Abstract
AIM
The introduction of calcineurin inhibitors (CNIs) ciclosporin (CsA) and tacrolimus (Tac) has improved the outcome of organ transplants, but complications such as new onset diabetes mellitus after transplantation (NODAT) cause impairment of survival rates. The relative contribution of each CNI to the pathogenesis and development of NODAT remains unclear. We sought to compare the impact of CsA and Tac on glucose metabolism in human subjects.
METHODS
Ten healthy men underwent 5 h infusions of CsA, Tac and saline in a randomized, double-blind, crossover study. During infusion glucose metabolism was investigated using following methods: a hyperinsulinaemic-euglycemic clamp, an intravenous glucose tolerance test (i.v.GTT), glucose-stimulated insulin concentration–time series and indirect calorimetry.
RESULTS
Clamp derived insulin sensitivity was increased by 25% during CsA (P < 0.0001) and 13% during Tac administration (P = 0.047), whereas first phase and pulsatile insulin secretion were unaffected. Coinciding with the CNI induced improved insulin sensitivity, glucose oxidation rates increased, while insulin clearance rates decreased, only non-significantly. Tac singularly lowered hsCRP concentrations, otherwise no changes were observed in circulating glucagon, FFA or adiponectin concentrations. Mean blood concentrations of CNIs were 486.9 ± 23.5 µg l−1 for CsA and 12.8 ± 0.5 µg l−1 for Tac.
CONCLUSIONS
Acute effects of i.v. CsA, and to a lesser degree Tac infusions, in healthy volunteers include increased insulin sensitivity, without any effect on first phase or pulsatile insulin secretion.
Keywords: ciclosporin, glucose clamp, insulin resistance, insulin secretion, new onset diabetes mellitus after transplantation, tacrolimus
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
New onset diabetes after transplantation is related to treatment with immunosuppressive medications. Clinical studies have shown that risk of new onset diabetes is greater with tacrolimus compared with ciclosporin. The diabetogenicity of ciclosporin and tacrolimus has been attributed to both beta cell dysfunction and impaired insulin sensitivity.
WHAT THIS STUDY ADDS
This is the first trial to investigate beta cell function and insulin sensitivity using gold standard methodology in healthy human volunteers treated with clinically relevant doses of ciclosporin and tacrolimus. We document that both drugs acutely increase insulin sensitivity, while first phase and pulsatile insulin secretion remain unaffected. This study demonstrates that ciclosporin and tacrolimus have similar acute effects on glucose metabolism in healthy humans.
Introduction
New onset diabetes after transplantation (NODAT) confers a high risk of cardiac events among renal transplant recipients [1], and is furthermore associated with more than a 60% increase in risk of graft failure and nearly a 90% increase in risk of death [2, 3]. With its frequent occurrence of up to 50% [4, 5], NODAT poses a great threat to transplant recipients and a great burden on clinicians dealing with it.
Risk factors for NODAT following renal transplantation include increasing age, obesity, non-Caucasian ethnicity, steroid use and type of calcineurin inhibitor (CNI) [2, 6]. Focusing on CNI treatment, diabetogenicity seems particularly enhanced with tacrolimus (Tac) compared with ciclosporin (CsA) [3–5, 7, 8]. However some studies have reported similar prevalence rates of NODAT in Tac- and CsA-treated kidney transplant recipients [2, 7–9]. The concomitant use of steroids has made it more difficult to define the precise role of CNIs in NODAT, as steroids themselves are associated with increased insulin resistance [10, 11].
Several in vitro and biopsy studies in man and animals indicate that CNIs impair pancreatic beta cell function in a dose dependent manner, through cell death [12], impaired insulin production [13] and/or diminished insulin secretion [14, 15]. Clinical studies so far have mainly corroborated these pre-clinical results, as both Tac and CsA have been shown to have detrimental effects on insulin secretion [16–18]. The possible impact of Tac and CsA on insulin sensitivity is less clearly defined [16–19]. Collectively previous studies are limited by study design, insufficient assessment methods or confounding factors such as concomitant steroid treatment. It is also important to bear in mind the ongoing attempts of immunotherapy for preventing or halting autoimmune diabetes. Indeed earlier investigations proved CsA to be very efficient in preserving beta cell function in patients with new-onset type 1 diabetes mellitus [20]. These findings only highlight the complexity of these drugs and their individual effects in different clinical conditions. Hence the underlying pathophysiological mechanisms and a direct comparison of the diabetogenicity of Tac and CsA have not been documented. We herein present the first sequence randomized, double-blind crossover study comparing the acute effects of stable and clinically relevant concentrations of CsA and Tac on glucose metabolism in healthy volunteers.
Methods
The studies were conducted in accordance with the Helsinki Declaration and following the approval by the local ethics committee (M-20080060), the Danish Medicines Agency and the Good Clinical Practice (GCP) unit of Aarhus University Hospital. According to the International Committee of Medical Journal Editors, the protocol was registered at Clinicaltrials.gov (identification study1: NCT00766909) before the onset of enrolment. Prior to entering the study, written consent was obtained after receipt of written and oral information.
Study subjects
Ten healthy men with a median age of 27 (21–52) years and mean body mass index (BMI) of 24.7 ± 0.5 kg m−2 volunteered for this study. All had a normal physical examination and haematological and renal function assessed by biochemical screening. Mean systolic and diastolic blood pressures were 126 ± 3 mmHg and 74 ± 2 mmHg respectively. Two had a family history of diabetes mellitus and two were smokers. None of the participants was taking medication regularly during the study period.
Study design
This study was a double-blind, placebo controlled, sequence randomized crossover trial. Upon inclusion sequence randomization, preparation of study medication and blinding was performed and ensured by the hospital pharmacy at Aarhus University Hospital. Each participant underwent three investigations on 3 separate days 4–6 weeks apart, receiving CsA, Tac or saline infusions. On each investigational day the participants arrived at the research unit at 08.00 h after a 10 h overnight fast. Cannulas were inserted in the antecubital veins on each side for infusion and sampling purposes, and an additional cannula was inserted in a heated dorsal hand vein for sampling of arterialized blood. At t = 0 min, bolus doses of CsA (0.34 mg kg−1), Tac (0.0024 mg kg−1) or saline were commenced and infused over a 20 min interval, followed by maintenance doses of CsA (0.155 mg kg−1 h−1), Tac (0.0012 mg kg−1 h−1) or saline until t = 285 min. Blood drug concentrations were measured at t = 0, 120, 165 and 285 min. Doses and administration modes were based on a targeted area under the curve, AUC(0,12 h), of 8000 µg l−1 h for CsA and 200 µg l−1 h for Tac, and aiming towards constant levels throughout the experiment [21, 22]. These targeted drug concentrations are comparable with respect to immunosuppressive efficacy in clinical practice. Doses were estimated from previous results at our own laboratory [23], tried and adjusted on a healthy volunteer. The following tests were then performed as outlined in Figure 1:
Figure 1.
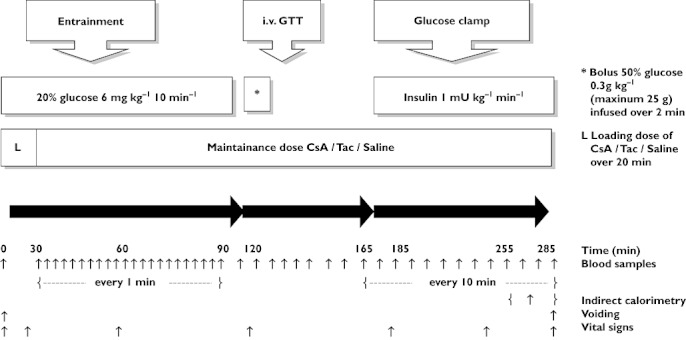
Study protocol. Please refer to Methods for further detalis
Glucose stimulated insulin concentration–time series
Pulsatile insulin secretion was assessed by collection of blood samples every minute for 60 min (t = 30–90 min) by the study procedure described previously [24]. The ability of the beta cell to sense and respond to minor glucose oscillations (entrainment) was evaluated during repeated punctuated glucose infusions every 10 min (pulse duration 1 min, glucose infusion rate 6 mg kg−1 min−1). The resultant insulin concentration time series were analyzed by deconvolution analysis and regularity statistics as described previously [25, 26].
Intravenous glucose tolerance test
At t = 120 min first phase insulin secretion was determined using an i.v. glucose bolus of 0.3 g kg−1 50% glucose (maximum 25 g) infused over 2 min. Blood was collected at t = 110, 120, 124, 126, 128, 130, 135, 150 and 165 min for determination of plasma glucose, serum insulin and C-peptide. First phase insulin secretion was calculated as AUC from 120 to 130 min and total insulin secretion was calculated as area under the curve AUC(120,165 min).
Hyperinsulinaemic euglycaemic clamp
A 2 h hyperinsulinaemic euglycaemic clamp (insulin 1 mU kg−1 min−1; Actrapid, Novo Nordisk, Denmark) was performed from t = 165–285 min. Plasma glucose was clamped at 5.0 mmol l−1 by adjusting the rate of infusion of 20% glucose according to plasma glucose measurements every 10 min. Insulin sensitivity was calculated from the glucose infusion rate (GIR) during the clamp. The M-value was obtained by calculating mean GIR during steady-state (final 30 min) of the euglycaemic clamp. The metabolic clearance rate of insulin (MCRINS) during the steady-state of the euglycaemic clamp was calculated as insulin infusion rate divided by the increase in insulin concentrations above basal.
Indirect calorimetry
Energy expenditure (EE) and respiratory exchange ratios (RQ) were assessed by indirect calorimetry with a ventilated hood (Deltatrack Metabolic Monitor, Datex, Helsinki, Finland) [27] during the last 30 min of the hyperinsulinaemic euglycemic clamp (t = 255–285 min). The equipment was calibrated using standard oxygen and CO2 gases before each measurement. Net lipid and glucose oxidation rates were computed and protein oxidation rates were estimated from urinary carbamide excretion. We used following equations [28]:
where VO2 is the O2 consumption (l min−1), VCO2 is the CO2 production (l min−1) and N is the urinary nitrogen excretion (g min−1).
Safety
Adverse events, vital signs, haematology, biochemistry and ECG were monitored throughout the study procedure and in between study days.
Assays
All biochemical analyses were performed in duplicate. Plasma glucose was measured immediately on a glucose analyzer (Beckman Instruments, Palo Alto, CA) using the glucose oxidase technique. All other serum and plasma samples were stored at −20°C (C-peptide at −80°C) until analyzed. Serum insulin was determined using a highly specific and sensitive two-site enzyme-linked immunosorbent assay (ELISA, DAKO Diagnostics, Cambridgeshire, UK) and serum C-peptide was measured by a two-site monoclonal-based ELISA with an intra-assay coefficient of variation of 5.1% (DAKO Diagnostics). Plasma glucagon was measured at t = 0, 165, 255 and 285 min using a radioimmunoassay kit (Linco Research). Serum free fatty acids (FFAs) were determined using a commercial kit (Wako Chemicals, Neuss, Germany). Serum adiponectin concentrations were measured at t = 285 min and determined by a validated in-house time-resolved immunofluorometric assay as described by Frystyk et al. [29]. All unknown samples were analyzed for adiponectin in duplicate, with an intra-assay coefficient of variation (CV) averaging < 5% and inter-assay CV < 7%. Serum CRP concentrations were measured at t = 285 min and determined by a high sensitive CRP (hsCRP) TRIFMA assay based on commercially available monoclonal antibodies (R & D systems), and the intra- and inter-assay variations (%CV) were below 5 and 6%, respectively. Blood Tac and CsA concentrations (µg l−1) were determined using Waters Micromass HPLC-MS/MS system.
Statistics
Results are expressed as means ± SEM for normally distributed data and otherwise medians with range are given. anova repeated measures were used to analyze GIR and glucose concentrations during the clamp and the interaction between time and treatment was considered the term of interest. Adjustments for randomization order and treatment period were included in the model. All other measurements of hormones, metabolites and calculated estimates were analyzed using a one way anova. To obtain normal distribution, data were transformed when necessary using natural logarithm and otherwise non-parametric Kruskall-Wallis tests were used for comparisons. Statistical analysis was performed using STATA 11.0 software.
Results
Insulin sensitivity and substrate metabolism
The clamp derived insulin sensitivity, given as the M-value, was significantly increased by 25% during i.v. CsA infusion (P < 0.0001) and by 13% (P = 0.047) during Tac infusion compared with saline. Glucose oxidation rates were increased during CNI treatment only non-significantly, together with slightly elevated non-oxidative glucose metabolism (Figure 2A,B and Table 1). A concommitant minor trend towards greater net fat synthesis (negative lipid oxidation rates) was present during Tac and CsA treatment, together with higher RQ values. Energy expenditure did not differ significantly between the treatments (Table 1). Serum insulin concentrations during the steady-state period of the clamp were slightly higher for CsA and Tac compared with saline, with lower insulin clearance rates during CNI treatment, but ultimately mean insulin and C-peptide concentrations as well as insulin clearance rates were not found to be significantly different between treatments (Figure 2C,D and Table 1). Total AUCs together with clamp steady-state mean concentrations of FFAs and glucagon were also comparable between treatments (Figure 2E and Table 1). Serum hsCRP concentrations were significantly lowered by Tac compared with saline (P = 0.02) and CsA (P = 0.016), while no significant changes were seen in serum concentrations of the anti-inflammatory adipokine, adiponectin (Table 1).
Figure 2.
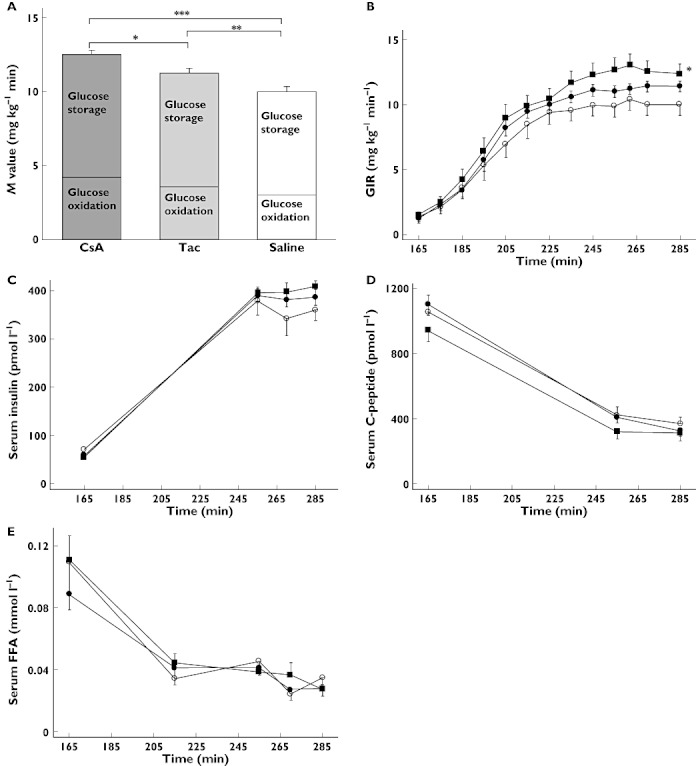
Hyperinsulinemic euglycaemic clamp investigations of 10 healthy men receiving CsA, Tac and saline infusions. (A) M-values (mean GIR during steady-state) were significantly increased for both CNIs: *P = 0.038 CsA vs. Tac, **P = 0.047 Tac vs. saline, ***P < 0.0001 CsA vs. saline, analyzed using anova, (B) glucose infusion rates were significantly increased during CsA treatment: *P = 0.003 CsA vs. saline, analyzed using anova repeated measures, (C) serum insulin values, (D) serum C-peptide values and (E) serum FFA values. CsA ( closed squares), Tac (• closed circles), Saline (○ open circles). Data are presented as means ± SEM
closed squares), Tac (• closed circles), Saline (○ open circles). Data are presented as means ± SEM
Table 1.
Parameters of glucose metabolism during saline, tacrolimus and ciclosporin infusion
| Placebo | Tacrolimus | Ciclosporin | P | |
|---|---|---|---|---|
| Fasting values | ||||
| Plasma glucose (mmol l−1) | 5.29 ± 0.12 | 5.19 ± 0.14 | 5.22 ± 0.07 | 0.82 |
| Serum insulin pmol l−1 | 40.55 ± 5.5 | 40.85 ± 8.9 | 37.75 ± 4.6 | 0.55 |
| Serum C-peptide (pmol l−1) | 480.7 ± 63.4 | 573.3 ± 94 | 472.5 ± 37.8 | 0.53 |
| Serum FFA (mmol l−1) | 0.27 ± 0.03 | 0.42 ± 0.08 | 0.33 ± 0.05 | 0.18 |
| Plasma glucagon (pg ml−1) | 28.5 ± 5.7 | 38 ± 12.2 | 35.6 ± 8 | 0.65 |
| Insulin sensitivity | ||||
| M (mg kg−1 min−1) | 9.9 ± 08 | 11.2 ± 0.4 | 12.4 ± 0.8 | <0.005* |
| Insulin clearance | ||||
| MCRINS (l min−1) | 1.72 (1.44–3.33) | 1.58 (1.41–2.74) | 1.52 (1.37–2.03) | 0.28 |
| Insulin secretion | ||||
| AUCins(120,130min) first phase (pmol l−1 min) | 2209 ± 228 | 2197 ± 167 | 2125 ± 114 | 0.84 |
| AUCins(120,165 min) (pmol l−1 min) | 5614 ± 717 | 4833 ± 335 | 4689 ± 307 | 0.32 |
| AUCC-peptide(120,130 min) first phase (pmol l−1 min) | 18186 ± 1991 | 19119 ± 1557 | 17153 ± 1171 | 0.19 |
| Regularity analysis | ||||
| Spectral density | 10.27 (3.11–12.49) | 10.07 (7.61–11.68) | 11.53 (7.21–12.01) | 0.6 |
| Autocorrelation coefficient | 0.54 (−0.1–0.75) | 0.52 (0.29–0.70) | 0.51 (0.26–0.72) | 0.99 |
| Deconvolution analysis | ||||
| Secretory burst mass (pmol l−1 pulse−1) | 33.35 ± 5.1 | 38.9 ± 6 | 34.68 ± 4.4 | 0.33 |
| Secretory burst amplitude (pmol l−1 min−1) | 13.3 ± 2.1 | 15.5 ± 2.4 | 13.8 ± 1.7 | 0.33 |
| Basal secretion (pmol l−1 min−1) | 2.7 ± 0.52 | 2.56 ± 0.71 | 2 ± 0.58 | 0.53 |
| Pulse fraction | 0.55 ± 0.08 | 0.64 ± 0.07 | 0.66 ± 0.08 | 0.17 |
| Substrate metabolism | ||||
| Glucose oxidation (mg kg−1 min−1) | 2.95 ± 0.19 | 3.47 ± 0.23 | 4.11 ± 0.99 | 0.48 |
| Lipid oxidation (mg kg−1 min−1) | −0.09 (−0.67–0.31) | −0.23 (−0.44–0.11) | −0.17 (−0.44–0.02) | 0.38 |
| Energy expenditure (kcal 24 h–1) | 1971 ± 63 | 1940 ± 58 | 1998 ± 62 | 0.41 |
| RQ (ratio O2 : CO2) | 0.94 ± 0.01 | 0.96 ± 0.01 | 0.95 ± 0.008 | 0.31 |
| Counter-regulatory response | ||||
| Serum FFA (mmol l−1) (t = 255–285 min) | 0.0349 ± 0.003 | 0.0321 ± 0.003 | 0.0343 ± .003 | 0.56 |
| Plasma glucagon (pg ml−1) (t = 255–285 min) | 17.25 ± 2.9 | 20.15 ± 5 | 17.85 ± 3 | 0.81 |
| Inflammatory markers | ||||
| Serum adiponectin (mg l−1) (t = 285 min) | 8.33 ± 1.12 | 8.58 ± 1.13 | 7.96 ± 1.05 | 0.18 |
| Serum hsCRP (mg l−1) (t = 285 min) | 0.22 (0.03–3.45) | 0.115 (0.02–3.14) | 0.285 (0.02–2.11) | 0.03** |
| Pharmacokinetics | ||||
| Drug concentration (µg l−1) | 12.8 ± 0.5 | 486.9 ± 23.5 |
Data are means ± SEM and medians with (range).
P = 0.038 CsA vs. Tac, P < 0.0001 CsA vs. placebo, P = 0.047 Tac vs. placebo.
P = 0.016 CsA vs. Tac, P = 0.02 Tac vs. placebo.
Insulin secretion
First phase insulin secretion was not affected by the CNIs, as AUCins(120,130 min) was comparable between treatments. Peak insulin concentrations during i.v.GTT were also unaffected and reached 267.9 ± 22.8 pmol l−1 for CsA, 259 ± 21.6 pmol l−1 for Tac and 276.1 ± 30.3 pmol l−1 for saline. Total AUCins(120,165 h) was, however, diminished to some degree during both Tac and CsA administration, yet this difference did not reach statistical significance (Figure 3A and Table 1). CNI treatment did not change first phase, peak or total C-peptide secretion during i.v.GTT (Figure 3C and Table 1). Pulsatile insulin secretion, as measured by insulin secretory burst mass and amplitude was unchanged during CNI infusions, which was also the case for basal insulin secretion. The fraction of insulin released in a pulsatile manner was slightly, yet non-significantly, increased during CNI treatment (Figure 4 and Table 1). Employing spectral and autocorrelation analyses to the insulin time series from the entrainment period showed no statistically significant difference between CNI and saline treatment, indicating no effect upon glucose entrainment of pancreatic beta cell function (Figure 4 and Table 1).
Figure 3.
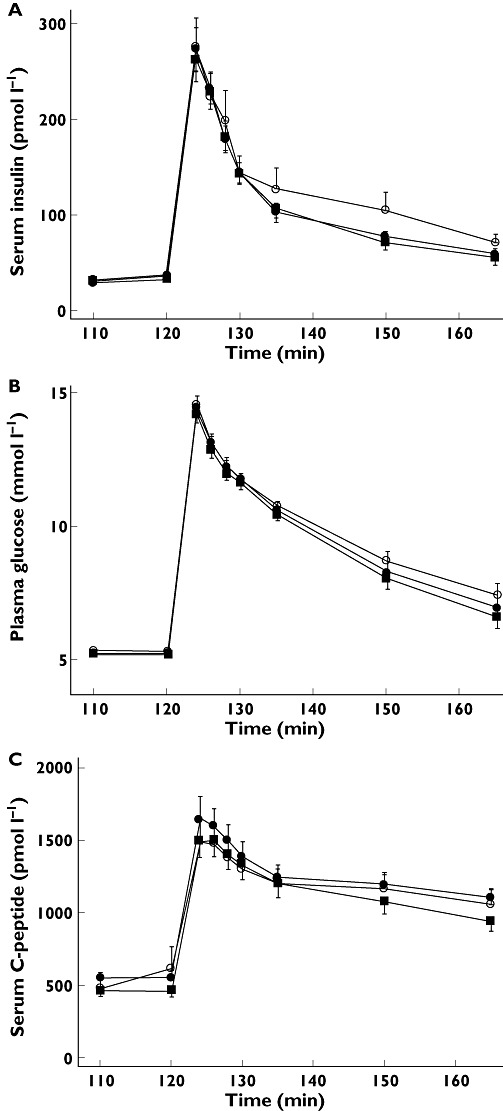
Intravenous glucose tolerance tests (i.vf.GTT) of 10 healthy men receiving CsA, Tac and saline infusions. (A) Serum insulin values. (B) Plasma glucose values. (C) Serum C-peptide values. CsA ( closed squares), Tac (• closed circles) and saline (○ open circles). Data are presented as means ± SEM
closed squares), Tac (• closed circles) and saline (○ open circles). Data are presented as means ± SEM
Figure 4.
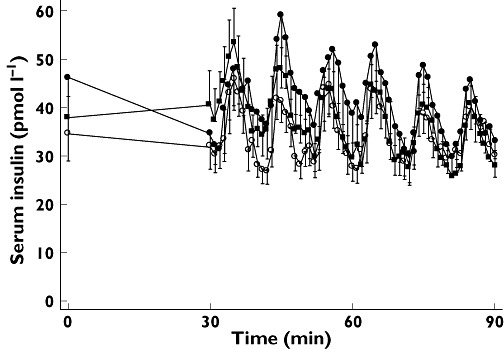
Glucose stimulated insulin concentration–time series investigations of 10 healthy men receiving CsA ( closed squares), Tac (•closed circles) and saline (○ open circles) infusions. Data are presented as means ± SEM
closed squares), Tac (•closed circles) and saline (○ open circles) infusions. Data are presented as means ± SEM
Immunosupression
Mean CsA and Tac concentrations during the 5 h infusion on study days are given in Table 1 and mean values at each time point during infusion are illustrated in Figure 5. We achieved 73% of the targeted AUCs for CsA and 76% for Tac. The achieved mean of 12.8 µg l−1 for Tac corresponds to a trough concentration of about 8 µg l−1 and the mean of 487 µg l−1 for CsA corresponds to a trough concentration of 172 µg l−1 or a C2 of 1020 µg l−1. These concentrations correspond to desired concentrations early after transplantation in clinical practice today.
Figure 5.
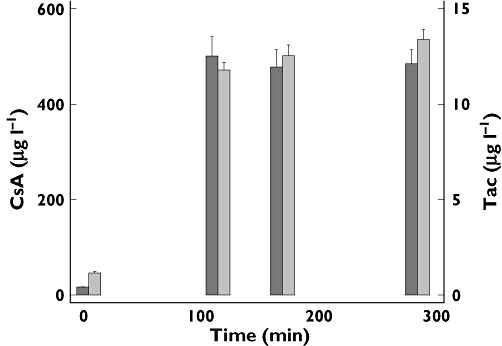
Blood drug concentrations (µg l−1) of CsA (black bars) and Tac (grey bars) during the study days. Data are presented as means ± SEM
Discussion
It is generally accepted that CsA and Tac impair insulin secretion and insulin action through dose-dependent, complex, and imperfectly understood mechanisms, ultimately contributing to the development of NODAT. These assumptions are however based on studies limited by their study design, insufficient assessment methods or confounding factors such as concomitant steroid treatment. This is the first randomized study to compare the acute effects of stable and clinically relevant concentrations of CsA and Tac on glucose metabolism, as assessed by the hyperinsulinaemic-euglycaemic clamp technique, i.v.GTT, glucose-stimulated insulin concentration–time series and indirect calorimetry in healthy volunteers.
The main and novel finding of the present study is that within 5 h of administering CsA and Tac insulin sensitivity was increased by 25% (P < 0.0001) and 13% (P = 0.047) respectively, whereas pulsatile and first phase insulin secretion were unchanged. These findings somewhat contradict previous studies indicating a detrimental effect on insulin secretion and insulin sensitivity [16–19], yet corroborate other studies documenting no impact on insulin secretion and beta cell function [30–32], but comparison with our study is generally hampered by differences in methodology and treatment regimens. Our contradictory results may be ascribable to the fact, that we have studied a younger and healthier population exposed only briefly to CNIs, whereas previous studies usually include longer exposure times and older populations. Hjelmesaeth and colleges found that 14 days of oral CsA treatment in nine haemodialysis patients impaired second phase insulin secretion, without affecting first phase insulin secretion or insulin sensitivity indexes, all assessed by a hyperglycaemic glucose clamp [18]. In a study by van Duijnhoven et al. 5 days of oral Tac treatment in 17 dialysis patients led to a decreased insulin secretion and sensitivity index assessed by i.v.GTTs, primarily due to impaired insulin secretion rather than sensitivity, yet only when Tac trough concentrations were above 15 ng ml−1[17]. When compared with our results, these previous studies suggest that the diabetogenicity of the CNIs may be time and dose dependent. Hence NODAT is thought to occur most frequently a few months following transplantation, ultimately supporting the notion of a distinct difference between acute vs. chronic exposure. More importantly they also highlight that increasing age next to a complex metabolic milieu residing in patients pre-transplantation, may facilitate more aggravating diabetogenic actions of CNIs. Finally, concurrent treatment with multiple medications especially steroids post transplantation may offer an additional explanation for the many ambiguous reports on CNI induced alterations in glucose metabolism. Indeed the importance of the potential interaction with steroids is highlighted by studies showing negligible or absent diabetogenicity when steroids are tapered from the treatment regimen in transplant recipients [7, 8, 33, 34] and when CNIs are administered as monotherapy in patients with multiple sclerosis and autoimmune diseases [30, 35].
The acute CNI response seen in this study could be mediated through impact on hormones involved in glucose metabolism. We found no significant differences in circulating glucagon or FFA concentrations between treatments. A few studies have shown a direct inhibitory effect of Tac and CsA on pancreatic alpha cells [36, 37], but the pathogenic relevance of glucagonaemia in overall glucose tolerance and insulin action is unclear [38]. Other counter-regulatory hormones include cortisol, epinephrine and growth hormone, encompassing actions that trigger lipolyis, glycolysis and insulin resistance within minutes to a few hours [39–41]. Although these hormones were not measured in this study, their actions would likely lead to deviations and differences in circulating glucose and FFA concentrations, which were not observed in this study. More importantly reports on CNI related effects on these neuroendocrine hormones are sparse and contradictory, mainly suggesting no or limited impact of the drugs [42–44].
Insulin clearance is inseparably linked to insulin action, and substantially decreased insulin clearance has been demonstrated in patients suffering from conditions characterized by insulin resistance [45]. Impaired ability to clear insulin from the circulation, may initially yield higher insulin concentrations reaching the periphery, but ultimately result in persistent hyperinsulinaemia with attendant insulin resistance. Accordingly we found that steady-state insulin concentrations tended to be slightly higher, while insulin clearance levels tended to be lower during CNI treatment compared with saline, albeit these differences were not statisically significant (Figure 2C and Table 1). The slightly increased insulin concentrations, possibly reflected in decreased clearance rates, may have contributed to the higher M-values during CNI treatment. Investigating whether sustained CNI exposure secondarily induces insulin resistance through failure of insulin removal, and to what extent the liver and kidney, as main insulin clearing organs, are involved is essential to advance our understanding of the pathogenesis of NODAT.
Finally the anti-inflammatory properties of CNIs could be responsible for the acutely improved insulin sensitivity, although the observed changes in hsCRP and adiponectin concentrations in this study do not entirely support this hypothesis (Table 1). Yet the role of CNIs on the inflammatory state is not fully known and existing data in this area have been obtained for the most part in small groups of transplant patients after conversion from one CNI to another [46, 47]. More importantly immunotherapy for preventing autoimmune diabetes has existed for decades. Thus it is still tempting to speculate that regulation of cytokines represents a possible mechanism for CNI mediated impact on glucose metabolism. The observed increases in glucose oxidation rates in our study propose the insulin receptor and the GLUT 4 transporter as additional candidates for such mediation [48, 49], rendering them prospects for future studies.
In conclusion acute effects of stable and clinically relevant doses of CsA and Tac in young and healthy human subjects are similar for the two immunosuppressive agents and include increased insulin sensitivity, without any effect on first phase or pulsatile insulin secretion. The findings are not ascribable to changes in circulating glucagon or FFA concentrations, but may partially be explained by an acute anti-inflammatory response of Tac. Further studies are warranted to evaluate the underlying pathophysiological mechanisms more substantially. The diabetogenicity of long-term treatment with these drugs remains controversial.
Acknowledgments
In memory of Melvin Madsen DMSc and Professor Ole Schmitz, who contributed in the most inspiring way to this study. We thank Annette Mengel M-Laboratory Aarhus University Hospital, Aarhus Sygehus, Denmark, Karin Hansen, Ilse Rasmussen and Birgitte Kildevaeld Sahl, C-Laboratory, Department of Nephrology, Aarhus University Hospital Skejby, Denmark for expert technical assistance. This work was supported in part by grants from The Research Foundation of the Danish Kidney Association, The Danish Society of Nephrology Research Foundation, The AP Moeller Foundation, Novartis a/s and Astellas Pharma a/s.
Competing Interests
LAØ, NM, CJ, MB, JC and JR: none. KAJ has received funds for research from Novartis a/s and Astellas Pharma a/s.
REFERENCES
- 1.Cosio FG, Kudva Y, van der Velde M, Larson TS, Textor SC, Griffin MD, Stegall MD. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415–21. doi: 10.1111/j.1523-1755.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–85. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El-Shahawy M, Budde K, Goto N DIRECT Investigators. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–14. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 4.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4:583–95. doi: 10.1046/j.1600-6143.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 5.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. 2002;25:583–92. doi: 10.2337/diacare.25.3.583. [DOI] [PubMed] [Google Scholar]
- 6.Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernandez D, Kasiske BL, Kiberd B, Krentz A, Legendre C, Marchetti P, Markell M, van der Woude FJ, Wheeler DC International Expert Panel. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75:SS3–24. doi: 10.1097/01.TP.0000069952.49242.3E. [DOI] [PubMed] [Google Scholar]
- 7.van Duijnhoven EM, Christiaans MH, Boots JM, Nieman FH, Wolffenbuttel BH, van Hooff JP. Glucose metabolism in the first 3 years after renal transplantation in patients receiving tacrolimus versus cyclosporine-based immunosuppression. J Am Soc Nephrol. 2002;13:213–20. doi: 10.1681/ASN.V131213. [DOI] [PubMed] [Google Scholar]
- 8.Gelens MA, Christiaans MH, van Hooff JP. Glucose metabolism before and after conversion from cyclosporine microemulsion to tacrolimus in stable renal recipients. Nephrol Dial Transplant. 2008;23:701–6. doi: 10.1093/ndt/gfm544. [DOI] [PubMed] [Google Scholar]
- 9.Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, Margreiter R, Hugo C, Grinyo JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF ELITE-Symphony Study. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–75. doi: 10.1056/NEJMoa067411. [DOI] [PubMed] [Google Scholar]
- 10.Hjelmesaeth J, Hartmann A, Kofstad J, Stenstrom J, Leivestad T, Egeland T, Fauchald P. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation. 1997;64:979–83. doi: 10.1097/00007890-199710150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Oterdoom LH, de Vries AP, Gansevoort RT, van Son WJ, van der Heide JJ, Ploeg RJ, de Jong PE, Gans RO, Bakker SJ. Determinants of insulin resistance in renal transplant recipients. Transplantation. 2007;83:29–35. doi: 10.1097/01.tp.0000245844.27683.48. [DOI] [PubMed] [Google Scholar]
- 12.Drachenberg CB, Klassen DK, Weir MR, Wiland A, Fink JC, Bartlett ST, Cangro CB, Blahut S, Papadimitriou JC. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68:396–402. doi: 10.1097/00007890-199908150-00012. [DOI] [PubMed] [Google Scholar]
- 13.Oetjen E, Baun D, Beimesche S, Krause D, Cierny I, Blume R, Dickel C, Wehner S, Knepel W. Inhibition of human insulin gene transcription by the immunosuppressive drugs cyclosporin A and tacrolimus in primary, mature islets of transgenic mice. Mol Pharmacol. 2003;63:1289–95. doi: 10.1124/mol.63.6.1289. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen JH, Mandrup-Poulsen T, Nerup J. Direct effects of cyclosporin A on human pancreatic beta-cells. Diabetes. 1986;35:1049–52. doi: 10.2337/diab.35.9.1049. [DOI] [PubMed] [Google Scholar]
- 15.Ozbay LA, Smidt K, Mortensen DM, Carstens J, Jorgensen KA, Rungby J. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS-1E beta-cells. Br J Pharmacol. 2011;62:136–46. doi: 10.1111/j.1476-5381.2010.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekstrand AV, Eriksson JG, Gronhagenriska C, Ahonen PJ, Groop LC. Insulin resistance and insulin deficiency in the pathogenesis of post transplantation diabetes in man. Transplantation. 1992;53:563–8. doi: 10.1097/00007890-199203000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Duijnhoven EM, Boots JM, Christiaans MH, Wolffenbuttel BH, Van Hooff JP. Influence of tacrolimus on glucose metabolism before and after renal transplantation: a prospective study. J Am Soc Nephrol. 2001;12:583–8. doi: 10.1681/ASN.V123583. [DOI] [PubMed] [Google Scholar]
- 18.Hjelmesaeth J, Hagen LT, Asberg A, Midtvedt K, Storset O, Halvorsen CE, Morkrid L, Hartmann A, Jenssen T. The impact of short-term ciclosporin A treatment on insulin secretion and insulin sensitivity in man. Nephrol Dial Transplant. 2007;22:1743–9. doi: 10.1093/ndt/gfl820. [DOI] [PubMed] [Google Scholar]
- 19.Kutkuhn B, Hollenbeck M, Heering P, Koch M, Voiculescu A, Reinhard T, Grabensee B. Development of insulin resistance and elevated blood pressure during therapy with cyclosporine A. Blood Press. 1997;6:13–7. doi: 10.3109/08037059709086440. [DOI] [PubMed] [Google Scholar]
- 20.Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, Du Rostu H, Rodier M, Sirmai J, Lallemand A. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2:119–24. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- 21.Undre NA, van Hooff J, Christiaans M, Vanrenterghem Y, Donck J, Heeman U, Kohnle M, Zanker B, Land W, Morales JM, Andres A, Schafer A, Stevenson P. Low systemic exposure to tacrolimus correlates with acute rejection. Transplant Proc. 1999;31:296–8. doi: 10.1016/s0041-1345(98)01633-9. [DOI] [PubMed] [Google Scholar]
- 22.Mahalati K, Belitsky P, West K, Kiberd B, Fraser A, Sketris I, Macdonald AS, McAlister V, Lawen J. Approaching the therapeutic window for cyclosporine in kidney transplantation: a prospective study. J Am Soc Nephrol. 2001;12:828–33. doi: 10.1681/ASN.V124828. [DOI] [PubMed] [Google Scholar]
- 23.Karamperis N, Povlsen JV, Hojskov C, Poulsen JH, Pedersen AR, Jorgensen KA. Comparison of the pharmacokinetics of tacrolimus and cyclosporine at equivalent molecular doses. Transplant Proc. 2003;35:1314–8. doi: 10.1016/s0041-1345(03)00481-0. [DOI] [PubMed] [Google Scholar]
- 24.Porksen N, Munn S, Steers J, Vore S, Veldhuis J, Butler P. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol. 1995;269:E478–88. doi: 10.1152/ajpendo.1995.269.3.E478. [DOI] [PubMed] [Google Scholar]
- 25.Juhl CB, Hollingdal M, Porksen N, Prange A, Lonnqvist F, Schmitz O. Influence of rosiglitazone treatment on beta-cell function in type 2 diabetes: evidence of an increased ability of glucose to entrain high-frequency insulin pulsatility. J Clin Endocrinol Metab. 2003;88:3794–800. doi: 10.1210/jc.2002-021181. [DOI] [PubMed] [Google Scholar]
- 26.Porksen N, Grofte T, Greisen J, Mengel A, Juhl C, Veldhuis JD, Schmitz O, Rossle M, Vilstrup H. Human insulin release processes measured by intraportal sampling. Am J Physiol Endocrinol Metab. 2002;282:E695–702. doi: 10.1152/ajpendo.00516.2000. [DOI] [PubMed] [Google Scholar]
- 27.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37:287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 28.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–34. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 29.Frystyk J, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia. 2005;48:1911–8. doi: 10.1007/s00125-005-1850-z. [DOI] [PubMed] [Google Scholar]
- 30.Robertson RP, Franklin G, Nelson L. Intravenous glucose tolerance and pancreatic islet beta-cell function in patients with multiple sclerosis during 2-yr treatment with cyclosporin. Diabetes. 1989;38:58–64. doi: 10.2337/diab.38.1.58. [DOI] [PubMed] [Google Scholar]
- 31.Konrad T, Steinmuller T, Vicini P, Toffolo G, Grewerus D, Schuller A, Bechstein WO, Usadel KH, Cobelli C, Neuhaus P. Regulation of glucose tolerance in patients after liver transplantation: impact of cyclosporin versus tacrolimus therapy. Transplantation. 2000;69:2072–8. doi: 10.1097/00007890-200005270-00017. [DOI] [PubMed] [Google Scholar]
- 32.Rickels MR, Mueller R, Teff KL, Naji A. {beta}-Cell secretory capacity and demand in recipients of islet, pancreas, and kidney transplants. J Clin Endocrinol Metab. 2010;95:1238–46. doi: 10.1210/jc.2009-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boots JM, van Duijnhoven EM, Christiaans MH, Wolffenbuttel BH, van Hooff JP. Glucose metabolism in renal transplant recipients on tacrolimus: the effect of steroid withdrawal and tacrolimus trough level reduction. J Am Soc Nephrol. 2002;13:221–7. doi: 10.1681/ASN.V131221. [DOI] [PubMed] [Google Scholar]
- 34.Midtvedt K, Hjelmesaeth J, Hartmann A, Lund K, Paulsen D, Egeland T, Jenssen T. Insulin resistance after renal transplantation: the effect of steroid dose reduction and withdrawal. J Am Soc Nephrol. 2004;15:3233–9. doi: 10.1097/01.ASN.0000145435.80005.1E. [DOI] [PubMed] [Google Scholar]
- 35.Strumph P, Kirsch D, Gooding W, Carroll P. The effect of FK506 on glycemic response as assessed by the hyperglycemic clamp technique. Transplantation. 1995;60:147–51. [PubMed] [Google Scholar]
- 36.Fernandez LA, Lehmann R, Luzi L, Battezzati A, Angelico MC, Ricordi C, Tzakis A, Alejandro R. The effects of maintenance doses of FK506 versus cyclosporin A on glucose and lipid metabolism after orthotopic liver transplantation. Transplantation. 1999;68:1532–41. doi: 10.1097/00007890-199911270-00017. [DOI] [PubMed] [Google Scholar]
- 37.Martin F, Bedoya FJ. Mechanisms of action of cyclosporin A on islet alpha- and beta-cells. Effects on cAMP- and calcium-dependent pathways. Life Sci. 1991;49:1915–21. doi: 10.1016/0024-3205(91)90293-k. [DOI] [PubMed] [Google Scholar]
- 38.D'Alessio DA. Taking aim at islet hormones with GLP-1: is insulin or glucagon the better target? Diabetes. 2010;59:1572–4. doi: 10.2337/db10-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christiansen JJ, Djurhuus CB, Gravholt CH, Iversen P, Christiansen JS, Schmitz O, Weeke J, Jorgensen JO, Moller N. Effects of cortisol on carbohydrate, lipid, and protein metabolism: studies of acute cortisol withdrawal in adrenocortical failure. J Clin Endocrinol Metab. 2007;92:3553–9. doi: 10.1210/jc.2007-0445. [DOI] [PubMed] [Google Scholar]
- 40.Bessey PQ, Brooks DC, Black PR, Aoki TT, Wilmore DW. Epinephrine acutely mediates skeletal muscle insulin resistance. Surgery. 1983;94:172–9. [PubMed] [Google Scholar]
- 41.Moller N, Jorgensen JO, Schmitz O, Moller J, Christiansen J, Alberti KG, Orskov H. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am J Physiol. 1990;258:E86–91. doi: 10.1152/ajpendo.1990.258.1.E86. [DOI] [PubMed] [Google Scholar]
- 42.Rickels MR, Schutta MH, Mueller R, Kapoor S, Markmann JF, Naji A, Teff KL. Glycemic thresholds for activation of counterregulatory hormone and symptom responses in islet transplant recipients. J Clin Endocrinol Metab. 2007;92:873–9. doi: 10.1210/jc.2006-2426. [DOI] [PubMed] [Google Scholar]
- 43.Lafuente A, Alvarez-Demanuel E, Blanco A, Garcia-Bonacho M, Esquifino AI. Effects of cyclosporine on circulating levels of prolactin, LH, FSH, TSH and GH in chronic hyperprolactinemic male rats. Rev Esp Fisiol. 1996;52:161–6. [PubMed] [Google Scholar]
- 44.Klein IH, Abrahams AC, van Ede T, Oey PL, Ligtenberg G, Blankestijn PJ. Differential effects of acute and sustained cyclosporine and tacrolimus on sympathetic nerve activity. J Hypertens. 2010;28:1928–34. doi: 10.1097/HJH.0b013e32833c20eb. [DOI] [PubMed] [Google Scholar]
- 45.Valera Mora ME, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr. 2003;22:487–93. doi: 10.1080/07315724.2003.10719326. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura K, Kishikawa H, Kato T, Kobayashi Y, Fujii N, Takahara S, Ichikawa Y. Tacrolimus and angiotensin receptor blockers associated with changes in serum adiponectin level in new-onset diabetes after renal transplantation: single-center cross-sectional analysis. Transpl Int. 2009;22:694–701. doi: 10.1111/j.1432-2277.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- 47.Lauzurica R, Pastor MC, Bayes B, Malumbres S, Homs M, Llopis MA, Bonet J, Romero R. Subclinical inflammation in renal transplant recipients: impact of cyclosporine microemulsion versus tacrolimus. Transplant Proc. 2007;39:2170–2. doi: 10.1016/j.transproceed.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 48.Behme MT, Dupre J, Stiller CR. Effect of cyclosporine on insulin binding to erythrocytes in type 1 diabetes mellitus of recent onset. Clin Invest Med. 1988;11:113–22. [PubMed] [Google Scholar]
- 49.Garcia-Roves PM, Jones TE, Otani K, Han DH, Holloszy JO. Calcineurin does not mediate exercise-induced increase in muscle GLUT4. Diabetes. 2005;54:624–8. doi: 10.2337/diabetes.54.3.624. [DOI] [PubMed] [Google Scholar]


