Abstract
Epithelial to mesenchymal transition (EMT) plays a critical role during normal development and in adult tissue repair. It is known that immortalized epithelial cells can undergo an EMT and become cancer stem cells, and that epithelial cells from mouse pancreatic islet and avian inner ear can acquire mesenchymal traits in vitro via EMT. However, it is unclear whether epithelial cells from mammalian sensory system can undergo an EMT and obtain features of stem/progenitor cells. In this study, we used mouse utricle sensory epithelial cells (MUCs) as a mammalian cell model to address this issue. When cultured on 2-dimensional substrates, dissociated MUCs gradually lost their columnar shape and started to expand on the substrate with downregulation of expression of epithelial junction markers and upregulation of genes and proteins that are widely shown in mesenchymal cells. Moreover, MUCs expressed genes and proteins that are usually presented in prosensory epithelial cells and stem cells. These MUCs showed potential to differentiate into epithelial cells via a reverse EMT when they were forced to suspend in culture medium. Our findings reveal that sensory epithelial cells from mammalian tissue can undergo an EMT to become cells expressing features of stem cells that can be induced to become epithelial cells via a reverse EMT. The outcomes of this study may provide a novel approach to generate epithelial progenitors for use in cell replacement therapy to treat a number of human diseases, such as hearing loss and vision loss.
Introduction
During development, prosensory cells in the otocyst develop into inner ear sensory epithelial cells. Inner ear sensory epithelia are mainly composed of supporting cells and hair cells, which are primarily responsible for detection of auditory and vestibular information. However, mammalian sensory epithelial cells are vulnerable to a number of insults leading to permanent degeneration that cause hearing loss, tinnitus, vestibular problems, and other inner ear disorders. Currently, 2 major approaches are proposed for sensory epithelia regeneration. The first strategy is to introduce exogenous cells into the inner ear with the hope of replacing the function of damaged auditory system [1,2]. The second approach is to activate local sensory epithelial progenitors to differentiate into new sensory epithelial cells [3–5]. Generation of an in vitro sensory epithelial progenitor model directly from inner ear tissues will, therefore, facilitate the study of activation, proliferation, and differentiation of sensory epithelial progenitor. Sphere-forming cells have been identified from mammalian inner ear, which have the potentials to proliferate and become cells expressing sensory epithelial markers [6,7]. However, sphere-forming cells are cultured in suspension medium with relatively limited proliferation ability. The present research focuses on inducing mammalian sensory epithelia to become proliferative progenitors in vitro.
Well-differentiated epithelial cells such as inner ear sensory epithelia usually lack the ability to proliferate, whereas mesenchymal cells retain the capability of dividing. During development, epithelial to mesenchymal transition (EMT) is essential in the formation of the body plan and generation of tissues and organs. In adults, EMT plays an important role in tissue repair, organ fibrosis, and carcinoma progression. These observations lead to the question: can in vitro cultured epithelial cells undergo an EMT to become mesenchymal-like cells with the capability of proliferation? Indeed, when epithelial cells from pancreatic islet are cultured on 2-dimensional (2D) substrates in vitro, they de-differentiate into mesenchymal-like cells that can be expanded to a large number for use in regeneration study [8,9]. In our previous efforts for inner ear regeneration, utricle epithelial cells from avian embryos are found to undergo an EMT to proliferate and subsequently become sensory epithelial cells through a reverse EMT, mesenchymal-to-epithelial transition (MET) [10]. Since avian inner ear sensory epithelium is known to be able to regenerate, it might not be surprising to observe that these cells are able to proliferate via EMT. It is more challenging to study whether sensory epithelial cells from adult mammals, which are usually unable to regenerate, can undergo a similar process of EMT and consequently obtain the ability to proliferate [11]. Therefore, our hypothesis is that cell phenotype change may contribute to the generation, proliferation, and differentiation of inner ear sensory epithelial progenitors.
Mesenchymal status has recently been observed to be essential for the acquisition and maintenance of multi-/pluri- potency of cancer stem cells and/or embryonic stem (ES) cells. In cancer study, it is found that immortalized epithelial cells acquire cancer stem cell-like properties via EMT that may also contribute to cancer metastasis [12,13]. EMT is observed in human ES (hES) cells grown on matrigels, in which hES cells start to express mesenchymal markers while retaining the expression of pluripotent markers such as Oct4 and Nanog [14]. However, it remains to be determined whether epithelial cells from mammalian sensory system are able to acquire features of stem/progenitor cell via EMT. This is the first step for an effective regeneration strategy. For the next step, we will use these stem/progenitor cells to produce large numbers of new sensory epithelial cells, such as inner ear hair cells and retinal rod cell.
In this study, we use adult mouse inner ear sensory epithelial cells as a model to address the following questions: (a) whether mammalian sensory epithelial cells are able to undergo an EMT, (b) whether these sensory epithelial cells can proliferate following EMT, (c) whether epithelial cells from the inner ear can obtain general properties of stem/progenitor cells via EMT, and (d) whether these mammalian cells acquire features specific for inner ear stem/progenitor cells via EMT. The aim of the study is to develop novel culture strategies via EMT to generate a number of sensory epithelial progenitors that can be used to study the generation and proliferation of these cells, which will be used to guide in vivo hearing regeneration in the future. The results will also be applicable to epithelial cell generation in other systems, such as retina.
Materials and Methods
Generation of sensory epithelial cell clones from mouse inner ear utricle
All animal procedures have been approved by local Institutional Animal Care and Use Committee. Pure sensory epithelial sheets were harvested from 3-month-old Swiss Webster female mice using previously described methods [10]. The pure sensory epithelial sheets were cut into 1–2 mm2 pieces that were then transferred into a new 15 mL centrifuge tube. Following dissociation with 1 mL papain mixture (Sigma) at 37°C for 1 h [15], the sensory epithelial pieces were treated with 9 mL Dulbecco's modified Eagle's medium (DMEM)/F12 with 10% fetal bovine serum (FBS, all from Invitrogen) to end dissociation. The cell suspension was centrifuged at 200 g for 5 min followed by removal of supernatant and resuspension of cells in 1 mL culture medium containing DMEM/F12 supplemented with 10% FBS. The cell suspension was gently triturated, filtered through a cell strainer (40 μm; BD), and the cell number was evaluated using a hemocytometer. The cells were serially diluted and plated into a 96-well plate precoated with 0.1% gelatin (Millipore) and containing prewarmed mouse utricle sensory epithelial cell (MUC) medium (DMEM/F12, 10% FBS, 1% insulin transferrin selenium (Invitrogen), 0.1% 2-mercaptoethanol (Invitrogen), and 0.1% ampicillin (Fishersci). MUCs were then cultured in humidified 5% CO2 and 95% air at 37°C. Half of the culture medium was replaced every 2–3 days. The primary culture was replicated 5 times using tissue from 5 different animals.
Generation of MUC cell lines and proliferation assay
When MUCs reached 70%–80% confluence in the culture dish, TryplE (Invitrogen) was used to dissociate cells and serum-containing medium was used to stop dissociation. The cell suspension was centrifuged at 200 g for 5 min, the supernatant was removed, and the cells were resuspended into 1 mL culture medium. The cell number was evaluated using a hemocytometer and the cells were plated into a large culture dish/flask at the density of ∼2,000 cells/cm2. MUCs were cultured and gradually passaged into larger culture dishes/flasks for 8 months. To maintain cell lines, some of the cells were frozen in culture medium supplemented with 5% DMSO. Samples of cells after passage 2 were used for experimental purposes.
Three methods were used to determine cell proliferation. First, cell number was evaluated with a hemocytometer at each passage and the doubling time was calculated using the following formula: td=(t1-t0)/log2(C1/C0). C1=cell number at time point t1, C0=cell number at time point t0, td=doubling time for cell population [10].
Second, to further characterize cell growth, MUCs (Passage 10) were cultured for 24 h in the presence of 3 μg/mL 5-bromo-2-deoxyuridine (BrdU; Sigma) at culture day 1, 3, 5, and 7. At the end of culture periods, MUCs were fixed in 4% paraformaldehyde followed by DNA denaturization in 1 M HCl for 30 min at room temperature. The samples were blocked in 10% donkey serum and 0.2% triton X-100 for 30 min before mouse anti-BrdU antibodies (BD) were applied overnight at 4°C. Dylight549 conjugated secondary antibodies (Jackson ImmunoResearch) were added to the samples to visualize cells using a Leica epifluorescence microscope with appropriate filters. 4′,6′-diamidino-2-phenylindole (DAPI) was used for detection of all nuclei in the samples. Three microscope fields of each sample were randomly selected, and the images were captured using a digital camera (Q Imaging). The total cell number and the number of cells stained with both BrdU and DAPI were counted in each field and the percentage of BrdU-positive cells was calculated at each culture period.
Third, cell proliferation was determined by fluorescence-detection-based CyQuant NF cell proliferation assay (Invitrogen) according to the manufacturer's instruction. This method can directly observe cell growth without the need for nuclear incorporation during cell growth. To evaluate the correlation between cell number and fluorescence intensity, a standard curve was initially set up using passage 18 MUCs with the manufacturer's instruction. To evaluate cell proliferation, ∼1,000 cells were seeded into each well of a 96-well plate. Cells were incubated for at least 4 h for cell attachment and the fluorescence intensity of the cells was evaluated at 4 h, 1, 2, 3, 4, 5, 7, 8, and 9 days. The culture medium was removed and 100 μL of CyQuant NF dye was applied to the cells for 40 min followed by fluorescence intensity detection using a microplate reader (Molecular devices). Cell number was evaluated using the aforementioned standard curve. Six replicates were used for each time point.
Suspension culture
From passage 14, ∼50,000 MUCs were plated into a 60 mm culture dish containing DMEM/F12, 1% B27, 2% N2, 20 ng/mL epidermal growth factor (EGF), and 20 ng/mL basic fibroblast growth factor (FGF2) (all from Invitrogen) and maintained for 8–12 days. Half of the culture medium was replaced every 3–4 days. The cultures were daily observed under a phase contrast microscope and the images were captured using a digital camera. At the end of the culture period, the samples were taken out for experimental purposes.
RNA preparation and reverse transcription polymerase chain reaction
Total RNA was extracted from samples of MUCs cultured in 2D and suspension cultures using an Rneasy mini extract kit (Qiagen). cDNA was synthesized using a Reverse transcription kit (Qiagen). All the procedures were performed according to the manufacturers' instruction. Primers and polymerase chain reaction (PCR) conditions are listed in Table 1.
Table 1.
Primers and Polymerase Chain Reaction Conditions
| Gene name | Forward | Reverse | Product length (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| Bmp4 | 5′-CATTCCGTAGTGCCATTCGG-3′ | 5′-GAATGACGGCGCTCTTGCTA-3′ | 366 | 55 |
| Cdkn1b (P27kip1) | 5′-AAGAAGCGAGTCAGCGCAAG-3′ | 5′-ACGAGTCAGGCATTTGGTCC-3′ | 255 | 55 |
| Lfng | 5′-AACGGATCAGCGAGCACAA-3′ | 5′-TCACCTGCTCATGAAGCTCG-3′ | 279 | 55 |
| Jag1 | 5′-CCTGTCCATGCAGAACGTGA-3′ | 5′-GATGCGATTACGGTCGTTGC-3′ | 244 | 55 |
| Isl1 | 5′-GCATAGGCTTCAGCAAGAACG-3′ | 5′-TGCAGCTGCTTCTCGTTGA-3′ | 334 | 55 |
| Hes1 | 5′-TCAACACGACACCGGACAA-3′ | 5′-TCGTTCATGCACTCGCTGA-3′ | 298 | 55 |
| Eya1 | 5′-AGGTTCAGCTCTCGGAAGCA-3′ | 5′-TCCGTCTTGATGCCATAGGAG-3′ | 237 | 55 |
| Pax8 | 5′-GCCTGGCAATGACAACAAGAG-3′ | 5′-AATGAGGATCTGCCACCACAG-3′ | 341 | 55 |
| Dlx5 | 5′-TCCACAGCCAATTCAGGCA-3′ | 5′-TAGTCGGCATAAGCCTTGGC-3′ | 346 | 55 |
| Six1 | 5′-TCCAGAGCAATATGGGCCA-3′ | 5′-GTGGTTCCTTTCTGCGCAA-3′ | 290 | 55 |
| Numb | 5′-GTCACAACTGCCACTGAGCAA-3′ | 5′-ACGAGCAAGCTGTTCAATTGG-3′ | 231 | 55 |
| Notch1 | 5′-TGGTGCTCTGATGGACGACA-3′ | 5′-AGCCATCTGGTCCTCGAACA-3′ | 239 | 55 |
| Pou5f1 (Oct4) | 5′-CGAACCTGGCTAAGCTTCCA-3′ | 5′-TCCACCTTCTCCAACTTCACG-3′ | 275 | 55 |
| Gfap | 5′-CGCTTCTCCTTGTCTCGAATG-3′ | 5′-GCTCGAAGCTGGTTCAGTTCA-3′ | 212 | 55 |
| Sox2 | 5′-AAACCACCAATCCCATCCAA-3′ | 5′-AGCAAGAACCCTTTCCTCGAA-3′ | 398 | 55 |
| Nes | 5′-GCCTGGATCTGGAAGTCAACA-3′ | 5′-TCTGGCATTCCCTGAGCAAC-3′ | 306 | 55 |
| Nanog | 5′-AAGCAGAAGATGCGGACTGTG-3′ | 5′-GGATAGCTGCAATGGATGCTG-3′ | 266 | 55 |
| Snai1 | 5′-CAAACCCACTCGGATGTGAAG-3′ | 5′-AGAGAGCCAAGCAGGAACCA-3′ | 245 | 55 |
| Snai2 | 5′-TTCGAACCCACACATTGCC-3′ | 5′-AGTAAACACTGGTTGCGCCAC-3′ | 296 | 55 |
| Zeb1 | 5′-TCTGCAGCAACAAGACACCG-3′ | 5′-CAGTGCACTTGAACTTGCGG-3′ | 344 | 55 |
| Vim | 5′-CGCCATCAACACTGAGTTCAA-3′ | 5′-CCTTGTCGTTGGTGAGCTGA-3′ | 236 | 55 |
| Ctnnb1 (β-catenin) | 5′-GAACGTGCATTGTGATTGGC-3′ | 5′-TCTTGTGATCCATTCGTGTGC-3′ | 217 | 55 |
| Fn1 | 5′-GCTCCTTCACTGATGTCCGAA-3′ | 5′-TTCGGTGGTGCAGGAATAGAA-3′ | 281 | 55 |
| Cdh2 (N-cadherin) | 5′-TTACCAGCTCGCTCTCATTGG-3′ | 5′-CGTGCACATCCTTCGGTAAGA-3′ | 339 | 55 |
| Cdh1 (E-cadherin) | 5′-AAGGTGAAGGCTTGAGCACAA-3′ | 5′-AACAACGAACTGCTGGTCAGG-3′ | 224 | 55 |
| Gapdh | 5′-ACAGTCAAGGCCGAGAATGG-3′ | 5′-GATCCACGACGGACACATTG-3′ | 559 | 55 |
Immunofluorescence
Immunofluorescence was performed on mouse utricle sheets and samples from MUCs cultured on 2D substrates or in suspension cultures. The samples were fixed with 4% paraformaldehyde for 15–30 min at room temperature. Several groups of primary antibodies were used in this study: (a) antibodies specific for epithelial cells, such as anti-E-cadherin (Santa Cruz), anti-ZO1 (Zymed), (b) antibodies specific for mesenchymal cells, such as anti-Snail2 [Developmental Studies Hybridoma Bank (DSHB)], Vimentin (DSHB), N-cadherin (DSHB), Fibronectin (Millipore), (c) antibodies specific for prosensory cells, such as anti-P27kip1 (Lab vision), Islet1 (DSHB), Bmp4 (Millipore), and (d) antibodies specific for pluripotent or multipotent stem cells/neural stem cells, such as Oct4 (R&D), SSEA1 (Santa Cruz), Nestin (Millipore), Sox2 (Abcam), GFAP (Covance). The samples were incubated in primary antibodies overnight at 4°C. Dylight488, Dylight549, or Dylight649 conjugated secondary antibodies (Jackson Immunoresearch) and appropriate filters were applied to observe the samples using Zeiss LSM 510 confocal microscopy or Leica epifluorescence microscopy.
Protein preparation and western blot
Protein samples were extracted using a RIPA buffer (Thermo pierce) with protease inhibitors followed by separation on a precasted gel (Biorad) and transferring onto a polyvinylidene fluoride membrane (Biorad). The membranes were blocked with 5% nonfat milk for 1 h and were incubated with the aforementioned primary antibodies overnight at 4°C. At the end of incubation, the membranes were washed with TBS (Biorad) with 0.05% Tween 20 (TBST), and were incubated with peroxidase-conjugated secondary antibodies (Santa Cruz) for 1 h. Enhanced chemilumescent substrates (Thermo pierce) and UVP image system (UVP) were used to detect the signals. Glyceraldehyde-3-phosphate dehydrogenase (Thermo pierce) was used as a loading control.
Results
Mammalian sensory epithelial cells underwent an EMT when cultured on 2D substrates
Generation of cell clones
After pure sensory epithelial sheets of utricles were dissociated and filtered through a cell strainer, solitary cells and a few small cell aggregates were observed. When plated into a 96-well plate, most of the cells were found to adhere to the cultured wells after overnight incubation. The solitary cells were identified in 10 wells and cells in 6 wells grew to small cell islands in 3–4 days (Fig. 1A, B). When cells reached ∼70%–80% confluence in the culture wells, they were dissociated and transferred to larger culture dishes or flasks. The cells of these 6 clones were expanded on 2D substrates in subsequent cultures for up to 55 passages. The cell clone and population expansion were repeated for 5 animals. When cultured on 2D substrates, MUCs started to de-differentiate and undergo the following morphological, genetic, and protein changes:
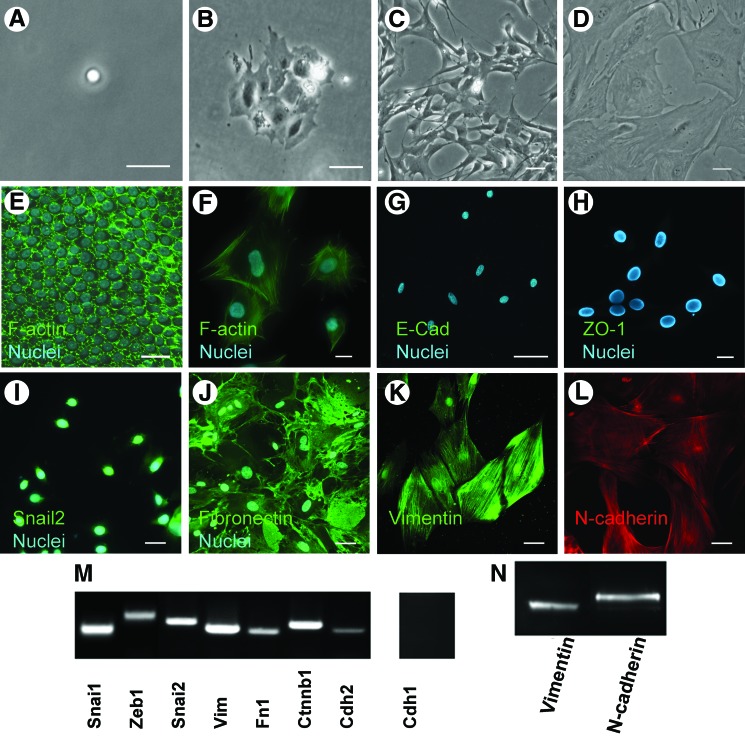
FIG. 1.
MUCs underwent EMT when cultured on 2D substrates. Solitary cells were obtained from dissociation of pure sensory epithelia of adult mouse utricle (A). The cell grew to a small cell island on a 2D substrate in 3–4 days (B), gradually lost columnar shape, and became flat on 2D substrates during passage 1 (C) and passage 2 (D). Phalloidin-labeled F-actin, which normally forms “actin ring” in utricle sensory epithelium (E), was changed into irregular “stress fibers” that are usually found in mesenchymal cells (F). Epithelial markers, such as E-cadherin (E-cad) and ZO-1 were not detected (G, H). Immunofluorescence study shows that MUCs expressed mesenchymal cell markers: Snail2, Fibronectin, Vimentin, and N-cadherin (I–L). RT-PCR data show that MUCs cultured on 2D substrates strongly expressed Snai1, Zeb1, Snai2, Vim (vimentin), Fn1 (fibronectin), Ctnnb1 (beta-catenin), Cdh2 (N-Cadherin) (M), which are widely shown in mesenchymal cells. Western blotting results revealed that mesenchymal markers vimentin and N-cadherin were detected in MUCs (N). Scale bar: 20 μm in A, E–H; 50 μm in B–D, I–L. EMT, epithelial to mesenchymal transition; MUC, mouse utricle sensory epithelial cell; 2D, 2-dimensional; RT-PCR, reverse transcription polymerase chain reaction. Color images available online at www.liebertonline.com/scd
Morphological changes
When cultured on 2D substrates, MUCs gradually lost their columnar shape and started to expand on the substrate from passage 1 to passage 2 (Fig. 1B–D). Phalloidin-labeled F-actin, which normally forms an “actin ring” in epithelial cells (Fig. 1E) [16], was observed to assemble into irregular “stress fibers” that are usually found in mesenchymal cells (Fig. 1F) [17].
Downregulation of expression of epithelial junction markers
E-cadherin, encoded by the Cdh1 gene, is located at the basolateral membrane in adherens junction and serves as one of the hallmarks of epithelial cells. Reverse transcription (RT)-PCR, immunofluorescence, and western blot data revealed that Cdh1 gene expression and E-cadherin protein expression were not detectable in MUCs after passage 2 (Fig. 1G, M). ZO1, a tight junction protein among epithelial cells [18], was not detectable in MUCs cultured on 2D substrates after passage 2 (Fig. 1H).
High expression of mesenchymal markers
RT-PCR showed that MUCs cultured on 2D substrates strongly expressed genes that are widely expressed in mesenchymal cells [19–21], such as Snai1 (snail1), Snai2 (snail2), Zeb1, Vim (vimentin), Fn1 (fibronectin), Ctnnb1 (beta-catenin), and Cdh2 (N-Cadherin) (Fig. 1M). Immunofluorescence and western blotting of MUCs also identified protein expression of mesenchymal cell markers, such as Snail2, Fibronectin, Vimentin, and N-cadherin (Fig. 1I–L, N).
Proliferation of MUCs
We found that MUCs proliferated in 2D cultures for up to 55 passages over 8 months. Three methods were used to characterize cell growth. First, the cell number was evaluated using a hemocytometer when plating and harvesting the cells at each passage. It is found that the total cell number increased and the doubling time of the cell population decreased with passage. The doubling time of the first 8 passages was ∼5–7 days, which shortened to 3–5 days for the later passages. Second, BrdU was added to the culture medium of passage 10 cells at culture day 1, 3, 5, and 7 for 24 h. Immunofluorescence results revealed that many cells incorporated BrdU, indicating that these cells had entered S-phase and possessed the potential to divide. Additionally, the percentage of cells incorporating BrdU increased from culture day 1 to 6, then decreased at day 7–8 (Fig. 2A–D). Third, CyQuant NF cell proliferation assay, a method that can directly observe cell growth without disturbing it, was performed. The cell population rapidly expanded during culture day 1–6 while cell growth rate mildly decreased during culture day 7–9 (Fig. 2E, F). The slow cell growth rate after culture day 6 may have been related to the space limitation in the culture dish.
FIG. 2.
Proliferation of MUCs. BrdU was added to culture medium of passage 10 MUCs at culture day 1, 3, 5, and 7 for 24 h. Immunofluorescence results revealed that many cells incorporated BrdU (A–C), indicating that these cells had entered S-phase and possessed the potential to divide. The percentage of cells incorporated BrdU increased from culture day 1 to 6, then decreased at day 7–8 (D). CyQuant NF cell proliferation assay showed that MUCs rapidly grew during culture day 1–6 while cell growth rate decreased during culture day 7–9 (E, F). The slow cell growth rate after culture day 6 may have been related to the space limitation in the culture dish. Scale bar: 20 μm in C, and 100 μm in E. BrdU, 5-bromo-2-deoxyuridine. Color images available online at www.liebertonline.com/scd
MUCs cultured on 2D substrates acquired properties of stem/progenitor cells via EMT
It is known that immortalized epithelial cells have the ability to become mesenchymal-like cells that express features of stem/progenitor cells via EMT [12]. However, it is unclear whether mammalian sensory epithelial cells from normal tissues have the same capability. To address this issue, samples of MUCs from passage 2–50 were obtained for detection of candidate markers for inner ear prosensory cells, which are located in the developing otocyst and develop into hair cells and supporting cells to form sensory epithelium in the inner ear. RT-PCR results revealed the expression of a number of prosensory genes, including Bmp4, Cdkn1b (P27kip1), Lfng, Jag1, Isl1, Hes1, Eya1, Pax8, Dlx5, Six1, Notch1, and Numb (Fig. 3A). Western blotting showed protein bands of Islet1 and P27kip1 (Fig. 3B). Moreover, immunofluorescence of the cells demonstrated the expression of transcriptional factor Islet1 and P27kip1 in the nucleus and the secreting protein BMP4 locating in the cytoplasm (Fig. 3C–K). These data show that MUCs expressed inner ear prosensory genes and proteins, indicating that MUCs may have the characteristics of prosensory cells and therefore the ability to differentiate into sensory epithelial cells.
FIG. 3.
MUCs acquired stem cell properties in 2D cultures via EMT. RT-PCR results of MUCs from passage 2–50 revealed the expression of a number of prosensory genes, including Bmp4, Cdkn1b (P27kip1), Lfng, Jag1, Isl1, Hes1, Eya1, Pax8, Dlx5, Six1, Notch1, and Numb (A). Western blotting data show that MUCs expressed proteins specific for prosensory cells, such as Islet1 and P27 kip1 (B). Immunofluorescence of MUCs demonstrated expression of transcriptional factor Islet1 and P27kip1 at nucleus and secreting protein BMP4 located in the cytoplasm (C–K). MUCs were studied with markers widely expressed in pluripotent/multipotent stem cells and it was found that MUCs expressed Pou5f1 (Oct4), SSEA1, Nanog, Gfap, Nes (Nestin), and Sox2, as evidenced by immunofluorescence (L–N, Q), RT-PCR (O), and western blotting studies (P). Scale bar: 50 μm in E, H, K–N, and Q. Color images available online at www.liebertonline.com/scd
To further explore whether MUCs shared general features of stem/progenitor cells, MUCs were studied with markers widely expressed in stem cells, such as Oct4, Nanog, SSEA1, Nestin, Sox2, and GFAP [22–26]. Indeed, RT-PCR results revealed that MUCs expressed Pou5f1 (Oct4), Nanog, Gfap, Nes (Nestin), and Sox2 (Fig. 3O). Protein expression of Oct4, SSEA1, GFAP, Sox2, and Nestin was also identified by western blotting and immunofluorescence (Fig. 3L–N, Q), indicating that these cells may have obtained some of the general features of stem cells.
Sensory epithelial progenitors shifted to epithelial cell fate via MET
Because MUCs expressed markers of stem/progenitor cell and prosensory cell, a suspension culture method was used for MUCs to test whether they can reverse their cell fate to epithelial phenotype. When samples of passage 14 to 55 MUCs were cultured in suspension medium, cells initiated the process of adhering to each other, and gradually developed into smooth-surface spheres. All MUCs remained solitary when plated into suspension culture medium (Fig. 4A), and then they aggregated following a regular progression of transformation over a 8–12 day period, highlighted by changes at the following times: 6 h after seeding in suspension media, 60%–70% of the cells started to adhere together to form small cell aggregates (Fig. 4B). By culture day 2–3, cells had aggregated together to form small spherical structures (Fig. 4C). At day 4–8, the spheres enlarged to a diameter of 100–200 μm and the surface of sphere became smooth (Fig. 4D). In the final stage (8–12 days), projections were found to extend from the surface of the cells, which were shown as bundle-like structures filled with F-actin labeled by fluorescence phalloidin (Fig. 4J).
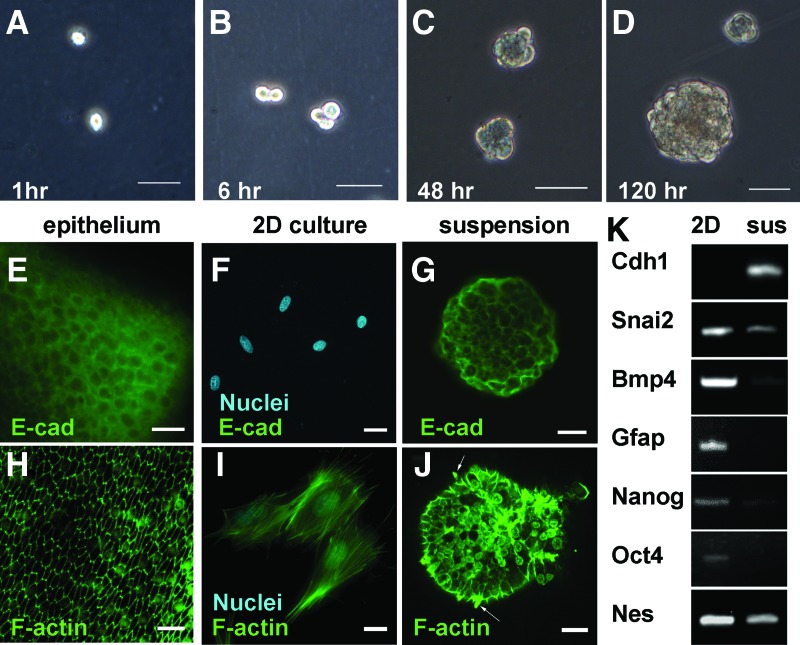
FIG. 4.
MUCs obtained features of epithelia via a reverse EMT when they were forced to suspend in a suspension culture system. When MUCs were plated into suspension culture medium, solitary cells were observed (A). Six h later, some of the cells started to adhere together to form small cell aggregates (B). During culture day 2–3, these cells aggregated together to form small spherical structures (C). At day 4–8, the spheres enlarged to a diameter of 100–200 μm and the surface of sphere became smooth (D). E-cadherin (E-cad), which was located at the plasma membrane to form cell-cell adherens junctions in mouse utricle epithelium (E), was not detectable in 2D-cultured MUCs (F). When MUCs were cultured in suspension culture for 10 days, E-cadherin was detected at the cell membrane (G). Consistently, the cells in the spheres obtained a columnar shaped morphology, as evidenced by rearrangement of phalloidin-labeled F-actin into an “actin ring” pattern, which is widely observed in epithelial cells (H–J). The cell projections, which were shown as bundle-like structures filled with F-action labeled by fluorescence phalloidin, were found to extend from the surface of the spheres cultured for 12 days in suspension medium (J, arrows). The epithelial specific gene Cdh1 encoding E-cadherin was found to be highly expressed in the cells from the spheres cultured for 10 days (K, 2D=2D culture, sus=suspension culture). In contrast to upregulation of epithelial markers, gene expression of mesenchymal marker Snai2 downregulated (K). Progenitor markers such as Bmp4, Gfap, Pou5f1 (Oct4), Nanog, and Nes (Nestin) were also found to downregulate in cells from the spheres (K). Scale bar: 100 μm in A–D; 10 μm in E–H; 20 μm in I and J. Color images available online at www.liebertonline.com/scd
RT-PCR and immunofluorescence were used to determine the genetic and protein properties of the MUCs in composition of the spheres at 8–12 days. The epithelial specific gene Cdh1 encoding E-cadherin was found to be highly expressed using RT-PCR (Fig. 4K). In immunofluorescence stained spheres, it was observed that E-cadherin was located at the plasma membrane to form cell-cell adherens junctions, which is a hallmark of epithelial cells (Fig. 4E–G). Further, the cells in the spheres obtained a columnar shaped morphology, as evidenced by rearrangement of phalloidin-labeled F-actin into an “actin ring” pattern, which is widely observed in epithelial cells (Fig. 4H–J). These results indicate that the cells in the sphere obtained an epithelial cell fate in suspension culture. In contrast to upregulation of epithelial markers, gene expression of mesenchymal marker Snai2 was downregulated (Fig. 4K). Progenitor markers such as Bmp4, Pou5f1 (Oct4), Nanog, and Nes (Nestin) were also found to downregulate in cells from the spheres (Fig. 4K).
Discussion
In birds and other nonmammalian vertebrates, damaged inner ear sensory epithelia can be replaced throughout life. However, the sensory epithelium in the mammalian inner ear is normally unable to, or has severely limited ability to regenerate after damage that leads to permanent inner ear disorders, including hearing loss and balance disability, that impede the daily activities of over 10% of the population. Therefore, generation of mammalian inner ear epithelial stem/progenitor cell is a critical challenge in the field. While studying the activation of inner ear sensory progenitor with the hope of regenerating inner ear cells, we successfully generated mammalian inner ear sensory epithelial progenitors via EMT. These progenitors can proliferate and expand in cell number continuously for at least 55 passages. These progenitors exhibit not only prosensory epithelial properties but also stem cell specific genes and proteins. Remarkably, these progenitors obtain epithelial features in a reverse EMT when they are forced to suspend in serum-free culture medium.
Recent studies indicate that EMT may contribute to in vitro expansion of epithelial cell lines [27,28]. Epithelial cells depend on sequential arrangement of adherens junctions, desmosomes, and tight junctions to produce close contacts with each other and maintain the apicobasal axis of polarity. EMT involves profound phenotypic changes including the loss of cell-cell adhesion, the loss of cell polarity, and the acquisition of mesenchymal traits, which involve many molecules and intracellular pathways. EMT may be activated by Snai1 that can bind and repress the activity of the E-cadherin promoter [29]. Generally, the loss of E-cadherin is considered as one of the critical initial events of EMT [30]. Snai1 may also activate other molecules involved in EMT such as Zeb1 and Snai2. Zeb1 not only represses E-cadherin [31], but also maintains the mesenchymal status, whereas Snai2 is mainly involved in the maintenance of mesenchymal status [32]. Mesenchymal markers such as vimentin, fibronectin, and N-cadherin were persistently upregulated during EMT to sustain a mesenchymal status. Additionally, F-actin, which is normally arranged in an “actin ring” pattern in epithelial cells, is rearranged to “stress fibers” that are usually observed in mesenchymal cells. Indeed, previous works and our observations indicate that changes in cell-cell contact and cell-matrix contact may contribute to cell fate determination. When MUCs were dissociated from normal epithelial sheet and cultured on 2D substrates, cells were observed to express Snai1, Snai2, and Zeb1 in conjunction with downregulation of the expression of E-cadherin. This is in accordance with the observation that MUCs lost columnar shape and cell-cell contacts, started to grow flat on the substrate from passage1, and F-actin was arranged into “stress fibers.” In addition to the loss of the epithelial markers, MUCs in 2D cultures expressed mesenchymal markers, such as Vim (vimentin), Fn1 (fibronectin), and Cdh2 (N-cadherin). The evidences from morphological, genetic, and protein expression levels suggest that adult mammalian sensory epithelial cells are able to undergo an EMT when they are cultured on 2D substrates. These results are similar to those of chick utricular cells [10]. However, N-cadherin, a mammalian mesenchymal marker, was observed in utricular sensory epithelial cells derived from chick embryos. The reason why embryonic chick epithelial cells express N-cadherin is unclear; however, this may be due to the variability of protein expression in different species. Additionally, it is likely that embryonic avian epithelial cells express mesenchymal proteins during development.
In our previous study, chick utricular cells were observed to undergo EMT when grown on 2D substrates. However, it is unclear whether these chick cells acquire features of stem/progenitor cells via EMT. In this study, MUCs, which have undergone EMT, have retained the traits of inner ear progenitors by expressing several genes widely expressed in inner ear development. The Notch ligand Jagged1 (Jag1), the Notch regulator Lunatic fringe (Lfng), and the secreted signaling molecule Bmp4 are considered as the 3 main candidates for the markers of prosensory domains in the otocyst during inner ear development [33]. In addition, other genes and proteins specific for prosensory cells, including Isl1 (islet1), Cdkn1b (P27kip1), Eya1, Dlx5, Notch1, Numb, Pax8, Six1, and Hes1 were observed to be expressed in our 2D-cultured MUCs, suggesting that MUCs obtained the properties of inner ear prosensory cells [34]. It is still controversial whether there is a specialized reserve pool of distinct stem/progenitor cells in adult mammalian sensory epithelia [35,36]. To determine whether MUCs come from existing stem/progenitor cells, primary culture of MUCs were used to study the expression of prosensory markers Islet1 and Bmp4 using immunocytochemistry. We found that primary cultured MUCs did not express these prosensory markers (data not shown), suggesting that MUCs less likely come from existing stem/progenitors. Our data indicate that MUCs likely originated from de-differentiation of inner ear epithelial cells via EMT.
Remarkably, we found that adult mammalian sensory epithelial cells could acquire properties of stem/progenitor cell through EMT, as evidenced by expression of several transcription factors that are usually shown in stem cells, such as Pou5f1(Oct4), Nanog, and Sox2 [22–26]. MUCs also expressed Nestin, an intermediate filament expressed in stem/precursor cells, and GFAP, which commonly expressed in neural progenitors and astrocytes [37,38]. Our observations may support the hypotheses that (a) mesenchymal status is related to acquirement and/or maintenance of pluri-/multi- potency, and (b) sensory epithelial cells from adult mammals can obtain stem cell features via EMT. We also established an in vitro de-differentiate model for mammalian sensory epithelial cells that can be used to identify the molecules that are critical to regulate cell fate determination in future attempts for a cell-based regeneration.
It is known that sensory epithelial cells from embryonic chick utricles can undergo EMT and grow for over 20 passages in the cultures [10]. In this study, we have addressed the question of whether mammalian sensory epithelial cells can proliferate after they have undergone EMT. Indeed, MUCs can be cultured for up to 55 passages over 8 months. They have the ability to proliferate, as evidenced by cell number counting, BrdU incorporation test, and CyQuant NF cell proliferation assay. The cloned cells have grown to millions of cells that can be used for inner ear sensory epithelium progenitor research. Moreover, we found that these cells showed stable mesenchymal and prosensory epithelial properties with appropriate morphological, genetic, and protein expressions.
Chick utricular cells differentiated into cells expressing hair cell markers when they were cultured in suspensions [10]. In this study, it is found that MUCs showed potential to differentiate into epithelial cells when they were forced to suspend in serum-free medium. In the suspension culture system, MUCs closely contacted their neighbors to form spheres, and hence established adherens junctions by upregulation of E-cadherin expression. In contrast to upregulation of E-cadherin expression, stem cell markers and mesenchymal markers were downregulated; F-actin was rearranged to “actin ring.” In addition, some bundle-like structures labeled by F-actin marker were observed in the surface of spheres. These data suggest that the cells from the spheres may obtain an epithelial phenotype via MET, which may provide the possibility of inducing mesenchymal cell-like stem/progenitor cells to differentiate into cell types needed in a cell replacement strategy. To characterize mammalian sensory hair cells generated via MET, thorough screening of hair cell markers, hair bundle markers, supporting cell markers, ultrastructural study of hair bundle using scanning and transmission electron microscopy, and functional evaluation of new hair cells using electrophysiology need to be conducted in future research.
In conclusion, adult mammalian sensory epithelial cells can undergo EMT when they are cultured on 2D substrates. During EMT, MUCs acquired the ability to proliferate, and expressed the properties of prosensory cells and stem cells. In a reverse EMT, MUCs obtained sensory epithelial characteristics when they were forced to suspend in serum-free culture medium. EMT is critical to the induction and proliferation of prosensory cells, which may provide a novel approach to expand progenitors in vitro and to study the activation of progenitors located within the inner ear sensory epithelium both in vitro and in vivo. Future research will be directed toward identifying the factors that can control the initiation of inner ear progenitor generation during EMT. Generation of the progenitor cells for inner ear hair cells and retina epithelial cells in vitro will be beneficial to guiding in vivo epithelia regeneration in a variety of systems in the future, and thus facilitate the development of a novel cell replacement therapy for hearing loss and vision loss.
Acknowledgments
The authors thank Dr. Paul G. Finlayson for valuable comments to the manuscript, Drs. Chunying Li, Yanning Wu, Shuo Wang, Hui Jiang, and Baofu Zhang for their technical support, and antibodies from the Developmental Studies Hybridoma Bank. This study is supported by the Deafness Research Foundation, the American Hearing Research Foundation, the American Academy of Audiology Foundation, and NIDCD/NIH (R03DC011597).
Author Disclosure Statement
Drs. Lei Zhang and Zhengqing Hu agree that there are no commercial associations that might create a conflict of interest in connection with this manuscript.
References
- 1.Hu Z. Ulfendahl M. Cell replacement therapy in the inner ear. Stem Cells Dev. 2006;15:449–459. doi: 10.1089/scd.2006.15.449. [DOI] [PubMed] [Google Scholar]
- 2.Hildebrand MS. Newton SS. Gubbels SP. Sheffield AM. Kochhar A. de Silva MG. Dahl HH. Rose SD. Behlke MA. Smith RJ. Advances in molecular and cellular therapies for hearing loss. Mol Ther. 2008;16:224–236. doi: 10.1038/sj.mt.6300351. [DOI] [PubMed] [Google Scholar]
- 3.Izumikawa M. Minoda R. Kawamoto K. Abrashkin KA. Swiderski DL. Dolan DF. Brough DE. Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 4.Zheng JL. Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 5.Sage C. Huang M. Karimi K. Gutierrez G. Vollrath MA. Zhang DS. Garcia-Anoveros J. Hinds PW. Corwin JT. Corey DP. Chen ZY. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- 6.Diensthuber M. Oshima K. Heller S. Stem/progenitor cells derived from the cochlear sensory epithelium give rise to spheres with distinct morphologies and features. J Assoc Res Otolaryngol. 2009;10:173–190. doi: 10.1007/s10162-009-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H. Liu H. Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 8.Gershengorn MC. Hardikar AA. Wei C. Geras-Raaka E. Marcus-Samuels B. Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 9.Gallo R. Gambelli F. Gava B. Sasdelli F. Tellone V. Masini M. Marchetti P. Dotta F. Sorrentino V. Generation and expansion of multipotent mesenchymal progenitor cells from cultured human pancreatic islets. Cell Death Differ. 2007;14:1860–1871. doi: 10.1038/sj.cdd.4402199. [DOI] [PubMed] [Google Scholar]
- 10.Hu Z. Corwin JT. Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition. Proc Natl Acad Sci U S A. 2007;104:16675–16680. doi: 10.1073/pnas.0704576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley MW. Has hair cell loss MET its match? Proc Natl Acad Sci U S A. 2007;104:16400–16401. doi: 10.1073/pnas.0708154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mani SA. Guo W. Liao MJ. Eaton EN. Ayyanan A. Zhou AY. Brooks M. Reinhard F. Zhang CC, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morel AP. Lievre M. Thomas C. Hinkal G. Ansieau S. Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eastham AM. Spencer H. Soncin F. Ritson S. Merry CL. Stern PL. Ward CM. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L. Jiang H. Hu Z. Concentration-dependent effect of NGF on cell fate determination of neural progenitors. Stem Cells Dev. 2011;20:1723–1731. doi: 10.1089/scd.2010.0370. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci. 2008;13:6662–6681. doi: 10.2741/3180. [DOI] [PubMed] [Google Scholar]
- 17.Pollack V. Scheiber K. Pfaller W. Schramek H. Loss of cytokeratin expression and formation of actin stress fibers in dedifferentiated MDCK-C7 cell lines. Biochem Biophys Res Commun. 1997;241:541–547. doi: 10.1006/bbrc.1997.7837. [DOI] [PubMed] [Google Scholar]
- 18.Sheth B. Fesenko I. Collins JE. Moran B. Wild AE. Anderson JM. Fleming TP. Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha+ isoform. Development. 1997;124:2027–2037. doi: 10.1242/dev.124.10.2027. [DOI] [PubMed] [Google Scholar]
- 19.Nieto MA. Sargent MG. Wilkinson DG. Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- 20.Franke WW. Grund C. Schmid E. Intermediate-sized filaments present in Sertoli cells are of the vimentin type. Eur J Cell Biol. 1979;19:269–275. [PubMed] [Google Scholar]
- 21.Weiss RE. Reddi AH. Role of fibronectin in collagenous matrix-induced mesenchymal cell proliferation and differentiation in vivo. Exp Cell Res. 1981;133:247–254. doi: 10.1016/0014-4827(81)90316-5. [DOI] [PubMed] [Google Scholar]
- 22.Nichols J. Zevnik B. Anastassiadis K. Niwa H. Klewe-Nebenius D. Chambers I. Scholer H. Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 23.Brambrink T. Foreman R. Welstead GG. Lengner CJ. Wernig M. Suh H. Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia AD. Doan NB. Imura T. Bush TG. Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 25.Lendahl U. Zimmerman LB. McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 26.Ellis P. Fagan BM. Magness ST. Hutton S. Taranova O. Hayashi S. McMahon A. Rao M. Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 27.Ulianich L. Garbi C. Treglia AS. Punzi D. Miele C. Raciti GA. Beguinot F. Consiglio E. Di Jeso B. ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J Cell Sci. 2008;121:477–486. doi: 10.1242/jcs.017202. [DOI] [PubMed] [Google Scholar]
- 28.Joglekar MV. Hardikar AA. Epithelial-to-mesenchymal transition in pancreatic islet beta cells. Cell Cycle. 2010;9:4077–4079. doi: 10.4161/cc.9.20.13590. [DOI] [PubMed] [Google Scholar]
- 29.Cano A. Perez-Moreno MA. Rodrigo I. Locascio A. Blanco MJ. del Barrio MG. Portillo F. Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 30.Batlle E. Sancho E. Franci C. Dominguez D. Monfar M. Baulida J. Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 31.Spaderna S. Schmalhofer O. Hlubek F. Berx G. Eger A. Merkel S. Jung A. Kirchner T. Brabletz T. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006;131:830–840. doi: 10.1053/j.gastro.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Pena C. Garcia JM. Garcia V. Silva J. Dominguez G. Rodriguez R. Maximiano C. Garcia de Herreros A. Munoz A. Bonilla F. The expression levels of the transcriptional regulators p300 and CtBP modulate the correlations between SNAIL, ZEB1, E-cadherin and vitamin D receptor in human colon carcinomas. Int J Cancer. 2006;119:2098–2104. doi: 10.1002/ijc.22083. [DOI] [PubMed] [Google Scholar]
- 33.Driver EC. Kelley MW. Specification of cell fate in the mammalian cochlea. Birth Defects Res C Embryo Today. 2009;87:212–221. doi: 10.1002/bdrc.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- 35.White PM. Doetzlhofer A. Lee YS. Groves AK. Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 36.Kelley MW. Talreja DR. Corwin JT. Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. J Neurosci. 1995;15:3013–3026. doi: 10.1523/JNEUROSCI.15-04-03013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smeti I. Savary E. Capelle V. Hugnot JP. Uziel A. Zine A. Expression of candidate markers for stem/progenitor cells in the inner ears of developing and adult GFAP and nestin promoter-GFP transgenic mice. Gene Expr Patterns. 11:22–32. doi: 10.1016/j.gep.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Hudspeth AJ. The cellular basis of hearing: the biophysics of hair cells. Science. 1985;230:745–752. doi: 10.1126/science.2414845. [DOI] [PubMed] [Google Scholar]