Abstract
AIMS
Although adjunctive cilostazol to dual antiplatelet therapy can reduce the risks of clinical events after percutaneous coronary intervention (PCI), whether genetic polymorphism can influence the pharmacodynamics of this regimen has not been evaluated.
METHODS
One hundred and twenty-seven patients treated with PCI and taking triple antiplatelet therapy (≥1 month) were enrolled. Platelet reactivity was assessed by conventional aggregometry and the VerifyNow P2Y12 assay. High on-treatment platelet reactivity (HPR) was defined as 5 µm ADP-induced maximal platelet reactivity (Aggmax) >46%. CYP3A5*3, CYP2C19*2/*3 and ABCB1 3435C > T were genotyped.
RESULTS
CYP3A5*3 and ABCB1 3435C > T variants did not affect the antiplatelet effect of triple antiplatelet therapy. For non-carriers, one and two carriers of the CYP2C19 loss-of-function (LOF) allele, Aggmax consecutively increased after the addition of 5 µm[mean (95% confidence intervals): 24.6% (20.8 to 28.5%) vs. 28.7% (25.4 to 32.0%) vs. 32.3% (25.8 to 38.7%), P = 0.062, respectively] and 20 µm ADP [34.2% (29.3 to 39.0%) vs. 41.7% (37.8 to 45.6%) vs. 44.9% (37.9 to 51.9%), P = 0.007, respectively]. Likewise, late platelet reactivity and P2Y12 reaction units proportionally changed according to the number of CYP2C19 LOF alleles. HPRs were observed in 9.2% of subjects: 6.3%, 7.4% and 20.0% with 0, 1 and 2 carriers of CYP2C19 LOF allele(s) (P = 0.099). In multivariate analysis, carriage of two CYP2C19 LOF alleles was a significant predictor for the prevalence of HPR (odds ratio 5.78, 95% CI 1.21, 27.78, P = 0.028).
CONCLUSION
Among PCI-treated patients, the effect of triple antiplatelet therapy is influenced by the CYP2C19 LOF allele. Its clinical benefit needs to be validated according to the CYP2C19 metabolic phenotype in future clinical trials. [Adjunctive Cilostazol Versus High Maintenance dose ClopidogrEL in Acute Myocardial Infarction Patients According to CYP2C19 Polymorphism (ACCEL-AMI-2C19), NCT00915733 and Adjunctive Cilostazol Versus High Maintenance-dose Clopidogrel According to Cytochrome 2C19 Polymorphism (ACCEL-2C19), NCT01012193].
Keywords: cilostazol, clopidogrel, genetic polymorphism, high on-treatment platelet reactivity, platelet
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Compared with standard dual antiplatelet therapy, adjunctive cilostazol to dual antiplatelet therapy ('triple antiplatelet therapy’) has a potential to reduce ischemic event occurrence after percutaneous coronary intervention.
The pharmacokinetic and pharmacodynamic effects of clopidogrel have been significantly influenced by the enzyme activity of the ABCB1 C3435T and the CYP2C19 system.
For the pharmacokinetics of cilostazol, genetic polymorphisms of the CYP3A5 and CYP2C19 have been associated with the substantial interindividual variability in healthy volunteers.
WHAT THIS STUDY ADDS
Loss-of-function polymorphism of the CYP2C19 gene, but not the ABCB1 C3435T and CYP3A5*3 genes, affects the antiplatelet effect of triple antiplatelet therapy.
Most of extensive and intermediate East Asian metabolizers (0 or 1 CYP2C19 loss-of-function allele) show adequate platelet inhibition when treated with triple antiplatelet therapy after percutaneous coronary intervention.
However, carriage of 2 CYP2C19 loss-of-function alleles is still associated with the risk of high platelet reactivity (defined by by 5 µM ADP-induced maximal platelet aggregation >46%), which clinical impact needs to be validated in future clinical trials.
Introduction
Dual antiplatelet therapy with aspirin and clopidogrel reduces ischaemic complications following percutaneous coronary intervention (PCI) or acute coronary syndrome (ACS) [1, 2]. Although this combination has been widely adopted in clinical practice, the recurrence of post PCI adverse cardiovascular events has still been observed in a proportion of patients. Numerous clinical factors can contribute to the development of cardiovascular events [3], and clopidogrel hyporesponsiveness and/or high on-treatment platelet reactivity (HPR) has been suggested as an important trigger of post PCI ischaemic event [4].
Cilostazol, a selective and reversible inhibitor of phosphodiesterase type 3 (PDE 3), has a unique property of platelet inhibition and vascular protection [5, 6]. A growing body of studies has demonstrated that adjunctive cilostazol to standard dual antiplatelet therapy (triple antiplatelet therapy) can reduce the risk of post PCI ischaemic events compared with standard antiplatelet therapy [6–15]. Although its beneficial role in recovery of endothelial dysfunction has been suggested as the underlying mechanism [7–9, 11], reduction of thrombotic events such as myocardial infarction and stent thrombosis [10, 12–14] may be related to a platelet-centric mechanism. To date, several translational studies demonstrated that adding cilostazol can enhance inhibition of adenosine diphosphate (ADP)-induced platelet aggregation [16–19].
On-clopidogrel platelet reactivity measured by multiple platelet function testing has been significantly associated with the enzyme activities of intestinal transporter and the hepatic cytochrome P-450 (CYP) system, especially the CYP2C19 isozyme [4]. Single nucleotide polymorphisms (SNPs) occurring in these genes can change the antiplatelet effect of clopidogrel [20–22]. Likewise, cilostazol is metabolized by the hepatic metabolism via the CYP enzyme, primarily CYP3A4/5 and, to a lesser extent, CYP2C19 [23]. Yoo et al. demonstrated that with regard to the pharmacokinetics of cilostazol, genetic polymorphisms of the CYP3A5*3 and CYP2C19*2/*3 can explain the substantial interindividual variability in healthy volunteers [24, 25]. However, there have been no data regarding the influence of genetic polymorphisms on the pharmacodynamics of adding cilostazol to dual antiplatelet therapy, especially the antiplatelet effect.
This analysis was performed to assess the achieved antiplatelet effect of triple antiplatelet therapy and the effect of SNPs on the pharmacodynamics among post PCI patients.
Methods
Patient population and study design
A total of 127 PCI-treated patients with available genotyping and platelet measures together were enrolled into the ACCEL-TRIPLE (Accelerated Platelet Inhibition by Triple Antiplatelet Therapy According to Gene Polymorphism) study (Figure 1). Patients were recruited from the Gyeongsang National University Hospital between January 2008 and June 2009. One hundred and two patients (80.3%) were collected from the triple therapy group in the ACCEL [Adjunctive Cilostazol vs. high maintenance dose (MD) ClopidogrEL] studies, which were performed to compare the magnitude of platelet inhibition by adjunctive cilostazol vs. high MD clopidogrel in high risk patients: HPR, diabetes, complex lesion PCI and acute myocardial infarction [17, 18, 26]. Patients receiving triple antiplatelet therapy were treated for 1 month. A minority (n = 25, 19.7%) of patients received post PCI triple antiplatelet therapy for 1 month at the attending physician's discretion. A minority (n = 25, 19.7%) of the patients was recruited from high risk patients who started adding cilostazol after PCI at the attending physician's discretion. Patient compliance to antiplatelet therapy was assessed by questionnaire, interview and tablet counting at the follow-up visit. Because pharmacokinetic and pharmacodynamic responses may vary profoundly during the initial days or weeks after antiplatelet therapy [16], only patients in the steady-state phase of triple antiplatelet therapy were included (≥1 month of cilostazol 100 mg twice a day, clopidogrel 75 mg once a day and aspirin 200 mg once a day).
Figure 1.
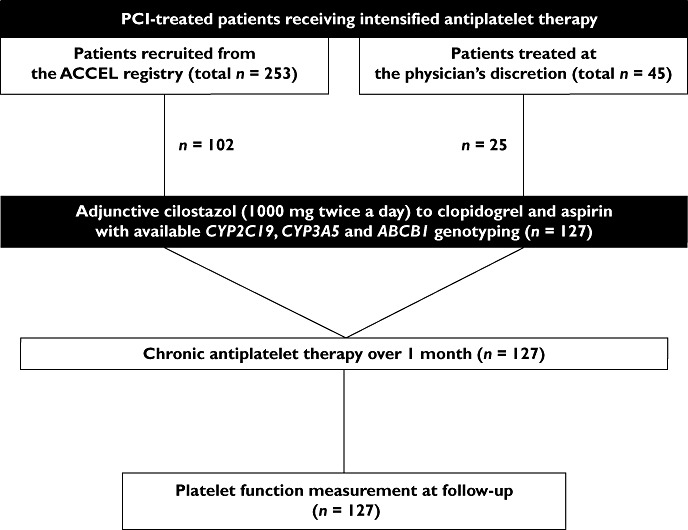
Flow diagram of the ACCEL-TRIPLE study. ABCB1, ATP-binding cassette sub-family B member 1; ACCEL, Adjunctive Cilostazol Versus High Maintenance-Dose Clopidogrel; CYP, cytochrome P450; PCI, percutaneous coronary intervention
Patients were eligible for enrolment if they were ≥18 years of age and had been treated with PCI for symptomatic coronary artery disease. The contraindications to triple antiplatelet therapy were known allergies to antiplatelet therapy, haemodynamic instability, active bleeding and bleeding diatheses, oral anticoagulation therapy with coumadin, left ventricular ejection fraction <30%, a leucocyte count <3000 mm3 and/or a platelet count <100 000 mm3, an aspartate aminotransferase (AST) concentration or an alanine aminotransferase (ALT) concentration ≥three times the upper normal limit, noncardiac disease with a life expectancy <1 year and the inability to receive the regimen. The study protocol was approved by the Institutional Ethics Committee of Gyeongsang National University Hospital, Korea and the patients provided written informed consent to participate in this study.
Platelet function measurements
Blood samples were obtained 2–4 h after the last intake of triple antiplatelet therapy. Blood samples were collected using the double-syringe technique and the first 2 to 4 ml of blood was discarded to avoid spontaneous platelet activation. The platelet functions were measured by light transmittance aggregometry (LTA) and the VerifyNow P2Y12 assay (Accumetrics Inc., San Diego, California). We have previously presented the correlation between the two methods at our laboratory [27].
LTA was performed according to a standard protocol and has been described in detail elsewhere [27]. Blood samples were drawn into Vacutainer tubes containing 0.5 ml of 3.2% sodium citrate (Becton-Dickinson, San Jose, California) and processed within 2 h. Platelet rich plasma (PRP) was obtained as a supernatant fluid after centrifuging the blood at 120 g for 10 min. The remaining blood was further centrifuged at 1200 g for 10 min to prepare platelet poor plasma (PPP). PRP was adjusted to platelet counts of 250 000/mm3 by adding PPP as needed. Platelet aggregation was assessed at 37°C using an AggRAM aggregometer (Helena Laboratories Corporation, Beaumont, Texas). Light transmission was adjusted to 0% with PRP and to 100% with PPP for each measurement. Platelet function tests were performed after the addition of 5 and 20 µm ADP and the curves were recorded for 10 min. Platelet reactivity was determined at maximal aggregation (Aggmax) and late aggregation at 5 min (Agglate).
The VerifyNow P2Y12 assay is a whole blood, point-of-care system that has been developed to assess responsiveness to P2Y12 antagonists [27]. Blood was drawn into a Greiner Bio-One 3.2% citrate Vacuette tube (Greiner Bio-One, Kremsmünster, Austria). The assay device contains fibrinogen-coated polystyrene beads and 20 µm ADP, which also contains 22 nm PGE1 to reduce the non-specific contribution of other pathways. The results are reported in P2Y12 reaction units (PRU).
Gene analysis and phenotype
We performed genotyping of the two CYP2C19 LOF alleles (*2 and *3), CYP3A5*3, and ABCB1 3435C > T variants on the basis of previous studies [20–25]. The base numbering and allele definitions follow the nomenclature of the Human CYP Allele Nomenclature Committee [28]. Genomic DNA was extracted from leucocytes of whole blood specimens with a commercially available kit (QIAamp DNA Blood Mini Kit, Qiagen, Hilden, Germany). All genotyped SNPs were in Hardy-Weinberg equilibrium (P > 0.05).
Because the frequencies of the CYP2C19*4,*5,*6,*7 and *8 LOF alleles are extremely rare in East Asians [29], genotyping for the CYP2C19*2 (rs4244285, c.681G > A) and CYP2C19*3 (rs4986893, c.636G > A) were investigated using the ABI SNaPshot (Applied Biosystems, Foster City, California) reaction. Polymerase chain reaction (PCR) was carried out by using the same primers. The PCR product was processed as per the ABI SNaPshot protocol, using primers designed for fluorescent dideoxy nucleotide termination. SNP analysis was carried out on the ABI 3100 genetic analyzer (Applied Biosystems, Foster City, California). Genotyping for the CYP3A5*3 (rs776746, g.6986A > G) and ABCB1 (rs1045642, c.3435C > T) was performed with the use of an allelic discrimination assay based on the TaqMan method (Applied Biosystems) and ABI PRISM 7900HT Sequence Detection System (SDS) (Applied Biosystems). The PCR amplification protocol for the TaqMan assays included denaturation at 95°C for 10 min, followed by 40 cycles at 92°C for 15 s, 60°C for 1 min and 72°C for 45 s, followed by elongation at 72°C for 5 min. The TaqMan assays were then read on a 7900HT Fast Real-Time PCR system and alleles were called using SDS software.
We classified every CYP2C19 phenotype by established nomenclature and its reported effect on enzymatic function according to published reports, extensive (EM), intermediate (IM) and poor (PM) metabolizers. For ABCB1 3435C > T, patients were classified as homozygous for the C allele (CC, high expression), heterozygous (CT, intermediate expression) and homozygous for the T allele (TT, low expression). In terms of the CYP3A5 phenotype, carriers of two CYP3A5*3 LOF alleles were defined as reduced metabolizers [30].
Endpoints and definitions
The primary endpoint was Aggmax according to metabolic phenotypes. The secondary endpoints were (i) Agglate, (ii) PRU and (iii) the prevalence and predictor of HPR according to metabolic phenotype. According to the consensus of the Working Group [4], HPR was defined as 5 µm ADP-induced Aggmax >46%.
Sample size calculation and statistical analysis
Under the assumption that carriage of the CYP2C19 LOF variant may influence platelet inhibition by triple antiplatelet therapy, we calculated the number of enrolled patients. If there was a 20% relative difference of ADP-induced Aggmax between the CYP2C19 LOF carriers vs. non-carriers, and the CYP2C19 LOF allele was observed in 60% of East Asians [29, 31], at least 120 patients (72 carriers and 48 non-carriers of the CYP2C19 LOF variant) were needed to guarantee a power of 90% to detect a significant difference with a two-sided α-level of 0.05 and SD of 0.3.
Continuous variables, presented as mean ± SD, were compared using Student's unpaired t-test or Mann-Whitney U test. Categorical variables, presented as numbers or percentages, were compared using chi-square test or Fisher's exact test as appropriate. Platelet measures and baseline characteristics according to metabolic phenotype were analyzed using one way analysis of variance (anova). After demonstration of significant differences among variables by one way anova, post hoc comparisons among the groups were made with the Student-Newman-Keuls procedure for multiple comparisons. To consider the gene–dose relationship, the Jonckheere-Terpstra test also was used. To determine predictors of HPR, a logistic regression analysis was performed using known variables, and odds ratio (OR) and 95% CI were also calculated. A value of P < 0.05 was considered to indicate a significant difference. All the statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, Illinois).
Results
Patient characteristics
Because of the characteristics of this analysis, the cohorts showed complete compliance with triple antiplatelet therapy for at least 30 days. No major cardiovascular and bleeding events were observed in any patient during follow-up period. One patient in the triple therapy group suffered from thrombolysis in myocardial infarction minor bleeding due to entry site aneurysm and haematoma. Although there were four cases of transient headache (3.1%) and two cases of palpitation (1.6%) in the triple therapy group in the early phase of treatment, all regimens were generally well tolerated. Baseline characteristics of the enrolled patients are shown in Table 1. The average age was 62.9 (SD 10.9) years, and about 70% of patients were men. Patients were comprised of relatively high risk cohorts: ACS 74.8%, diabetes 35.4%, and drug-eluting stent implantation 96.8%.
Table 1.
Baseline characteristics according to CYP2C19 metabolic phenotype
| Variables n (%) | Total population (n = 127) | Extensive metabolizer (n = 48) | Intermediate metabolizer (n = 54) | Poor metabolizer (n = 25) | P value |
|---|---|---|---|---|---|
| Age (years) | 62.9 ± 10.9 | 61.3 ± 12.3 | 63.4 ± 9.8 | 65.1 ± 10.3 | 0.351 |
| Male | 89 (70.1) | 33 (68.8) | 38 (70.4) | 18 (72.0) | 0.795 |
| BMI (kg m–2) | 24.3 ± 3.1 | 24.0 ± 3.0 | 24.7 ± 3.3 | 24.1 ± 3.2 | 0.531 |
| Clinical presentation | |||||
| Stable angina | 32 (25.2) | 10 (20.8) | 16 (29.6) | 6 (24.0) | 0.259 |
| Unstable angina | 40 (31.5) | 14 (29.2) | 16 (29.6) | 10 (40.0) | |
| NSTEMI | 29 (22.8) | 12 (25.0) | 10 (18.5) | 7 (28.0) | |
| STEMI | 26 (20.5) | 12 (25.0) | 12 (22.2) | 2 (8.0) | |
| Risk factors | |||||
| Diabetes mellitus | 45 (35.4) | 11 (22.9) | 23 (42.6) | 11 (44.0) | 0.045 |
| Hypertension | 68 (53.5) | 23 (47.9) | 29 (53.7) | 16 (64.0) | 0.229 |
| Hypercholesterolaemia | 32 (25.2) | 11 (22.9) | 11 (20.4) | 10 (40.0) | 0.214 |
| Current smoking | 56 (44.1) | 21 (43.8) | 23 (42.6) | 12 (18.0) | 0.810 |
| Chronic kidney disease | 17 (13.4) | 7 (14.6) | 8 (14.8) | 2 (8.0) | 0.599 |
| History | |||||
| Previous MI | 67 (52.8) | 29 (60.4) | 26 (48.1) | 12 (48.0) | 0.279 |
| Previous CABG | 4 (3.1) | 1 (2.1) | 1 (1.9) | 2 (8.0) | 0.310 |
| Previous stroke | 4 (3.1) | 1 (2.1) | 2 (3.7) | 1 (4.0) | 0.737 |
| Concomitant medications | |||||
| Statin | 116 (91.3) | 46 (95.8) | 46 (85.2) | 24 (96.0) | 0.832 |
| CYP 3A4 metabolized | 102 (80.3) | 37 (77.1) | 42 (77.8) | 23 (92.0) | 0.227 |
| β-adrenoceptor blocker | 107 (84.7) | 39 (81.3) | 46 (85.2) | 22 (88.0) | 0.512 |
| Angiotensin blockade | 106 (91.3) | 48 (100.0) | 45 (83.3) | 23 (92.0) | 0.438 |
| Calcium channel blocker | 22 (17.3) | 8 (16.7) | 12 (22.2) | 2 (8.0) | 0.533 |
| Proton pump inhibitor | 4 (3.1) | 2 (4.2) | 2 (3.7) | 0 (0.0) | 0.508 |
| LV ejection fraction ≤45% | 22 (17.3) | 12 (25.0) | 7 (13.0) | 3 (12.0) | 0.116 |
| Haemoglobin (g dl–1) | 12.9 ± 1.4 | 13.0 ± 1.7 | 12.6 ± 1.3 | 13.0 ± 1.2 | 0.254 |
| Platelet count (× 103 mm–3) | 285.0 ± 91.4 | 283.4 ± 86.7 | 288.2 ± 100.8 | 281.0 ± 81.7 | 0.940 |
| Hb A1C (%) | 6.5 ± 1.2 | 6.3 ± 0.9 | 6.5 ± 1.4 | 6.6 ± 1.3 | 0.716 |
| GFR (MDRD (ml min–1 1.73 m–2) | 85.3 ± 23.5 | 82.6 ± 23.6 | 87.0 ± 25.1 | 86.8 ± 19.8 | 0.609 |
| Total cholesterol (mg dl–1) | 135.6 ± 26.4 | 136.9 ± 25.2 | 133.6 ± 27.4 | 137.3 ± 27.0 | 0.778 |
| Target artery | |||||
| Left anterior descending | 69 (54.3) | 28 (58.3) | 28 (51.9) | 13 (52.0) | 0.352 |
| Right coronary | 33 (26.0) | 12 (25.0) | 13 (24.1) | 8 (32.0) | |
| Left circumflex | 21 (16.5) | 6 (12.5) | 11 (20.4) | 4 (16.0) | |
| Left main | 4 (3.1) | 2 (4.2) | 2 (3.7) | 0 (1.0) | |
| Intervention method | |||||
| Drug-eluting stent | 123 (96.8) | 46 (95.8) | 53 (98.1) | 24 (96.0) | 0.838 |
| Bare-metal stent | 2 (1.6) | 1 (2.1) | 0 (0.0) | 1 (4.0) | |
| Ballooning only | 2 (1.6) | 1 (2.1) | 1 (1.9) | 0 (0.0) | |
| Multivessel intervention | 37 (29.1) | 11 (22.9) | 19 (35.2) | 7 (28.0) | 0.510 |
| Stent diameter (mm) | 3.1 ± 0.4 | 3.2 ± 0.4 | 3.1 ± 0.4 | 3.1 ± 0.3 | 0.487 |
| Stents per patient | 2.1 ± 1.2 | 1.9 ± 1.1 | 2.2 ± 1.3 | 2.2 ± 1.2 | 0.531 |
| Total stent length (mm) | 50.0 ± 32.9 | 47.0 ± 28.0 | 51.7 ± 36.3 | 51.8 ± 35.1 | 0.742 |
Values are expressed as mean ± SD or as number of patients (%). BMI, body mass index; CABG, coronary artery bypass grafting; CYP, cytochrome P450; GFR, glomerular filtration rate; Hb A1C, haemoglobin A1C; LV, left ventricular; MDRD, Modification of Diet in Renal Disease; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Table 2 shows distributions according to allele, genotype and metabolic phenotype. There was a high prevalence of the CYP2C19 LOF phenotype (IMs and PMs: 42.5% and 19.7%, respectively). Moreover, there were 22 carriers of the CYP2C19*3 LOF allele (17.3%). There were no differences in platelet measures between the CYP2C19*2 vs.*3 LOF allele whether these were present in IMs or PMs (data not shown). Baseline characteristics did not differ across the CYP3A5 and ABCB1 3435C > T phenotype (data not shown), and they were well matched between the CYP2C19 groups, except for a higher prevalence of diabetes in IMs and PMs (Table 1).
Table 2.
Distributions according to allele, genotype and metabolic phenotype
| Gene | Allele | Frequency, % | Genotype | Distribution, n (%) | Predicted phenotype |
|---|---|---|---|---|---|
| CYP2C19 | *1 | 59.1 | *1/*1 | 48 (37.8) | Extensive metabolizer |
| *2 | 31.5 | *1/*2 | 42 (33.1) | Intermediate metabolizer | |
| *3 | 9.4 | *1/*3 | 12 (9.4) | Intermediate metabolizer | |
| *2/*2 | 15 (11.8) | Poor metabolizer | |||
| *2/*3 | 8 (6.3) | Poor metabolizer | |||
| *3/*3 | 2 (1.6) | Poor metabolizer | |||
| CYP3A5 | *1 | 24.8 | *1/*1 | 10 (7.9) | Normal metabolizer |
| *3 | 75.2 | *1/*3 | 43 (33.9) | Normal or reduced metabolizer | |
| *3/*3 | 74 (58.3) | Reduced metabolizer | |||
| ABCB1 3435C > T | C | 65.4 | CC | 52 (40.9) | High expression |
| T | 34.6 | CT | 62 (48.8) | Intermediate expression | |
| TT | 13 (10.2) | Low expression |
CYP, cytochrome P450: ABCB1, ATP-binding cassette sub-family B member 1.
Platelet function measurements according to metabolic phenotype
The means of 5 and 20 µm ADP-induced Aggmax were 27.9 ± 13.5% and 39.5 ± 16.2%, respectively. Agglate values after the addition of 5 and 20 µm ADP were 15.7 ± 12.5% and 25.0 ± 18.0%, respectively. The PRU value from the VerifyNow P2Y12 assay was 172 ± 87. Phenotypes of the CYP3A5 and ABCB1 alleles did not affect the levels of platelet reactivity. Compared with non-carriers, carriers of the CYP2C19 LOF allele showed significant differences of platelet measures (data not shown).
The number of the CYP2C19 LOF carriage alleles increased proportionally Aggmax after the addition of 5 µm[24.6 ± 13.3% (95% CI 20.8, 28.5%) vs. 28.7 ± 12.2% (95% CI 25.4, 32.0%) vs. 32 ± 15.7% (95% CI 25.8, 38.7%), P = 0.062] and 20 µm ADP [34.2 ± 16.7% (95% CI 29.3, 39.0%) vs. 41.7 ± 14.2% (95% CI 37.8, 45.6%) vs. 44.9 ± 17.0% (95% CI 37.9, 51.9%), P = 0.007] (Table 3, Figure 2). Values of 5 and 20 µM ADP-induced Agglate were increased depending on the number of the CYP2C19 LOF alleles (P = 0.021 and 0.002 after adjustment for diabetes, respectively) (Table 3, Figure 3). PRU consecutively increased according to the number of the CYP2C19 LOF alleles (P < 0.001 after adjustment for diabetes) (Table 3, Figure 4).
Table 3.
Platelet reactivity and rate of HPR according to metabolic phenotype
| CYP2C19 | CYP3A5 | ABCB1 3435C > T | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Extensive metabolizer (n = 48) | Intermediate metabolizer (n = 54) | Poor metabolizer (n = 25) | *1/*1 (n = 10) | *1/*3 (n = 43) | *3/*3 (n = 74) | CC (n = 52) | CT (n = 62) | TT (n = 13) | |
| light transmittance aggregometry | |||||||||
| 5 µm ADP-aggmax (%) | |||||||||
| Mean ± SD | 24.6 ± 13.3 | 28.7 ± 12.2 | 32.3 ± 15.7 | 27.0 ± 14.9 | 28.4 ± 13.6 | 27.7 ± 13.5 | 27.4 ± 13.8 | 27.9 ± 13.7 | 29.7 ± 12.5 |
| 95% CI | 20.8, 28.5 | 25.4, 32.0 | 25.8, 38.7 | 16.3, 37.6 | 24.2, 32.6 | 24.5, 30.8 | 23.5, 31.2 | 24.4, 31.4 | 22.1, 37.3 |
| P value (anova) | 0.062 | 0.934 | 0.855 | ||||||
| P value (Jonckheere-Terpstra) | 0.016 | 0.678 | 0.538 | ||||||
| 5 µM ADP-Agglate (%) | |||||||||
| Mean ± SD | 12.1 ± 12.2 | 16.4 ± 11.1 | 21.0 ± 14.0 | 14.9 ± 10.2 | 15.9 ± 13.8 | 15.6 ± 12.1 | 15.4 ± 13.2 | 16.2 ± 12.6 | 14.1 ± 9.2 |
| 95% CI | 8.6, 15.7 | 13.3, 19.4 | 15.2, 26.7 | 7.6, 22.2 | 11.7, 20.2 | 12.8, 18.4 | 11.8, 19.1 | 13.0, 19.4 | 8.5, 19.7 |
| P value (anova) | 0.021 | 0.972 | 0.846 | ||||||
| P value (Jonckheere-Terpstra) | 0.002 | 0.933 | 0.859 | ||||||
| 20 µm ADP-Aggmax (%) | |||||||||
| Mean ± SD | 34.2 ± 16.7 | 41.7 ± 14.2 | 44.9 ± 17.0 | 41.4 ± 19.6 | 40.2 ± 16.5 | 38.8 ± 15.7 | 39.3 ± 16.5 | 38.7 ± 16.3 | 44.1 ± 14.6 |
| 95% CI | 29.3, 39.0 | 37.8, 45.6 | 37.9, 51.9 | 27.3, 55.4 | 35.1, 45.3 | 35.2, 42.5 | 34.7, 43.9 | 34.5, 42.8 | 35.3, 52.9 |
| P value (anova) | 0.007 | 0.845 | 0.551 | ||||||
| P value (Jonckheere-Terpstra) | 0.003 | 0.601 | 0.596 | ||||||
| 20 µm ADP-Agglate (%) | |||||||||
| Mean ± SD | 18.5 ± 17.4 | 27.1 ± 16.8 | 32.9 ± 18.1 | 28.8 ± 21.4 | 24.3 ± 19.7 | 24.8 ± 16.7 | 25.0 ± 18.6 | 24.1 ± 17.6 | 28.7 ± 18.4 |
| 95% CI | 13.4, 23.5 | 22.5, 31.6 | 25.4, 40.4 | 13.5, 44.1 | 18.2, 30.4 | 21.0, 28.7 | 19.8, 30.2 | 19.7, 28.6 | 17.6, 39.8 |
| P value (anova) | 0.002 | 0.775 | 0.713 | ||||||
| P value (Jonckheere-Terpstra) | <0.001 | 0.990 | 0.696 | ||||||
| VerifyNow P2Y12 assay | |||||||||
| P2Y12 reaction units | |||||||||
| Mean ± SD | 125 ± 76 | 188 ± 75 | 226 ± 88 | 164 ± 75 | 173 ± 92 | 172 ± 86 | 172 ± 88 | 169 ± 86 | 186 ± 91 |
| 95% CI | 103, 147 | 168, 209 | 190, 262 | 111, 218 | 145, 201 | 152, 192 | 147, 196 | 147, 191 | 131, 241 |
| P value (anova) | <0.001 | 0.958 | 0.824 | ||||||
| P value (Jonckheere-Terpstra) | <0.001 | 0.975 | 0.880 | ||||||
| HPR (5 µM ADP-induced Aggmax >46%) | |||||||||
| n (%) | 3 (6.3) | 4 (7.4) | 5 (20.0) | 1 (10.0) | 5 (11.6) | 6 (8.1) | 7 (13.5) | 4 (6.5) | 1 (7.7) |
| P value | 0.099 | 0.620 | 0.279 | ||||||
CYP, cytochrome P450; ABCB1, ATP-binding cassette sub-family B member 1; ADP, adenosine diphosphate; Aggmax, maximal platelet aggregation; Agglate, late platelet aggregation at 5 min; HPR, high on-treatment platelet reactivity.
Figure 2.
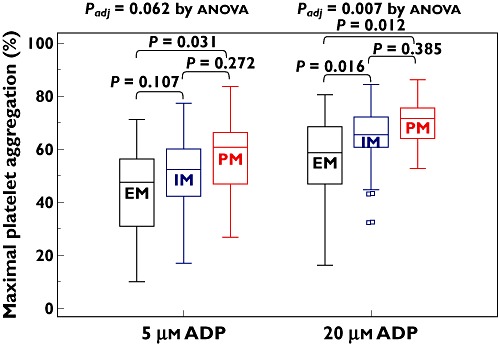
Maximal platelet aggregation according to CYP2C19 metabolic phenotype. EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer; ADP, adenosine diphosphate. The central box represents the values between the lower and upper quartiles and the middle line is the median. The vertical line extends from the minimum to the maximum value, excluding outside values, which are displayed as separate points
Figure 3.
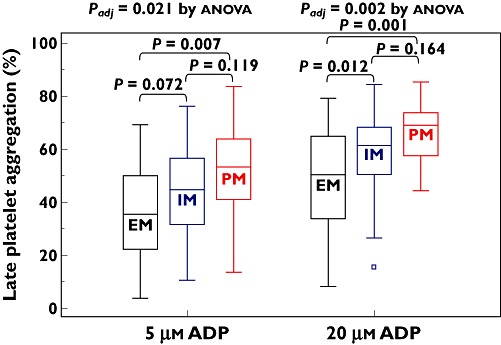
Late platelet aggregation according to CYP2C19 metabolic phenotype. EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer; ADP, adenosine diphosphate. The central box represents the values between the lower and upper quartiles and the middle line is the median. The vertical line extends from the minimum to the maximum value, excluding outside values, which are displayed as separate points
Figure 4.
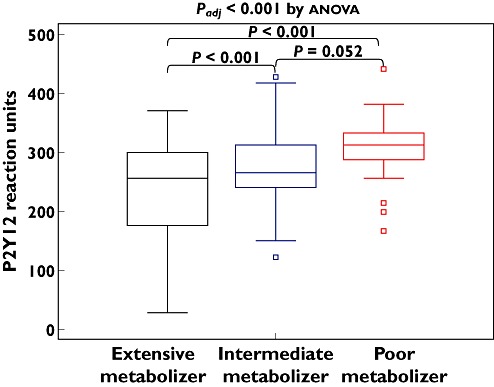
P2Y12 reaction units according to CYP2C19 metabolic phenotype. The central box represents the values between the lower and upper quartiles and the middle line is the median. The vertical line extends from the minimum to the maximum value, excluding outside values, which are displayed as separate points
Prevalence and predictors of HPR
Twelve patients (9.4%) of all cohorts receiving triple platelet therapy met the criteria of HPR. The CYP3A5*3 and ABCB1 3435C > T polymorphisms were not correlated with the increased risk of HPR (Table 3). The CYP2C19 PMs tended to have a higher prevalence of HPR than the CYP2C19 EMs and IMs: 6.3%, 7.4% and 20.0% in 0, 1 and 2 carriers of CYP2C19 LOF allele(s) (P = 0.099 after adjustment for diabetes) (Figure 5). To determine the predictors of HPR, a logistic multivariate regression analysis was performed using the known covariates, gender, age, body mass index (BMI), the CYP2C19 phenotype, ACS presentation at index PCI, smoking status, hypertension, diabetes mellitus, chronic kidney disease, left ventricular ejection fraction ≤45% and use of a CYP3A4-metabolized statin, calcium channel blocker and proton pump inhibitor. Carriage of two CYP2C19 LOF alleles (PMs) was only a significant predictor for HPR while taking triple antiplatelet therapy (OR 5.78, 95% CI 1.21, 27.78, P = 0.028).
Figure 5.
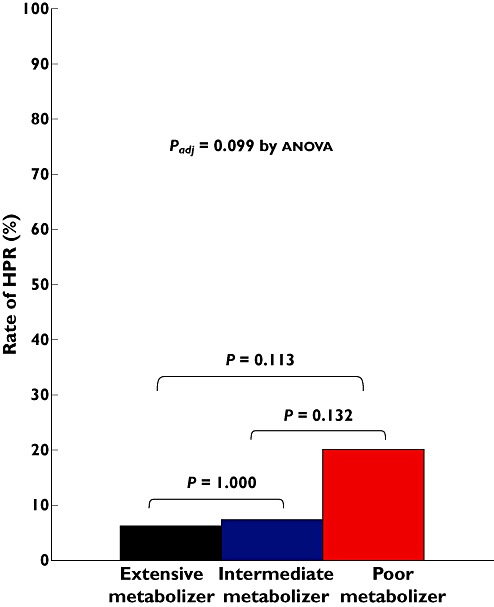
Rate of HPR according to CYP2C19 metabolic phenotype. HPR indicates high on-treatment platelet reactivity (5 µ ADP-induced maximal platelet aggregation >46%)
Discussion
The present study demonstrates the interaction between candidate polymorphisms and the pharmacodynamic effect of triple antiplatelet therapy in high-risk PCI-treated patients. Observed findings were as follows: (i) on the basis of consensus-defined HPR, more than 90% of the patients showed adequate platelet inhibition during triple antiplatelet therapy, (ii) about 60% of the patients carried the CYP2C19 LOF allele (*2 or *3), which are representative of an East Asian population [28], (iii) SNPs of the CYP2C19 gene, but not the ABCB1 3435C > T and CYP3A5 genes could affect the antiplatelet effect of triple antiplatelet therapy and (iv) a proportion of the CYP2C19 PMs still exhibited low platelet inhibition with triple antiplatelet therapy (∼20%).
During antiplatelet therapy, persistent HPR can increase the risk of occurrence of ischaemic events, whereas enhanced platelet inhibition may increase the risk of bleeding events [32]. Therefore, it may be important to identify the major attributable factors to antiplatelet response. Although clinical risk factors can affect the effect of antiplatelet therapy and the density of the platelet P2Y12 receptor [4, 33], enzymatic activity of genes encoding for intestinal absorption and hepatic metabolism has shown a significant and consistent association with the effect of antiplatelet therapy or clinical outcomes [20–25].
ADP-induced platelet inhibition by triple antiplatelet therapy may be mainly derived from the additive increase of cyclic adenosine monophosphate by both clopidogrel and cilostazol [16–18]. For clopidogrel response, the CYP2C19 LOF allele has been consistently linked with pharmacokinetic and pharmacodynamic profiles [4, 20–22], and the ABCB1 3435C > T and the CYP3A5 polymorphism also have reduced the antiplatelet response of clopidogrel in some cases [20, 21, 30]. Meanwhile, for the cilostazol effect, the CYP3A5 and CYP2C19 LOF alleles could explain the substantial variability in the pharmacokinetics of cilostazol in healthy subjects [24, 25]. However, in the present study, the ABCB1 3435C > T and CYP3A5*3 variant did not significantly influence the effect of triple antiplatelet therapy.
In addition to cilostazol itself, its metabolites also can inhibit platelet activation with different potency, which may implicate that the pharmacodynamics of cilostazol can be different from the pharmacokinetics of cilostazol. OPC-13015 (dehydro-cilostazol) and OPC-13213 (monohydroxy-cilostazol) are the main metabolites of cilostazol [23]. OPC-13015 is mainly produced by the CYP3A4 system, whereas OPC-13213 is generally produced by the CYP3A5 and CYP2C19 pathways. Because of its different potency (OPC-13015 : cilostazol : OPC-13213 = 9:3:1), the pharmacodynamics of cilostazol are less influenced by the CYP3A5 and CYP2C19 LOF polymorphisms. Therefore, the association between the antiplatelet effect of triple antiplatelet therapy and the CYP2C19 LOF allele carriage in the present study may be mainly explained by the interaction between the CYP2C19 LOF allele and clopidogrel.
In the ACCEL-DOUBLE (Accelerated Platelet Inhibition by a Double Dose of Clopidogrel According to Gene Polymorphism) study [34], we reported 21.1% prevalence of HPR (5 µm ADP-induced Aggmax >50%) in PCI-treated patients taking a double dose of clopidogrel (150 mg day–1). If we adapted the consensus-defined criteria of HPR (5 µm ADP-induced Aggmax >46%), the frequency of HPR reached 29.4%: 13.0%, 33.3% and 55.0% in 0, 1 and 2 carriers of the CYP2C19 LOF allele(s) (P < 0.001). These findings may explain partly the modest effect of double dose clopidogrel on the risk of HPR and clinical outcomes in the GRAVITAS (Gauging Responsiveness with A VerifyNow assay-Impact on Thrombosis And Safety) trial [35]. In this ACCEL-TRIPLE study, ∼ 20% of the CYP2C19 PMs were stratified into HPR even though they were receiving triple antiplatelet therapy. However, because the prevalence of the CYP2C19 PMs among African and Caucasian is much less (<5%) [31, 36], the estimated risk may be low in these populations. Moreover, some studies have suggested that East Asians could have a different cutoff of HPR (≥275 PRU) for predicting post PCI ischaemic events [37, 38]. If we apply this cutoff of HPR for the ACCEL-TRIPLE study, most of the patients achieved adequate platelet inhibition (∼95%).
Compared with clopidogrel, new P2Y12 receptor antagonists have shown the consistently predictable and potent pharmacokinetic and pharmacodynamic profiles and impacting clinical outcomes, irrespective of genetic polymorphisms [21, 39, 40]. However, P2Y12 receptors play a limited role in ADP-induced platelet aggregation [41] and post PCI ischaemic event recurrence has still been observed during potent P2Y12 receptor inhibitor administration. Furthermore, clinical introduction of these regimens is inevitably accompanied by the increased risk of major bleeding and bleeding risk seems higher in Asians according to the subanalysis [42]. More interestingly, adjuctive cilostazol with dual antiplatelet therapy did not increase the risk of major bleeding in several registries and prospective trials [7–15]. The endothelium-targeted antithrombotic effect and reversible antiplatelet properties (such as ticagrelor) of cilostazol may explain the safe profile for bleeding [5]. In addition, pleiotropic effects of cilostazol including endothelium [6–9, 11, 12, 15, 43], inflammation and ischaemia-reperfusion injury [44, 45] may also influence clinical outcomes in PCI-treated patients. Therefore, when we take into account both post-PCI efficacy and safety, adjunctive cilostazol with standard antiplatelet therapy can be a considerable antiplatelet regimen.
However, this concept of adjunctive cilostazol has not been supported by the result of the CILON-T (Influence of CILostazol-based triple antiplatelet therapy ON ischaemic complication after drug-eluting stenT implantation) prospective trial [46]. This study enrolled relatively low risk patients treated with a drug-eluting stent (∼10%: positive cardiac enzyme), and the 6 month composite of cardiac death, nonfatal myocardial infarction and ischaemic stroke was quiet low (∼2.8%). In addition, adjunctive cilostazol could not reduce the risk of restenosis. Although the GRAVITAS and CURRENT-OASIS 7 (double dose vs. standard dose clopidogrel and high dose vs. low dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes) trials including PCI-treated patients compared the efficacy and safety of the same regimens (standard dose vs. double dose clopidogrel) [36, 47], the latter only could suggest the beneficial effect of double dose clopidogrel in reduction of ischaemic events. Contrary to the GRAVITAS trial, the CURRENT-OASIS 7 trials enrolled ACS patients with high risk profiles, and started double dose therapy before coronary angiography, implicating that clinical outcome in antiplatelet therapy trials can be changed depending on the study design. Future clinical trials using cilostazol need to incorporate a study design including high risk patients and evaluation of front-loaded effect.
To date, there have been no definite guidelines of P2Y12 blockade in the CYP2C19 PMs. The GIFT (Genotype Information and Functional Testing) demonstrated that doubling of clopidogrel dose cannot overcome the influence of CYP LOF allele, especially in PMs, based on pharmacodynamic and clinical outcomes [48]. New potent P2Y12 inhibitors can be used as alternative drugs in PMs [49, 50]. However, exploratory analysis including non-ST elevation ACS patients showed no difference in the occurrence of ischaemic events with prasugrel vs. clopidogrel in carriers of the CYP2C19 LOF allele (HR 0.98, 95% CI 0.80, 1.20) [49]. The present study also suggested that adding cilostazol may be a useful regimen in PMs. Pharmacodynamic profiles of several antiplatelet regimens currently have been extrapolated into clinical scenarios. Because clinical benefits of theses regimens cannot be explained by their antiplatelet effect alone, superiority of one regimen over another must be determined based on large-scale clinical trials.
The present study has several limitations. First, because this observational, single centre study included only a small number to compare the genetic influences on platelet reactivity, it may be underpowered to elucidate significant differences of clinical efficacy and safety. Second, this study was performed using candidate gene analysis and other genetic variants may be relevant in risk stratification. Third, the sample size was calculated solely based on the hypothesized influence of CYP2C19 alleles without considering the other two genetic variants. However, the results of this analysis justified our assumption. Finally, this study enrolled East Asians only, and the pharmacodynamics of triple antiplatelet therapy may be different in other ethnic groups. Contrary to other clinical risk factors, there are remarkable differences in BMI and the CYP2C19 genotype between Caucasians and East Asians. Among East Asians, low BMI (about 24 kg m–2 in this study) can increase the antiplatelet effect of clopidogrel, whereas the influence of the CYP2C19 LOF allele can decrease the effect. In addition, carriage of the CYP2C19*17 gain-of-function allele seems uncommon among the Korean population (∼2%) [37]. Taken together, the antiplatelet effect of clopidogrel appears to be low in East Asians, and response to triple antiplatelet therapy may be somewhat greater in Western populations [16].
In conclusion, among PCI-treated high risk patients, the antiplatelet effect of triple antiplatelet therapy is influenced by carriage of the CYP2C19 LOF allele. Its clinical benefit in PMs needs to be validated in future large scale trials.
Acknowledgments
This study was partly supported by grants from Gyeongsang National University Hospital and the Institute of the Health Sciences, Gyeongsang National University.
Competing Interests
Dr Jeong received honoraria for lectures from Sanofi-Aventis, Daiichi Sankyo Inc and Otsuka. Other authors declared no conflict of interest.
REFERENCES
- 1.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–33. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC, Jr, Salette G, Contant CF, Massaro JM, Steg PG REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–7. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 4.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes D, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–33. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 5.Goto S. Cilostazol: potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl. 2005;6:3–11. doi: 10.1016/j.atherosclerosissup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Douglas JS., Jr Pharmacologic approaches to restenosis prevention. Am J Cardiol. 2007;100:10K–16K. doi: 10.1016/j.amjcard.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Singh I, Shafiq N, Pandhi P, Reddy S, Pattanaik S, Sharma Y, Malhotra S. Triple antiplatelet therapy vs. dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: an evidence-based approach to answering a clinical query. Br J Clin Pharmacol. 2009;68:4–13. doi: 10.1111/j.1365-2125.2009.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas JS, Jr, Holmes DR, Jr, Kereiakes DJ, Grines CL, Block E, Ghazzal ZM, Morris DC, Liberman H, Parker K, Jurkovitz C, Murrah N, Foster J, Hyde P, Mancini GB, Weintraub WS Cilostazol for Restenosis Trial (CREST) Investigators. Coronary stent restenosis in patients treated with cilostazol. Circulation. 2005;112:2826–32. doi: 10.1161/CIRCULATIONAHA.104.530097. [DOI] [PubMed] [Google Scholar]
- 9.Tamhane U, Meier P, Chetcuti S, Chen KY, Rha SW, Grossman MP, Gurm H. Efficacy of cilostazol in reducing restenosis in patients undergoing contemporary stent based PCI: a meta-analysis of randomised controlled trials. Eurointervention. 2009;5:384–93. doi: 10.4244/v5i3a60. [DOI] [PubMed] [Google Scholar]
- 10.Lee SW, Park SW, Hong MK, Kim YH, Lee BK, Song JM, Han KH, Lee CW, Kang DH, Song JK, Kim JJ, Park SJ. Triple versus dual antiplatelet therapy after coronary stenting: impact on stent thrombosis. J Am Coll Cardiol. 2005;46:1833–7. doi: 10.1016/j.jacc.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, Hong MK, Kim HS, Ko JK, Park JH, Lee JH, Choi SW, Seong IW, Cho YH, Lee NH, Kim JH, Chun KJ, Park SJ. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES Trial (A Randomized Comparison of Triple Antiplatelet Therapy with Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in Diabetic Patients) J Am Coll Cardiol. 2008;51:1181–7. doi: 10.1016/j.jacc.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Lee SW, Park SW, Yun SC, Kim YH, Park DW, Kim WJ, Lee JY, Lee CW, Hong MK, Kim JJ, Park SJ. Triple antiplatelet therapy reduces ischemic events after drug-eluting stent implantation: Drug-Eluting stenting followed by Cilostazol treatment REduces Adverse Serious cardiac Events (DECREASE registry) Am Heart J. 2010;159:284–91.e1. doi: 10.1016/j.ahj.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Chen KY, Rha SW, Li YJ other Korea Acute Myocardial Infarction Registry (KAMIR) investigators. Triple versus dual antiplatelet therapy in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. 2009;119:3207–14. doi: 10.1161/CIRCULATIONAHA.108.822791. [DOI] [PubMed] [Google Scholar]
- 14.Han Y, Li Y, Wang S, Jing Q, Wang Z, Wang D, Shu Q, Tang X. Cilostazol in addition to aspirin and clopidogrel improves long-term outcomes after percutaneous coronary intervention in patients with acute coronary syndromes: a randomized, controlled study. Am Heart J. 2009;157:733–9. doi: 10.1016/j.ahj.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, Kang SJ, Park SJ, Lee JH, Choi SW, Seong IW, Lee NH, Cho YH, Shin WY, Lee SJ, Lee SW, Hyon MS, Bang DW, Choi YJ, Kim HS, Lee BK, Lee K, Park HK, Park CB, Lee SG, Kim MK, Park KH, Park WJ DECLARE-LONG II Study Investigators. A randomized, double-blind, multicenter comparison study of triple antiplatelet therapy with dual antiplatelet therapy to reduce restenosis after drug-eluting stent implantation in long coronary lesions results from the DECLARE-LONG II (drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with long coronary lesions) trial. J Am Coll Cardiol. 2011;57:1264–70. doi: 10.1016/j.jacc.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Angiolillo DJ, Capranzano P, Goto S, Aslam M, Desai B, Charlton RK, Suzuki Y, Box LC, Shoemaker SB, Zenni MM, Guzman LA, Bass TA. A randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual antiplatelet therapy: results of the OPTIMUS-2 study. Eur Heart J. 2008;29:2202–11. doi: 10.1093/eurheartj/ehn287. [DOI] [PubMed] [Google Scholar]
- 17.Jeong YH, Lee SW, Choi BR, Kim IS, Seo MK, Kwak CH, Hwang JY, Park SW. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity: results of the ACCEL-RESISTANCE (adjunctive cilostazol versus high maintenance dose clopidogrel in patients with clopidogrel resistance) randomized study. J Am Coll Cardiol. 2009;53:1101–9. doi: 10.1016/j.jacc.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Jeong YH, Hwang JY, Kim IS, Park Y, Hwang SJ, Lee SW, Kwak CH, Park SW. Adding cilostazol to dual antiplatelet therapy achieves greater platelet inhibition than high maintenance-dose clopidogrel in patients with acute myocardial infarction: results of the ACCEL-AMI study. Circ Cardiovasc Interv. 2010;3:17–26. doi: 10.1161/CIRCINTERVENTIONS.109.880179. [DOI] [PubMed] [Google Scholar]
- 19.Lee K, Kim JY, Yoo BS, Yoon J, Hong MK, Ahn MS, Choe H, Lee SH. Adding cilostazol further inhibits the platelet aggregation compared to the standard or high dose of clopidogrel in clopidogrel low responders. J Thromb Haemost. 2010;8:2577–9. doi: 10.1111/j.1538-7836.2010.04019.x. [DOI] [PubMed] [Google Scholar]
- 20.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, Steg PG, Ferrières J, Danchin N, Becquemont L French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 21.Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, Antman EM, Braunwald E, Sabatine MS. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312–9. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–62. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 23.Hiratsuka M, Hinai Y, Sasaki T, Konno Y, Imagawa K, Ishikawa M, Mizugaki M. Characterization of human cytochrome P450 enzymes involved in the metabolism of cilostazol. Drug Metab Dispos. 2007;35:1730–2. doi: 10.1124/dmd.107.016758. [DOI] [PubMed] [Google Scholar]
- 24.Yoo HD, Park SA, Cho HY, Lee YB. Influence of CYP3A and CYP2C19 genetic polymorphisms on the pharmacokinetics of cilostazol in healthy subjects. Clin Pharmacol Ther. 2009;86:281–4. doi: 10.1038/clpt.2009.90. [DOI] [PubMed] [Google Scholar]
- 25.Yoo HD, Cho HY, Lee YB. Population pharmacokinetic analysis of cilostazol in healthy subjects with genetic polymorphisms of CYP3A5, CYP2C19 and ABCB1. Br J Clin Pharmacol. 2010;69:27–37. doi: 10.1111/j.1365-2125.2009.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong YH, Park Y, Kim IS, Yun SE, Kang MK, Hwang SJ, Kwak CH, Hwang JY. Enhanced platelet inhibition by adjunctive cilostazol to dual antiplatelet therapy after drug-eluting stent implantation for complex lesions. Thromb Haemost. 2010;104:1286–9. doi: 10.1160/TH10-04-0239. [DOI] [PubMed] [Google Scholar]
- 27.Kim IS, Jeong YH, Kang MK, Koh JS, Park Y, Hwang SJ, Kwak CH, Hwang JY, Kim S. Correlation of high post-treatment platelet reactivity assessed by light transmittance aggregometry and the VerifyNow P2Y12 assay. J Thromb Thrombolysis. 2010;30:486–95. doi: 10.1007/s11239-010-0484-2. [DOI] [PubMed] [Google Scholar]
- 28.Home Page of the Human Cytochrome P450 (CYP) Allele Nomenclature Database. Available at http://www.cypalleles.ki.se (last accessed 7 February 2012)
- 29.Man M, Farmen M, Dumaual C, Teng CH, Moser B, Irie S, Noh GJ, Njau R, Close S, Wise S, Hockett R. Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. J Clin Pharmacol. 2010;50:929–40. doi: 10.1177/0091270009355161. [DOI] [PubMed] [Google Scholar]
- 30.Suh JW, Koo BK, Zhang SY, Park KW, Cho JY, Jang IJ, Lee DS, Sohn DW, Lee MM, Kim HS. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ. 2006;174:1715–22. doi: 10.1503/cmaj.060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim IS, Choi BR, Jeong YH, Kwak CH, Kim S. The CYP2C19*2 and CYP2C19*3 polymorphisms are associated with high post-treatment platelet reactivity in Asian patients with acute coronary syndrome. J Thromb Haemost. 2009;7:897–9. doi: 10.1111/j.1538-7836.2009.03319.x. [DOI] [PubMed] [Google Scholar]
- 32.Sibbing D, Steinhubl SR, Schulz S, Schömig A, Kastrati A. Platelet aggregation and its association with stent thrombosis and bleeding in clopidogrel-treated patients: initial evidence of a therapeutic window. J Am Coll Cardiol. 2010;56:317–8. doi: 10.1016/j.jacc.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Gladding P, Panattoni L, Webster M, Cho L, Ellis S. Clopidogrel pharmacogenomics: next steps: a clinical algorithm, gene-gene interactions, and an elusive outcomes trial. JACC Cardiovasc Interv. 2010;3:995–1000. doi: 10.1016/j.jcin.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Jeong YH, Kim IS, Park Y, Kang MK, Koh JS, Hwang SJ, Kwak CH, Hwang JY. Carriage of cytochrome 2C19 polymorphism is associated with risk of high post-treatment platelet reactivity on high maintenance-dose clopidogrel of 150 mg/day: results of the ACCEL-DOUBLE (Accelerated Platelet Inhibition by a Double Dose of Clopidogrel According to Gene Polymorphism) study. JACC. 2010;3:731–41. doi: 10.1016/j.jcin.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillablower ME, Aragon JR, Kandzari DE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 36.Zhou SF, Di YM, Chan E, Du YM, Chow VD, Xue CC, Lai X, Wang JC, Li CG, Tian M, Duan W. Clinical pharmacogenetics and potential application in personalized medicine. Curr Drug Metab. 2008;9:738–84. doi: 10.2174/138920008786049302. [DOI] [PubMed] [Google Scholar]
- 37.Park KW, Park JJ, Jeon KH, Kang SH, Oh IY, Yang HM, Cho HJ, Lee HY, Kang HJ, Koo BK, Oh BH, Park YB, Kim HS. Enhanced clopidogrel responsiveness in smokers. Smokers' paradox is dependent on cytochrome P450 CYP1A2 status. Arterioscler Thromb Vasc Biol. 2011;31:665–71. doi: 10.1161/ATVBAHA.110.217182. [DOI] [PubMed] [Google Scholar]
- 38.Ko YG, Suh JW, Kim BH, Lee CJ, Kim JS, Choi D, Hong MK, Seo MK, Youn TJ, Chae IH, Choi DJ, Jang Y. Comparison of 2 point-of-care platelet function tests, VerifyNow Assay and Multiple Electrode Platelet Aggregometry, for predicting early clinical outcomes in patients undergoing percutaneous coronary intervention. Am Heart J. 2011;161:383–90. doi: 10.1016/j.ahj.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, Becker RC PLATO Investigators. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–8. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 40.Gurbel PA, Bliden KP, Antonino MJ, Stephens G, Gretler DD, Jurek MM, Pakyz RE, Shuldiner AR, Conley PB, Tantry US. The effect of elinogrel on high platelet reactivity during dual antiplatelet therapy and the relation to CYP2C19*2 genotype: first experience in patients. J Thromb Haemost. 2010;8:43–53. doi: 10.1111/j.1538-7836.2009.03648.x. [DOI] [PubMed] [Google Scholar]
- 41.Goto S, Tamura N, Eto K, Ikeda Y, Handa S. Functional significance of adenosine 5′-diphosphate receptor (P2Y12) in platelet activation initiated by binding of von Willebrand factor to platelet GP Ibα induced by conditions of high shear rate. Circulation. 2002;105:2531–6. doi: 10.1161/01.cir.0000016703.93845.af. [DOI] [PubMed] [Google Scholar]
- 42.Mak KH, Bhatt DL, Shao M, Hankey GJ, Easton JD, Fox KA, Topol EJ. Ethnic variation in adverse cardiovascular outcomes and bleeding complications in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study. Am Heart J. 2009;157:658–65. doi: 10.1016/j.ahj.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 43.Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, Ouchi Y. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1634–9. doi: 10.1161/ATVBAHA.108.164368. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal NK, Maiti R, Dash D, Pandey BL. Cilostazol reduces inflammatory burden and oxidative stress in hypertensive type 2 diabetes mellitus patients. Pharmacol Res. 2007;56:118–23. doi: 10.1016/j.phrs.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Manickavasagam S, Ye Y, Lin Y, Perez-Polo RJ, Huang MH, Lui CY, Hughes MG, McAdoo DJ, Uretsky BF, Birnbaum Y. The cardioprotective effect of a statin and cilostazol combination: relationship to Akt and endothelial nitric oxide synthase activation. Cardiovasc Drugs Ther. 2007;21:321–30. doi: 10.1007/s10557-007-6036-0. [DOI] [PubMed] [Google Scholar]
- 46.Suh JW, Lee SP, Park KW, Lee HY, Kang HJ, Koo BK, Cho YS, Youn TJ, Chae IH, Choi DJ, Rha SW, Bae JH, Kwon TG, Bae JW, Cho MC, Kim HS. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (Influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J Am Coll Cardiol. 2011;57:280–9. doi: 10.1016/j.jacc.2010.08.631. [DOI] [PubMed] [Google Scholar]
- 47.Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S. CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–43. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 48.Price MJ. Primary results from genotype information and functional testing (GIFT): A prospective pharmacogenomic analysis of clopidogrel therapy. 2011. Presented at American College of Cardiology Meeting 2011 at New Orleans, USA.
- 49.Sorich MJ, Vitry A, Ward MB, Horowitz JD, McKinnon RA. Prasugrel vs. clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI 38 trial data. J Thromb Haemost. 2010;8:1678–84. doi: 10.1111/j.1538-7836.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- 50.Bliden KP, Tantry US, Storey RF, Jeong YH, Gesheff M, Wei C, Gurbel PA. The effect of ticagrelor versus clopidogrel on high on-treatment platelet reactivity: combined analysis of the ONSET/OFFSET and RESPOND studies. Am Heart J. 2011;162:160–5. doi: 10.1016/j.ahj.2010.11.025. [DOI] [PubMed] [Google Scholar]


