virus (HIV) was identified as the pathogen of acquired immune deficiency syndrome (AIDS) in the 1980s, extensive efforts have been made to study this virus. Because the cellular immune response is indispensable in antiviral immunity,1, 2, 3 current HIV vaccines are aimed to promote cellular immunity to eradicate HIV. Cytokine adjuvants including interleukin-2 (IL-2), IL-12, IL-15 and IL-21 have been used in combination with these vaccines to enhance anti-HIV cellular immunity.4, 5, 6, 7 IL-15 belongs to the four-α-helix bundle cytokine family and is secreted by antigen presenting cells such as dendritic cells and monocytes during the early stages of the immune response. Its receptor shares the common γ-chain with those of IL-2, -3, -7, -9 and -21. Therefore, IL-15 exhibits biological effects similar to those of IL-2, promoting T-cell and natural killer cell proliferation.8, 9
A recent study showed that the function and survival of CD8+ T cells were promoted in vivo by coimmunization with optimized IL-15 plasmid and HIV vaccine after CD4+ T cells were depleted.10 Furthermore, studies in mice have shown that HIV-specific cellular and humoral immunity and the frequency of central memory CD8+ T cells in the spleen were enhanced by coimmunization with IL-15 plasmid.11 Here we report a study in rhesus monkeys to assess the effects of IL-15 plasmid on CD8+ T cells.
Materials and methods
Vaccines
A multiple epitope HIV vaccine was constructed by the National Center for AIDS/STD Control and Prevention, China CDC (Beijing, China). This HIV vaccine was constructed in the form of a DNA plasmid containing the gp140, gag, pol, tat, nef and rev genes or a recombinant Tiantan strain vaccinia virus (rTV) expressing the env, gag and pol genes of HIV strain B/C. IL-15 cDNA plasmid was constructed in our laboratory as previously described.11
Animal immunization
Eight 3- to 4-year-old rhesus monkeys (four males and four females) weighing 4–5 kg were bred and fed at the Institute of Medical Biological Science, Chinese Academy of Medical Sciences (Beijing, China). The rhesus monkey immunization and assay schedule is shown in Figure 1. Briefly, animals were immunized intramuscularly using 4-mg HIV DNA plasmid (Group C) or 4-mg HIV DNA plasmid and 1-mg IL-15 cDNA plasmid (Group T) on weeks 0, 4 and 8 and boosted with 1×107 plaque-forming unit rTV on week 22. On weeks 6, 10, 18, 23, 26 and 30, blood from each animal was sampled and examined. All animal studies were carried out in accordance with the standards set forth in the Guide for the Care and Use of Laboratory Animals (published by the National Academy of Science, National Academy Press, Washington, DC, USA).
Figure 1.
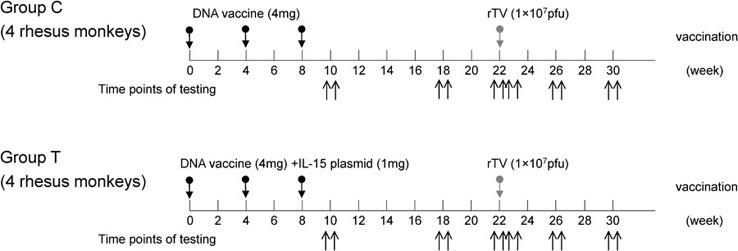
Rhesus monkey immunization protocol. Monkeys were injected intramuscularly with 2 mg/ml HIV DNA and IL-15 plasmid in a volume of 0.5 ml/plasmid. rTV was administered subcutaneously (50 µl of 108 pfu/ml rTV). The animals were divided into two groups: Group C was primed with HIV DNA and boosted with rTV, and Group T was primed with HIV DNA and IL-15 plasmid and boosted with rTV. Peripheral blood was collected 10, 18, 22, 23, 26 and 30 weeks after the first immunization. pfu, plaque-forming unit; rTV, recombinant Tiantan strain vaccinia virus.
Enzyme-linked immunospot assay
The monkey IFN-γ ELISPOT kit(U-Cytech, Utrecht, The Netherlands) was used according to the manufacturer's instructions. Plates were coated overnight with 50 µl of anti-interferon (IFN)-γ coating antibodies per well at 4 °C. Blocking buffer (200 µl/well, 1×) was then added, and plates were incubated for 1 h at 37 °C. Rhesus monkey peripheral blood mononuclear cells were plated at a density of 2×105 cells/well and stimulated for 24 h at 37 °C in 5% CO2 with 5 µg/ml of HIV peptide pools (grant from NIH) or phorbyl myristate acetate (positive control). After a 1-h incubation at 37 °C with 100 µl of biotinylated anti-IFN-γ detection antibodies, 50 µl of properly diluted γ-aminobutyric acid solution was added to each well, and plates were incubated for 1 h at 37 °C. After this incubation, 30 µl of freshly prepared activator I/II solution was added to each well, and plates were incubated at room temperature in the dark. When clear spots developed, reactions were stopped by rinsing the wells with deionized water. The spots were counted using an immunospot image analyzer.
Antibody detection
Plates were coated with 0.2 µg/well HIV-1 env antigen for 2 h at 37 °C. After blocking with phosphate-buffered saline containing 10% bovine serum albumin for 1 h at 37 °C, 100 µl of serially diluted sera was added to each well and incubated for 1 h at 37 °C. The plates were then incubated at 37 °C for 1 h with a 1∶5000 dilution of goat anti-mouse IgG antibody conjugated to horseradish peroxidase. After a 10- to 15-min incubation with substrate solution (100 µl/well), the reaction was stopped by adding 2 M H2SO4, and optical density was measured at 450 nm.
Flow cytometry
Peripheral blood mononuclear cells were incubated for 30 min at 4 °C with anti-human CD8-PE (RPA-T8), CD45RA-Pecy5 (5H9) or purified anti-human CCR7 (2H4) antibody (BD Pharmingen, San Diego, CA, USA). Cells were washed, anti-mouse IgM was added and samples were incubated at 4 °C for 30 min. After washing, samples were resuspended in phosphate-buffered saline and immediately analyzed using a FACSort flow cytometer (Becton Dickinson, San Jose, CA, USA).
Statistical analysis
Statistical analyses were performed with SPSS 11.5 software (SPSS Inc., Chicago, IL, USA). Immune responses in the two groups of rhesus monkeys were compared using two-tailed t-tests. In all cases, P values <0.05 were considered significant.
Results
IL-15 increased the frequency of effector memory CD8+ T cells in peripheral blood in rhesus monkeys
Although enzyme-linked immunospot assays showed no significant differences in IFN-γ production between peripheral blood mononuclear cells from rhesus monkeys that were immunized with both HIV DNA plasmid and IL-15 cDNA plasmid (Group T) and those from monkeys that were immunized only with HIV DNA plasmid (Group C) (data not shown), changes in the frequency of CD8+ T cells were observed in the group that received IL-15 plasmid coimmunization (Group T). As shown in Figure 2, the frequency of effector memory CD8+ T cells (TEM) in the peripheral blood in rhesus monkeys was higher in Group T than in Group C at most time points. In addition, the difference between Group T and Group C was statistically significant 8 weeks after rTV boosting (P<0.05). Furthermore, the frequency of central memory CD8+ T cells (TCM) in the peripheral blood was higher in Group T than in Group C 10 weeks after HIV DNA vaccine immunization.
Figure 2.
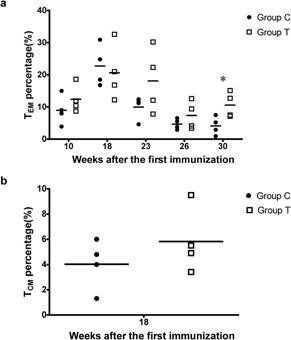
Increased frequency of CD8+ TEM among CD8+ T cells in rhesus monkey peripheral blood. (a) The frequency of CD8+ TEM among CD8+ T cells in rhesus monkey peripheral blood. (b) The frequency of CD8+ TCM among CD8+ T cells in rhesus monkey peripheral blood. The CD8+/CD45RA−/CCR7− cells were defined as effector CD8+ memory T cells (TEM), and CD8+/CD45RA−/CCR7+ cells were defined as central CD8+ memory T cells (TCM)12 (*P<0.05).
IL-15 enhanced the humoral immune response in rhesus monkeys
As shown in Table 1, anti-env antibody titers 1, 4 and 8 weeks after rTV boosting were higher in Group T than in Group C, and this increase in anti-env antibody titers was statistically significant 1 week after rTV boosting (P<0.05), indicating that plasmid IL-15 could enhance the long-term humoral immune response in rhesus monkeys.
Table 1. Anti-env antibody titer after rTV boosting.
| Group C | Group T | |||||||
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | T1 | T2 | T3 | T4 | |
| 1 week after rTV boosting | 1∶16 | 1∶32 | 1∶32 | 1∶32 | 1∶4 | 1∶512 | 1∶512 | 1∶16 |
| 4 weeks after rTV boosting | 1∶16 | 1∶4 | 1∶8 | 1∶32 | 0 | 1∶64 | 1∶64 | 1∶32 |
| 8 weeks after rTV boosting | 1∶4 | 1∶2 | 0 | 1∶4 | 0 | 1∶8 | 1∶8 | 1∶4 |
Abbreviation: rTV, recombinant Tiantan strain vaccinia virus.
Discussion
HIV vaccines have remained a major topic of interest in the fields of immunology, virology and clinical medicine since the identification of HIV in the last century; however, little clinically significant progress was made in HIV research until one clinical trial carried out by the United States and Thailand indicated that the ALVAC-HIV and AIDSVAX B/E vaccine regimens may marginally reduce the risk of HIV infection.13
IL-15 plays an important role in the generation and maintenance of memory CD8+ T cells,8, 14, 15, 16, 17 and it is thus a good candidate for enhancing the cellular immune response. At the time points examined in this study, the frequency of effector memory CD8+ T cells was higher in monkeys immunized with HIV DNA plasmid and IL-15 cDNA plasmid than in monkeys immunized only with HIV DNA plasmid, indicating that IL-15 promotes the conversion of effector T cells into memory T cells. When re-exposed to the same antigen, specific effector memory CD8+ T cells can respond to the antigen and convert to CTL rapidly; thus the ability of IL-15 to enhance the conversion of effector T cells into memory T cells is helpful for the long-term immune function of the vaccine. Furthermore, the frequency of central memory CD8+ T cells increased 10 weeks after DNA immunization, suggesting that the effector memory CD8+ T cells induced by IL-15 were converted to central memory CD8+ T cells.
It has been recently reported that high doses of IL-15 decrease the production of IFN-γ and the proliferation of CD4+ and CD8+ T cells and the frequency of TCM among proliferating CD4+ and CD8+ T cells in a non-human primate influenza model.18 IL-15 did not significantly enhance the HIV-specific CD8+ cellular immune response in our study, suggesting that the effect of IL-15 on cellular immunity may be related to the dose of IL-15 plasmid. Studies with larger sample sizes and dose ranges will be necessary to reach a definite conclusion.
Our study suggests that IL-15 increases the frequency of effector memory CD8+ T cells in rhesus macaque peripheral blood and enhances the long-term immune response to vaccine to some extent. Meanwhile, a recent report demonstrated in non-human primates that IL-15 administration expands memory CD8+ and CD4+ T cells and natural killer cells in the peripheral blood with minimal increases in CD4+CD25+Foxp3+ regulatory T cells, and intermittent administration of IL-15 for more than 3 weeks has been shown to be safe.19 Together, these findings suggest that with further optimization of the dose and dosing schedule, IL-15 may be an effective adjuvant for HIV vaccine.
Acknowledgments
This work is sponsored by a grant (2006CB504205) from the National Program for Key Basic Research Project, Ministry of Science and Technology, China, and a grant from NIH CIPRA (1U19AI51915-02) to Dr Wei He. We are grateful to Dr Yiming Shao (Division of Virology and Immunology, National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention) for the gift of pDRVISV1.0 plasmid.
References
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JD, Ogg GS, Allen RL, Davis C, Shaunak S, Downie J, et al. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS. 2000;14:225–233. doi: 10.1097/00002030-200002180-00003. [DOI] [PubMed] [Google Scholar]
- Safrit JT, Andrews CA, Zhu T, Ho DD, Koup RA. Characterization of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope. J Exp Med. 1994;179:463–472. doi: 10.1084/jem.179.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Santra S, Steenbeke TD, Zheng XX, Perry HC, Davies ME, et al. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J Immunol. 1998;161:1875–1882. [PubMed] [Google Scholar]
- Bolesta E, Kowalczyk A, Wierzbicki A, Eppolito C, Kaneko Y, Takiguchi M, et al. Increased level and longevity of protective immune responses induced by DNA vaccine expressing the HIV-1 Env glycoprotein when combined with IL-21 and IL-15 gene delivery. J Immunol. 2006;177:177–191. doi: 10.4049/jimmunol.177.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halwani R, Boyer JD, Yassine-Diab B, Haddad EK, Robinson TM, Kumar S, et al. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA+IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J Immunol. 2008;180:7969–7979. doi: 10.4049/jimmunol.180.12.7969. [DOI] [PubMed] [Google Scholar]
- Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- Carson W, Caligiuri MA. Interleukin-15 as a potential regulator of the innate immune response. Braz J Med Biol Res. 1998;31:1–9. doi: 10.1590/s0100-879x1998000100001. [DOI] [PubMed] [Google Scholar]
- Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, Choe PY, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175:112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- Li W, Li S, Hu Y, Tang B, Cui L, He W. Efficient augmentation of a long-lasting immune responses in HIV-1 gag DNA vaccination by IL-15 plasmid boosting. Vaccine. 2008;26:3282–3290. doi: 10.1016/j.vaccine.2008.03.081. [DOI] [PubMed] [Google Scholar]
- Koesters SA, Alimonti JB, Wachihi C, Matu L, Anzala O, Kimani J, et al. IL-7Ra expression on CD4+ T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur J Immunol. 2006;36:336–344. doi: 10.1002/eji.200535111. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. New Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Kim HR, Hwang KA, Park SH, Kang I. IL-7 and IL-15: biology and roles in T-cell immunity in health and disease. Crit Rev Immunol. 2008;28:325–339. doi: 10.1615/critrevimmunol.v28.i4.40. [DOI] [PubMed] [Google Scholar]
- Yin J, Dai A, Laddy DJ, Yan J, Arango T, Khan AS, et al. High dose of plasmid IL-15 inhibits immune responses in an influenza non-human primates immunogenicity model. Virology. 2009;393:49–55. doi: 10.1016/j.virol.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]


