Abstract
The chemokine stromal cell-derived factor (SDF)-1α/CXCL12 and its receptor CXC chemokine receptor 4 (CXCR4) play a crucial role in the homing/engraftment and retention of hematopoietic stem/progenitor cells (HSPCs) in the bone marrow. It has been shown using the viral gene transfer technique that CXCR4 overexpression on human CD34+ HSPC significantly improves their engraftment in murine models. However, clinical trials with gene therapy have revealed safety concerns related to the immunogenicity of the viral carriers, due to the random integration of viral genes into the host genome. Therefore, a method for CXCR4 gene delivery into HSPC that is safe, nonviral, and highly efficient is needed to improve clinical transplantation and gene therapies. In this work, we investigated the nonviral CXCR4 gene delivery into HSPC using the cationic liposome agent IBAfect. We used CD34+ cells from cord blood and the models of immature hematopoietic cells expressing CD34 antigen, namely, leukemic cell lines KG-1a and KG-1. Transfection efficiency was determined by flow cytometric analysis 12, 24, 48, and 72 h after transfection, and the viability of cells analyzed by trypan blue exclusion and MTS assays. The functional response of CXCR4-transfected HSPC toward an SDF-1α gradient was determined by chemotaxis assay. We found that ∼25% transfection is achieved for KG-1a and KG-1 cells and 20% for HSPC, and that the viability of CXCR4-transfected HSPC is not significantly altered. More importantly, overexpression of CXCR4 using IBAfect significantly increased the chemotaxis of KG-1 cells and HSPC toward SDF-1α. However, we tested 2 other commercially available cationic liposomes (Lipofectamine 2000 and 1,2-dioleoyl-3-trimethylammonium-propane [DOTAP]) in parallel, and we found that they failed to deliver the CXCR4 gene into cells under the same conditions. These results suggest that IBAfect-mediated in vitro gene delivery to overexpress CXCR4 on HSPC is a safe and efficient technique with great potential for improving the efficacy of HSPC transplantation and gene therapy protocols.
Introduction
Human umbilical cord blood (CB) is an attractive alternative to bone marrow (BM) and mobilized peripheral blood as a source of transplantable hematopoietic stem/progenitor cells (HSPCs), and a recent target of ex vivo genetic modification, due to CB's availability, ease of collection, higher content of more primitive progenitors, proliferative potential, and lower risk of severe graft-versus-host disease [1–3]. However, the low numbers of HSPC present in collected CB units have been associated with failed or delayed engraftment, restricting its use in adult patients [4]. Thus, strategies to improve the efficacy of HSPC homing and engraftment could enhance the outcome of clinical transplantation and gene therapy protocols.
Homing and engraftment of HSPC to the BM is a multi-step process orchestrated by the interplay between adhesion molecules, chemokines, growth factors, and regulatory cofactors [5–7]. Recent studies have highlighted the pivotal role of stromal cell-derived factor (SDF)-1α/CXC chemokine receptor 4 (CXCR4) signaling in the regulation of HSPC homing, retention, and subsequent engraftment [8–11]. The chemotactic effect of SDF-1α is mediated through its G protein-coupled CXCR4. SDF-1α is constitutively produced by BM stromal and endothelial cells, as well as osteoblasts, and its cognate receptor CXCR4 is expressed by HSPC [8,12,13].
Proper functioning of the SDF-1α/CXCR4 axis is essential for directing the homing and engraftment of HSPC into BM after transplantation. Specifically, increasing the responsiveness of HSPC to an SDF-1 gradient could enhance their homing after transplantation. In fact, we have previously reported that CXCR4 signaling could be enhanced by small molecules, such as complement cleavage fragments [14,15], fibrinogen, fibronectin [16], hyaluronic acid [17], platelet-derived microparticles [18], and valproic acid [19]. In addition, we and others reported that the rate of engraftment or hematopoietic recovery after HSPC transplantation appears to be dependent on surface CXCR4 level in HSPC [20,21]. In support of the pivotal role of surface CXCR4 levels of HSPC for their homing and engraftment into the BM, highly efficient lentivirus and retrovirus transduction-mediated overexpression of CXCR4 have been shown to significantly enhance HSPC marrow repopulation [22,23]. However, the undesirable consequences of the viral integration process, the development of unwanted immune responses against vectors, and high cost for producing large amounts of high-titer viral stocks for clinical use have raised concerns and ruled out the clinical use of viral vectors [24]. Therefore, the development of a nonviral system for efficient and safe CXCR4 delivery into CB HSPC is required for improving the clinical transplantation and gene therapies that are potentially life-saving in a variety of disorders.
Nonviral cationic liposomal delivery has emerged as a valuable alternative to gene therapy using viral vectors because of low toxicity, lack of immunogenicity after in vivo administration, low cost, and relative ease in creating nucleic acid/liposome complexes in large scale for clinical use [25]. One of the most critical drawbacks of nonviral delivery systems has been the low levels of delivery and gene expression [26], but recent advances have improved the transfection efficiencies of cationic liposomes. In fact, cationic liposomal transfection has been proven to be an efficient method that is routinely used to transfect human cancer cells and hard-to-transfect human neural progenitor cells for identifying specific genes and cell signaling pathways in these cells [27,28]. In a recent study, it has been reported that cationic liposomes are also promising transfection reagents of human mesenchymal stem cells (MSCs) [29] and, importantly, for gene and small interfering RNAs (siRNAs) delivery in HSPC [30,31]. Hence, in this work we evaluated the efficiency and cytotoxicity of cationic liposome-mediated CXCR4 gene delivery into HSPC in vitro for improving the efficacy of clinical transplantation and gene therapy.
Materials and Methods
Cells
CB was collected immediately after delivery in a sterile tube containing heparin (1,000 IU/mL), and with the informed consent of the mother in accordance with the institutional guidelines approved by the Health Research Ethics Board of the University of Alberta. Light-density cells from CB were obtained by Percoll density gradient centrifugation and enriched for CD34+ cells by immunoaffinity selection with MACS paramagnetic beads (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions as described previously [19]. The purity of isolated CB CD34+ cells was more than 90% as determined by fluorescence-activated cell sorter (FACS) analysis.
The myeloid leukemia cell lines KG-1 (CD34+ CD38+) and KG-1a (CD34+ CD38−) were purchased from American Type Culture Collection (Rockville, MD). All cells were then maintained in Iscove's modified Dulbecco's medium (IMDM; GibcoBRL, Long Island, NY) supplemented with 20% bovine growth serum (BGS; Hyclone, Logan, UT).
Preparation of plasmid DNA
Plasmid containing pcDNAI-CXCR4 (kindly provided by NIH AIDS Research and Reference Reagent Program, Germantown, MD) was propagated in transformed Escherichia coli cells. E. coli cells were grown in standard Luria Bertani medium at pH 7.0 to a cell density of ∼3 to 4×109 cells/mL. Cells were harvested by centrifugation and the plasmid DNA was extracted and purified using a Qiagen Plasmid Maxi Kit (Qiagen, Santa Clarita, CA) as per the manufacturer's recommended protocols. In brief, the plasmid purification procedure involved alkaline hydrolysis of the cells and isolation of the plasmid by binding to an anion-exchange resin of proprietary composition. The concentration of the purified plasmid preparation was determined using a NanoDrop 1000 UV-visible spectrophotometer (Thermo Fisher Scientific, Wilmington, MA) using optical density at wavelength 260 nm (OD260). The OD260/OD280 ratio was about 1.9, indicating that the plasmid preparation was sufficiently pure and could be used for transfection purposes. Standard agarose gel electrophoresis on a 0.8% wt/wt agarose gel was conducted to investigate the plasmid structural integrity and revealed 2 major bands. The high-mobility band was attributed to the most compact or supercoiled form of plasmid DNA. The other band with low mobility indicated the overall nonsupercoil content in the plasmid preparation.
Transfection of cells
About 2×105 CD34+ cells were plated in poly-d-lysine (0.5 μg/cm2)-coated 24-well plates, whereas KG-1 and KG-1a cells were plated in poly-l-lysine (10 μg/mL)-coated 24-well plates at a density between 1×105 and 2×105 cells in 0.5 mL phosphate buffer saline, and incubated for 45 min at 37°C, 5% CO2. After cell attachment, 0.5 mL IMDM supplemented with 20% BGS was added to each well for both control and transfection groups. Cells were transfected with cationic liposomal transfection reagents IBAfect (Promokine, San Francisco, CA), Lipofectamine 2000 (Invitrogen, San Francisco, CA), and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) (kindly provided by Dr. Hasan Uludag at the University of Alberta) following protocol from the manufacturer. Briefly, 0.6 μg plasmid DNA in 25 μL of serum-free IMDM was added to different concentrations of IBAfect (1.8, 2.4, 3.6, and 4.2 μL IBAfect in 25 μL of serum-free IMDM), 0.8 μg plasmid DNA in 50 μL of serum-free OPTIMEM1 medium was added to different concentrations of Lipofectamine 2000 (1 and 5 μL Lipofectamine 2000 in 50 μL of serum-free OPTIMEM1), and 1 μg plasmid DNA in 50 μL of 10 mM HEPES was added to different concentrations of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) (5 and 10 μg DOTAP in 50 μL of 10 mM HEPES), gently mixed, and incubated for 30 min at room temperature. About 50 and 100 μL of these mixtures were added to the culture medium for each well and incubated for 12–72 h. The cells were harvested, counted, and analyzed for transfection efficiency.
Cell viability
Cell viability was assessed by the trypan blue exclusion assay and 3-(4,5-dimethylthiazol-2-yl)-5-(3carboxymethoxhenyl)-2-(4sulfophenyl)-2H-tetrazolium (MTS) assay (CellTiter 96 AQueous One Solution; Promega, Madison, WI). The MTS assay utilizes a novel tetrazolium compound that metabolically active cells and convert to a water-soluble formazan by the action of cellular dehydrogenases, which is measured by absorbance at 490 nm using a colorimetric microtiter plate reader (Bio-Tek, Winooski, VT). Background absorbance was subtracted from each sample.
FACS analysis
The cells were stained with PE-anti-CXCR4, PE-anti-CD34, and FITC-anti-CD38 monoclonal antibodies (BD Biosciences, Oakville, Ontario, Canada). After the final wash, cells were fixed in 1% paraformaldehyde before FACS analysis (FACScalibur, Becton-Dickinson, San Jose, CA) using PE-goat-anti-mouse and FITC-goat-anti-mouse IgG as the isotype control.
Confocal microscopy
CXCR4 surface expression was also visualized by confocal microscopy. KG-1 cells were seeded at a density of 1×105 cells/cm2 on microslides previously coated with poly-L-lysine (10 μg/mL) for 45 min at 37°C, 5% CO. Cells were stained with primary mouse anti-human CXCR4 monoclonal antibody (12G5; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:100 dilution for 1 h. Cells were washed and incubated with a secondary antibody goat anti-mouse conjugated with Texas Red (Santa Cruz Biotechnology, Inc.) at 1:200 dilution for 1 h. After immunostaining, the cells were counterstained with DAPI to visualize nuclei. The cells were fixed in 3.7% paraformaldehyde, stored overnight at 4°C, and examined under an inverted confocal laser scanning microscope (Carl Zeiss LSM510, Toronto, Canada) equipped with an imaging software (LSM 5 Image Browser, Carl Zeiss).
Colony forming unit assay
The untransfected CB CD34+ cells and CB CD34+ cells transfected with IBAfect were plated in triplicate into methylcellulose complete medium containing cytokines (Methocult GF H4434; Stem Cell Technologies, Vancouver, British Columbia, Canada) at a concentration of 1×103/mL. The plates were incubated at 37°C in a humidified incubator with 5% CO2 as previously described [21]. After 14 days of incubation, colonies were identified and enumerated using an inverted microscope.
Chemotaxis assay
Chemotaxis was assayed using modified Boyden chambers (Neuro Probe, Inc., Cabin John, MD) with 5-μm pore size polycarbonate filters as described previously [19]. Prewarmed serum-free IMDM containing SDF-1α (200 ng/mL; Biochemical Research Centre, University of British Columbia, Vancouver, BC, Canada) was added to the lower chambers. Aliquots of the cell suspension (1×105 cells/100 μL) were loaded onto the upper chambers and incubated for 3 h (37°C, 5% CO2). Cells from the lower chambers were recovered and counted, and percentage cell migration was calculated from the ratio of the number of cells recovered from the lower compartment to the total number of cells loaded in the upper compartment. Each experiment was performed at least 2 independent times using no < 4 chambers for each cell sample.
Statistical analysis
Results were expressed as means with their standard errors. Mann–Whitney U-test was used for comparison of data between control and IBAfect pcDNAI/CXCR4-transfected groups. A P value ≤0.05 was considered to represent a significant difference.
Results
Cationic liposome-mediated transfection of the CXCR4 gene into KG-1 and KG-1a cells
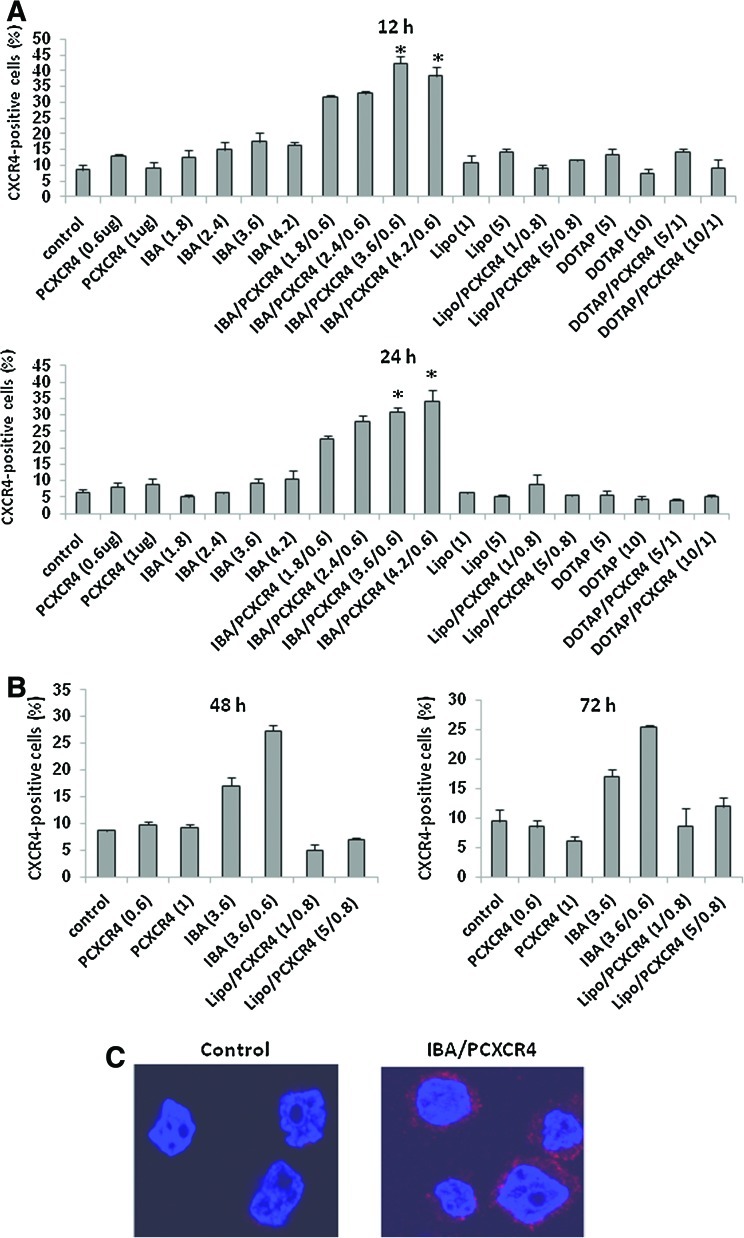
Three cationic liposomal transfection reagents—IBAfect, Lipofectamine 2000, and DOTAP—were tested in our study to determine CXCR4 gene transfection because they have been shown to be effective transfection and delivery reagents in hard-to-transfect cells such as neural progenitor cells, MSC, and HSPC [28–31]. Cationic liposome-mediated transfection of plasmid pcDNAI/CXCR4 into HSPC was first investigated in models of immature hematopoietic cells, namely, the myeloid leukemic cell lines KG-1 and KG-1a cells. We used these cell lines because they highly express CD34 with very low (∼8% for KG-1) or nearly negative (2% for KG-1a) surface CXCR4 expression. To optimize cationic liposome-mediated CXCR4 gene transfection, KG-1 cells were exposed to different concentrations of IBAfect (1.8, 2.4, 3.6, and 4.2 μL in 25 μL media), Lipofectamine 2000 (1 and 5 μL in 50 μL media), DOTAP (5 and 10 μg in 50 μL media), and pcDNAI/CXCR4 complexes (0.6, 0.8, and 1 μg, respectively) as described in Materials and Methods. After 12, 24, 48, and 72 h incubations, transfection efficiencies were evaluated by FACS. Among 3 commercial cationic liposomal transfection reagents, IBAfect was the only effective reagent for delivering the CXCR4 gene into KG-1 cells. At 12 h incubation, IBAfect-mediated transfection reached 10%, 18%, 24%, and 21% for 1.8, 2.4, 3.6, and 4.2 μL, respectively (Fig. 1A). We observed that cationic liposomes alone also gave a background fluorescence signal (IBAfect: between 4% and 10%; Lipofectamine 2000 and DOTAP: between 2% and 5.7%) with increasing concentrations. This is expected as it has been reported that some cationic liposome transfection reagents could lead to autofluorescence in flow cytometry analysis [32]. Therefore, the fluorescence signals of IBAfect-transfected samples were corrected for background fluorescence accordingly. The transfection efficiencies of IBAfect-transfected cells did not significantly drop after 24 h incubation, whereas we did not obtain any CXCR4 transfection into the cells even after 24 h incubation using both Lipofectamine 2000 and DOTAP (Fig. 1A). We also found that the IBAfect-mediated transfection of KG-1 cells after 12 h incubation dropped from 24% to 10% and 8.5% after 48 and 72 h incubations, respectively (Fig. 1A, B). There was still no CXCR4 transfection into KG-1 cells after 48 and 72 h incubations using Lipofectamine 2000 (Fig. 1B). We did not obtain FACS data using DOTAP, since most of the cells were dead after 48 and 72 h incubations. This clearly indicated that only cationic liposomal IBAfect reagent delivered the CXCR4 gene into KG-1 cells and the transfection rate in KG-1 cells reached its peak 12 h after the transfection. Confocal analysis also revealed the efficient IBAfect-mediated pcDNAI/CXCR4 transfection into KG-1 cells at 12 h incubation compared to control KG-1 cells, which did not show any evidence of red fluorescence, most likely due to a very weak fluorescence signal (CXCR4 level ∼8%; Fig. 1C).
FIG. 1.
Cationic liposome-mediated CXCR4 gene delivery into KG-1 cells. (A) The percentage of CXCR4-expressing KG-1 cells exposed to different concentrations of IBAfect (IBA): 1.8, 2.4, 3.6, and 4.2 μL; Lipofectamine 2000 (Lipo): 1 and 5 μL; DOTAP: 5 and 10 μg alone and 0.6 and 1 μg pcDNAI/CXCR4 (PCXCR4) alone and IBA, Lipo, DOTAP/PCXCR4 complexes at 12 and 24 h after the transfection. (B) % CXCR4 of KG-1 cells at 48 and 72 h after transfection (3.6 μL IBAfect; 1 and 5 μL Lipofectamine 2000; 0.6 and 1 μg PCXCR4). Results represent the mean±SEM of 3 independent experiments. *P≤0.05, statistically significant compared with untreated cells (Mann–Whitney U-test). (C) Confocal microscopy of IBA/PCXCR4-transfected KG-1 cells (3.6 μL IBAfect and 0.6 μg pcDNAI/CXCR4) at 12 h after delivery. Texas Red: Surface CXCR4 expression; Blue (DAPI): nucleus. CXCR4, CXC chemokine receptor 4; SEM, standard error of the mean; DAPI, 4′,6-diamidino-2-phenylindole; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane. Color images available online at www.liebertonline.com/scd
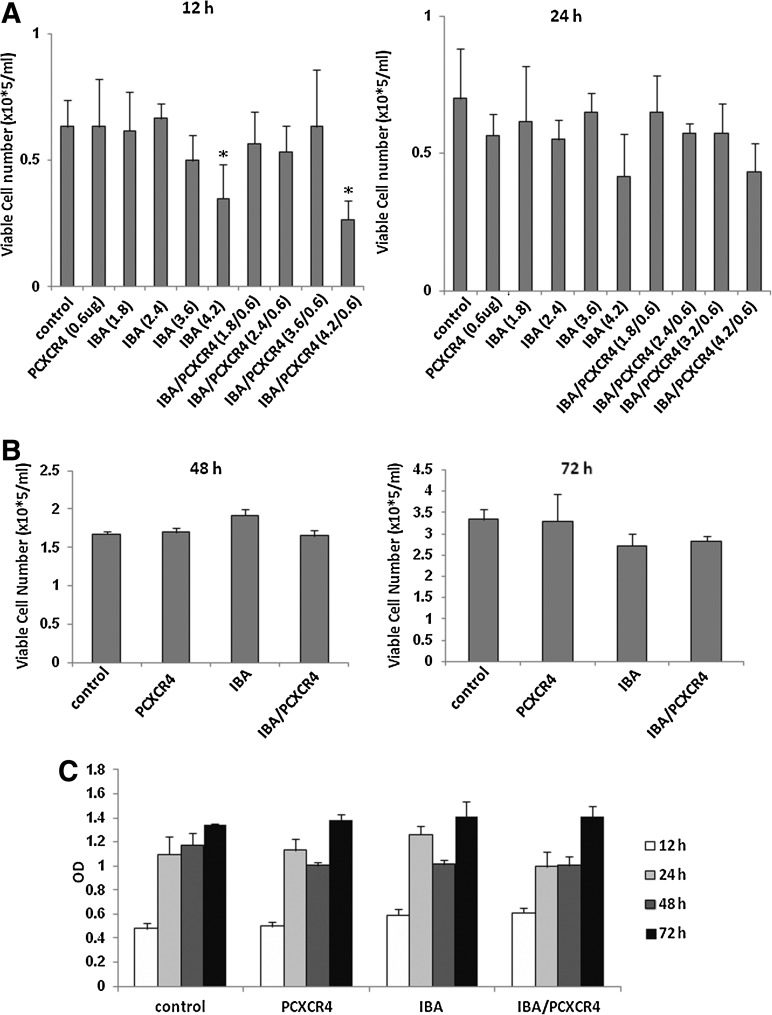
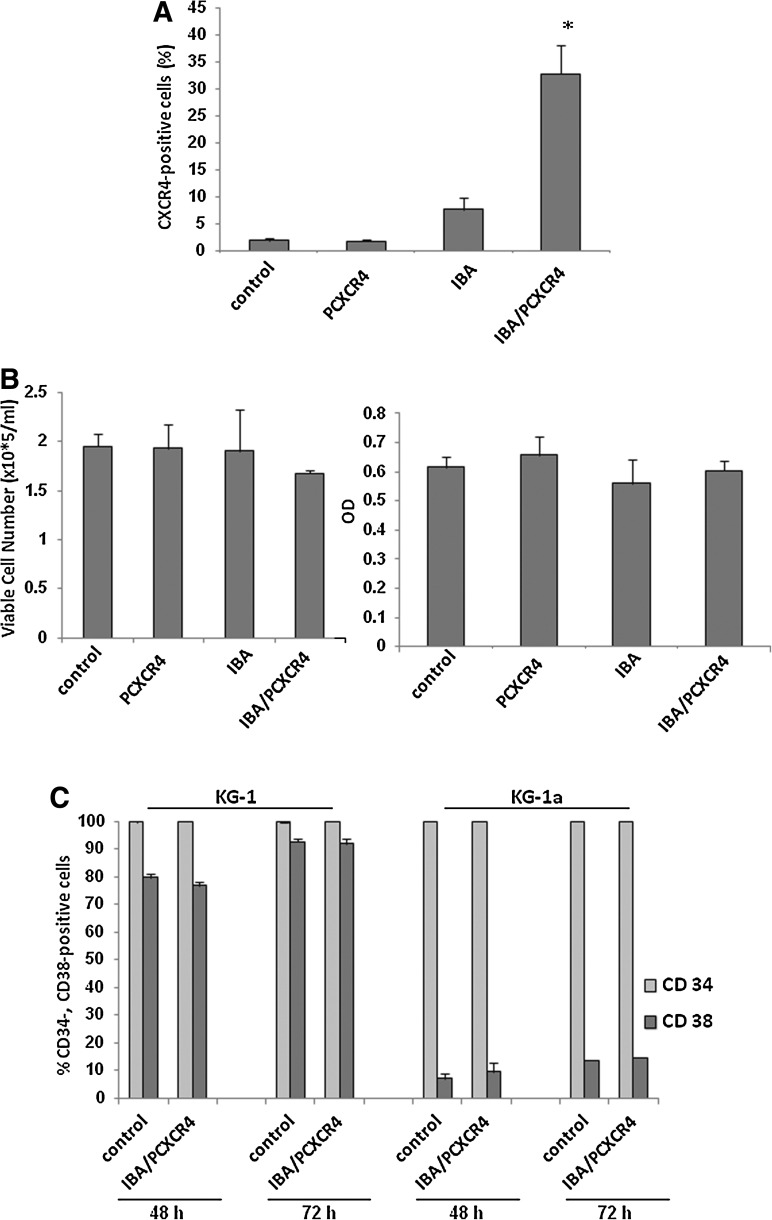
To investigate the cytotoxicity of IBAfect-mediated transfection, we studied the viability of KG-1 cells 12, 24, 48, and 72 h post-transfection by trypan blue exclusion and MTS assays. The viability of CXCR4-transfected KG-1 cells was not compromised except for with a high dose of IBAfect (4.2 μL) 12 h after the transfection (Fig. 2A, B). Nevertheless, cells were recovered after 24 h incubation. The viability data were also confirmed by MTS assay (Fig. 2C). On the other hand, the viability of the cells dropped with high dose of DOTAP even after 24 h incubation (OD=0.6; Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd) and nearly all the cells were dead at 48 and 72 incubation for both low and high dose of DOTAP (OD 48–72 h<0; Supplementary Fig. S1). Next we evaluated transfection efficiency in even more immature (CD34+CD38−), CXCR4-negative KG-1a cells. We selected an IBAfect:plasmid ratio of 3.6:0.6, which yielded the highest transfection efficiency with no concomitant toxicity. CXCR4 transfection efficiency was about 25% in KG-1a cells 12 h after the transfection (Fig. 3A) after correcting for about 6% of background signal from IBAfect alone. The cell viability was not affected by the IBAfect-mediated CXCR4 transfection (Fig. 3B). In addition, very similar results to KG-1 cells were observed in KG-1a cells with respect to transfection efficiencies 24, 48, and 72 h after transfection (data not shown).
FIG. 2.
Viability of IBA/PCXCR4-transfected KG-1 cells (3.6 μL IBAfect and 0.6 μg pcDNAI/CXCR4) was not compromised. The number of viable cells as assessed by trypan blue exclusion A, at 12 and 24 h after CXCR4 delivery. (B) At 48 and 72 h after CXCR4 delivery and (C) optical density (OD) at 490 nm, directly proportional to the number of viable cells, was determined according to manufacturer's instructions. Results represent the mean±SEM of 3 independent experiments. *P≤0.05, statistically significant compared with untreated cells (Mann–Whitney U-test).
FIG. 3.
IBAfect-mediated CXCR4 gene transfection into KG-1a cells. (A) The percentage of CXCR4-expressing KG-1a cells at 12 h after transfection. (B) Viable cell number (left panel) and OD (right panel) of IBA/PCXCR4-transfected KG-1a cells at 12 h after transfection. (C) % CD34- and CD38-positive KG-1 and KG-1a cells at 48 and 72 h after transfection. Results represent the mean±SEM of 3 independent experiments. *P≤0.05, statistically significant compared with untreated cells (Mann–Whitney U-test).
IBAfect-mediated CXCR4 transfection into HSPC
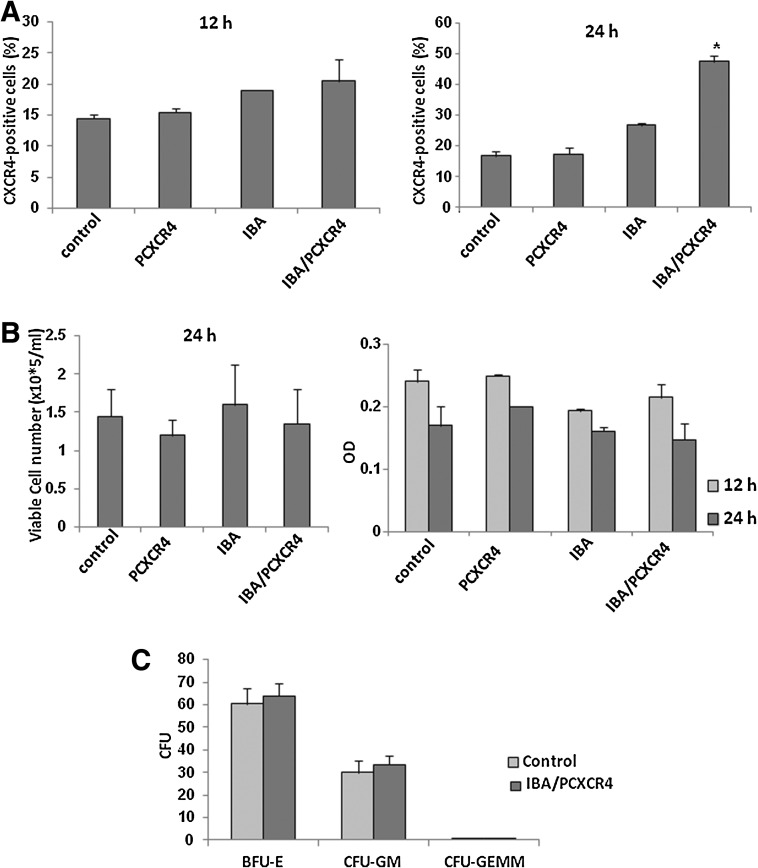
Next we investigated IBAfect-mediated CXCR4 transfection into CB HSPC. In clinical settings and gene transfer applications, a shorter culture period is favorable because it will be possible to transfect and manipulate as many stem cells as possible without inducing their differentiation [33]. In fact, culture periods before or during gene delivery applications are generally kept short, usually 1 day or less for lentiviral vectors [34]. Therefore, we evaluated the efficacy of IBAfect-mediated CXCR4 gene delivery 12 and 24 h after the transfection in CB CD34+ cells. We found that CB CD34+ cells were not transfected at 12 h incubation (Fig. 4A), probably due to their slow growth rate in comparison to KG-1 cells. On the other hand, 20% of CB CD34+ cells exhibited detectable expression of CXCR4 24 h after the transfection, without compromising viability (Fig. 4A, B). In addition, we observed that the transfection efficiency of CD34+ cells did not decrease after 48 h incubation, but for 72 h incubation there was a marked decrease in the percentage of CXCR4-expressing CD34+ cells (Supplementary Fig. S2); a similar trend was obtained in KG-1 cells.
FIG. 4.
Efficiency, cytotoxicity, and differentiation of IBA/PCXCR4-transfected cord blood (CB) CD34+ cells. (A) The percentage of CXCR4-expressing CB CD34+ cells at 12 and 24 h after transfection. (B) Viable cell count (left panel) and OD (right panel) of IBA/PCXCR4-transfected CB CD34+ cells 24 h after delivery. (C) Colony forming unit of IBA/PCXCR4-transfected CB CD34+ cells at 24 h after transfection. Results represent the mean±SEM of 3 independent experiments. *P≤0.05, statistically significant compared with untreated cells (Mann–Whitney U-test).
In order to investigate the effect of IBAfect-mediated transfection on proliferation and differentiation of CB CD34+ cells, we performed a colony forming-unit (CFU) assay of IBAfect-CXCR4-transfected cells after 24 h incubation. Colonies of CFU-granulocyte/macrophage (GM), CFU of GM-erythroid-megakaryocyte, and burst forming unit of erythrocyte were identified and enumerated on day 14. Neither proliferation nor differentiation was affected in IBAfect-CXCR4-transfected CB CD34+ cells as there was no difference in colony number or type between transfected and control CB CD34+ cells (Fig. 4C). These observations suggest that IBAfect-mediated CXCR4 gene delivery is not only efficient, but also did not compromise proliferation and differentiation potential of HSPC.
Enhanced migration of CXCR4-transfected KG-1 cells and HSPC toward SDF-1α
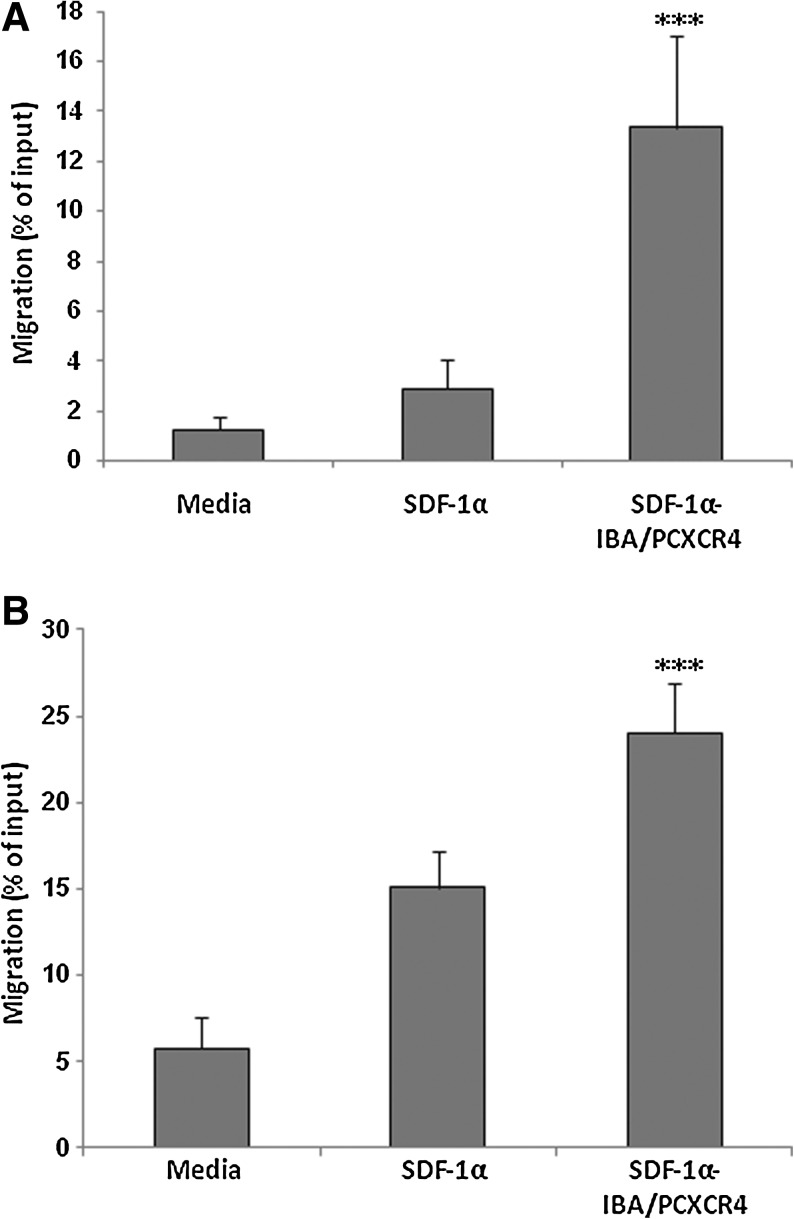
It has been previously shown that CXCR4 overexpression on HSPC cells using lentiviral and retroviral gene transfer system greatly enhanced their migration toward SDF-1α [22,35]. To evaluate whether IBAfect-mediated exogenous overexpression of CXCR4 translates to an enhanced functional response, we examined the in vitro migration of CXCR4-transfected KG-1 cells and CB CD34+ cells toward an SDF-1α gradient in a chemotaxis assay. CXCR4 modification of KG-1 cells and CB CD34+ cells significantly enhanced cell migration toward SDF-1α in both cell types (Fig 5A, B). The percent of migrating CXCR4-transfected KG-1 cells and CB CD34+ cells was 5- and 2-fold greater than the percent of migrating untransfected cells, respectively (P<0.0001; Fig 5A, B), indicating that the overexpression of CXCR4 enhances the ability of KG-1 cells and HSPC to respond to SDF-1α-induced chemotaxis.
FIG. 5.
IBAfect-mediated CXCR4 overexpression enhanced the migration of KG-1 cells and CB CD34+ cells toward SDF-1α gradient. Percentage cell migration was calculated from the ratio of the number of cells migrating toward SDF-1α to the total number of cells loaded. Migration toward SDF-1α gradient (200 ng/mL) of (A) IBAfect-CXCR4-transfected KG-1 cells (3.6 μL IBAfect and 0.6 μg pcDNAI/CXCR4) at 12 h after transfection. (B) IBAfect-CXCR4-transfected CB CD34+ cells (3.6 μL IBAfect and 0.6 μg pcDNAI/CXCR4) at 24 h after transfection. Results represent the mean±SEM of 3 independent experiments. ***P<0.0001, statistically highly significant compared with untreated cells (Mann–Whitney U-test). SDF-1α, stromal cell-derived factor-1α.
Discussion
Clinical application of genetically modified HSPC for transplantation has rapidly become an area of intensive investigation. Gene therapy requires an efficient and safe procedure for gene delivery into HSPC without loss of their multipotency. However, gene transfection into HSPC has been very difficult to achieve due to the quiescent nature of the most primitive HSPC [24,36]. Although some viral vectors have been shown to incorporate foreign DNA into HSPC with high efficiency [37,38], many clinical gene therapy trials described poor engraftment and impaired homing of virally transduced HSPC [39,40]. To overcome this drawback, both retroviral and lentiviral-mediated CXCR4 delivery into HSPC have been attempted with high efficiency, and CXCR4 overexpression greatly enhanced homing/engraftment of HSPC into murine BM [22,23]. However, the possibility of viral infection and immunogenicity are still major concerns. Here we showed for the first time a nonviral, cationic liposomal reagent (IBAfect)-mediated CXCR4 gene delivery into KG-1 cells, KG-1a cells, and CB HSPC, without compromising their viability. More importantly, CXCR4 transfection using this cationic liposome resulted in a significantly increased chemotactic response toward an SDF-1α gradient. Although the transfection efficiency of IBAfect is not as high as viral vector-mediated transfection [22,23], the use of liposome-mediated CXCR4 delivery into HSPC might be clinically more relevant due to its greater safety, lack of immunogenicity, lower cost, easier scale-up, and better quality control. Furthermore, the other cationic liposomes we tested (Lipofectamine 2000 and DOTAP) failed to transfect KG-1 cells under the same conditions as IBAfect. In agreement with our data, it has been shown that Lipofectamine-mediated transfection of HSPC and hematopoietic cell lines are inefficient when cells were adhered to retronectin-coated plates by physical means during the transfection [30]. However; there was a significant increase in the Lipofectamine-mediated transfection of these cells grown in adherence to stroma or fibroblast monolayers [30]. Furthermore, it has been shown that Lipofectamine 2000 is a promising transfection reagent with respect to human MSC, most likely due to the adherent nature of MSC versus HSPC [29]. On the other hand, Lipofectamine 2000 was recently shown to be ineffective for siRNA delivery into HSPC [31]. In contrast to our data, DOTAP has been recently reported to deliver siRNA into resting and differentiating HSPC more efficiently in comparison to other chemical reagents [31]. This siRNA delivery into HSPC using DOTAP was very successful in comparison to our CXCR4 transfection, which was most likely due to the different genetic material delivery and presence of cytokines. We did not stimulate HSPC using cytokines in cationic liposome-mediated transient transfection due to the dynamic regulation of CXCR4 by cytokines [22,41]. In addition to CXCR4 transfection efficiency, IBAfect was superior to the DOTAP reagent with regard to cell viability after 24, 48, and 72 h incubation. In fact, all cells were dead using both low and high doses of DOTAP after 48 and 72 h incubations (Supplementary Fig. S1). On the other hand, Martino et al. did not observe a reduction in cell viability 24 h after transfection with lower concentration of DOTAP [31]. Moreover, IBAfect-mediated transfection in HSPC is superior to physical methods such as electroporation due to the high cell viability [42], indicating the value of the IBAfect transfection reagent in gene therapy protocols for transient transfection.
Our present data suggest that the enhancement of in vitro HSPC migration to the SDF-1-rich microenvironment of the BM could be accomplished by cationic liposome-mediated CXCR4 gene delivery. We previously reported that small molecules such as complement cleavage fragments, fibrinogen, fibronectin, hyaluronic acid, platelet-derived microparticles, and valproic acid prime the responsiveness of HSPC to an SDF-1α gradient by an enhanced incorporation of CXCR4 into lipid rafts, resulting in efficient and rapid engraftment [14–19]. IBAfect-mediated CXCR4 delivery might be even more efficient in cells with very low or negative CXCR4 surface expression, since we have achieved a more profound effect in KG-1a cells with a very low surface CXCR4 level. In addition, rapid transfection of CXCR4 into HSPC would be particularly beneficial in clinical transplantation and gene therapy protocols using CB in adult patients. Although the CXCR4 transfection is transient and drops after 48 h, this is likely to be sufficient to enhance homing of HSPC into BM, which occurs within the first 24 h of transplantation and is a critical step in engraftment and initiation of marrow reconstitution. However, further investigation is needed to evaluate the homing/engraftment efficiency of CXCR4-transfected HSPC in vivo. Moreover, whether the transfection efficiency in HSPC with IBAfect could be further enhanced with a combination of physical methods (such as ultrasound exposure) or other chemical methods (such as gold nanoparticle-mediated delivery) needs to be evaluated. There has been increasing interest in using both liposome/ultrasound exposure and liposome/gold nanoparticle hybrid system for gene delivery into various cell types [43–46]. The novel cationic liposome-mediated system for CXCR4 gene delivery into HSPC may serve as an efficient platform to improve clinical transplantation and gene therapy protocols by enhancing HSPC homing and engraftment in the treatment of hematologic disorders. Furthermore, MSC have also become a potential source of cells for gene and cell-based therapies for the regeneration of various organs and tissues [47]. However, the low ability of ex vivo expanded MSC to respond to homing signals emanating from the BM or damaged tissues/organs due to the surface CXCR4 level may limit their clinical benefits [48,49]. Therefore, enhanced responsiveness of MSC to SDF-1α signaling through cationic-liposome-mediated CXCR4 delivery might also improve the outcome of MSC-based gene therapies.
Although advances have been made in gene therapy for the immunological reconstitution of patients with severe combined immune deficiency using HSPC transduced with viral vectors, some patients subsequently develop leukemia, probably due to the undesired activation of an oncogene [50,51]. Therefore, we suggest that cationic liposome-mediated delivery technology would overcome these drawbacks related to viral vectors in certain conditions despite its low and transient transfection efficiency. Cationic liposome-mediated delivery technology could also be used for expressing therapeutic genes safely for a short period or enhancing HSPC expansion, differentiation, and survival by overexpressing or silencing of specific genes. However, if HSPC are used to treat genetic disorders and must express the gene long term, integrating viruses would be advantageous due to their capacity for permanent expression.
In conclusion, safe and efficient in vitro IBAfect-mediated CXCR4 transfection into CB HSPC justifies recommendation of cationic liposomal delivery to improve the efficacy of gene and stem cell-based therapies in the treatment of both malignant and nonmalignant hematopoietic disorders. However, further optimization of cationic liposomal complexes for in vitro delivery in stem cells is needed.
Supplementary Material
Acknowledgments
The authors would like to acknowledge funding support from the Natural Sciences and Engineering Research Council (NSERC) and Canadian Institutes of Health Research (CIHR) joint CHRP grant 365569-09, the IRAP program of National Research Council (NRC), Canada, and the Canadian Blood Services/CIHR grant. The authors would like to thank Dr. Hasan Uludag for supplying the DOTAP liposomal transfection reagent. The authors would also like to acknowledge the valuable discussions with Dr. Eric Swanson, Director of the National Research Council (IRAP program), Edmonton, Canada.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Broxmeyer HE. Insights into the biology of cord blood stem/progenitor cells. Cell Prolif. 2011;44(Suppl 1):55–59. doi: 10.1111/j.1365-2184.2010.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman E. History of cord blood transplantation. Bone Marrow Transplant. 2009;44:621–626. doi: 10.1038/bmt.2009.280. [DOI] [PubMed] [Google Scholar]
- 3.Rocha V. Broxmeyer HE. New approaches for improving engraftment after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:S126–S132. doi: 10.1016/j.bbmt.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Eapen M. Rubinstein P. Zhang MJ. Stevens C. Kurtzberg J. Scaradavou A. Loberiza FR. Champlin RE. Klein JP. Horowitz MM. Wagner JE. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 5.Kucia M. Reca R. Miekus K. Wanzeck J. Wojakowski W. Janowska-Wieczorek A. Ratajczak J. Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1–CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 6.Lapidot T. Dar A. Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 7.Marquez-Curtis LA. Turner AR. Sridharan S. Ratajczak MZ. Janowska-Wieczorek A. The ins and outs of hematopoietic stem cells: studies to improve transplantation outcomes. Stem Cell Rev. 2011;7:590–607. doi: 10.1007/s12015-010-9212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peled A. Petit I. Kollet O. Magid M. Ponomaryov T. Byk T. Nagler A. Ben-Hur H. Many A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 9.Lapidot T. Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2mnull mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 10.Kucia M. Jankowski K. Reca R. Wysoczynski M. Bandura L. Allendorf DJ. Zhang J. Ratajczak J. Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;3:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 11.Broxmeyer HE. Orschell CM. Clapp DW. Hangoc G. Cooper S. Plett PA. Liles WC. Li X, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiuti A. Webb IJ. Bleul C. springer T. Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratajczak MZ. Zuba-Surma E. Kucia M. Reca R. Wojakowski W. Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 14.Reca R. Mastellos D. Majka M. Marquez L. Ratajczak J. Franchini S. Glodek A. Honczarenko M. Spruce LA. Janowska-Wieczorek A. Lambris J. Ratajczak MZ. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784–3793. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- 15.Jalili A. Marquez-Curtis L. Shirvaikar N. Wysoczynski M. Ratajczak MZ. JanowskaWieczorek A. Complement C1q enhances homing-related responses of hematopoietic stem/progenitor cells. Transfusion. 2010;50:2002–2010. doi: 10.1111/j.1537-2995.2010.02664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wysoczynski M. Reca R. Ratajczak J. Kucia M. Shirvaikar N. Honczarenko M. Mills M. Wanzeck J. Janowska-Wieczorek A. Ratajczak MZ. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 17.Shirvaikar N. Marquez-Curtis LA. Ratajczak MZ. Janowska-Wieczorek A. Hyaluronic acid and thrombin upregulate MT1-MMP through PI3K and Rac-1 signaling and prime the homing-related responses of cord blood hematopoietic stem/progenitor cells. Stem Cells Dev. 2011;20:19–30. doi: 10.1089/scd.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Janowska-Wieczorek A. Majka M. Kijowski J. Baj-Krzyworzeka M. Reca R. Turner AR. Ratajczak J. Emerson SG. Kowalska MA. Ratajczak MZ. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 19.Gul H. Marquez-Curtis LA. Jahroudi N. Lo J. Turner AR. Janowska-Wieczorek A. Valproic acid increases CXCR4 expression in hematopoietic stem/progenitor cells by chromatin remodeling. Stem Cells Dev. 2009;18:831–838. doi: 10.1089/scd.2008.0235. [DOI] [PubMed] [Google Scholar]
- 20.Spencer A. Jackson J. Baulch-Brown C. Enumeration of bone marrow homing haemopoietic stem cells from G-CSF-mobilised normal donors and influence on engraftment following allogeneic transplantation. Bone Marrow Transplant. 2001;28:1019–1022. doi: 10.1038/sj.bmt.1703289. [DOI] [PubMed] [Google Scholar]
- 21.Marquez-Curtis LA. Turner AR. Larratt LM. Letcher B. Lee SF. Janowska-Wieczorek A. CD34+ cell responsiveness to stromal cell-derived factor-1alpha underlies rate of engraftment after peripheral blood stem cell transplantation. Transfusion. 2009;49:161–169. doi: 10.1111/j.1537-2995.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- 22.Kahn J. Byk T. Jansson-Sjostrand L. Petit I. Shivtiel S. Nagler A. Hardan I. Deutsch V. Gazit Z, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 23.Brenner S. Whiting-Theobald N. Kawai T. Linton GF. Rudikoff AG. Choi U. Ryser MF. Murphy PM. Sechler JM. Malech HL. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells. 2004;22:1128–1133. doi: 10.1634/stemcells.2003-0196. [DOI] [PubMed] [Google Scholar]
- 24.Papapetrou EP. Zoumbos NC. Athanassiadou A. Genetic modification of hematopoietic stem cells with nonviral systems: past progress and future prospects. Gene Therapy. 2005;12:118–130. doi: 10.1038/sj.gt.3302626. [DOI] [PubMed] [Google Scholar]
- 25.Ewert KK. Ahmad A. Bouxsein NF. Evans HM. Safinya CR. Non-viral gene delivery with cationic liposome-DNA complexes. Methods Mol Biol. 2008;433:159–175. doi: 10.1007/978-1-59745-237-3_10. [DOI] [PubMed] [Google Scholar]
- 26.Patil SD. Rhodes DG. Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J. 2005;7:E61–E77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irshad S. Mahul-Mellier AL. Kassouf N. Lemarie A. Grimm S. Isolation of ORCTL3 in a novel genetic screen for tumor-specific apoptosis inducers. Cell Death Differ. 2009;16:890–898. doi: 10.1038/cdd.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sartori da Silva MA. Tee JM. Paridaen J. Brouwers A. Runtuwene V. Zivkovic D. Diks SH. Guardavaccaro D. Peppelenbosch MP. Essential role for the d-Asb11 cul5 Box domain for proper notch signaling and neural cell fate decisions in vivo. PLoS One. 2010;5:e14023. doi: 10.1371/journal.pone.0014023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madeira C. Mendes RD. Ribeiro SC. Boura JS. Aires-Barros MR. da Silva CL. Cabral JM. Nonviral gene delivery to mesenchymal stem cells using cationic liposomes for gene and cell therapy. J Biomed Biotech. 2010;2010:735349. doi: 10.1155/2010/735349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marit G. Cao Y. Froussard P. Ripoche J. Dupouy M. Elandaloussi A. Lacombe F. Mahon FX. Keller H, et al. Increased liposome-mediated gene transfer into haematopoietic cells grown in adhesion to stromal or fibroblast cell line monolayers. Eur J Haematol. 2000;64:22–31. doi: 10.1034/j.1600-0609.2000.80230.x. [DOI] [PubMed] [Google Scholar]
- 31.Martino S. di Girolamo I. Tiribuzi R. D'Angelo F. Datti A. Orlacchio A. Efficient siRNA delivery by the cationic liposome DOTAP in human hematopoietic stem cells differentiating into dendritic cells. J Biomed Biotechnol. 2009;2009:410260. doi: 10.1155/2009/410260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo B. Pearce AG. Traulsen KE. Rintala AC. Lee H. Fluorescence produced by transfection reagents can be confused with green fluorescent proteins in mammalian cells. Biotechniques. 31:314–321. doi: 10.2144/01312st02. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira L. Karp JM. Nobre L. Langer R. New opportunities: the use of nanotechnologies to manipulate and track stem cells. Cell Stem Cell. 2008;3:136–146. doi: 10.1016/j.stem.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Pfeifer A. Brandon EP. Kootstra N. Gage FH. Verma IM. Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc Natl Acad Sci U S A. 2001;98:11450–11455. doi: 10.1073/pnas.201415498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porecha NK. English K. Hangoc G. Broxmeyer HE. Christopherson KW., 2nd Enhanced functional response to CXCL12/SDF-1 through retroviral overexpression of CXCR4 on M07e cells: implications for hematopoietic stem cell transplantation. Stem Cells Dev. 2006;15:325–333. doi: 10.1089/scd.2006.15.325. [DOI] [PubMed] [Google Scholar]
- 36.Brenner MK. Emerging applications of gene transfer in the hematopoietic cancers. J Pediatr Hemat Oncol. 1997;19:1–6. doi: 10.1097/00043426-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Baum C. Eckert HG. Stockschlader M. Just U. Hegewisch-Becker S. Hildinger M. Uhde A. John J. Ostertag W. Improved retroviral vectors for hematopoietic stem cell protection and in vivo selection. J Hematother. 1996;5:323–329. doi: 10.1089/scd.1.1996.5.323. [DOI] [PubMed] [Google Scholar]
- 38.Frey BM. Hackett NR. Bergelson JM. Finberg R. Crystal RG. Moore MA. Rafii S. High-efficiency gene transfer into ex vivo expanded human hematopoietic progenitor and precursor cells by adenovirus vectors. Blood. 1998;91:2781–2792. [PubMed] [Google Scholar]
- 39.Kang EM. Hanazano Y. Frare P. Vanin EF. De Witte M. Metzger M. Liu JM. Tisdale JF. Persistent low-level engraftment of rhesus peripheral blood progenitor cells transduced with the Fanconi anemia C gene after conditioning with low-dose irradiation. Mol Ther. 2001;3:911–919. doi: 10.1006/mthe.2001.0337. [DOI] [PubMed] [Google Scholar]
- 40.Hall KM. Horvath TL. Abonour R. Cornetta K. Srour EF. Decreased homing of retrovirally transduced human bone marrow CD34+ cells in the NOD/SCID mouse model. Exp Hematol. 2006;34:433–442. doi: 10.1016/j.exphem.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Aiuti A. Tavian M. Cipponi A. Ficara F. Zappone E. Hoxie J. Peault B. Bordignon C. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29:1823–1831. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 42.Li LH. McCarthy P. Hui SW. High-efficiency electrotransfection of human primary hematopoietic stem cells. FASEB J. 2001;15:586–588. doi: 10.1096/fj.00-0447fje. [DOI] [PubMed] [Google Scholar]
- 43.Feril LB., Jr Ogawa R. Kobayashi H. Kikuchi K. Kondo T. Ultrasound enhances liposome-mediated gene transfection. Ultrason Sonochem. 2005;12:489–493. doi: 10.1016/j.ultsonch.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki R. Takizawa T. Negishi Y. Utoguchi N. Maruyama K. Effective gene delivery with liposomal bubbles and ultrasound as novel non-viral system. J Drug Target. 2007;15:531–537. doi: 10.1080/10611860701499789. [DOI] [PubMed] [Google Scholar]
- 45.Rhim WK. Kim JS. Nam JM. Lipid-gold-nanoparticle hybrid-based gene delivery. Small. 2008;4:1651–1655. doi: 10.1002/smll.200800628. [DOI] [PubMed] [Google Scholar]
- 46.Li D. Li G. Li P. Zhang L. Liu Z. Wang J. Wang E. The enhancement of transfection efficiency of cationic liposomes by didodecyldimethylammonium bromide coated gold nanoparticles. Biomaterials. 2010;31:1850–1857. doi: 10.1016/j.biomaterials.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 47.Fritz V. Noel D. Bouquet C. Opolon P. Voide R. Apparailly F. Louis-Plence P. Bouffi C. Drissi H, et al. Antitumoral activity and osteogenic potential of mesenchymal stem cells expressing the urokinase-type plasminogen antagonist amino-terminal fragment in a murine model of osteolytic tumor. Stem Cells. 2008;26:2981–2990. doi: 10.1634/stemcells.2008-0139. [DOI] [PubMed] [Google Scholar]
- 48.Wynn RF. Hart CA. Corradi-Perini C. O'Neil L. Evans CA. Wraith JE. Fairbairn LJ. Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 49.Son BR. Marquez-Curtis LA. Kucia M. Wysoczynski M. Turner AR. Ratajczak J. Ratajczak MZ. Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells is regulated by stromal-derived factor-1–CXCR4 and hepatocyte growth factor–c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 50.Kohn DB. Sadelain M. Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 51.Woods NB. Bottero V. Schmidt M. von Kalle C. Verma IM. Gene therapy: therapeutic gene causing lymphoma. Nature. 2006;440:1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.