Abstract
AIM
To evaluate the association between the in vitro sensitivity of peripheral blood mononuclear cells (PBMCs) to methylprednisolone (MP) and the presence of genetic polymorphisms involved in glucocorticoid (GC) response.
METHODS
In vitro MP inhibition of the proliferation of lymphocytes stimulated with concanavalin A was determined. Non linear regression of dose–response data was performed computing the MP concentration required to reduce proliferation to 50% (IC50). The maximum inhibition achievable at the highest MP concentration (Imax) was also calculated. Moreover, the Taqman technique was used to analyze the BclI polymorphism in the NR3C1 gene and the Leu155His polymorphism in the NALP1 gene.
RESULTS
A significant association between the BclI mutated genotype and an increased in vitro sensitivity to GCs was observed.
CONCLUSIONS
The a priori evaluation of the BclI polymorphism, associated with a lymphocyte proliferation assay, could represent a useful diagnostic tool for the optimization of steroid treatment.
Keywords: BclI polymorphism, glucocorticoid sensitivity, lymphocyte proliferation assay
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
In vitro lymphocyte steroid sensitivity has been suggested as a useful tool to predict in vivo response to glucocorticoid treatment in different inflammatory chronic diseases.
A correlation between genetic polymorphisms and clinical response to glucocorticoids has been demonstrated in these patients.
WHAT THIS STUDY ADDS
The BclI polymorphism in the glucocorticoid receptor (NR3C1) gene is associated with higher methylprednisolone potency in vitro.
The combined evaluation of the in vitro sensitivity to methylprednisolone and BclI polymorphism could represent an aid for physicians to adjust therapy a priori.
Introduction
Glucocorticoids (GCs) are a well-accepted therapy for inflammatory and autoimmune diseases in transplant patients and in the treatment of leukaemia and lymphomas [1]. However, despite their large clinical impact and justified use, the benefits of these agents are often narrowed by a great inter-individual variability that might potentially lead to treatment failure or drug induced toxicity.
Polymorphisms in genes involved in the molecular effects of these hormones could be important in the observed differences in efficacy. In recent studies conducted in our laboratory [2, 3], among various polymorphisms considered, the BclI polymorphism in the GC receptor gene (NR3C1), and the Leu155His polymorphism in the NALP1 gene (NLRP1: NACHT, LRR and PYD domain-containing protein 1), were associated with GC response. The BclI polymorphism consists in a C > G substitution 646 nucleotides downstream from exon 2, and the mutated allele has been associated with hypersensitivity to GCs [4, 5], and with a better response to these hormones in paediatric patients with inflammatory bowel disease (IBD) [2, 3]. NALP1 belongs to a group of cytoplasmic pattern recognition receptors that stimulate innate immunity and promote the maturation of cytokines [6]. Jin et al. [7] have recently shown that variants in the NALP1 gene, in particular Leu155His, confer susceptibility to autoimmune and auto-inflammatory diseases, probably related to an altered cytokine activation. Moreover, paediatric patients with IBD, carriers of the NALP1 homozygous variant, exhibit a higher probability of non response to GC therapy [2].
In vitro tests based on the proliferation of mononuclear cells exposed to GCs have been correlated with clinical response in different diseases such as rheumatoid arthritis [8], systemic lupus erythematosus [9], bronchial asthma [10], renal transplant rejection [11] and ulcerative colitis [12]. These findings, however, have not always been reproduced and a bioassay that could be used to predict GC responsiveness in clinical practice is still lacking. To evaluate individual response to GCs, a pharmacodynamic approach using patients' peripheral blood mononuclear cells (PBMC), together with a pharmacogenetic approach with the evaluation of polymorphisms involved in the GC response, could be an efficient strategy. The final goal of our study was therefore to set up a simple and reproducible assay to evaluate in vitro methylprednisolone (MP) individual sensitivity and to correlate this with the presence of the BclI and Leu155His polymorphisms.
Methods
Subjects
An in vitro proliferation assay with MP and genetic analyses were performed on PBMCs obtained from 42 blood donors.
Samples were obtained between September 2010 and March 2011 from the Transfusion Center, Azienda Ospedaliera Universitaria, Trieste. Blood was obtained by venipuncture between 08.00 a.m. and 10.00 a.m. to minimize any variability due to circadian rhythms, and immediately processed. Written informed consent was obtained from each subject and the local ethics commission gave permission for this study. A total of 9 ml of buffy coats was used for the isolation of PBMCs.
Drug/molecular target nomenclature conforms to the Guide to Receptors and Channels [13].
In vitro proliferation assay
The effect of MP on proliferation of PBMCs was determined by labelling metabolically active cells with [methyl-3H] thymidine (Perkin Elmer, Milan, Italy). PBMCs were collected by density gradient centrifugation on Ficoll Paque™ Plus (Healthcare, Milan, Italy), resuspended in complete RPMI-1640 medium containing concanavalin-A (5 µg ml−1) and seeded into a 96 well round bottom plate (2 × 105 cells/well) in the presence of MP (range from 54 µm to 0.05 nm). After 50 h of incubation, cells were pulsed with [methyl-3H] thymidine (2.5 µCi ml−1 well−1) and the incubation was continued for an additional 22 h. The radioactivity of the samples was determined by a liquid scintillation analyzer (Wallac 1450 Microbeta liquid scintillation counter, Perkin Elmer, Milan, Italy). Raw counts per minute (counts min−1) data were converted and normalized to percent of maximal survival for each experimental condition (counts min−1 MP/counts min−1 control × 100). Non linear regression of dose–response data was performed using GraphPad Prism version 4.00 for computing IC50, the MP concentration required to reduce proliferation to 50%. Imax was also calculated and defined as the maximum inhibition achievable at the highest concentration of MP (54 µm).
The calculated coefficient of variation was 15% and the limit of determination of this assay was calculated at 1 nCi ml−1 of [methyl-3H] thymidine.
Genetic analysis
Total genomic DNA was isolated from peripheral blood using a commercial kit (Gene Elute Blood Genomic DNA kit, Sigma Aldrich, Milan, Italy) and genetic polymorphisms were determined using TaqMan® genotyping technologies (Applied Biosystems, Bedford, UK) on an ABI7900 HT sequence detection system device.
Statistical analysis
Any possible association between MP IC50 and Imax and the studied polymorphisms was investigated by the non-parametric Mann-Whitney and Kruskal-Wallis tests. On the basis of previous results [2, 3], BclI homozygous carriers were compared with a group of both heterozygous and wild type carriers. However a dose allele effect was also studied and results are presented in Figure S1.
Statistical analysis was performed using the software R.
Results and discussion
The in vitro lymphocyte sensitivity to MP was evaluated in 42 healthy blood donors (mean age 41.8, range 18–60 years; 16.7% female and 83.3% male) and a wide interindividual variation in IC50 and Imax was evident (IC50 median value 1.43 × 10−7m, range 7.43 × 10−10m–2.94 × 10−4m; Imax median value 91.5%, range 50.0–98.0%) and comparable with that reported in the literature [14, 15]. Preliminary work conducted in our laboratory revealed that, within a given individual, relatively little variation, both in IC50 and Imax, was observed. MP was employed in this in vitro study as it is one of the steroids of choice in chronic diseases.
Among the possible causes of a variable response to GCs, genetic polymorphisms can be important. Two variants in genes coding for proteins involved in the pharmacodynamics of these agents, the BclI polymorphism in the NR3C1 gene, and a polymorphism in the NALP1 gene, have been shown to be particularly relevant in previous studies from our laboratory [2, 3]. Therefore, the presence of these polymorphisms was evaluated in this study. The genotype distribution was in Hardy-Weinberg equilibrium (BclI P = 0.64, Leu155His P = 0.71) and is presented in Table 1.
Table 1.
Frequencies of genotype polymorphisms involved in GC action and Hardy-Weinberg (HW) equilibrium
| Gene | Polymorphisms | Wild type | Heterozygous | Mutated | HW equilibrium (P value) |
|---|---|---|---|---|---|
| NR3C1 | BclI (n° (%)) | 20 (47.6) | 17 (40.4) | 5 (12.0) | 0.64 |
| NALP1 | Leu155His (n° (%)) | 8 (19.0) | 22 (52.4) | 12 (28.6) | 0.71 |
An increased GC in vitro sensitivity was observed in lymphocytes with the mutated BclI genotype. Indeed this genotype was associated with a lower MP IC50 (median 2.39 × 10−9m, range 7.43 × 10−10m–1.46 × 10−7m) compared with non mutated carriers (wild-type and heterozygous; median 2.76 × 10−7m, range 2.03 × 10−9m–2.94 × 10−4m, P = 0.0058 Mann-Whitney test; Figure 1 and Figure S1). Selected inhibition curves are presented in Figure S2. The mutated BclI genotype was also associated with significantly higher Imax values than non mutated (mutated: median 95%, range 95–98%; wild-type and heterozygous: median 90%, range 50–98%, P = 0.0078 Mann Whitney test; Figure 1 and Figure S1), revealing the presence of a subgroup of unresponsive cells in non mutated patients. The present data confirm that the BclI polymorphism in the NR3C1 gene, already associated with a better GC response in pediatric patients with IBD [2, 3], is an important marker of increased sensitivity to GCs.
Figure 1.
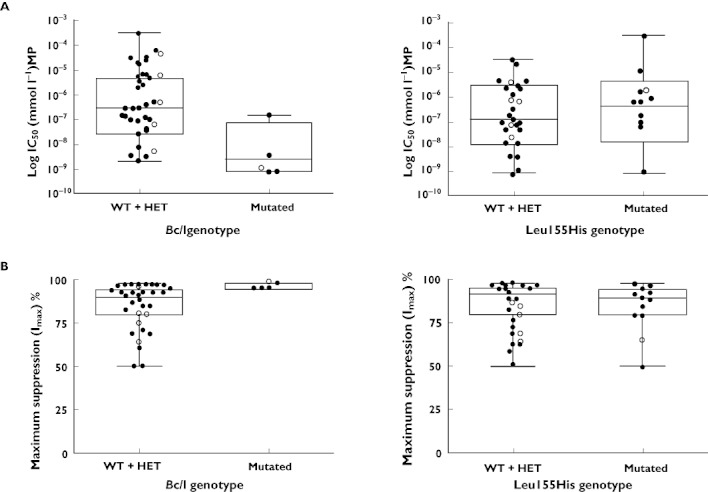
A) IC50 of non mutated genotype (wild type: WT and heterozygous: HET) compared with mutated genotype for BclI genotype in the NR3C1 gene (P = 0.0058) and for Leu155His in the NALP1 gene (P = 0.5544, Mann-Whitney test). Close circles indicate males and open circles indicate females. B) Imax of non mutated genotype (wild type: WT and heterozygous: HET) compared with mutated genotype for BclI genotype in the NR3C1 gene (P = 0.0078) and for Leu155His in the NALP1 gene (P = 0.56, Mann-Whitney test). Close circles indicate males and open circles indicate females
No association was observed in our study between the Leu155His polymorphism in the NALP1 gene with MP IC50 and Imax (IC50 mutated: median 3.78 × 10−7m, range 7.60 × 10−10m–2.93 × 10−4m; wild-type and heterozygous: median 1.25 × 10−7m, range 7.43 × 10−10m–2.94 × 10−5m, P = 0.5544; Imax mutated: median 89.5%, range 50–98%; wild-type and heterozygous: median 92.5%, range 50–98%, P = 0.56 Mann Whitney test; Figure 1 and Figure S1). We can hypothesize that, due to the role of NALP1 in the activation of cytokines, genetic polymorphisms of this gene become relevant only in inflammatory conditions such as IBD. Our study was, on the contrary, performed on lymphocytes obtained from healthy subjects, and this could explain this somewhat unexpected result.
Literature data [12, 14] indicate that measurement of in vitro PBMC steroid sensitivity is a predictor of response to treatment in inflammatory chronic diseases. Our results, on lymphocytes obtained from healthy donors, suggest that the evaluation of the BclI polymorphism, associated with a lymphocyte proliferation assay could represent a small step in the identification of subjects with a reduced probability of response to GCs. The in vitro prediction of GC response before the start of treatment would have important clinical implications, allowing to adjust therapy a priori, avoiding the use of these agents in patients who would probably not respond and reducing dosages in those who are hypersensitive, and hence at risk of toxicity. A limitation of this study is that only 42 healthy subjects were enrolled, a low number for an association study with polymorphisms. Therefore further studies are needed to confirm these results in a larger number of subjects and also in patients affected by chronic diseases.
Acknowledgments
Eva Cuzzoni is the recipient of a fellowship from the Department of Life Sciences, Trieste.
Competing Interests
There are no competing interests to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1
A) IC50 of wild type (WT) genotype comparedwith heterozygous (HET) and mutated genotype for BclIgenotype in the NR3C1 gene (P = 0.018) and forLeu155His in the NALP1 gene (P = 0.75, Kruskal-Wallistest). B) Imax of wild type (WT) genotype compared withheterozygous (HET) and mutated genotype for BclI genotype inthe NR3C1 gene (P = 0.02) and for Leu155His in theNALP1 gene (P = 0.77, Kruskal-Wallis test)
Figure S2
Selected inhibition curves in a steroid sensitive (BclImutated) (top) and a steroid resistant patient (BclI wildtype) (bottom). Points represent the mean values of triplicate dataand vertical bars represent standard errors
REFERENCES
- 1.Riccardi C, Bruscoli S, Migliorati G. Molecular mechanisms of immunomodulatory activity of glucocorticoids. Pharmacol Res. 2002;45:361–8. doi: 10.1006/phrs.2002.0969. [DOI] [PubMed] [Google Scholar]
- 2.De Iudicibus S, Stocco G, Martelossi S, Londero M, Ebner E, Pontillo A, Lionetti P, Barabino A, Bartoli F, Ventura A, Decorti G. Genetic predictors of glucocorticoid response in pediatric patients with inflammatory bowel diseases. J Clin Gastroenterol. 2011;45:e1–7. doi: 10.1097/MCG.0b013e3181e8ae93. [DOI] [PubMed] [Google Scholar]
- 3.De Iudicibus S, Stocco G, Martelossi S, Drigo I, Norbedo S, Lionetti P, Pozzi E, Barabino A, Decorti G, Bartoli F, Ventura A. Association of BclI polymorphism of the glucocorticoid receptor gene locus with response to glucocorticoids in inflammatory bowel disease. Gut. 2007;56:1319–20. doi: 10.1136/gut.2006.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Blasio AM, van Rossum EF, Maestrini S, Berselli ME, Tagliaferri M, Podesta F, Koper JW, Liuzzi A, Lamberts SW. The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol (Oxf) 2003;59:68–74. doi: 10.1046/j.1365-2265.2003.01798.x. [DOI] [PubMed] [Google Scholar]
- 5.Panarelli M, Holloway CD, Fraser R, Connell JM, Ingram MC, Anderson NH, Kenyon CJ. Glucocorticoid receptor polymorphism, skin vasoconstriction, and other metabolic intermediate phenotypes in normal human subjects. J Clin Endocrinol Metab. 1998;83:1846–52. doi: 10.1210/jcem.83.6.4828. [DOI] [PubMed] [Google Scholar]
- 6.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 7.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–25. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 8.Kirkham BW, Corkill MM, Davison SC, Panayi GS. Response to glucocorticoid treatment in rheumatoid arthritis: in vitro cell mediated immune assay predicts in vivo responses. J Rheumatol. 1991;18:821–5. [PubMed] [Google Scholar]
- 9.Seki M, Ushiyama C, Seta N, Abe K, Fukazawa T, Asakawa J, Takasaki Y, Hashimoto H. Apoptosis of lymphocytes induced by glucocorticoids and relationship to therapeutic efficacy in patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:823–30. doi: 10.1002/1529-0131(199805)41:5<823::AID-ART8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Hirano T, Homma M, Oka K, Tsushima H, Niitsuma T, Hayashi T. Individual variations in lymphocyte-responses to glucocorticoids in patients with bronchial asthma: comparison of potencies for five glucocorticoids. Immunopharmacology. 1998;40:57–66. doi: 10.1016/s0162-3109(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 11.Langhoff E, Ladefoged J, Jakobsen BK, Platz P, Ryder LP, Svejgaard A, Thaysen JH. Recipient lymphocyte sensitivity to methylprednisolone affects cadaver kidney graft survival. Lancet. 1986;1:1296–7. doi: 10.1016/s0140-6736(86)91220-1. [DOI] [PubMed] [Google Scholar]
- 12.Hearing SD, Norman M, Probert CS, Haslam N, Dayan CM. Predicting therapeutic outcome in severe ulcerative colitis by measuring in vitro steroid sensitivity of proliferating peripheral blood lymphocytes. Gut. 1999;45:382–8. doi: 10.1136/gut.45.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander S, Mathie A, Peter J. Guide to receptors and channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl. 1):S1–254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano T, Akashi T, Kido T, Oka K, Shiratori T, Miyaoka M. Immunosuppressant pharmacodynamics on peripheral-blood mononuclear cells from patients with ulcerative colitis. Int Immunopharmacol. 2002;2:1055–63. doi: 10.1016/s1567-5769(02)00077-2. [DOI] [PubMed] [Google Scholar]
- 15.Briggs WA, Gao ZH, Scheel PJ, Jr, Burdick JF, Gimenez LF, Choi MJ. Differential glucocorticoid responsiveness of dialysis patients' lymphocytes. Perit Dial Int. 1996;16:406–11. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


