Abstract
AIMS
To investigate the influence of ABCB1 (1236-2677-3435) polymorphisms on nortriptyline pharmacokinetics and nortriptyline-induced postural hypotension in healthy volunteers.
METHODS
Genetic screening of 67 healthy volunteers identified eight CGC homozygotes and nine TTT homozygotes of ABCB1 (1236-2677-3435), who were administered a single dose of nortriptyline 25 mg. Plasma exposure of nortriptyline and its active metabolites, E- and Z-10-hydroxynortriptyline, was determined over 72 h. Heart rate and blood pressure responses to posture change (active standing and passive head-up tilt) were measured continuously using finger plethysmography.
RESULTS
There were no differences in plasma exposure between ABCB1 haplotype groups, as the geometric mean (95% CI) AUC(0,72 h) ratios were 0.98 (0.94, 1.03), 1.02 (0.96, 1.09) and 0.95 (0.80, 1.10) for nortriptyline, E- and Z-10-hydroxynortriptyline, respectively. The pre dose heart rate response to standing was greater in the TTT than CGC homozygotes (mean (95% CI) difference 7.4 (1.5, 13.4) beats min–1, P = 0.02). At tmax at 8 h post dose, nortriptyline increased the heart rate response to posture change in all subjects with mean (95% CI) Δ heart rate values of 7.4 (3.6, 11.3) beats min–1 on active standing (P = 0.0009) and 4.8 (2.0, 7.6) beats min–1 on head-up tilt (P = 0.002), but no difference was observed between haplotype groups. There was no difference in blood pressure response to posture change in either group.
CONCLUSION
The association between ABCB1 polymorphisms and nortriptyline-induced postural hypotension found in the previous study could not be confirmed. The results raise the possibility of a predisposition in heart rate response in the TTT homozygotes rather than an effect of nortriptyline.
Keywords: ABCB1, nortriptyline, P-glycoprotein, postural hypotension, tricyclic antidepressants
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
A single nucleotide polymorphism in ABCB1, which encodes P-glycoprotein, has retrospectively been associated with symptoms of nortriptyline-induced postural hypotension in depressed patients.
This finding needs to be replicated in independent studies before recommendations regarding pharmacogenetic testing can be made.
WHAT THIS STUDY ADDS
In a prospective study of healthy volunteers homozygous for ABCB1 (1236-2677-3435, TTT/TTT or CGC/CGC), a single dose of nortriptyline was administered, plasma exposure was determined and blood pressure and heart rate were monitored during posture change.
No differences between ABCB1 haplotype groups were found in plasma exposure of nortriptyline and its active metabolites, E- and Z-10-hydroxynortriptyline. The heart rate response to posture change was increased with nortriptyline, whereas there was no difference in blood pressure response. However, no differences between haplotype groups were observed except that the pre dose heart rate response to standing was greater in the TTT than CGC homozygotes.
The association between ABCB1 polymorphisms and nortriptyline-induced postural hypotension found in a previous study could not be confirmed. The results raise the possibility of a predisposition in heart rate response in the TTT homozygotes rather than an effect of nortriptyline.
Introduction
Nortriptyline is a tricyclic antidepressant (TCA) that was developed in the 1960s and remains in common use. While nortriptyline is used on its own, it is also produced as an active demethylated metabolite of amitriptyline. Its mechanism of action involves re-uptake inhibition of neurotransmitters, primarily of norepinephrine and serotonin to a lesser extent [1, 2]. Nortriptyline is metabolized via a phase I reaction in the liver by the cytochrome P450 isoenzyme 2D6 (CYP2D6) to mainly E-10-hydroxynortriptyline and to a minor extent the stereoisomer Z-10-hydroxynortriptyline. Both metabolites are active re-uptake inhibitors of norepinephrine, with E-10-hydroxynortriptyline having the greatest activity of the two, equivalent to approximately 50% of nortriptyline [1–3].
Adverse effects are a major clinical issue with nortriptyline and other TCAs and occur in as many as 20% of patients [4]. Anticholinergic side effects are common and include dry mouth, constipation, urinary retention and blurred vision while antagonism of histamine receptors causes sedation. The most common cardiovascular complication of TCAs is postural hypotension (fall in blood pressure when changing from a supine to a standing position, also termed orthostatic hypotension) caused largely by α1-adrenoceptor blockade [1, 2, 4]. This is especially problematic in elderly patients, who become more prone to injury through falls. Other cardiac side effects are tachycardia (increased heart rate) and arrhythmias, which are at least partially caused by nortriptyline's anticholinergic effects [1, 2, 4].
Interindividual variability in predisposition to TCA-induced postural hypotension could in part be genetically determined. In a previous study [5] we found that a single nucleotide polymorphism (SNP) in ABCB1(3435C>T), the gene that encodes for P-glycoprotein, was associated with symptoms of nortriptyline-induced postural hypotension in depressed patients. Patients homozygous for the 3435T allele were more likely to experience symptoms of postural hypotension with nortriptyline than those with the 3435C allele (OR = 1.37, 95% CI 1.01, 1.86) [5]. This study received considerable attention in the literature and if the finding is confirmed by independent replication, it implies that a pharmacogenetic test could predict the risk of TCA-induced postural hypotension. This would be of considerable value, as patients at risk may then be preferentially prescribed antidepressants other than TCAs.
The ABCB1 gene is a member of the ATP-binding cassette (ABC) superfamily. The gene is also known as the multi-drug-resistence 1 (MDR1) gene and its product is a transmembrane active efflux pump called P-glycoprotein (P-gp). P-gp has been shown to be important in reducing the absorption of drugs from the intestinal lumen, providing active secretion of drugs into the bile or urine, and extrusion of drugs from vital organs such as the brain [6, 7]. Nortriptyline and its active metabolites E- and Z-10-hydroxynortriptyline are substrates for P-gp and have been shown to have enhanced penetration into the brain in P-gp knock-out mice compared with wild-type [8–11]. Polymorphisms in ABCB1 may be associated with different levels of P-gp expression and function [7]. A proposed explanation for the finding that ABCB1 polymorphisms were associated with symptoms of postural hypotension during nortriptyline therapy could be that accumulation of nortriptyline and/or its active metabolites within the brain occurs in subjects with lower expression of P-gp leading to higher rates of centrally mediated adverse effects [5].
The SNPs 1236C>T (G412G), 2677G>T (A893S), 3435C>T (I1145I) are the most common variants in the open reading frame of ABCB1 and together define the two most prevalent haplotypes (ABCB1*1 1236C-2677G-3435C; and ABCB1*13 1236T-2677T-3435T) [12]. In the recent past there was a tendency for studies investigating the association of ABCB1 with disease susceptibility or drug response, to focus solely on ABCB1 3435C>T. This approach has yielded inconsistent results and raises the question as to whether 1236C>T and 2677G>T, which are in strong linkage disequilibrium with 3435C>T, also make a significant contribution to observed phenotype. This assertion is supported by the findings of recent pharmacogenetic studies that report stronger and more consistent associations of ABCB1 with drug efficacy or toxicity when haplotype analysis is conducted rather than single marker testing [6, 7].
As our previous study was retrospective in terms of the ABCB1 polymorphism and not designed to investigate postural hypotension specifically, we wished to confirm or refute this finding in a prospective study designed with this objective. The aim of this study was to determine if the extent of postural hypotension with nortriptyline is greater in individuals with the 1236-2677-3435TTT/TTT vs. the 1236-2677-3435CGC/CGC haplotype pair of ABCB1. A secondary aim was to determine if any differences exist between haplotypes in the pharmacokinetics of nortriptyline and its main active metabolites, E- and Z-10-hydroxynortriptyline. As these metabolites are formed by CYP2D6, which is subject to genetic polymorphism, individuals with no functional alleles of this enzyme were excluded from the study, so as to leave only the effects of ABCB1 haplotypes.
Methods
Subjects
A total of 67 Caucasian healthy volunteers with Caucasian parents and grandparents underwent genetic screening for ABCB1 and CYP2D6. Inclusion criteria were; age of 18–50 years, BMI of 18.5–30 kg m–2, normotensive (sitting systolic/diastolic BP between 90/60 and 140/90 mm Hg), non-smoking, caffeine intake of ≤300 mg day–1 (≤three standard caffeine-containing beverages), no intake of systemic medications except oral contraceptives and no history of syncope or seizures. The health of the subjects was ascertained by medical history, physical examination, electrocardiogram and blood tests for renal and liver function, electrolytes and blood count. Ethical approval (no. URB/07/06/017) was obtained from the Upper South B Regional Ethics Committee, New Zealand and informed written consent was obtained from all participants after full explanation of what was involved.
DNA extraction and genotyping
Genomic DNA was collected from 5 ml peripheral blood samples using phenol chloroform extraction [13, 14]. A two tube allele-specific PCR was used simultaneously to test subjects for the ABCB1 SNPs 1236C>T (rs1128503), 2677G>T/A (rs2032582) and 3435C>T (rs1045642). Since the variant ABCB1 2677A occurs at a very low frequency (<2%) in Caucasians [15], and was therefore unlikely to occur in this study (n = 17), only primers for the detection of 2677G and 2677T were included in this assay. As 2677A was not tested for, we cannot rule out the possibility that one or more of the volunteers included in the single dose study were CGC/CAC or TTT/TAT, instead of homozygous for the CGC or TTT haplotypes. However, given the rarity of the ABCB1 2677A allele this is very unlikely. For ease of resolution, common primers were positioned so that all allele-specific PCR products were between 150 bp and 350 bp and differed in size by 100 bp (primer sequences available on request). To ensure specificity all allele-specific primers were 30 bases and the second base from the 3′ end of each primer was changed to the corresponding base in the complementary sequence. These 3′ penultimate mismatches destabilized the primers, preventing mis-priming, and thereby ensured that amplification only occurred in the presence of the correct allele. The primers β2Mf and β2Mr were included in each reaction to amplify a 557 bp region of the beta-2-microglobulin gene (β2M) which served as an ABCB1-independent internal control for PCR failure. Each reaction was performed in a total volume of 10 µl containing 200 µm dNTPs, 3 mm MgCl2, 0.5 µm of the primers β2Mf and β2Mr, 0.5 µm of each of the three common primers, 1 U of platinum Taq DNA polymerase (5 U µl−1) (Invitrogen, CA, USA) and ∼50 ng of DNA. The allele-specific primers were split across the two reactions. Thermal cycling conditions comprised an initial denaturation of 2 min at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 65°C, 30 s at 72°C and a final extraction of 2 min at 72°C. Reactions were visualized using 3% agarose gel electrophoresis. The accuracy of genotyping was checked by repeat analysis of 5% of samples and by sequencing another 5% of samples and no inconsistencies were found. ABCB1 haplotypes were inferred from SNP genotypes.
Study participants who were homozygotes for the ABCB1 1236-2677-3435 TTT or CGC haplotype were screened for common CYP2D6 non-functional alleles and reduced activity alleles using a previously described two step allele-specific PCR [16]. Briefly, a long-range PCR was performed in the first step of this assay to test simultaneously for the CYP2D6*5 (deletion) allele, whole gene duplications, and to isolate CYP2D6 from the CYP2D gene cluster to avoid subsequent mis-genotyping as a result of co-amplification of related pseudogene sequences. In the second step the 4.7 kb CYP2D6-specific PCR product was diluted and used as a template in a two tube allele-specific PCR designed to detect the non-functional alleles CYP2D6*3, *4, *6 and the reduced activity alleles CYP2D6*9, *10, *41[16]. Study participants who tested negative for these variant alleles were assumed to be homozygous for the wild type alleles (CYP2D6*1). CYP2D6 activity scores [17] were calculated with the functional allele (CYP2D6*1) assigned a value of 1, reduced activity alleles (CYP2D6*9, *10 and *41) assigned a value of 0.75 and non-functional alleles (CYP2D6*3, *4, *5 and *6) assigned a value of 0. The activity score of an individual was obtained by adding the activity values of the two alleles carried by that individual. Subjects with a CYP2D6 activity score <1 (i.e. poor metabolizers of CYP2D6) were excluded from the study.
Study design
The eligible subjects received a single oral dose of nortriptyline 25 mg (Norpress, Mylan New Zealand Ltd) with 150 ml of water in the morning of the study day. The subjects were asked to abstain from caffeine 12 h before the study and throughout the study day. The subjects had finished breakfast at least 2 h before the start of the study, a yoghurt was served 2 h after nortriptyline administration, a standard lunch after 4 h and a piece of fruit after 6 h. At 0 h (pre dose), 2, 4, 6 and 8 h post dose, heart rate (HR) and beat-to-beat blood pressure (BP) were monitored non-invasively from the right middle finger at heart level using finger plethysmography (Finapres, Ohmeda, Englewood, CO, USA) [18]. Passive head-up tilt (HUT) to the 70° position was performed on a hydraulic tilt table with foot support. For HUT, the time to get from horizontal to head-up position was approximately 20 s and for active standing (AS) less than 5 s. Recordings were undertaken continuously during the following sequence: 1) 5 min at rest in horizontal supine position, 2) 5 min of HUT, 3) 5 min at rest after return to the horizontal position and 4) 2 min immediately after AS. For the supine position, the recordings were averaged over the last 120 s before HUT or AS. For HUT, 60 s epochs were averaged from the 2nd and 5th min. For AS, maximum HR and minimum BP (occurring about 10–12 s after standing) and minimum HR and maximum BP (occurring about 20 s after standing) were averaged from 3 s epochs. After AS, 15 s epochs, centred at the end of the 1st and 2nd min were averaged. During AS, the BP signal was more subject to movement artefact and interruption by calibration inflations than during passive HUT and therefore shorter time intervals were sampled.
Determination of nortriptyline and metabolite concentrations
Venous blood samples (8 ml) were taken at 0 (pre dose) and 2, 4, 6, 8, 24, 48 and 72 h post dose (after the BP and HR measurements for the 0–8 h samples). The blood samples were collected in K2EDTA tubes and centrifuged within 30 min. Plasma samples were stored at −80°C until analysis. A liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay was developed to determine nortriptyline, E- and Z-10-hydroxynortriptyline concentrations in plasma [19]. Briefly, plasma samples were precipitated with acetonitrile, separated on a C18 column with a mobile phase consisting of 0.1% formic acid in an acetonitrile gradient over 6 min and detected by MS/MS. Nortriptyline-D3 was used as internal standard and the assay was validated according to FDA guidelines. Standard curves over the range 0.2–40 µg l−1 for nortriptyline and 0.5–40 µg l−1 for the metabolites were used. Each plasma sample was analyzed in triplicate and outlier analysis of triplicate values was based on a 95% confidence interval (CI) and 10% interday CV. QC and calibration samples for all analytes were all within 15% CV and bias. Concentration ranges in the post dose samples were within the ranges 0.4–15 µg l−1 for nortriptyline, 0.6–29 µg l−1 for E-10-hydroxynortriptyline and 0.5–3.9 µg l−1 for Z-10-hydroxynortriptyline.
Pharmacokinetics
Pharmacokinetic parameters were calculated for nortriptyline, E- and Z-10-hydroxynortriptyline. The peak plasma concentration (Cmax) and time to Cmax (tmax) were estimated directly from the data. The areas under the plasma concentration–time curve from 0–72 h (AUC(0,72 h)) were calculated using the linear trapezoidal rule. The AUC from 0 h to infinity (AUC(0,∞)) was calculated as AUC(0,72 h) + Ct/λz, where Ct was the last measured plasma concentration and λz was determined using linear regression analysis of the logarithm-linear part of the plasma concentration–time curve.
Statistical analysis
Results are expressed as mean (SEM) values or with 95% CIs. Values for Cmax and AUC were normalized to the mean weight-adjusted nortriptyline dose and logarithmically transformed. The geometric mean ratio between haplotype groups was calculated for AUC values. Differences between groups were assessed using paired or unpaired two-tailed Student's t-tests as appropriate. For tmax comparisons Mann-Whitney tests (non-parametric) were used. For all statistical analyses, a P value <0.05 was considered significant. GraphPad Prism ver. 4.0 software was used for statistical analysis.
Results
A total of 67 Caucasian healthy volunteers underwent genetic screening for ABCB1. The subjects who were homozygotes for the 1236-2677-3435TTT haplotype (n = 15, i.e. 22%) or the 1236-2677-3435CGC haplotype (n = 9, i.e. 13%) were then genotyped for CYP2D6. Four of these subjects (17%, three TTT and one CGC homozygote) were poor metabolizers of CYP2D6 and were excluded from the study to avoid major differences in CYP2D6 metabolism. Three volunteers were subsequently excluded; two had started antidepressant therapy and one had moved overseas, resulting in nine TTT homozygotes and eight CGC homozygotes participating in the study. The demographic characteristics of the study participants are shown in Table 1. There were no statistically significant differences between haplotype groups in any of these parameters. All subjects had at least one functional allele of CYP2D6. The mean CYP2D6 activity score was 1.7 (0.1) and did not differ between haplotype groups (P = 0.61).
Table 1.
Mean (SEM) demographic characteristics of all healthy volunteers (n = 17) and within the ABCB1 haplotype pairs TTT/TTT (n = 9) and CGC/CGC (n = 8)
| Characteristic | All | TTT/TTT | GCG/CGC | P-value* |
|---|---|---|---|---|
| Age (years) | 33 (2) | 30 (3) | 37 (4) | 0.18 |
| Weight (kg) | 74 (4) | 72 (4) | 77 (7) | 0.60 |
| Height (cm) | 170 (12) | 168 (3) | 172 (3) | 0.38 |
| BMI (kg m–2) | 25 (1) | 25 (1) | 26 (2) | 0.96 |
| CLCr (ml min−1)† | 94 (4) | 93 (8) | 97 (7) | 0.66 |
| Gender (male/female) | 5/12 | 2/7 | 3/5 | 0.62 |
TTT/TTT vs. CGC/CGC, unpaired t-test, Fisher's exact test for gender.
Cockcroft & Gault.
The mean weight-adjusted dose of nortriptyline was 0.35 (0.02) mg kg−1, which did not differ between the two haplotype groups (P = 0.79). The exposure to nortriptyline and its active metabolites E- and Z-hydroxynortriptyline over 72 h was determined for each of the subjects. Plasma concentration–time curves are depicted in Figure 1. The results for dose-normalized Cmax and area under the curve (AUC(0,72 h), AUC(0,∞)) values are given in Table 2 along with tmax and t1/2 values. The geometric mean AUC ratios obtained from the TTT/TTT and CGC/CGC haplotypes differed ≤5% in all cases and no statistically significant differences were found for any of the pharmacokinetic parameters between the haplotypes. No influence of gender on the pharmacokinetic parameters was found, nor were there any trends. The overall median tmax value for nortriptyline and its active metabolites was 7.8 h. This corresponds with the time (8 h) that the largest effects of nortriptyline were observed and the 8 h data are detailed below. The 6 h response was similar.
Figure 1.
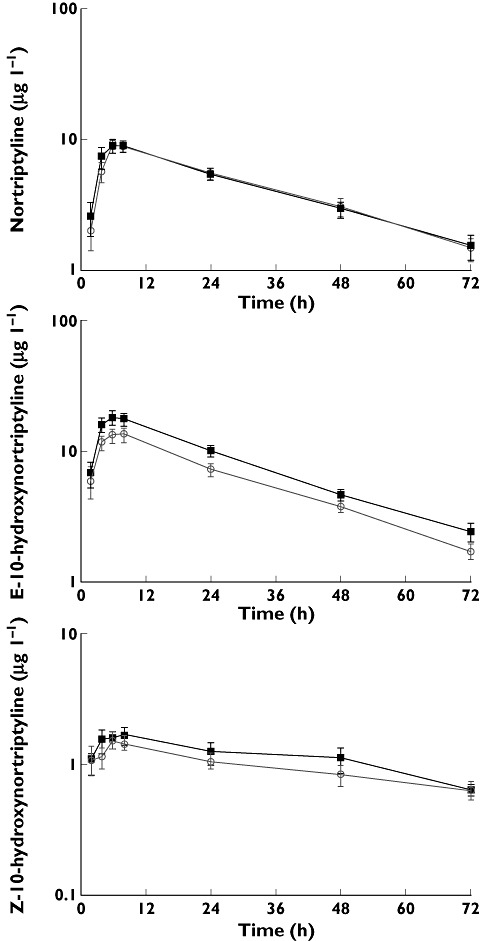
Mean ± SEM plasma concentration–time curves following a single oral dose of nortriptyline 25 mg to healthy volunteers with the ABCB1 haplotype pairs TTT/TTT ( , n = 9) and CGC/CGC (
, n = 9) and CGC/CGC ( , n = 8)
, n = 8)
Table 2.
Pharmacokinetic variables following administration of a single oral dose of nortriptyline 25 mg to healthy volunteers with the ABCB1 haplotype pairs TTT/TTT (n = 9) and CGC/CGC (n = 8)
| ABCB1 haplotype | Cmax (µg l−1) | tmax (h) | t1/2 (h) | AUC(0,72 h) (µg l−1 h) | AUC(0,∞) (µg l−1 h) |
|---|---|---|---|---|---|
| Nortriptyline | |||||
| TTT/TTT | 9.4 (7.4, 11.8) | 7.8 (3.8–8.0) | 24 (20, 28) | 281 (232, 340) | 350 (286, 429) |
| CGC/CGC | 9.5 (8.0, 11.1) | 7.4 (4.0–8.0) | 24 (20, 28) | 313 (279, 351) | 364 (315, 419) |
| Mean ratio | 0.98 (0.94, 1.03) | 0.99 (0.95, 1.04) | |||
| P value | 0.94 | 0.89 | 0.82 | 0.38 | 0.77 |
| E-10-hydroxynortriptyline | |||||
| TTT/TTT | 17.0 (12.8, 22.6) | 7.8 (5.9–8.0) | 31 (22, 39) | 504 (385, 660) | 665 (524, 842) |
| CGC/CGC | 14.0 (10.0, 19.4) | 6.0 (4.0–7.9) | 23 (20, 26) | 440 (343, 566) | 500 (390–641) |
| Mean ratio | 1.02 (0.96, 1.09) | 1.05 (0.99, 1.11) | |||
| P value | 0.39 | 0.32 | 0.16 | 0.49 | 0.13 |
| Z-10-hydroxynortriptyline | |||||
| TTT/TTT | 1.6 (1.2, 2.1) | 7.8 (3.8–8.0) | 37 (25, 49) | 47 (31, 71) | 91 (58, 142) |
| CGC/CGC | 1.6 (1.4, 1.9) | 7.9 (5.1–8.1) | 31 (25, 36) | 57 (44, 76) | 88 (69, 112) |
| Mean ratio | 0.95 (0.80, 1.10) | 1.01 (0.87, 1.15) | |||
| P value | 0.89 | 0.30 | 0.43 | 0.47 | 0.90 |
These data are geometric mean (95% CI) for Cmax and AUC values (normalized to the mean nortriptyline dose of 0.35 mg kg–1), geometric mean ratio (95% CI) of AUC values for TTT/TTT over CGC/CGC, median (range) for tmax, mean (95% CI) for t1/2. P values obtained using unpaired t-test, Mann-Whitney test for tmax.
The changes in HR and BP responses to postural change were measured before and after nortriptyline. Figure 2 (A,C,E) shows the response in HR and BP in the first 2 min following changing from supine to active standing (AS) in all subjects pre (0 h) and post (8 h) treatment with nortriptyline. A normal response to AS was seen [18], with an initial decrease in BP, followed immediately by a baroreflex-mediated increase in HR and a BP overshoot, after which the HR and diastolic BP remained raised, whereas systolic BP gradually returned to baseline. The greatest effect of nortriptyline was observed in the early phase of stabilization (1–2 min), and the 1 and 2 min data were therefore averaged to represent the AS response. For passive head-up tilt (HUT) the changes were smaller and more gradual as expected [18] and the 2 and 5 min results were averaged to represent the HUT response (Figure 2B,D,F).
Figure 2.
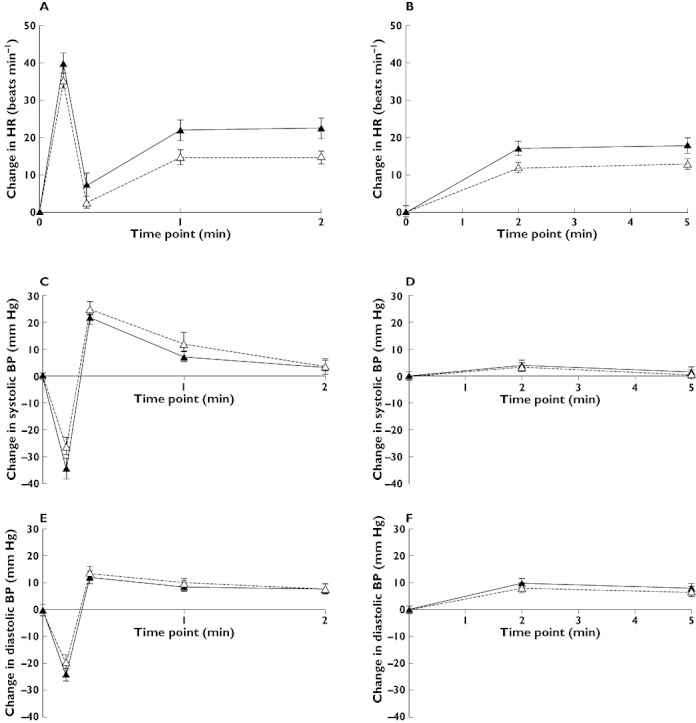
Mean ± SEM change in heart rate (HR) and systolic and diastolic blood pressure (BP) when changing from supine position to active standing (A,C,E) and 70° head-up tilt (B,D, F) at 0 h (▵) and 8 h after nortriptyline 25 mg (▴)
The postural changes in HR and BP after AS and HUT both pre (0 h) and post (8 h) treatment with nortriptyline, along with Δ values between pre and post dose, are shown for all subjects and each of the ABCB1 haplotype groups in Table 3. No differences were observed in the pre dose responses, except in the HR response to AS, which was greater in the TTT than CGC homozygotes (mean difference 7.4 (1.5, 13.4) beats min–1, P = 0.02). Nortriptyline resulted in an augmented HR response in all subjects with mean (95% CI) Δ HR values of 7.4 (3.6, 11.3) beats min–1 on AS (P = 0.0009) and 4.8 (2.0, 7.6) beats min–1 on HUT (P = 0.002). However, no differences could be shown between ABCB1 haplotype groups in Δ HR values as the mean differences (95% CI) were 0.5 (–7.6, 8.5) beats min–1 on AS and 0.7 (–5.1, 6.5) beats min–1 on HUT. There were no significant differences in BP in response to AS or HUT within either group or between groups. No influence of gender on the postural response was found, nor were there any trends. For completeness, the absolute values of HR and BP in supine, AS and HUT positions are shown in Table 4. There were no differences within either group or between groups.
Table 3.
Mean (95% CI) changes in heart rate (HR), systolic and diastolic blood pressure (BP) upon change from supine to active standing and supine to 70° head-up tilt position, pre and post treatment with nortriptyline 25 mg in all healthy volunteers (n = 17) and within ABCB1 haplotype pairs TTT/TTT (n = 9) and CGC/CGC (n = 8)
| Change in HR (beats min–1) | Pre dose (0 h) | Post dose (8 h) | Δ (0 h to 8 h) | P value (Δ)‡ |
|---|---|---|---|---|
| Supine to active standing | ||||
| All | 15.0 (11.5, 18.5) | 22.4 (16.9, 28.0) | 7.4 (3.6, 11.3) | 0.0009 |
| TTT/TTT | 18.5 (15.2, 21.8) | 26.2 (19.9, 32.5) | 7.7 (2.5, 12.8) | 0.009 |
| CGC/CGC | 11.1 (5.1, 17.0) | 18.3 (8.0, 28.5) | 7.2 (−0.2, 14.6) | 0.06 |
| Mean difference* | 7.4 (1.5, 13.4) | 7.9 (−2.8, 18.6) | 0.5 (−7.6, 8.5) | |
| P value† | 0.02 | 0.14 | 0.90 | |
| Supine to head-up tilt | ||||
| All | 13.0 (9.9, 16.0) | 17.8 (13.4, 22.1) | 4.8 (2.0, 7.6) | 0.002 |
| TTT/TTT | 15.1 (11.3, 18.9) | 20.3 (15.5, 25.0) | 5.1 (0.3, 9.9) | 0.04 |
| CGC/CGC | 10.8 (5.7, 16.0) | 15.3 (7.0, 23.5) | 4.4 (0.2, 8.7) | 0.04 |
| Mean difference | 4.3 (−1.5, 10.1) | 5.0 (−3.7, 13.7) | 0.7 (−5.1, 6.5) | |
| P value | 0.13 | 0.24 | 0.80 |
| Change in systolic BP (mmHg) | ||||
|---|---|---|---|---|
| Supine to active standing | ||||
| All | 7.2 (3.3, 11.2) | 4.1 (−0.8, 9.0) | −3.1 (−9.7, 3.6) | 0.33 |
| TTT/TTT | 4.8 (0.4, 9.2) | 5.8 (−2.0, 13.6) | 0.9 (−6.5, 8.4) | 0.78 |
| CGC/CGC | 9.9 (2.5, 17.4) | 2.2 (−5.2, 9.6) | −7.7 (−20.4, 5.0) | 0.19 |
| Mean difference | −5.1 (−12.7, 2.5) | 3.6 (−6.3, 13.5) | 8.6 (−4.4, 21.7) | |
| P value | 0.17 | 0.45 | 0.18 | |
| Supine to head-up tilt | ||||
| All | 2.1 (−0.9, 5.0) | 2.9 (−0.4, 6.0) | 0.8 (−3.8, 5.4) | 0.72 |
| TTT/TTT | 1.6 (−2.4, 5.5) | 3.0 (−2.2, 8.3) | 1.4 (−4.6, 7.5) | 0.60 |
| CGC/CGC | 2.6 (−3.0, 8.2) | 2.7 (−2.3, 7.7) | 0.1 (−8.8, 8.9) | 0.99 |
| Mean difference | −1.1 (−7.2,5.0) | 0.3 (−6.4, 7.0) | 1.4 (−6.5, 9.3) | |
| P value | 0.71 | 0.92 | 0.76 |
| Change in diastolic BP (mmHg) | ||||
|---|---|---|---|---|
| Supine to active standing | ||||
| All | 9.1 (5.6, 12.5) | 8.3 (4.6, 12.0) | −0.7 (−5.2, 3.8) | 0.73 |
| TTT/TTT | 9.7 (6.9, 12.5) | 8.8 (3.0, 14.7) | −0.8 (−6.0, 4.5) | 0.72 |
| CGC/CGC | 8.4 (0.7, 16.0) | 7.7 (1.7, 13.7) | −0.7 (−10.0, 8.7) | 0.87 |
| Mean difference | 1.3 (−5.7, 8.4) | 1.1 (−6.5, 8.8) | −0.1 (−9.6, 4.4) | |
| P value | 0.70 | 0.76 | 0.98 | |
| Supine to head-up tilt | ||||
| All | 7.2 (5.7, 8.6) | 8.9 (5.5, 12.2) | 1.7 (−1.5, 4.9) | 0.28 |
| TTT/TTT | 7.7 (5.3, 10.0) | 8.2 (4.6, 11.8) | 0.5 (−2.7, 3.8) | 0.71 |
| CGC/CGC | 6.6 (4.3, 8.9) | 9.6 (2.6, 16.6) | 3.0 (−3.8, 9.8) | 0.33 |
| Mean difference | 1.0 (−1.9, 4.1) | −1.4 (−8.3, 5.5) | −2.5 (−9.1, 4.1) | |
| P value | 0.77 | 0.67 | 0.43 |
Mean difference (95% CI) between TTT/TTT and CGC/CGC
unpaired t-test
paired t-test.
Table 4.
Mean (SEM) absolute values of heart rate (HR), systolic and diastolic blood pressure (BP) at supine, active standing and 70° head-up tilt position, pre- (0 h) and post- (8 h) treatment with nortriptyline 25 mg in healthy volunteers with ABCB1 haplotype pairs TTT/TTT (n = 9) and CGC/CGC (n = 8)
| Absolute HR (bpm) | Absolute Sys BP (mmHg) | Absolute Dia BP (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ABCB1 haplotype | Pre-dose | Post-dose | P-value* | Pre-dose | Post-dose | P-value | Pre-dose | Post-dose | P-value |
| Supine (before AS) | |||||||||
| TTT/TTT | 64.6 (3.5) | 60.7 (3.2) | 0.21 | 122.0 (3.3) | 127.0 (4.5) | 0.18 | 66.0 (1.4) | 67.8 (1.6) | 0.14 |
| CGC/CGC | 60.1 (5.1) | 57.9 (2.3) | 0.49 | 128.4 (3.9) | 128.0 (3.0) | 0.92 | 67.6 (2.7) | 69.3 (0.8) | 0.51 |
| P-value† | 0.47 | 0.50 | 0.22 | 0.86 | 0.54 | 0.45 | |||
| Active standing (AS) | |||||||||
| TTT/TTT | 82.8 (3.5) | 86.5 (3.6) | 0.26 | 126.7 (4.4) | 132.8 (3.8) | 0.05 | 75.6 (1.9) | 76.6 (2.0) | 0.63 |
| CGC/CGC | 70.9 (6.8) | 75.6 (5.8) | 0.09 | 138.3 (5.0) | 130.1 (4.7) | 0.15 | 75.9 (2.8) | 77.1 (2.7) | 0.66 |
| P-value | 0.13 | 0.13 | 0.10 | 0.66 | 0.93 | 0.92 | |||
| Supine (before HUT) | |||||||||
| TTT/TTT | 68.8 (3.5) | 62.8 (3.1) | 0.11 | 123.3 (3.9) | 126.1 (3.7) | 0.48 | 67.9 (1.3) | 71.1 (1.9) | 0.09 |
| CGC/CGC | 61.0 (4.8) | 61.0 (2.7) | 1.00 | 123.6 (3.4) | 126.3 (4.1) | 0.60 | 67.0 (2.7) | 70.6 (3.0) | 0.49 |
| P-value | 0.20 | 0.67 | 0.96 | 0.98 | 0.75 | 0.88 | |||
| Head-up tilt (HUT) | |||||||||
| TTT/TTT | 83.3 (3.1) | 83.1 (3.8) | 0.68 | 124.9 (5.0) | 129.1 (4.4) | 0.35 | 75.6 (2.1) | 79.3 (2.3) | 0.16 |
| CGC/CGC | 71.8 (6.3) | 76.3 (5.1) | 0.09 | 126.3 (2.2) | 128.9 (4.1) | 0.47 | 73.6 (2.5) | 80.2 (1.7) | 0.06 |
| P-value | 0.11 | 0.30 | 0.82 | 0.98 | 0.55 | 0.76 | |||
paired t-test
unpaired t-test.
Discussion
This study was undertaken to see if the association found in a previous study between a SNP in ABCB1 and symptoms of postural hypotension in depressed patients on nortriptyline therapy [5] could be confirmed by objective measurements of HR and BP responses in a prospective study designed to investigate postural hypotension specifically. The previous study suggested that patients, who were on nortriptyline, were more likely to experience symptoms of postural hypotension if they were homozygous for the T allele of ABCB1(3435C>T).
The current study was performed in healthy volunteers, who were on no other systemic drugs, to minimize the risk of other medications influencing the results. Although elderly patients are more prone to postural hypotension and the signal may be greater in this group, the previous study involved patients with a mean age of 30 years, which is similar to that of the volunteers in the current study (mean age 33 years). To ensure genetic uniformity, all volunteers were of one ethnicity (third generation Caucasians), had homozygous haplotypes of ABCB1 (1236-2677-3435) and none was a CYP2D6 poor metabolizer. The ABCB1 haplotype frequencies observed in the screening of participants for this study were comparable with those reported in other Caucasian populations [12, 20, 21] whereas the CYP2D6 poor metabolizer frequency was slightly higher [22].
There was no difference between the ABCB1 haplotype groups in the pharmacokinetic variables for nortriptyline and its active metabolites E- and Z-10-hydroxynortriptyline. The 95% CIs of the AUC ratios of haplotype groups were all within 0.80, 1.15 (Table 2). It is generally accepted that the 90% CI for this measure should be within 0.80, 1.25 for treatments to be considered bioequivalent [23]. The study was thus adequately powered to show differences well below what would be considered clinically relevant and we therefore conclude that polymorphism of ABCB1 did not influence nortriptyline pharmacokinetics. This is similar to what was found in most other studies as reviewed in Leschziner et al. [7].
The use of continuous monitoring of HR and BP using finger plethysmography is an improvement over previous studies on nortriptyline, which have either relied on reported symptoms [5] or intermittent manual measurements [24–31]. Haemodynamic changes over short time intervals could be discerned and normal responses in HR and BP were observed pre dose [18] indicating adequate sensitivity of the method. Although technically easier, passive HUT was found not to offer increased sensitivity as compared with AS. This was also observed by Scalco et al. [30].
The healthy volunteers were administered a single dose of 25 mg nortriptyline, which is a common starting dose for nortriptyline therapy. It is possible that responses may differ after a single dose compared with multiple dosing. Arguably, effects might be expected to be small compared with previous studies in patients on regular nortriptyline therapy. The augmented postural HR response after nortriptyline seen in this study has not been detected in previous patient studies, whereas an increase in supine HR was seen [26, 27, 31]. This may reflect the use of different methodologies. For the BP response to upright posture, no effect of nortriptyline was observed in this study. This is similar to some other reports [25–27] but in others, of predominantly older patients, nortriptyline was found to augment an orthostatic drop in systolic BP [24, 28–31]. In some cases this occurred upon initiation of nortriptyline therapy and did not correlate with nortriptyline plasma concentrations [24, 29, 30].
The only difference observed between haplotype groups for HR and BP responses was in the pre dose HR response, which was greater in the TTT than CGC homozygotes. This would suggest that this group may be somehow predisposed to postural hypotension, which may explain the symptoms observed in the previous study [5]. Following nortriptyline, both groups had an almost identical increase in orthostatic HR response. The 95% CI of the mean difference between haplotype groups (−7.6, 8.5 beats min–1 on AS and −5.1, 6.5 beats min–1 on HUT) indicate that the study was sufficiently powered to pick up a difference of at least 10 beats min–1, which from a clinical perspective, would be of relevance. For BP no statistically significant change was seen when subjects changed to the upright position and this was similar for both haplotype groups. If anything, there was a trend towards a decrease in systolic BP on active standing in the CGC homozygotes following nortriptyline. This goes against the hypothesis that TTT homozygotes would be more prone to postural hypotension. We therefore conclude that there was no evidence in this study that the ABCB1 TTT haplotype was associated with nortriptyline-induced postural hypotension as observed in our previous study [5].
In a recent comprehensive review of ABCB1 genotypes and therapeutic drug response by Leschziner et al. [7] it was concluded that although extensive research has been conducted on ABCB1, the observed effects have generally been small, few findings have been replicated and no clear conclusions have been drawn to date. One explanation offered was that the effect size of functional variants in ABCB1 may be of such a small magnitude that very large samples sizes are required to detect associations, thus limiting the likely clinical value. While the number of subjects in this study was small, the method was sensitive enough to pick up effects of nortriptyline, but there was no change and no trend to support our hypothesis of an enhanced effect in the TTT haplotypes. Thus, in this case, greater numbers would be unlikely to confirm our hypothesis.
In conclusion, this study showed that nortriptyline increased the HR response to AS and HUT in both homozygous haplotype groups of ABCB1(TTT/TTT and CGC/CGC) but no difference was seen between groups. There was no difference in BP response between the haplotype groups. The association between ABCB1 polymorphism and nortriptyline-induced postural hypotension found in the previous patient study could thus not be confirmed in this study in healthy volunteers following a single oral dose of nortriptyline. The results raise the possibility of a predisposition in HR response in the TTT homozygotes rather than an effect of nortriptyline.
Acknowledgments
Financial support for this study is gratefully acknowledged from the Canterbury Medical Research Foundation, New Zealand. BPJ is the recipient of a Carney Centre Research Fellowship and RLR is the recipient of a Sir Charles Hercus Health Research Fellowship from the Health Research Council of New Zealand.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Baldessarini RJ. Drug therapy of depression and anxiety disorders. In: Brunton L, Lazo J, Parker K, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th. New York, NY: McGraw-Hill Companies, Inc; 2006. pp. 429–59. [Google Scholar]
- 2.Dollery C, editor. Therapeutic Drugs. 2nd. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- 3.Breyer-Pfaff U. The metabolic fate of amitriptyline, nortriptyline and amitriptylinoxide in man. Drug Metab Rev. 2004;36:723–46. doi: 10.1081/dmr-200033482. [DOI] [PubMed] [Google Scholar]
- 4.Aronson J, editor. Meyler's Side Effects of Drugs. 15th. Amsterdam: Elsevier; 2006. [Google Scholar]
- 5.Roberts RL, Joyce PR, Mulder RT, Begg EJ, Kennedy MA. A common P-glycoprotein polymorphism is associated with nortriptyline-induced postural hypotension in patients treated for major depression. Pharmacogenomics J. 2002;2:191–6. doi: 10.1038/sj.tpj.6500099. [DOI] [PubMed] [Google Scholar]
- 6.Hodges LM, Markova SM, Chinn LW, Gow JM, Kroetz DL, Klein TE, Altman RB. Very important pharmacogene summary. Pharmacogenet Genomics. 2011;21:152–61. doi: 10.1097/FPC.0b013e3283385a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leschziner GD, Andrew T, Pirmohamed M, Johnson MR. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J. 2007;7:154–79. doi: 10.1038/sj.tpj.6500413. [DOI] [PubMed] [Google Scholar]
- 8.Ejsing T, Hasselstrøm J, Linnet K. The influence of P-glycoprotein on cerebral and hepatic concentrations of nortriptyline and its metabolites. Drug Metab Drug Interact. 2006;21:139–62. doi: 10.1515/dmdi.2006.21.3-4.139. [DOI] [PubMed] [Google Scholar]
- 9.Grauer MT, Uhr M. P-glycoprotein reduces the ability of amitriptyline metabolites to cross the blood brain barrier in mice after a 10-day administration of amitriptyline. J Psychopharmacol. 2004;18:66–74. doi: 10.1177/0269881104042831. [DOI] [PubMed] [Google Scholar]
- 10.Uhr M, Grauer MT, Yassouridis A, Ebinger M. Blood-brain barrier penetration and pharmacokinetics of amitriptyline and its metabolites in p-glycoprotein (ABCB1AB) knock-out mice and controls. J Psychiatr Res. 2007;41:179–88. doi: 10.1016/j.jpsychires.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Uhr M, Steckler T, Yassouridis A, Holsboer F. Penetration of amitriptyline, but not of fluoxetine, into brain is enhanced in mice with blood-brain barrier deficiency due to MDR1a P-glycoprotein gene disruption. Neuropsychopharmacology. 2000;22:380–7. doi: 10.1016/S0893-133X(99)00095-0. [DOI] [PubMed] [Google Scholar]
- 12.Kroetz DL, Pauli-Magnus C, Hodges LM, Huang CC, Kawamoto M, Johns SJ, Stryke D, Ferrin TE, DeYoung J, Taylor T, Carlson EJ, Herskowitz I, Giacomini KM, Clark AG. Sequence diversity and haplotype structure in the human ABCB1 (MDR1, multidrug resistance transporter) gene. Pharmacogenetics. 2003;13:481–94. doi: 10.1097/00008571-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Eickbush TH, Moudrianakis EN. The compaction of DNA helices into either continuous supercoils or folded-fiber rods and toroids. Cell. 1978;13:295–306. doi: 10.1016/0092-8674(78)90198-8. [DOI] [PubMed] [Google Scholar]
- 14.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, Schaeffeler E, Eichelbaum M, Brinkmann U, Roots I. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69:169–74. doi: 10.1067/mcp.2001.114164. [DOI] [PubMed] [Google Scholar]
- 16.Roberts RL, Kennedy MA. Rapid detection of common cytochrome P450 2D6 alleles in Caucasians. Clin Chim Acta. 2006;366:348–51. doi: 10.1016/j.cca.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83:234–42. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 18.Wieling W, Karemaker J. Measurement of heart rate and blood pressure to evaluate disturbances in neurocardiovascular control. In: Mathias CJ, Bannister R, editors. Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. 4th. Oxford: Oxford University Press; 1999. pp. 196–209. [Google Scholar]
- 19.Bonke G, Jensen BP. Fast and sensitive LC-MS/MS assay for quantification of nortriptyline and its active metabolites E- and Z-10-hydroxynortriptyline in human plasma. Bioanalysis. 2010;2:1553–60. doi: 10.4155/bio.10.89. [DOI] [PubMed] [Google Scholar]
- 20.Woillard JB, Rerolle JP, Picard N, Rousseau A, Guillaudeau A, Munteanu E, Essig M, Drouet M, Le Meur Y, Donor MP. P-gp polymorphisms strongly influence renal function and graft loss in a cohort of renal transplant recipients on cyclosporine therapy in a long-term follow-up. Clin Pharmacol Ther. 2010;88:95–100. doi: 10.1038/clpt.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergmann TK, Brasch-Andersen C, Green H, Mirza M, Pedersen RS, Nielsen F, Skougaard K, Wihl J, Keldsen N, Damkier P, Friberg LE, Peterson C, Vach W, Karlsson MO, Brosen K. Impact of CYP2C8*3 on paclitaxel clearance: a population pharmacokinetic and pharmacogenomic study in 93 patients with ovarian cancer. Pharmacogenomics J. 2011;11:113–20. doi: 10.1038/tpj.2010.19. [DOI] [PubMed] [Google Scholar]
- 22.Sistonen J, Sajantila A, Lao O, Corander J, Barbujani G, Fuselli S. CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics. 2007;17:93–101. doi: 10.1097/01.fpc.0000239974.69464.f2. [DOI] [PubMed] [Google Scholar]
- 23.The European Agency for the Evaluation of Medicinal Products. Note for Guidance on the Investigation of Bioavailability and Bioequivalence. London: Committee for Proprietary medicinal Products; 2001. [Google Scholar]
- 24.Georgotas A, McCue R, Friedman E. A placebo-controlled comparison of the effect of nortriptyline and phenelezine on orthostatic hypotension in elderly depressed patients. J Clin Psychopharmacol. 1987;7:413–6. [PubMed] [Google Scholar]
- 25.Kiev A, Masco HL, Wenger TL, Johnston A, Batey SR, Holloman LC. The cardiovascular effecs of bupropion and nortriptyline in depressed outpatients. Ann Clin Psychiatry. 1994;6:107–15. doi: 10.3109/10401239409148989. [DOI] [PubMed] [Google Scholar]
- 26.Freyschuss U, Sjöqvist F, Tuck D, Asberg M. Circulatory effects in man of nortriptyline, a tricyclic antidepressant drug. Eur J Clin Pharmacol. 1970;2:68–71. [Google Scholar]
- 27.Thayssen P, Bjerre M, Kragh-Sørensen P, Møller M, Petersen OL, Kristensen CB, Gram LF. Cardiovascular effects of imipramine and nortriptyline in elderly patients. Psychopharmacology. 1981;74:360–4. doi: 10.1007/BF00432748. [DOI] [PubMed] [Google Scholar]
- 28.Nair NPV, Amin M, Holm P, Katona C, Klitgaard N, Ng Ying Kin NM, Kragh-Sørensen P, Kühn H, Leek CA, Stage KB. Moclobemide and nortriptyline in elderly depressed patients. A randomized, multicentre trial against placebo. J Affect Disord. 1995;33:1–9. doi: 10.1016/0165-0327(94)00047-d. [DOI] [PubMed] [Google Scholar]
- 29.Giardina E-GV, Johnson LL, Vita J, Bigger JT, Brem RF. Effect of imipramine and nortriptyline on left ventricular function and blood pressure in patients treated for arrhythmias. Am Heart J. 1985;109(5 Part 1):992–8. doi: 10.1016/0002-8703(85)90240-6. [DOI] [PubMed] [Google Scholar]
- 30.Scalco MZ, de Almeida OP, Hachul DT, Castel S, Serro-Azul J, Wajngarten M. Comparison of risk of orthostatic hypotension in elderly depressed hypertensive women treated with nortriptyline and thiazides versus elderly depressed normotensive women treated with nortriptyline. Am J Cardiol. 2000;85:1156–8. doi: 10.1016/s0002-9149(00)00717-7. [DOI] [PubMed] [Google Scholar]
- 31.Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT, Pollock BG, Gaffney A, Narayan M, Finkel MS, McCafferty J, Gergel I. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA. 1998;279:287–91. doi: 10.1001/jama.279.4.287. [DOI] [PubMed] [Google Scholar]


