Abstract
Cyclin dependent kinase inhibitors (CDKIs) influence proliferation of hematopoietic progenitor cells (HPCs), but little is known of how they influence proliferative responsiveness of HPCs to colony stimulating factors (CSFs), alone and in combination with other hematopoietically active factors, such as the potent co-stimulating cytokine stem cell factor (SCF), or inhibition by myelosuppressive chemokines. Using mice with deletions in p18INK4c, p21CIP1/WAF1, or p27KIP1 genes, and in mice with double gene deletions for either p18/p21 or p18/p27, we determined effects of absence of these CDKIs and their interactions on functional HPC numbers in vivo, and HPC proliferative responsiveness in vitro. There is a decrease in bone marrow HPC proliferation in p18−/− mice commensurate with decreased numbers of HPC, suggesting a positive role for p18 on HPC in vivo, similar to that for p21. These positive effects of p18 dominate negative effects of p27 gene deletion. Moreover, the CDKIs differentially regulate responsiveness of granulocyte macrophage (GM) progenitors to synergistic cell proliferation in response to GM-CSF plus SCF, which is considered important for normal hematopoiesis. Responsiveness of HPCs to inhibition by myelosuppressive chemokines is directly related to the capacity of HPCs to respond to synergistic stimulation, and their cell cycle status. P18INK4c gene deletion rescued the loss of chemokine suppression of synergistic proliferation due to deletion of p21CIP1/WAF1. These findings underscore the complex interplay of cell cycle regulators in HPC, and demonstrate that loss of one can sometimes be compensated by loss of another CDKI in both, a pro- or anti-proliferative context.
Introduction
Cell cycle regulation is key to numbers, proliferative capacity, and function of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) in vivo and in vitro and to proper hematopoiesis [1,2]. The cell cycle is mediated in part by cyclin dependent kinases (CDKs) and CDK inhibitors (CDKIs). Seven CDKIs have been identified, belonging to the INK family of closely related ankyrin-repeat containing genes (p16INK4a, p15INK4b, p18INK4c, and p18INK4d), or to the CIP1/KIP family (p21CIP1/WAF1, p27KIP1, and p57KIP2). Of these, p21CIP1/WAF1 [1–7], p27KIP1 [3,6,8–10], and p18INK4c [11,12] have been implicated in the regulation of different functions for HSC/HPC populations. Functional deletion of p21CIP1/WAF1 gene in mice (p21−/−) results in increases in HSC proliferation and absolute numbers in bone marrow. They also have impaired self-renewal capacity after serial transplantation [7], although recently a more limited role for p21CIP1/WAF1 in maintaining normal HSC function has been reported [13]. In contrast, immature subsets of HPC of p21−/− mice have decreased HPC proliferation and reduced absolute numbers [3], whereas over expression has the opposite effect [4]. Deletion of p27KIP1 (p27−/−) results in enhanced proliferation and numbers of HPC [8] without effecting HSC number, cell cycling, or self-renewal [9]. Loss of p18INK4c (p18−/−) results in increased long-term engraftment and increased self-renewal of HSC [9]. Interestingly, absence of p18INK4c counteracts HSC exhaustion of p21−/− cells after serial transplantation [11]. Thus, CDKIs differentially modulate HSC/HPC function in positive and negative ways.
Events mediating regulation of HPCs are perhaps as important as those that regulate HSCs, as these cells are intermediaries in the production of mature blood cells originating from the HSC compartment. Inherent in this regulation of hematopoietic progenitors is their response to cytokine stimulation of their proliferation [2]. Some colony stimulating factors (CSFs) stimulate one specific progenitor cell type, whereas others stimulate a number of different progenitors. Although granulocyte (G)-CSF will induce proliferation of mainly or only granulocyte progenitors (CFU-G), and macrophage (M)-CSF mainly or only stimulate macrophage progenitors (CFU-M), GM-CSF can stimulate proliferation of granulocyte macrophage (CFU-GM), in addition to CFU-G and/or CFU-M. Erythropoietin (EPO) is an example of a CSF which stimulates only colony formation by erythroid progenitors (BFU-E). HPCs responsive to a single cytokine are considered to be more mature subsets of HPCs [2]. In addition to the CSFs, there are also potent co-stimulating cytokines such as stem cell factor (SCF) and Flt3-ligand (FL), which act respectively through the tyrosine kinase receptors c-kit and Flt3, which have little or no CSF activity on their own. However, when a CSF is combined with one or both co-stimulating cytokines, SCF and/or FL, the responsive HPCs form larger colonies and are considered to be more immature HPCs than those stimulated by only a single CSF. We [2–4] and others [1] have been intrigued by the concept of cytokine synergy for proliferation of HPCs. Based on our previous interest in the role of the CDKI, p21CIP1/WAF1 in cytokine synergy, we evaluated our hypothesis that p18INK4c plays a role in HPC proliferation and function, both alone, and also in combination with p21CIP1/WAF1 and p27KIP1. We evaluated p18−/−, and mice double-deleted for p18/21 (p18−/−-p21−/−) and p18/p27 (p18−/−-p27−/−), in comparison with control (+/+), p21−/−, and p27−/− mice for: absolute numbers and cycling status of bone marrow and spleen granulocyte macrophage (CFU-GM), erythroid (BFU-E), and multipotential (CFU-GEMM) progenitor cells, responsiveness of CFU-GM to synergistic stimulation in vitro by the combination of granulocyte macrophage (GM) CSF and SCF, and to inhibition of HPC proliferation by selected members of the chemokine family.
Materials and Methods
Mice
These studies utilized normal control C57Bl/6 mice purchased from Jackson Laboratories (Bar Harbor, ME) and a number of CDKI knock-out (−/−) mice: p21−/− [3], p27−/− [8], p18−/− [14,15], and dual p18−/−-p21−/− [14], in addition to p18−/−-p27−/− [15] and their littermate controls. The p21−/− mice were originally obtained from Chuxia Deng, NIDDK, NIH (Bethesda, MD), and bred at the Indiana University School of Medicine. All other CDKI knock-out mice were supplied by coauthor D.S. Franklin. All mouse studies followed IACUC guidelines.
Cells
Femoral bone marrow and spleen cells were isolated and absolute numbers of HPC (CFU-GM, BFU-E, and CFU-GEMM) per femur and spleen, and the cycling status of the HPC (equals percent HPC in S-phase of the cell cycle as determined by high specific activity tritiated thymidine kill technique) were calculated as reported [16–18].
Culture conditions
For studies evaluating absolute numbers and cycling status, bone marrow and spleen cells were plated respectively at 5×104 and 5×105 cells/mL in 1% methylcellulose culture medium in 30% fetal bovine serum (FBS; Hyclone, Inc., Logan, Utah) in the presence of 1 U/mL recombinant (r) human EPO (Epo; Amgen Corp., Thousand Oaks, CA), 5% vol/vol pokeweed mitogen mouse spleen cell conditioned medium [18], 50 ng/mL r mouse (m) SCF (R&D Systems, Minneapolis, MN), and 0.1 mM hemin (Eastman Kodak, Rochester, NY). For synergy studies, colony formation stimulated by rm GM-CSF (R&D Systems) was compared with that stimulated by the combination of GM-CSF and SCF. Chemokines (lymphotactin, XCL1; MCP-1, CCL2; IL-8, CXCL8; and MIG, CXCL9) were purchased from R&D Systems. The inhibitor effects of these myelosuppressive chemokines [19,20] were assessed on colony formation by bone marrow CFU-GM of normal C57Bl/6 mice at time zero, or after 24 h culture in suspension in the presence of rm FL (100 ng/mL; R&D Systems), rm GM-CSF (10 ng/mL), rm IL-6 (10 ng/mL; R&D Systems), and rm SCF (50 ng/mL) to increase the cycling of the CFU-GM. Cells in suspension culture for 24 h were washed prior to plating in semi-solid cultures. The cells were thus plated at time 0 or after 24 h in the absence or presence of the specified chemokine and in the presence of Epo, PWMSCM, and SCF plus hemin. All plates were incubated at lowered (5%) O2 tension, to allow for optimal colony formation [21], in a humidified chamber at 5% CO2.
Statistics
Each experimental point was set up in triplicate and significant differences determined by 2 tailed students t-test, with a P value of at least <0.05 considered statistically significant.
Results
Effects of CDKIs on HPC numbers and proliferation
Different CDKIs differentially effect proliferation of HPCs [1–13]. Functional deletion of p21CIP1/WAF1 results in decreased numbers and cycling status of the immature subsets of mouse bone marrow HPC [4,5], those HPC that respond to synergistic proliferative stimulation when exposed to a CSF plus the potent co-stimulating/augmenting cytokine, SCF [21]. In contrast, loss of p27KIP1 results in enhanced proliferation and numbers of HPCs [8].
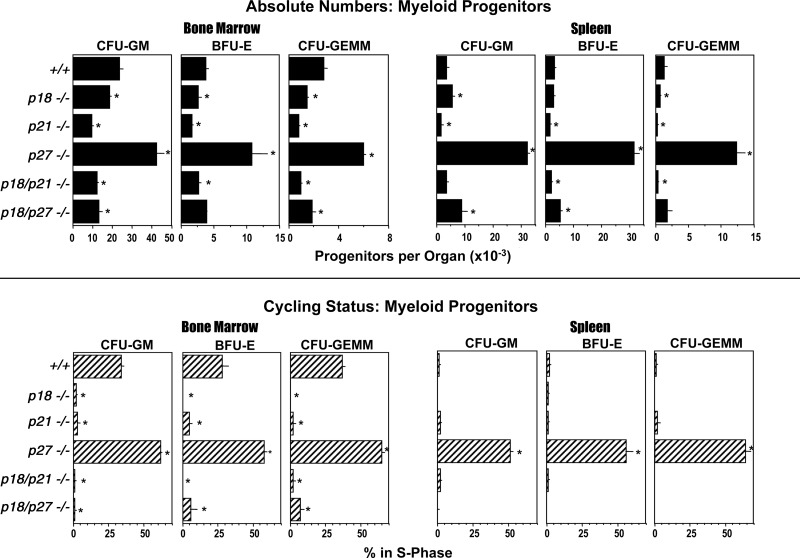
In the mouse, the spleen is an active hematopoietic organ, but not all reports evaluate both marrow and spleen for hematopoiesis and hematopoietic effects, and usually not as side-by-side comparisons within the same mouse. Also, there are few reports of assessment of differential effects on subsets of HPCs such as CFU-GM, BFU-E, and CFU-GEMM, or whether the effects seen are the same for the immature subsets of each [responsive to CSF(s) plus SCF], or the more mature subsets of these cells (responsive to only a CSF) [2]. Since such evaluations can enhance information of effects of CDKIs, we assessed numbers and cycling of immature subsets of CFU-GM, BFU-E, and CFU-GEMM in marrow and spleen (Fig. 1). Consistent with what has been reported for total bone marrow HPCs of mice deleted of p21CIP1/WAF1 [3], immature subsets of CFU-GM, BFU-E, and CFU-GEMM of p21−/− significantly decreased in bone marrow and spleen (Fig. 1). Moreover, the cycling status (% HPCs in S-Phase of the cell cycle) of these cells in bone marrow and spleen significantly decreased, even though cycling status of HPCs of control mice were already in a slow cycling state (Fig. 1). In contrast, absolute numbers of bone marrow and spleen HPC (CFU-GM, BFU-E, and CFU-GEMM) in the marrow and spleen of p27KIP1−/− mice significantly increased, and also the cell cycle status for the 3 HPC subsets was enhanced. Thus, p21CIP1/WAF1 and p27KIP1 act respectively as positive and negative regulators of immature subsets of HPC numbers and proliferation in vivo in bone marrow and spleen.
FIG. 1.
Influence of CDKI−/− on absolute numbers and cycling status (% in S phase) of bone marrow and spleen CFU-GM, BFU-E, and CFU-GEMM. Results are shown as the mean±1SEM of 6 individually assessed mice, per point from a total of 2 experiments. *P<0.05 compared to cells from +/+ mice. CDKI, cyclin dependent kinase inhibitor; GM, granulocyte macrophage.
Characteristics of p18−/− bone marrow HPCs were quite similar to that of p21−/− HPCs in that absolute numbers and cycling status of bone marrow CFU-GM, BFU-E, and CFU-GEMM significantly decreased compared with control progenitors, although the decrease in numbers of p18−/− HPCs was not as great as that of the p21−/− HPCs (Fig. 1). Splenic p18−/− CFU-GEMM numbers decreased less than those from p21−/− mice; splenic p18−/− BFU-E did not decrease at all; and splenic p18−/− CFU-GM very modestly but significantly increased compared to +/+ and p21−/− cells. Thus, the effects of 3 CDKI family members (p21CIP1/WAF1, p27KIP1, and p18INK4C) had not only overlapping but also unique effects on HPCs.
It has been reported that p18INK4c and p21CIP1/WAF1 functionally interact with each other in terms of HSC exhaustion after serial transplantation [12]. We thus evaluated p18INK4c for interacting effects either with p21CIP1/WAF1 or with p27KIP1 in terms of numbers and proliferative status of immature subsets of marrow and spleen HPCs by using dual p18−/−-p21−/− and dual p18−/−-p27−/− mice in comparison with +/+, p18−/−, p21−/−, and p27−/− single gene-deleted mice (Fig. 1). Deletion of both the p18INK4c and p21CIP1/WAF1 genes did not further effect decreased hematopoiesis noted in either of the single CDKI −/− genotypes. Decreased numbers of HPC of dual p18−/−-p21−/− mice were either equal to that of the p21−/− mice or midway between that of the p18−/− and p21−/− mice, whereas the cells from these 3 groups of knock-out mice were in a slow or noncycling state. These results with HPC are different from the impressive effect of p18−/− on p21−/− that others have shown for serial repopulation of HSC [11,12], highlighting differences in effects of CDKIs on the HSC and HPC compartments. In contrast, the combination of deletion of both p18INK4c and p27KIP1 had a strong modifying effect on HPC numbers and proliferation in the marrow and spleen. Dual p18−/−-p27−/− mice significantly manifested suppressed numbers and cycling status of bone marrow CFU-GM, BFU-E, and CFU-GEMM compared with that of p27−/− mice with the marrow CFU-GM and CFU-GEMM numbers of the dual p18−/−-p27−/− significantly decreased compared to both the +/+ and p27−/− mice. The very large enhancement in spleen CFU-GM, BFU-E, and CFU-GEMM numbers and cycling status seen in the p27−/− mice was greatly reduced in the dual p18−/−-p27−/− mice. This demonstrates the profound modulatory influences of p18INK4c and p27KIP1 on each other in terms of their effects on marrow and spleen HPC numbers and cell cycling.
Effects of CDKIs on synergistic proliferative responses of CFU-GM to GM-CSF and SCF
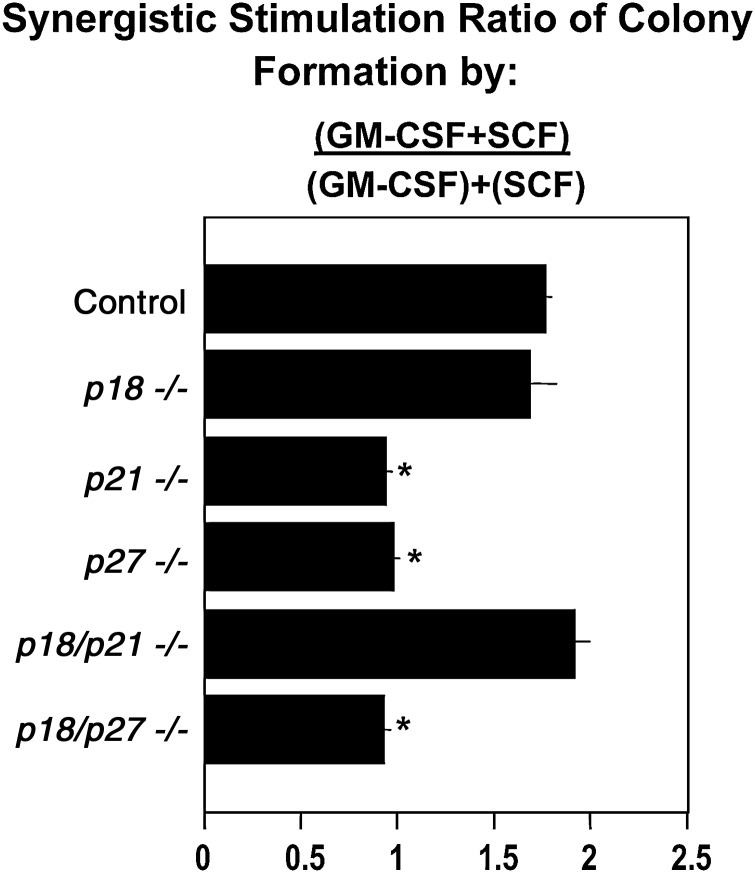
The capacity of HPC to synergistically respond to the proliferative inducing effects of a CSF and a potent co-stimulating cytokine is a characteristic of immature subsets of HPC [2], events likely relevant to proper regulation of in vivo hematopoiesis where HPC can be simultaneously subjected to multiple cytokines. The combination of a CSF, such as GM-CSF, and a potent co-stimulating cytokine such as SCF, which has little capacity to stimulate colony formation on its own, results in enhanced numbers of colonies in vitro (Fig. 2; shown as a ratio of colony number stimulated by GM-CSF plus SCF, divided by the additive colony formation of cells stimulated by only GM-CSF or only SCF), and increased numbers of cells per colony (Data not shown). We previously reported that p21CIP1/WAF1 has a role in HPC proliferation since p21−/− CFU-GM did not synergistically respond to the combined effects of GM-CSF and SCF [3], events reproduced in the current study (Fig. 2). Interestingly, here we found that p27−/−, but not p18−/−, CFU-GM respond in a manner similar to that of p21−/− cells (Fig. 2), suggesting that both p27KIP1 and p21CIP1/KIP1, but not p18INK4c, are involved in the synergistic proliferative response of CFU-GM to the combination of GM-CSF and SCF. These differential effects were not due to the cell cycling characteristics of the CFU-GM prior to cytokine stimulation, as p27−/− cells, which were not responsive to synergistic stimulation (Fig. 2), had a large percentage of CFU-GM in cycle prior to stimulation (Fig. 1) compared with p21CIP1/WAF1 cells which were in a slow or noncycling state (Fig. 1). The p18−/− CFU-GM that did synergistically respond to GM-CSF and SCF (Fig. 2) were in a slow or noncycling state before stimulation by cytokines (Fig. 1). That both p21−/− and p18−/− HPC could be induced into cycle by exogenous stimulation with cytokines is shown in Fig. 3, and is clear from the fact that these cells did form colonies (Fig. 1). The evidence for interactions between different CDKIs effects on proliferative responses of CFU-GM to synergistic stimulation is demonstrated by the responses of dual p18−/−-p21−/− and p18−/−-p27−/− HPC (Fig. 2). The dual p18−/−-p21−/− HPC synergistically responded to GM-CSF and SCF, demonstrating that p18INK4c was dominant over p21CIP1/WAF1 in its effects, whereas the dual p18−/−-p27−/− HPC did not respond to the synergistic effects of GM-CSF and SCF, showing that the p27KIP1 effects were dominant over the p18INK4c effects. These results were not due to percent of these dual CDKI−/− cells in S-phase before they were stimulated in vitro, as the cells of both dual CDKI−/− were in a slow or noncycling state before stimulation (Fig. 1).
FIG. 2.
Effect of CDKI−/− on the synergistic response of bone marrow CFU-GM to stimulation by the combination of GM-CSF plus SCF, compared with the additive effects of GM-CSF or SCF, each used alone to stimulate colony formation. Results are shown as the mean±1SEM as a stimulus ratio, and are based on studies from bone marrow cells of 6 individually assessed mice from a total of 2 experiments. *P<0.05 compared to control cells. CSF, colony stimulating factors; SCF, stem cell factor.
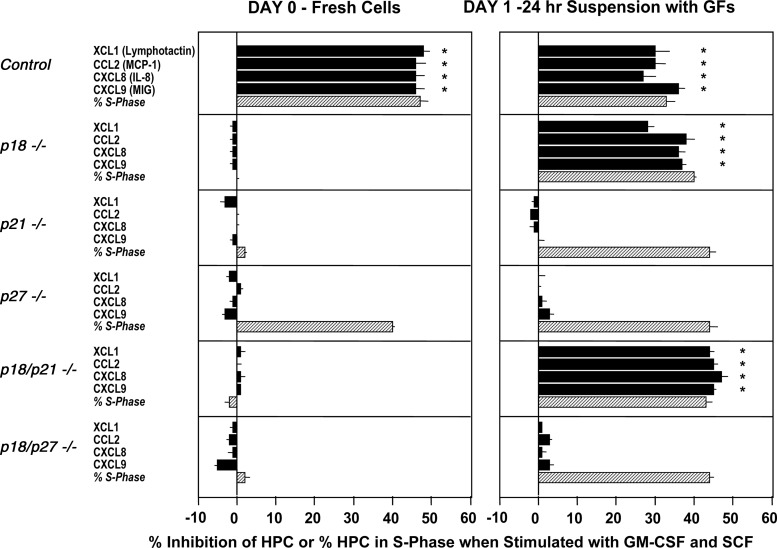
FIG. 3.
Suppressive effects of chemokines on colony formation of control (+/+) and CDKI−/− bone marrow CFU-GM stimulated by GM-CSF and SCF, at time 0 (=freshly isolated cells), or after 24 h. suspension culture in the presence of SCF, GM-CSF, FL, and IL-6 (=growth factors [GFs]). Also shown is the cycling status of CFU-GM in the absence of chemokines at time 0, or after 24 h in suspension culture for 24 h with GFs. Cells in suspension culture were washed 2×, prior to plating with GM-CSF and SCF with or without chemokines. Results are shown as mean±1SEM for bone marrow cells of 6 individually assessed mice from a total of 2 experiments. *P<0.05 compared to without chemokine.
Effects of CDKIs on responses of multi-cytokine stimulated CFU-GM to inhibition by suppressive chemokines
A number of the members of the CC, CXC, and C chemokine families demonstrate suppressive effects on HPC in vitro and in vivo [19,20]. This includes the C chemokine XCL1 (lymphotactin), the CC chemokine CCL2 (MCP-1), and the CXC chemokines CXCL8 (IL-8) in addition to CXCL9 (MIG). As shown in Fig. 3 (left panel), CFU-GM from +/+, but not from p18−/−, p21−/−, p27−/−, dual p18−/−-p21−/−, or dual p18−/−-p27−/−, responded to the suppressive effects of the 4 members of the chemokine family. However, HPC must be in active cell cycle before the addition of growth factors (GFs) and chemokines for them to be responsive to the inhibitory effects of suppressive chemokines [19,20]. Lack of chemokine suppression of p27−/− CFU-GM was not due to these CFU-GM not being in cycle, as these cells were in rapid cell cycle with a high percentage of cells in S-phase. To see if lack of responsiveness of p18−/−, p21−/−, and the dual p18−/−-p21CIP1−/− and p18−/−-p27−/− HPC to inhibition by chemokines was solely due to the percent of HPC in S-phase before the addition of cytokines and chemokines, we placed the CFU-GM from these CDKI−/− mice into rapid cell cycle by exposing them to a combination of cytokines for 24 h, before washing the cells and placing them in the presence of cytokines and chemokines. As shown in the right panel of Fig. 3, placing the p18−/− HPC into cycle restored their responsiveness to inhibition by these chemokines. However, enhancement of the percent of CFU-GM in S-phase did not restore the responsiveness of either p21−/− or p27−/− HPC to chemokine inhibition. Thus, having CFU-GM in active cell cycle before the addition of GFs and chemokines is alone not sufficient for the chemokines to cause their suppressive effects. However, the p18−/− component in the dual p18−/−-p21−/− cells was capable of restoring the responsiveness of the p21−/− cells to chemokine inhibition, but was not capable of restoring the responsiveness to inhibition of p27−/− HPC in the dual p18−/−-p27−/− cells. That the dual p18−/−-p21−/−, but not the p18−/−-p27−/− HPC responded to synergistic stimulation suggest that the HPC must not only be in active cell cycle for them to respond to suppression by chemokines, but these HPC must also be responsive to synergistic stimulation of their proliferation.
Discussion
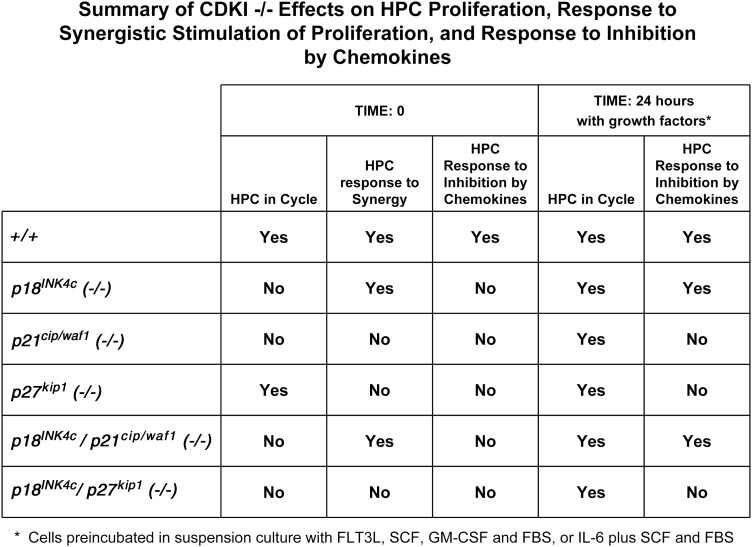
CDKIs have effects on the proliferation of a number of different cell systems. Some have been implicated in proliferation of HPCs and HSCs (2–13), T-cell proliferation [22], myogenesis [23], and tumorigenesis (14,15,24–28). There is still much to be learned regarding a role for CDKIs in hematopoiesis. This is especially true for the interacting/overlapping roles of CDKIs in this regulation. In the present paper, we focused on a role for CDKIs on HPCs, an intermediary cell between HSCs and more mature blood cells, for which little information is currently available. We compared effects of p21CIP1/WAF1, p27KIP1, and p18INK4C on in vivo and in vitro proliferation of HPCs, and also the modifying effects of p18INK4C with either that of p21CIP1/WAF1, or p27KIP1 on this proliferation, by using CDKI −/− mice. Although there was previous information available on loss of p21CIP1/WAF1, which results in decreased numbers of HPC [3], and p27KIP1 which results in increased numbers of HPC [8], there was no information on p18−/− on these intermediary immature subsets of HPC. Nor was there any information available on how p18−/− interfaced with loss of p21CIP1/WAF1 or p27KIP1. Our present results demonstrate potent effects of p18−/− on HPC proliferation and responsiveness to stimulation by GFs, and of inhibition by certain members of the chemokine family. Additionally, there were modifying effects noted in these functions of HPCs when dual p18−/−-p21−/− and p18−/−-p27−/− genotypes were assessed. Although it is always difficult to translate findings in vitro to those in vivo, a number of the studies we show in vitro are consistent with our findings in the mice themselves. Figure 4 presents a summary of these effects which demonstrate the following: First, there was decreased HPC proliferation in p18−/− mice, suggesting a positive role for p18INK4C similar to that we previously reported for p21CIP1/WAF1 [3,4]. Second, the positive effects of p18INK4C dominated over the negative effects of p27KIP1 on HPC proliferation. Third, CDKIs differentially regulated responses of CFU-GM to synergistic proliferation in response to GM-CSF and SCF, an observation of potential in vivo relevance as synergy between cytokines in vivo is likely to be an important physiological response since HPCs in vivo can be subjected to a number of different cytokines at the same time. In this context, it is possible that the sequence of specific receptor occupancies and receptor cross-talk in the cell may dictate the intracellular signals of relevance and ultimate HPC response(s). Fourth, the responsiveness of HPC to inhibition by myelosuppressive chemokines from 3 different subfamilies of chemokines was directly related to the capacity of HPC to respond to synergistic stimulation. Also, the cycling status of HPC was apparently under control by CDKIs. Exactly how the different CDKIs mechanistically regulate these different responses of HPCs to respective positive and negative cytokine or chemokine effects remains to be determined. A structure-based approach, as noted for p18INK4C [29], may be helpful in this regard in the future. Once we know exactly how CDKIs act downstream, the upstream events that modulate CDKIs, each alone, and in combination through possible cross-talk with each other in both normal homeostatic nonstressed, and in stressed conditions, we may eventually be able to either accelerate or suppresses proliferation of HPC and/or HSCs for clinical advantage. It is possible that CDKIs may play a role in the progression of nonmalignant and malignant hematological disorders that interfere with the balance needed for normal hematopoiesis under nondisease homeostatic conditions. If so, intervention at the level of CDKIs may also be useful in this context. Further studies on the CDKIs, HPCs, HSCs, and hematopoiesis under normal and disease conditions are thus warranted.
FIG. 4.
Summary of studies assessing CDKI−/− effects on proliferation of HPC (from Fig. 1), responsive of HPC to synergistic stimulation by GM-CSF plus SCF (from Fig. 2), and also to chemokine inhibition, on freshly isolated bone marrow cells or cells first incubated in suspension culture for 24 h with GFs and then washed (from Fig. 3). HPC, hematopoietic progenitor cell.
Acknowledgments
These studies were supported by the U.S. Public Health Service Grants R01 HL056416, R01 HL067384, and P30 DK090948 from the National Institutes of Health to H.E.B.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Steinman RA. Cell cycle regulators and hematopoiesis. Oncogene. 2002;21:3403–3413. doi: 10.1038/sj.onc.1205325. [DOI] [PubMed] [Google Scholar]
- 2.Shaheen M. Broxmeyer HE. The humoral regulation of hematopoiesis. In: Hoffman R, editor; Benz EJ Jr., editor; Shattil SJ, editor; Furie B, editor; Silberstein LE, editor; McGlave P, editor; Heslop H, editor; Anastasi J, editor. Hematology: Basic Principles and Practice. 5th. Elsevier Churchill Livingston; Philadelphia, PA: 2009. pp. 253–275. Part III, Chapter 24. [Google Scholar]
- 3.Mantel C. Luo Z. Canfield J. Braun S. Deng C. Broxmeyer HE. Involvement of p21cip-1 and p27kip-1 in the molecular mechanisms of steel factor-induced proliferative synergy in vitro and of p21cip-1 in the maintenance of stem/progenitor cells in vivo. Blood. 1996;88:3710–3719. [PubMed] [Google Scholar]
- 4.Braun SE. Mantel C. Rosenthal M. Cooper S. Liu L. Robertson KA. Hromas R. Broxmeyer HE. A positive effect of p21cip1/waf1 in the colony formation from murine myeloid progenitor cells as assessed by retroviral-mediated gene transfer. Blood Cells Mol Dis. 1998;24:138–148. doi: 10.1006/bcmd.1998.0181. [DOI] [PubMed] [Google Scholar]
- 5.Mantel C. Braun SE. Reid S. Henegariu O. Liu L. Hangoc G. Broxmeyer HE. p21(cip-1/waf-1) deficiency causes deformed nuclear architecture, centriole overduplication, polyploidy, and relaxed microtubule damage checkpoints in human hematopoietic cells. Blood. 1999;93:1390–1398. [PubMed] [Google Scholar]
- 6.Taniguchi T. Endo H. Chikatsu N. Uchimaru K. Asano S. Fujita T. Nakahata T. Motokura T. Expression of p21(Cip1/Waf1/Sdi1) and p27(Kip1) cyclin-dependent kinase inhibitors during human hematopoiesis. Blood. 1999;93:4167–4178. [PubMed] [Google Scholar]
- 7.Cheng T. Rodrigues N. Shen H. Yang Y. Dombkowski D. Sykes M. Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 8.Fero ML. Rivkin M. Tasch M. Porter P. Carow CE. Firpo E. Polyak K. Tsai LH. Broudy V, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 9.Cheng T. Rodrigues N. Dombkowski D. Stier S. Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27(kip1) Nat Med. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 10.Walkley CR. Fero ML. Chien WM. Purton LE. McArthur GA. Negative cell-cycle regulators cooperatively control self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2005;7:172–178. doi: 10.1038/ncb1214. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Y. Shen H. Franklin DS. Scadden DT. Cheng T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol. 2004;6:436–442. doi: 10.1038/ncb1126. [DOI] [PubMed] [Google Scholar]
- 12.Yu H. Yuan Y. Shen H. Cheng T. Hematopoietic stem cell exhaustion impacted by p18 INK4C and p21 Cip1/Waf1 in opposite manners. Blood. 2006;107:1200–1206. doi: 10.1182/blood-2005-02-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van OR. Kamminga LM. Ausema A. Bystrykh LV. Draijer DP. van PK. Dontje B. de HG. A Limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells. 2007;25:836–843. doi: 10.1634/stemcells.2006-0631. [DOI] [PubMed] [Google Scholar]
- 14.Franklin DS. Godfrey VL. O'Brien DA. Deng C. Xiong Y. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol Cell Biol. 2000;20:6147–6158. doi: 10.1128/mcb.20.16.6147-6158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin DS. Godfrey VL. Lee H. Kovalev GI. Schoonhoven R. Chen-Kiang S. Su L. Xiong Y. CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev. 1998;12:2899–2911. doi: 10.1101/gad.12.18.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christopherson KW. Hangoc G. Mantel CR. Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 17.Broxmeyer HE. Orschell CM. Clapp DW. Hangoc G. Cooper S. Plett PA. Liles WC. Li X. Graham-Evans B, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper S. Broxmeyer HE. Measurement of interleukin-3 and other hematopoietic growth factors, such as GM-CSF, G-CSF, M-CSF, erythropoietin and the potent co-stimulating cytokines steel factor and Flt-3 ligand. In: Coligan JE, editor; Kruisbeek AM, editor; Margulies DH, editor; Shevach EM, editor; Strober W, editor; Coico R, editor. Current Protocols in Immunology. Suppl 18. John Wiley & Sons, Inc.; New York: 1996. pp. 6.4.1–6.4.12. [Google Scholar]
- 19.Kim CH. Qu CK. Hangoc G. Cooper S. Feng GS. Broxmeyer HE. Abnormal chemokine-induced responses of immature and mature hematopoietic cells from motheaten mice implicates the protein tyrosine phosphatase SHP-1 in chemokine responses. J Exp Med. 1999;190:681–690. doi: 10.1084/jem.190.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broxmeyer HE. Youn BS. Kim CH. Hangoc G. Cooper S. Mantel C. Chemokine regulation of hematopoiesis and the involvement of pertussis toxin-sensitive Gαi proteins. NY Acad Sci. 2001;938:117–128. doi: 10.1111/j.1749-6632.2001.tb03580.x. [DOI] [PubMed] [Google Scholar]
- 21.Broxmeyer HE. Cooper S. Lu L. Miller ME. Langefeld CD. Ralph P. Enhanced stimulation of human bone marrow macrophage colony formation in vitro by recombinant human macrophage colony stimulatng factor in agarose medium at low oxygen tension. Blood. 1990;76:323–329. [PubMed] [Google Scholar]
- 22.Kovalev GI. Franklin DS. Coffield VM. Xiong Y. Su L. An important role of CDK inhibitor p18(INK4c) in modulating antigen receptor-mediated T cell proliferation. J Immunol. 2001;167:3285–3292. doi: 10.4049/jimmunol.167.6.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin DS. Xiong Y. Induction of p18INK4c and its predominant association with CDK4 and CDK6 during myogenic differentiation. Mol Biol Cell. 1996;7:1587–1599. doi: 10.1091/mbc.7.10.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damo LA. Snyder PW. Franklin DS. Tumorigenesis in p27/p53- and p18/p53-double null mice: functional collaboration between the pRb and p53 pathways. Mol Carcinog. 2005;42:109–120. doi: 10.1002/mc.20068. [DOI] [PubMed] [Google Scholar]
- 25.Dib A. Peterson TR. Raducha-Grace L. Zingone A. Zhan F. Hanamura I. Barlogie B. Shaughnessy J., Jr. Kuehl WM. Paradoxical expression of INK4c in proliferative multiple myeloma tumors: bi-allelic deletion vs increased expression. Cell Div. 2006;1:23. doi: 10.1186/1747-1028-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi PP. Kulkarni MV. Yu BK. Smith KR. Norton DL. van VW. Hoppener JW. Franklin DS. Simultaneous downregulation of CDK inhibitors p18(Ink4c) and p27(Kip1) is required for MEN2A-RET-mediated mitogenesis. Oncogene. 2007;26:554–570. doi: 10.1038/sj.onc.1209811. [DOI] [PubMed] [Google Scholar]
- 27.van VW. van Gasteren CJ. Acton DS. Franklin DS. Berger R. Lips CJ. Hoppener JW. Synergistic effect of oncogenic RET and loss of p18 on medullary thyroid carcinoma development. Cancer Res. 2008;68:1329–1337. doi: 10.1158/0008-5472.CAN-07-5754. [DOI] [PubMed] [Google Scholar]
- 28.Kool J. Uren AG. Martins CP. Sie D. de RJ. Turner G. van UM. Matentzoglu K. Lagcher W, et al. Insertional mutagenesis in mice deficient for p15Ink4b, p16Ink4a, p21Cip1, and p27Kip1 reveals cancer gene interactions and correlations with tumor phenotypes. Cancer Res. 2010;70:520–531. doi: 10.1158/0008-5472.CAN-09-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkataramani RN. MacLachlan TK. Chai X. El-Deiry WS. Marmorstein R. Structure-based design of p18INK4c proteins with increased thermodynamic stability and cell cycle inhibitory activity. J Biol Chem. 2002;277:48827–48833. doi: 10.1074/jbc.M208061200. [DOI] [PubMed] [Google Scholar]