Abstract
Voltage-sensing domains (VSDs) of Kv channels control ionic conductance through coupling of the movement of charged residues in the S4 segment to conformational changes at the cytoplasmic region of the pore domain, that allow K+ ions to flow. Conformational transitions within the VSD are induced by changes in the applied voltage across the membrane field. However, several other factors not directly linked to the voltage-dependent movement of charged residues within the voltage sensor impact the dynamics of the voltage sensor, such as inactivation, ionic conductance, intracellular ion identity, and block of the channel by intracellular ligands. The effect of intracellular ions on voltage sensor dynamics is of importance in the interpretation of gating current measurements and the physiology of pore/voltage sensor coupling. There is a significant amount of variability in the reported kinetics of voltage sensor deactivation kinetics of Kv channels attributed to different mechanisms such as open state stabilization, immobilization, and relaxation processes of the voltage sensor. Here we separate these factors and focus on the causal role that intracellular ions can play in allosterically modulating the dynamics of Kv voltage sensor deactivation kinetics. These considerations are of critical importance in understanding the molecular determinants of the complete channel gating cycle from activation to deactivation.
Keywords: ion channel, potassium channel, gating current, immobilization, inactivation
Introduction
Voltage-gated potassium channels (Kv) sense membrane voltage and underlie the repolarization of electrically excitable cells (Hille, 2001). This task is achieved by the division of the function of the channels into voltage-sensing domain (VSD) and ion conducting pore domain as shown in Figure 1A (Long et al., 2005b). Charged residues in the S4 transmembrane segment of the VSD move in response to changes in the membrane electrical field and this motion is translated through a coupling mechanism involving S4–S5 linker contacts with the bottom of S6-lined pore domain to open or close the intracellular gate of ionic conductance (Lee et al., 2009). The tight coupling between the two domains allows movements in one domain to be translated rapidly into movements in the other thus creating highly sensitive voltage-gated channels.
Figure 1.
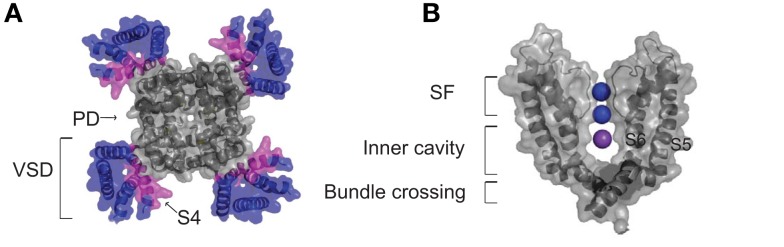
Overview of Kv channel structure. (A) A top down view of the Kv1.2 open state channel tetramer (PDB: 2A79) with the voltage-sensing domains (S1–S4) in color. The charge carrying S4 segment of the voltage sensor domain is highlighted magenta and the pore domain S5–S6 segments are gray. (B) Side view of two pore forming subunits of the Kv1.2 illustrating the selectivity filter containing two K+ ions of a possible four in positions 2 and 4 shown in blue and a K+ ion residing in the intracellular cavity in magenta.
Once K+ conductance has been activated the process must be reversed once the membrane is restored back to resting potentials so that excitable cell membranes do not become permanently hyperpolarized and unable to generate action potentials again. This is accomplished by deactivation and inactivation mechanisms, both of which serve to shut down channel conductance. Deactivation specifically refers to the voltage-dependent closure of the pore intracellular gate, whereas inactivation can occur under the conditions of continued depolarization and typically involves conformational changes in other regions of the channel. Inactivation can be broadly classed kinetically into fast inactivation, conferred by inactivation peptides either attached to the N-terminus of the channel protein or an accessory subunit that can enter the open channel at the intracellular gate and block conductance or slow inactivation which is caused by conformational rearrangements in the outer pore and selectivity filter region of the channel. Both inactivation and pore gating have effects on voltage sensor dynamics. Because there are several mechanisms that can impact on voltage sensor deactivation kinetics, the objective of this review is to present a clear description of these different factors which can overlap in their functional effects, and expand on the mechanism by which intracellular ions can influence these kinetics and thus the physiology of voltage sensing in Kv channels.
Basis for Voltage Sensing in Kv Channels
Kv channels are tetrameric transmembrane proteins with each subunit consisting of six transmembrane segments (S1–S6). The VSD of each subunit comprises of S1–S4, with the key determinant of voltage sensing being a series of positively charged arginine residues that line S4 (Figure 1A). The VSD is coupled to the pore domain, shown in Figure 1B, which comprises S5–S6 of each subunit, and comes together to form a S6-lined conduit for K+ ions to flow across the membrane (Long et al., 2005a). At the extracellular end of the pore, selectivity for K+ ions is conferred by a highly conserved structural element in the pore, the selectivity filter, that allows efficient dehydration and stabilization of dehydrated K+ ions over Na+ ions as the ions transport across the membrane (Heginbotham et al., 1994). On the cytosolic side of the selectivity filter a water filled central cavity is found where hydrated ions can reside before entering the filter (Zhou et al., 2001). Figure 1B highlights the locations of two ions (purple) occupying two of four possible binding sites in the SF and an ion residing in the inner cavity (blue) of the Kv1.2 channel crystal structure, which is in the open state (Long et al., 2005b). In the closed state of the channel this cavity is isolated from the bulk cytosol by a constriction formed by a narrow bundle crossing of the pore-lining S6 helices (Figure 1B), which does not allow hydrated ions to permeate (Liu et al., 1997). Opening of this gate is initiated by depolarization of the membrane. This drives the positively charged S4 segment outward and induces a conformational shift at the bottom of the S6 segments to dilate the bundle crossing. Hydrated ions can then diffuse freely into the cavity and through the selectivity filter (Lu et al., 2002; Long et al., 2005b).
Much progress in understanding voltage gating has relied upon the fact that voltage sensor movement can be examined separately from pore opening and closing by recording the transient currents (gating currents), that are associated with the charged residues in the S4 segment moving within the applied field (for review, see Bezanilla, 2000). Kv channel gating currents are of the order of 1% or less of the magnitude of ionic currents, and therefore can only be resolved when recorded from channels in the absence of larger contaminating ionic currents (Armstrong and Bezanilla, 1974). Experimentally, ionic conductance can be eliminated by the removal of permeant ions from the recording solutions (Zagotta et al., 1994b), block of conductance (Bezanilla et al., 1991; Schoppa et al., 1992), or mutation of channels to render them non-conductive (Perozo et al., 1993; Wang et al., 1999).
Mechanisms of Voltage Sensor Immobilization
Early studies of gating currents revealed that intracellular applied quaternary ammonium (QA) ions severely slow the recovery of ionic and gating currents by preventing pore gate closure (Armstrong, 1971; Choi et al., 1993; Melishchuk and Armstrong, 2001). This phenomenon was also observed to result from the fast inactivation process in Na+ channels and was referred to as “charge immobilization” (Armstrong and Bezanilla, 1977). Because all the charge that has been moved by depolarization eventually returns if given an adequate recovery period the term immobilization really refers to a slowing of gating charge return.
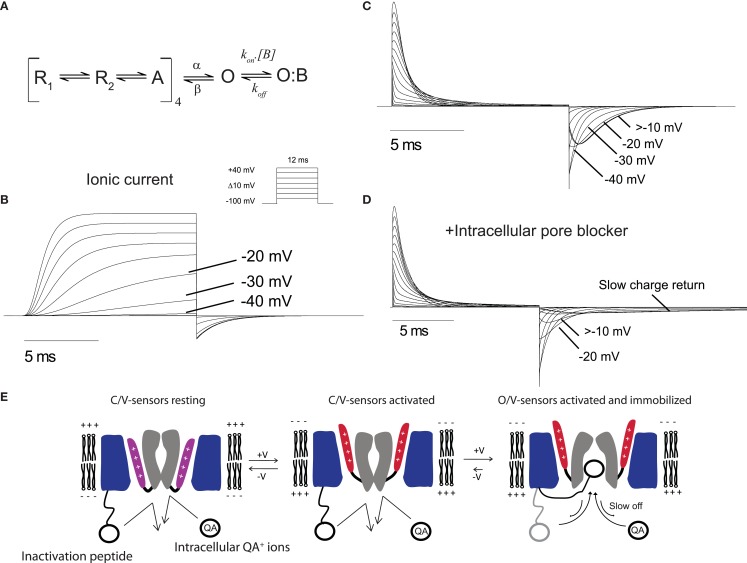
For the purposes of the review we have used an established model of ShakerIR gating to simulate typical ionic and gating currents from a channel to illustrate typical immobilized gating charge. Figure 2 illustrates currents simulated using an established model of shaker gating (Zagotta et al., 1994a). Figure 2A shows a simplified shaker gating scheme in which voltage sensors in the each four subunits undergo transitions between resting and activated states upon depolarization, followed by a sequential opening step that represents the pore opening. The transitions between resting and active carry the majority of gating charge. In Figure 2B the simulated ionic currents show a rapid increase in the open probability of the channel at depolarizing potentials more positive than −40 mV. The gating currents correlating to the same gating model are displayed in Figure 2C and show a mild immobilization of gating charge return, which is a typical feature of Shaker gating currents. The immobilization is seen in the return of gating charge (OFF gating currents) that display a rapid return after depolarizations to potentials where the channel has not opened (<−40 mV) followed by a slowing of the gating charge return after openings to potentials with significant channel probability of opening (>−20 mV). This slowing of OFF gating currents observed experimentally can be clearly linked to the channel opening process as it occurs only after the channel has opened.
Figure 2.
Gating currents demonstrating immobilization. (A) A model of Shaker gating used to simulate currents (Zagotta et al., 1994a). The transitions from R to A carry the majority of gating charge and the channel opens once all four subunits have transitioned to A. The extra state O:B represents the an intracellular blocking particle as discussed in the text. Ionic (B) and gating (C) currents generated from the model in response to 12 ms depolarization from a holding potential of −100 mV with the program Ionchannellab (De Santiago-Castillo et al., 2010). (D) Gating currents display severe immobilization when the model is run in the presence of an intracellular blocker [B] according to the scheme shown in (A). (E) Cartoon representation of the mechanism by which the N-terminus inactivation peptide or QA ions can cause an immobilization of gating charges. The S1–S3 segments of the VSD is colored blue, S4 shown in purple (resting) or red (activated), and the pore domain S5–S6 grey. At resting negative membrane potentials the pore is closed at the bundle crossing and the S4 charge carrying segments of the VSD are in a resting state. Upon depolarization the S4 segments move outwards into the activated position before the pore opens at the intracellular gate. Once the pore is opened the N-terminus inactivation peptide or QA ions can enter the cavity. The pore is unable to close when these particles in the cavity and has the effect of slowing the voltage sensors return from the activated state.
In early studies with cloned Shaker channels it was found that the N-terminus fast inactivating peptide caused slowing of gating charge return when opened channels were bound with the inactivation peptide (Bezanilla et al., 1991). As the binding site for internal QA ions and the Shaker inactivation peptide has been identified as residing deep in the inner cavity region of the channel pore these observations demonstrate that occupancy of this site can cause voltage sensor immobilization through the prevention of pore closure (Choi et al., 1993; Gonzalez et al., 2011). To illustrate a more severe immobilization, such as that observed when intracellular QA ions or N-terminus inactivation peptides are present, an extra blocked state O:B was added to the open state of the model shown in Figure 2A. The gating currents from this model in the presence of a blocking particle (B) are shown in Figure 2D. The OFF gating currents show a severe slowing of charge return after depolarization to potentials that open the channel significantly, which equates to a strong immobilization due to the occupancy of the open pore by B preventing the first open to closed transition and the subsequent voltage sensor return to its resting state. In this case the degree of immobilization will be dependent upon the affinity of the blocker for the pore of the channel where high affinity blockers would induce a stronger immobilization. This type of immobilization is not limited to channels such as Shaker that posses N-terminus inactivation peptides as Kv channels often associate with β-subunits that can confer inactivation properties to the channel. For example, Kv1.2 associates with the Kvβ1.2 subunit which confers N-type inactivation and has also been shown to slow voltage sensor return (Peters et al., 2009).
The slow return of gating charges is structurally mediated through the coupling of the pore to the voltage sensor S4 through inter-subunit interactions between the S4–S5 linker of one subunit and the inner end of the S6 from a neighboring subunit (Batulan et al., 2010). This observation indicates that an open pore can slow voltage sensor return from the activated state as a result of the tight coupling between the two domains.
A summary of the mechanism by which pore occupancy by QA ions or N-terminus peptides can occupy the pore is shown in Figure 2E. Depolarization that is sufficiently positive to open the channel are usually associated with an mild immobilization of the voltage sensors in the open state and has been postulated to reflect an intrinsic stability of the pore in the open confirmation but could also result from the occupancy of the pore by the particular ions used in the internal solution. This effect is very dramatic in the presence of intracellular blockers or fast inactivation peptides which can occupy the inner pore cavity with a high affinity and stabilize the open state thus limiting the rate of return of the voltage sensors and associated gating charge.
Inactivation and Immobilization
In the absence of fast inactivation peptides or QA ions, immobilization of charge is still observed in Kv channels. This indicates that there are mechanisms other than pore block that underlie the process of immobilization. Gating currents have been recorded in the presence of K+ ions from mutant Shaker channels that have been rendered non-conducting by a mutation W434F in the pore helix that causes the collapse of the selectivity filter (Perozo et al., 1993). These experiments were conducted using the non-conducting channel that also contained an N-terminus deletion of residues 6–46 that removes any fast inactivation process (named inactivation removed, ShakerIR), which causes voltage sensor immobilization (Bezanilla et al., 1991), yet gating currents from the ShakerIR-W434F recorded with K+ internal solutions still displayed a slowing of gating current return after depolarization to potentials at which the channels open (Perozo et al., 1993). In addition, wild-type (WT) channels recorded with solutions using ion replacement to deplete conductive intracellular ions with non-permeant cationic molecules such as NMG+ (Zagotta et al., 1994b) or Tris+ (Varga et al., 2002) also display a slowing of voltage sensor return after depolarization to potentials where channels open. These studies clearly implicate the open pore as a critical determinant in the slowing of charge return, however it is not the only determinant. In experiments with Kv1.5 channels using NMG+ to replace K+ and Na+ and reveal gating currents it was demonstrated that long depolarizations that persist past the full activation time course of the channels induced a greater slowing of the charge return upon repolarization than shorter pulses (Fedida et al., 1996). This indicated that an immobilization process was occurring that was not only mediated by the pore opening. To further examine this effect the authors used the drug 4AP, which stabilizes the closed pore conformation. 4AP was found to remove the development of immobilization, along with the slow inactivation properties of the channel, suggesting that the immobilization of the gating charge was linked to slow inactivation (Fedida et al., 1996). This pointed to inactivation processes directly mediating an immobilization of the voltage sensors in Kv1.5. Similarly, in the Shaker channel lacking fast inactivation (ShakerIR) prolonged depolarization was found to immobilize the voltage sensor (Olcese et al., 1997). The immobilization in these experiments was reflected by a left shift of the voltage dependence of charge return compared with charge activation which indicated that more energy was required to return the voltage sensor to its resting configuration (Olcese et al., 1997). Interestingly, immobilization occurred in ShakerIR channels recorded in cut-open oocyte configuration where the conducting ions were replaced with NMG+ as well as in the non-conducting mutant channels ShakerIR-W434F recorded in the presence of intracellular K+ ions, suggesting the effect was not just related to open pore occupancy by the intracellular ions. Furthermore, the time course of the development of charge immobilization in the ShakerIR-W434F channel correlated with the time course of inactivation of ShakerIR channels (Olcese et al., 1997). These experiments implied an important distinction between inactivated states – that the W434F channel represents a pore inactivated state (P-type), thought to be due to a localized collapse of the selectivity filter, that can still undergo further conformational rearrangements that stabilize the voltage sensor on the same time scale as slow inactivation. This observation indicated that the W434F mutation, whilst collapsing the selectivity filter and preventing conductance, had not prevented other conformational rearrangements that involved the voltage sensor and correlated with slow inactivation. Despite these correlations the study could not conclude whether the P-type inactivation was causally linked with the conformational changes that immobilized the voltage sensors. A separate study investigated ShakerIR gating using voltage-clamp fluorometry, in which an environmentally sensitive fluorophore was attached to an engineered cysteine residue at the top of the S4 or the pore region to track conformational rearrangements of the two regions (Loots and Isacoff, 1998). It was found that the fluorescent probes in both regions tracked the onset and recovery of slow inactivation which suggested that pore inactivation alters the interaction between the two domains. Specifically, the study found two conformational rearrangements during ShakerIR channel inactivation – the first shuts the channel at the selectivity filter and represents the transition into the inactivated state found in the W434F P-type inactivated channel whereas the second rearrangement involves changes around S4 having the effect of stabilizing the inactivated state and making it more difficult for the voltage sensor to return to its resting position (Loots and Isacoff, 1998).
Intracellular Ion Effects on Immobilization
The experimental conditions required for the resolution of gating currents (mutations, block, and permeant ion depletion) create a significant hurdle when attempting to interpret the relevance of these measurements to physiological gating, in which conducting channels permeate small ions unimpeded. A significant advance came with the development of methodology using the patch clamp technique that enabled precise control of intracellular ionic concentrations and enabled Kv1.5 gating currents to be recorded in the presence of permeating ions Cs+ and K+ (Chen et al., 1997). OFF gating currents at −100 mV were recorded in the presence of a low concentration of internal K+ or the poorly permeant Cs+ ion which avoided contamination of the gating currents by large ionic conductance. These experiments uncovered that the rate of gating charge return was accelerated by the presence of permeant ions in these channels when compared with recordings in the absence of permeant ions using NMG+ as a replacement cation (Chen et al., 1997). This implied that the very presence of permeating ions can influence the voltage sensor dynamics and led to the proposition of an allosteric modulatory site where permeant ions may interact to slow entry into a state from which gating charge is slow to return or to impair the entry into a slow inactivated state. Following this study the authors utilized a non-conducting Kv1.5-W472F mutant channel and found that replacing extracellular and intracellular NMG+ with small group 1 intracellular cations also had the effect of relieving the charge immobilization, with intracellular effects being 10-fold more prominent (Wang et al., 1999). The differences in rates of charge recovery were apparent after depolarization more positive than −20 mV consistent with voltages at which the channel open probability rapidly increases. These studies implied that the effect was strongly dependent upon an intracellular site that was accessible when the channel opens and was hypothesized to regulate entry of the channel into the slow inactivated state which caused immobilization of charge. These two studies combined indicated that the role of ions was not intrinsically linked to their fluxing through the channel as the non-conducting mutant Kv1.5-W472F could also be modulated by varying intracellular ionic composition, but did not precisely locate the ion interaction sites involved.
Insight into the location of the intracellular interaction site for ions to modulate charge return came from studies on ShakerIR channels in which the size of the internal cavity was increased by a mutation at isoleucine 470 (Melishchuk and Armstrong, 2001). This residue is located approximately mid way along the S6 helix lining the intracellular cavity. Mutation from isoleucine to cysteine had the effect of accelerating charge return of gating currents recorded with intracellular Cs+ or NMG+. This observation led the authors to propose that the limiting step in charge return was pore closure, which in turn was rate limited by the exit rate of ions from inner cavity binding sites. In this case, because the study utilized short (15 ms) depolarizations, the charge immobilization was considered separately from any conformational changes in the tertiary structure of the protein that occurs over the time course of slow inactivation processes which are typically of a much longer time constant. This model proposed by Melishchuk and Armstrong (2001) was consistent with and further supported the classical “occupancy hypothesis” that was based on the observation that increasing the external concentration of permeant ions such as K+, Cs+, and Rb+ ions slows the deactivation kinetics of Kv channels as the pore is prevented from closing when occupied (Swenson and Armstrong, 1981; Matteson and Swenson, 1986).
An insightful study using a variety of different permeant and non-permeant ions that compared ShakerIR conducting and ShakerIR-W4343F non-conducting channels attempted to clarify the mechanistic differences in immobilization in these channels (Varga et al., 2002). The study first sought to examine the relationship between ionic deactivation and gating charge return in the WT channel. To achieve this without masking the gating currents the experiments were recorded with low concentrations of permeant ions on either side of the membrane (mM) 1 K+, 1 Rb+, or a higher concentration of the poorly permeant Cs+ (115 mM). The gating currents under these conditions displayed ionic deactivation kinetics that were faster than charge recovery. This indicated that ion occupancy at these concentrations did not rate limit the voltage sensor return. However, because low concentrations of the highly permeant ions K+ and Rb+ had to be used to avoid contamination of the charge recovery measurements (this was not necessary for Cs+ due to its lower permeability allowing it to be used at 115 mM) the result was not directly informative of more physiological conditions. To enable the comparisons to be made with high internal concentrations (115 mM) of K+ and Rb+ the experiments were conducted using the non-conducting W434F channel. These recordings using W434F displayed a slower rate of charge recovery than the WT channel with low concentrations of K+ and Rb+. This indicated a potential concentration dependent effect, where higher concentrations of ions had the effect of slowing charge recovery, consistent with occupancy slowing pore closure. Further insight came as a byproduct of the interesting observation that “non-conducting” W434F channels can in fact conduct K+ in specific ionic conditions. When the extracellular solution was set at 115 mM K+ and the internal contained 115 mM Tris+, a K+ conductance was observed. This K+ conductance had the effect of slowing charge return more than when W434F was recorded in the absence of any permeating ions. This suggested that increasing the occupancy of the inner cavity with permeating K+ ions lowered the rate of pore closure, also supporting the occupancy hypothesis. Taken together, these results suggested that gate closure was not rate-limiting for voltage sensor return in the WT channel in the presence of low concentrations of permeant ions, and that the gate could close before the voltage sensors returned to resting. Conversely, in high concentrations of intracellular permeant ions occupancy of the cavity was rate-limiting pore closure in the W434F channel.
A further observation from this study was that a slow component of charge return was also present in almost all conditions, and was dependent upon the ion species present. The authors postulated that this slowing of gating charge return was caused by the presence of varying degrees of inactivation accumulating over the depolarizing pulses used to evoke gating currents. Because inactivation is associated with conformational processes that slow charge return (Olcese et al., 1997; Loots and Isacoff, 1998), the varied effects with different ion species is likely to be partly due to the different occupancy of a site that slows entry into the inactivated states consistent with previous studies on immobilization that develops after the channel pore is open (Fedida et al., 1999; Wang et al., 1999).
From the studies discussed up to this point it is apparent that occupancy of the cavity is a critical factor in regulating voltage sensor immobilization. The main factors determining the dwell time of an ion in a binding site at a constant membrane potential are the interactions of the ion with the binding site and the potential electrostatic interaction with other ions nearby. Experiments using the divalent ion Ba2+ have been used to probe some of the electrostatic effects of ion–ion interactions. A key feature of Ba2+ ions in these studies is the deep binding site they occupy in the SF of K+ channels, adjacent to the inner cavity site highlighted in Figure 1B (Neyton and Miller, 1988; Jiang and MacKinnon, 2000). The application of external Ba2+ was shown to accelerate charge return in ShakerIR-WT and -W434F, specifically after depolarizations to potentials where the channel opens, leading to the hypothesis that Ba2+ binding was destabilizing the open state of the channel (Hurst et al., 1997). This effect could well be mediated through a repulsive effect of the Ba2+ ions in the deep SF site with the positively charged K+ ions residing in inner cavity sites. Some experimental support for this hypothesis also came from the detailed study by Varga et al. It was hypothesized that external Ba2+ ions would have effects on ions in the cavity from the deep site and influence the occupancy and thus exit rate of intracellular ions in the cavity. To test this hypothesis they applied external Ba2+ and observed an accelerated charge return in the W434F channel, which was faster when K+ ions were in the cavity than with internal Na+ ions, and had little effect with Tris+ internal (Varga et al., 2002). These data indicated that K+ ions interacted strongly and repulsively with Ba2+ but that the Na+ and Tris+ ions were sufficiently different in size and physico-chemical structure that they did not occupy a site that was directly affected by Ba2+ binding in the filter. The implication of this experiment was that K+ occupancy of the inner cavity binding site could be destabilized by the Ba2+ ion binding presumably at a deep site in the SF (correlated with the lower blue sphere in Figure 1B), close enough to exert a significant electrostatic destabilization. This is however likely a simplified picture as it is likely that there is more than one binding site for ions within the intracellular cavity and that these sites can exert influence on each other (Thompson and Begenisich, 2001, 2003).
When discussing the intracellular ion binding sites it should be noted that these sites will be occupied by hydrated K+ ions as the cavity also contains water molecules. Because a hydrated K+ ion has a diameter of around 5 Å, with a hydration energy of −85 kcal/mol (Hille, 2001), it is likely that the steric bulk of the hydration sphere of the ion significantly contributes to an energetic barrier to channel pore closure. Generally the effects of ions in the cavity described have been considered to be steric, and the bulk of hydrated K+ ions support this kind of hypothesis, as does the observation that large ions such as QA ions immobilize charge more severely than small ions. In addition, recent high level studies using all atom molecular dynamics simulations to simulate a complete gating cycles indicated that the open cavity dewetted before the pore collapses due to the hydrophobic nature of the cavity (Jensen et al., 2010, 2012). Hydrated ions must therefore exit before the pore can dewet fully before shutting, supporting the hypothesis that ionic occupancy in the cavity slows pore closure.
Voltage Sensor Relaxation – A Third Mechanism of Charge Immobilization?
Stabilization of the voltage sensor in the activated state after depolarization has also been observed in non-inactivating channels such as HCN channels (Bruening-Wright and Larsson, 2007), and the voltage-sensing phosphatase, ciVSP which has no pore (Villalba-Galea et al., 2008). Slowing of charge return in a voltage sensor without a pore is clearly a different process that cannot be dependent upon pore inactivation or ionic occupancy and suggests that the voltage sensor might possess an intrinsic property to “relax,” representing the adoption of a distinct stable conformational state (Villalba-Galea et al., 2008, 2009). This process has also been proposed to occur in ShakerIR channels during prolonged depolarizing pulses as tracked by the entry into a state from which charge return is slow (Lacroix et al., 2011). Although relaxation may represent an intrinsic property of the voltage sensor, it is currently difficult to separate the observed slowing of charge return from those related to inactivation discussed above, that also show ion dependence (Shirokov, 2011). It follows that if voltage sensor relaxation is independent of pore-related inactivation processes then it should have little dependence on ionic conditions. Future studies that advance the structural information available for these states will ultimately allow the clear separation of inactivation and relaxation. Because relaxation of voltage sensors occurs over a much longer time scale than the slowing of charge return that can be observed developing with channel opening kinetics it is certainly not likely to impact directly on the rapid immobilization of charge caused by ions occupying the inner cavity.
Conclusion
The studies reviewed here have shown that charge immobilization can be caused by a combination of ion occupancy of the pore and conformational rearrangements associated with inactivation or relaxation, which can be broadly classed as intrinsic (related to protein tertiary structural rearrangements) and extrinsic (caused by external factors such as ion block of pore closure or inactivation) factors.
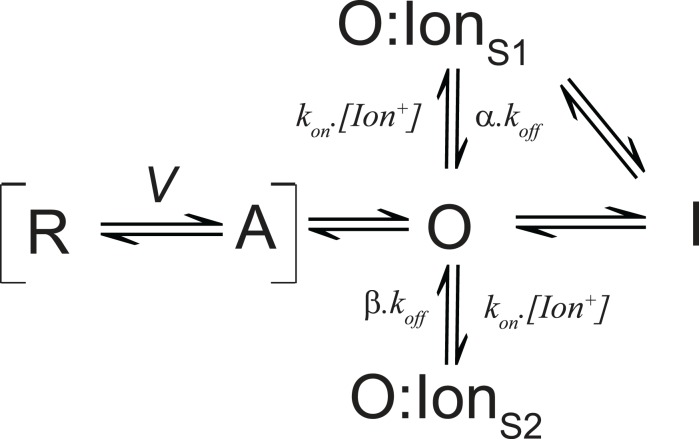
The various pathways affected by intracellular ions have been summarized by the simplified state scheme shown in Figure 3. The scheme assigns the majority of the charge carrying transitions of all the four subunits voltage sensors to the transition between R (resting) and A (activated). Depolarization causes the voltage sensors to shift from R to A, a transition which accounts for the gating charge measured on activation. After activation the pore domain opens sequentially and must shut before the reverse transition from A to R representing charge recovery. Slowing the transition from O to A will have the effect of slowing charge return. After opening, ions may enter the pore and occupy a site (O:IonS1) that prevents closure of the pore and will have the knock-on effect of slowing deactivation kinetics and, if slower than the A to R transition, the rate of charge return will also be slower. The rate of slowing of charge return is subject to modulation of the stability of O:IonS1, which is dependent upon the proximity of other ions and the intrinsic affinity for the pore cavity that the specific ion may have which will be dependent upon size, charge, and solvation. Once opened, or bound with an ion at S1, the channel might also enter an inactivated state (I) associated with a conformational rearrangement that stabilizes the voltage sensor and causes immobilization. The rate of entry into this inactivated state may be impaired by intracellular ions occupying a site that antagonizes inactivation (O:IonS2) – probably through a mechanism similar to the “foot in the door” attenuation of inactivation described for conducting channels (Baukrowitz and Yellen, 1995, 1996). The off rate for each ion site are modified by the multipliers α and β where α ( β, which results in occupancy of S2 impairing the entry into the inactivated state without affecting the return of charge significantly. Alternatively, the entry into the inactivated state could be accelerated by reducing binding at the “foot in the door” site S2 through the depletion of appropriate binding ions by non-permeant cations which favor site S1; an experimental condition necessary to record gating currents from conducting channels which would increase the degree of immobilization observed.
Figure 3.
State model summarizing the effects of intracellular ions on voltage sensor dynamics. The model is described in the text.
In this review we have attempted to identify and separate some important mechanisms that can contribute to voltage sensor immobilization and the ways in which those processes are subject to modulation by intracellular ions. It is apparent that these effects are not always easily separated and that often simultaneous immobilizing mechanisms are operating at the same time, dependent upon the particular recording solutions used and the durations of the depolarizing pulses. Despite these uncertainties it is clear that, especially after short depolarizations, that would not be considered to induce significant inactivation or relaxation, occupancy of the intracellular cavity by ions does affect the dynamics of the voltage sensor. This effect of intracellular ions could be thought of as allosteric in the sense that ion binding at pore sites influences the stability of the voltage sensor, a structurally distinct entity (Figure 1A). Considering that ions fluxing through the pore exert an influence on the deactivation voltage gating it is of physiological significance to understand the precise mechanisms by which these effects are mediated. It is conceivable that different channel subtypes have evolved around the ion species they carry and that ion occupancy effects on deactivation kinetics could have exerted some selective pressure depending on the particular channel subtype, which contributes to their particular deactivation gating phenotype. As more channels are investigated and high resolution structures are solved for Kv channels in different states a clearer picture will emerge of the ions role in gating as well as permeation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Armstrong C. M. (1971). Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58, 413–437 10.1085/jgp.58.4.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. (1974). Charge movement associated with the opening and closing of the activation gates of the Na channels. J. Gen. Physiol. 63, 533–552 10.1085/jgp.63.5.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. (1977). Inactivation of the sodium channel. II. Gating current experiments. J. Gen. Physiol. 70, 567–590 10.1085/jgp.70.5.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batulan Z., Haddad G. A., Blunck R. (2010). An intersubunit interaction between S4–S5 linker and S6 is responsible for the slow off-gating component in Shaker K+ channels. J. Biol. Chem. 285, 14005–14019 10.1074/jbc.M109.097717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T., Yellen G. (1995). Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron 15, 951–960 10.1016/0896-6273(95)90185-X [DOI] [PubMed] [Google Scholar]
- Baukrowitz T., Yellen G. (1996). Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science 271, 653–656 10.1126/science.271.5249.653 [DOI] [PubMed] [Google Scholar]
- Bezanilla F. (2000). The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80, 555–592 [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Perozo E., Papazian D. M., Stefani E. (1991). Molecular basis of gating charge immobilization in Shaker potassium channels. Science 254, 679–683 10.1126/science.1948047 [DOI] [PubMed] [Google Scholar]
- Bruening-Wright A., Larsson H. P. (2007). Slow conformational changes of the voltage sensor during the mode shift in hyperpolarization-activated cyclic-nucleotide-gated channels. J. Neurosci. 27, 270–278 10.1523/JNEUROSCI.3801-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. S., Steele D., Fedida D. (1997). Allosteric effects of permeating cations on gating currents during K+ channel deactivation. J. Gen. Physiol. 110, 87–100 10.1085/jgp.110.2.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. L., Mossman C., Aubé J., Yellen G. (1993). The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron 10, 533–541 10.1016/0896-6273(93)90340-W [DOI] [PubMed] [Google Scholar]
- De Santiago-Castillo J. A., Covarrubias M., Sánchez-Rodríguez J. E., Perez-Cornejo P., Arreola J. (2010). Simulating complex ion channel kinetics with IonChannelLab. Channels (Austin) 4, 422–428 10.4161/chan.4.5.13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Bouchard R., Chen F. S. (1996). Slow gating charge immobilization in the human potassium channel Kv1.5 and its prevention by 4-aminopyridine. J. Physiol. (Lond.) 494(Pt 2), 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D., Maruoka N. D., Lin S. (1999). Modulation of slow inactivation in human cardiac Kv1.5 channels by extra- and intracellular permeant cations. J. Physiol. (Lond.) 515(Pt 2), 315–329 10.1111/j.1469-7793.1999.315ac.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Lopez-Rodriguez A., Srikumar D., Rosenthal J. J., Holmgren M. (2011). Editing of human K(V)1.1 channel mRNAs disrupts binding of the N-terminus tip at the intracellular cavity. Nat. Commun. 2, 436. 10.1038/ncomms1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., Lu Z., Abramson T., MacKinnon R. (1994). Mutations in the K+ channel signature sequence. Biophys. J. 66, 1061–1067 10.1016/S0006-3495(94)80887-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. (2001). Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- Hurst R. S., Roux M. J., Toro L., Stefani E. (1997). External barium influences the gating charge movement of Shaker potassium channels. Biophys. J. 72, 77–84 10.1016/S0006-3495(97)78648-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. O., Borhani D. W., Lindorff-Larsen K., Maragakis P., Jogini V., Eastwood M. P., Dror R. O., Shaw D. E. (2010). Principles of conduction and hydrophobic gating in K+ channels. Proc. Natl. Acad. Sci. U.S.A. 107, 5833–5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. O., Jogini V., Borhani D. W., Leffler A. E., Dror R. O., Shaw D. E. (2012). Mechanism of voltage gating in potassium channels. Science 336, 229–233 10.1126/science.1216533 [DOI] [PubMed] [Google Scholar]
- Jiang Y., MacKinnon R. (2000). The barium site in a potassium channel by x-ray crystallography. J. Gen. Physiol. 115, 269–272 10.1085/jgp.115.3.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J. J., Labro A. J., Bezanilla F. (2011). Properties of deactivation gating currents in Shaker channels. Biophys. J. 100, L28–L30 10.1016/j.bpj.2010.12.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Banerjee A., MacKinnon R. (2009). Two separate interfaces between the voltage sensor and pore are required for the function of voltage-dependent K(+) channels. PLoS Biol. 7, e47 10.1371/journal.pbio.1000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Holmgren M., Jurman M. E., Yellen G. (1997). Gated access to the pore of a voltage-dependent K+ channel. Neuron 19, 175–184 10.1016/S0896-6273(00)80350-5 [DOI] [PubMed] [Google Scholar]
- Long S. B., Campbell E. B., Mackinnon R. (2005a). Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 10.1126/science.1116269 [DOI] [PubMed] [Google Scholar]
- Long S. B., Campbell E. B., Mackinnon R. (2005b). Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science 309, 903–908 10.1126/science.1116269 [DOI] [PubMed] [Google Scholar]
- Loots E., Isacoff E. Y. (1998). Protein rearrangements underlying slow inactivation of the Shaker K+ channel. J. Gen. Physiol. 112, 377–389 10.1085/jgp.112.4.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Klem A. M., Ramu Y. (2002). Coupling between voltage sensors and activation gate in voltage-gated K+ channels. J. Gen. Physiol. 120, 663–676 10.1085/jgp.20028677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Swenson R. P., Jr. (1986). External monovalent cations that impede the closing of K channels. J. Gen. Physiol. 87, 795–816 10.1085/jgp.87.5.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melishchuk A., Armstrong C. M. (2001). Mechanism underlying slow kinetics of the OFF gating current in Shaker potassium channel. Biophys. J. 80, 2167–2175 10.1016/S0006-3495(01)76189-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Miller C. (1988). Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+ -activated K+ channel. J. Gen. Physiol. 92, 569–586 10.1085/jgp.92.5.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcese R., Latorre R., Toro L., Bezanilla F., Stefani E. (1997). Correlation between charge movement and ionic current during slow inactivation in Shaker K+ channels. J. Gen. Physiol. 110, 579–589 10.1085/jgp.110.5.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E., MacKinnon R., Bezanilla F., Stefani E. (1993). Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron 11, 353–358 10.1016/0896-6273(93)90190-3 [DOI] [PubMed] [Google Scholar]
- Peters C. J., Vaid M., Horne A. J., Fedida D., Accili E. A. (2009). The molecular basis for the actions of KVbeta1.2 on the opening and closing of the KV1.2 delayed rectifier channel. Channels (Austin) 3, 314–322 10.4161/chan.3.5.9558 [DOI] [PubMed] [Google Scholar]
- Schoppa N. E., McCormack K., Tanouye M. A., Sigworth F. J. (1992). The size of gating charge in wild-type and mutant Shaker potassium channels. Science 255, 1712–1715 10.1126/science.1553560 [DOI] [PubMed] [Google Scholar]
- Shirokov R. (2011). What’s in gating currents? Going beyond the voltage sensor movement. Biophys. J. 101, 512–514; discussion 515–516. 10.1016/j.bpj.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr., Armstrong C. M. (1981). K+ channels close more slowly in the presence of external K+ and Rb+. Nature 291, 427–429 10.1038/291427a0 [DOI] [PubMed] [Google Scholar]
- Thompson J., Begenisich T. (2001). Affinity and location of an internal K+ ion binding site in shaker K channels. J. Gen. Physiol. 117, 373–384 10.1085/jgp.117.5.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Begenisich T. (2003). Functional identification of ion binding sites at the internal end of the pore in Shaker K+ channels. J. Physiol. (Lond.) 549(Pt 1), 107–120 10.1113/jphysiol.2002.038646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Rayner M. D., Starkus J. G. (2002). Cations affect the rate of gating charge recovery in wild-type and W434F Shaker channels through a variety of mechanisms. J. Gen. Physiol. 119, 467–485 10.1085/jgp.20028520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C. A., Sandtner W., Dimitrov D., Mutoh H., Knöpfel T., Bezanilla F. (2009). Charge movement of a voltage-sensitive fluorescent protein. Biophys. J. 96, L19–L21 10.1016/j.bpj.2008.12.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea C. A., Sandtner W., Starace D. M., Bezanilla F. (2008). S4-based voltage sensors have three major conformations. Proc. Natl. Acad. Sci. U.S.A. 105, 17600–17607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhang X., Fedida D. (1999). Gating current studies reveal both intra- and extracellular cation modulation of K+ channel deactivation. J. Physiol. (Lond.) 515(Pt 2), 331–339 10.1111/j.1469-7793.1999.331ac.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. (1994a). Shaker potassium channel gating. III: evaluation of kinetic models for activation. J. Gen. Physiol. 103, 321–362 10.1085/jgp.103.2.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Dittman J., Aldrich R. W. (1994b). Shaker potassium channel gating. II: transitions in the activation pathway. J. Gen. Physiol. 103, 279–319 10.1085/jgp.103.2.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Morais-Cabral J. H., Kaufman A., MacKinnon R. (2001). Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature 414, 43–48 10.1038/35102009 [DOI] [PubMed] [Google Scholar]