Abstract
Flavobacterium columnare is a bacterial pathogen causing high mortality rates for many freshwater fish species. Fish vaccination with a safe and effective vaccine is a potential approach for prevention and control of fish disease. Here, in order to produce bacterial ghost vaccine, a specific Flavobacterium lysis plasmid pBV-E-cat was constructed by cloning PhiX174 lysis gene E and the cat gene with the promoter of F. columnare into the prokaryotic expression vector pBV220. The plasmid was successfully electroporated into the strain F. columnare G4cpN22 after curing of its endogenous plasmid. F. columnare G4cpN22 ghosts (FCGs) were generated for the first time by gene E-mediated lysis, and the vaccine potential of FCG was investigated in grass carp (Ctenopharyngodon idellus) by intraperitoneal route. Fish immunized with FCG showed significantly higher serum agglutination titers and bactericidal activity than fish immunized with FKC or PBS. Most importantly, after challenge with the parent strain G4, the relative percent survival (RPS) of fish in FCG group (70.9%) was significantly higher than FKC group (41.9%). These results showed that FCG could confer immune protection against F. columnare infection. As a nonliving whole cell envelope preparation, FCG may provide an ideal alternative to pathogen-based vaccines against columnaris in aquaculture.
1. Introduction
Flavobacterium columnare, a Gram-negative-gliding bacterium, is the causative agent of columnaris disease, one of the most important bacterial diseases of freshwater fish species [1]. This bacterium is ubiquitous in aquatic environments, affecting wild and cultured fish as well as ornamental fish in aquaria [2]. The onset of columnaris disease is characterized by external infections in the fish body surface, gills, or fins. The disease often ends in death, leading to large economic losses in the fish farming industry.
In recent years, control of fish diseases using chemotherapy results in emergence of antibiotic-resistant microbes and negative impacts on environment and human beings [3–6]. Therefore, vaccination has become an increasingly important prevention strategy against infectious agents in farmed fishes [7]. Several attempts have been made to induce protection against columnaris disease with formalin- or heat-inactivated preparations of F. columnare, yet no protection or only partial protection was achieved following immersion or injection immunization [8–10]. Currently, a modified live columnaris vaccine has been developed and commercialized in the United States [11], and it is efficacious for prevention of columnaris disease in channel catfish and largemouth bass fry [12, 13]. However, live vaccines may bear the danger of reversion.
Bacterial ghosts have been given increasing attention as a promising new approach in nonliving vaccine technology. The potential usefulness of the technology has been reported in a wide variety of mammalian pathogenic Gram-negative bacteria [14–16] and in a fish pathogenic bacterium, Edwardsiella tarda [17, 18]. Generally, Bacterial ghosts are produced by the controlled expression of bacteriophage PhiX174 lysis gene E, and the E protein leads to the formation of small transmembrane pores through which cytoplasmic contents are expelled [14–19]. The resultant bacterial ghosts retain the functional and antigenic determinants of the envelope with their living counterparts; therefore they possess good immunogenicity and bioadhesive properties and are able to induce effective immunoprotection [20, 21].
In this study, F. columnare ghosts have been generated for the first time by gene E-mediated lysis, and immune protection against infection of F. columnare was detected in Grass carp (Ctenopharyngodon idellus) by intraperitoneal immunization with the ghosts.
2. Materials and Methods
2.1. Bacterial Strains, Culture Conditions, and Plasmids
The highly virulent pathogenic strain Flavobacterium columnare G4, isolated from a severe natural outbreak of gill rot disease in cultured cyprinid fishes, was kindly provided by Institute of Hydrobiology, Chinese Academy of Sciences. The strain was identified by biochemical diagnostic methods [22] and species-specific PCR [23]. It is resistant to ampicillin and tobramycin but highly sensitive to chloramphenicol. Shieh broth [24] was used as the basic culture medium for F. columnare G4 which was grown at 26°C and supplemented with tobramycin (Sigma, USA) at a concentration of 1 μg/mL when needed. E. coli DH5α kept in this laboratory was cultured in Luria-Bertani medium. Incubation temperatures for repression and expression of the lysis gene in transformants were 26°C and 42°C, respectively.
The plasmid pBV220 was a kind gift from Professor Hui Wang of Academy of Military Medical Sciences, Beijing, China. It is a prokaryotic nonfusion expression vector with an ampicillin resistance gene and a λpL/pR-cI857 temperature-sensitive system that can express target gene inserted in multiple cloning sites (MCS) at 42°C. The plasmid pLysS with a chloramphenicol resistant gene (cat gene) was extracted from the strain E. coli BL21 (DE3)-pLysS (TransGen Biotech, Beijing, China) using the GeneJET Plasmid Maxiprep Kit (Fermentas, Canada). Bacteriophage PhiX174 RF1 DNA was purchased from Fermentas.
2.2. Construction of Gene E-Mediated Lysis Vector with Cat Gene
The lysis gene E was amplified by PCR from PhiX174 RF1 DNA with oligonucleotide primers E-F (5′-ATCAGAATTCATGGTACGCTGGACTTTGTG-3′) and E-R (5′-GCCTGCTGCAGTACATCACTCCTTC-3′) containing the EcoRI and PstI restriction enzyme sites (underlined), respectively. The amplifications were performed for 1 cycle of 5 min at 94°C, 28 cycles of 45 s at 94°C, 45 s at 56°C, 1 min at 72°C, with a final extension step at 72°C for 10 min. PCR products were visualized on 1% trisacetate agarose gels stained with ethidium bromide, purified with GeneJET Gel Extraction Kit (Fermentas), cloned into pMD-18T vector (TaKaRa, Dalian, China) via T/A cloning, and designated as pT-E. The gene E was excised from the pT-E plasmid by EcoRI and PstI (Fermentas) digestion and then inserted into the plasmid pBV220 which was predigested with the same enzymes. The resulting plasmid was designated as pBV-E.
The cat gene was amplified from the plasmid pLysS with primers Cat-F (5′-GCCGATATCATGGAGAAAAAAATC-3′, EcoRV site underlined) and Cat-R (5′- TTATCATTACGCCCCGCCCTGCCA-3′) in a standard PCR protocol. The cat gene (675 bps) was purified and cloned into pMD-18T via T/A cloning, as pT-cat. A regulating sequence including promoter was amplified from the genomic DNA of F. columnare G4 with the Pfu DNA polymerase (Fermentas) which produces blunt ended PCR products and primers Catpro-F (5′-GATAGAATAGAAAAAAGAAAATGTA-3′) and Catpro-R (5′-GGCGATTTGCCTTTTTTATAAAAT-3′). The primers were designed according to the upstream sequence of acetyl-coenzyme, a synthetase gene of F. columnare in Genbank (AY387595.2). The DNA fragment including promoter (167 bps) was subcloned into pT-cat which had been digested with EcoRV, as pT-pro-cat. The sequence including the promoter and cat gene was amplified from pT-pro-cat with the Pfu DNA polymerase and the primers Catpro-F and Cat-R and ligated into pBV-E which was predigested with ScaI. The resulting plasmid was designated as pBV-E-cat (Figure 1).
Figure 1.
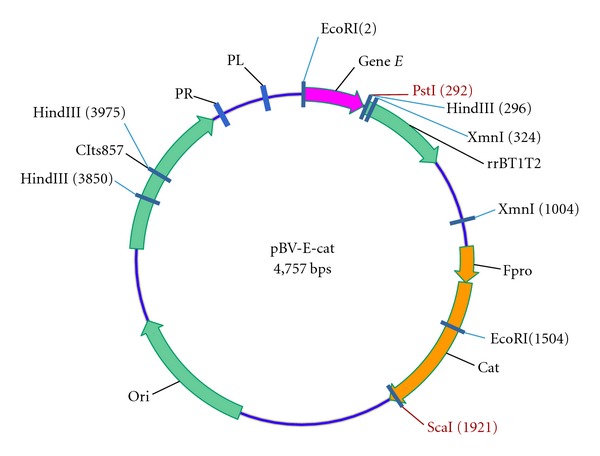
Physical map of the lysis plasmid pBV E cat. Gene E, under transcriptional control of the temperature-sensitive repressor; rrBT1T2: ribosome rrnB gene providing translation stop signal terminator sequence; pro: the regulating sequence including promoter; cat: chloramphenicol acetyl transferase gene; ori: replication origin; cIts857: restraining gene of lambda bacteriophage adapted to heat induced expression.
2.3. Curing of an Endogenous Plasmid from F. columnare G4
F. columnare G4 was cured of its endogenous plasmid by use of the intercalating dye acridine orange (AO, Sigma, USA). Basically, the filter-sterilized AO was added into the logarithmically growing culture (Tobramycin 1 μg/mL, OD600 = 0.4) of F. columnare G4 at concentrations ranging from 25 μg/mL to 450 μg/mL in 18 groups. The culture was incubated at the optimum growth temperature 26°C for 26 h. Then the culture containing the highest concentration of AO which still allows growth was used as a source of inoculum for plating cells on Shieh agar plate at 26°C until colony growth. Individual colonies were detected by plasmid purification and identified by primer-specific PCR with primers Fcp510-F (5′-GTGACGGTGACGATAAGT-3′), Fcp510-R (5′-CCTTCTTGCTGGTTCTGT-3′), Fcp1152-F (5′-GGTTCGGCGTTCTATGG-3′), and Fcp1152-R (5′-GGTGGTGAGTCGTTATACAA-3′) according to the sequence of the plasmid (Another study processing). After large-scale screening, 28 strains which have stably lost the endogenous plasmid were obtained and designated as F. columnare G4cpN1-N28.
2.4. Electroporation of F. columnare G4cpN1-N28 and Generation of F. columnare G4cpN22 Ghosts
The above strains were inoculated into 100 mL Shieh broth and grown at 26°C to an OD600 of 0.3 with agitation of 150 rpm. The cells were washed in ice-cold sucrose electroporation buffer (137 mM sucrose, 1 mM Hepes, 10% (v/v) glycerol, pH 8.0) and then suspended in ice-cold sucrose buffer at 1/100 of the original volume. The lysis plasmid pBV-E-cat DNA (3-4 μg) was mixed with 100 μL of the cell suspension, transferred to a precooling 1 mm cuvette and electroporated using a Gene Pulser (Bio-Rad, CA, USA) and Pulse controller (1.5 kV, 25 μF, 200 Ω) producing a time constant of ±4.0 ms. Immediately after electroporation, 800 μL of Shieh medium was added to cells and incubated for 1 h at 26°C. After incubation, cells were plated onto Shieh agar plates containing 10 μg/mL chloramphenicol and grown at 26°C for 24–36 h.
F. columnare G4cpN22 was the only one strain which had the ability to accept and maintain the pBV-E-cat. When the cultures reached an OD600 of 0.3 at 26°C, the expression of the gene E was induced by a temperature upshift to 42°C immediately. The number of cells was determined using a 6 × 6 drop plate method [25] before induction, with the exception that Shieh agar plates were used and incubated at 26°C for 36 h. At different time points after induction, an optical density was measured until no further decrease was detected, and viable cell counts were determined by plating serial dilutions on Shieh agar plates. At the end of the lysis process, 100 μL of cultures was inoculated on Shieh agar plate in order to examine whether or not there were any surviving cells. Efficiency of ghost induction was expressed by lysis rate which was calculated using the following formula: lysis rate = (1 − CFU of lysis completed/CFU before induction) × 100%. Finally, the harvested F. columnare G4cpN22 ghosts (FCGs) were washed twice with ice-cold phosphate-buffered saline (PBS, pH 7.3), lyophilized, and stored at 4°C until further use. The lyophilized FCG preparations were reconstituted with PBS to a bacterial concentration of 1 × 108 cells/mL prior to immunization.
2.5. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
The FCGs for SEM (S-500, Hitachi, Japan) were fixed with 2.5% glutaraldehyde in 0.01 M PBS (pH 7.0) at 4°C for 2 h. Cells were rinsed 3 times with the same buffer and postfixed in 1% osmium tetroxide for 1.5 h at 4°C. Subsequently, the cells were dehydrated with a graded series of ethyl alcohol and isoamyl acetate. Following final dehydration, cells were dried with liquid carbon dioxide and sputtered with platinum tetroxide using a pure platinum coater before scanning.
Ghost preparations for TEM (H-800, Hitachi) were obtained the same way, except that fixation was done for 3 h. Then the samples were washed as above and fixed with 2% osmic acid for 2 h, followed by washing three times with 0.1 M PBS before microscopy.
2.6. Experimental Fish Immunization and Challenge Test
2.6.1. Fish and Rearing Conditions
Healthy grass carps weighing 45 ± 5 g were obtained from Freshwater Fishery Research Institute of Shandong Province, China. Prior to vaccination, fish were acclimated for at least two weeks in 400 L aquarium supplied with specific pathogen-free aerated fresh water treated with ultraviolet light. Light cycle was held constant at 12 h light/12 h dark per day and water temperature was maintained at 24 ± 2°C. Fish were fed with a commercial pelleted diet at 2% body weight per day throughout the study. Fish were anaesthetized by immersion into 90 mg/L tricaine methanesulfonate (MS-222, Sigma, USA) prior to experiments involving injection, blood collection, or sacrifice. Before each of the infection and vaccination experiments, fish were randomly sampled for the examination of bacterial recovery from blood, liver, and spleen, and no bacteria could be detected in all the fish examined.
2.6.2. Generation of Formalin-Killed F. columnare
F. columnare G4 was inoculated into Shieh broth and incubated at 26°C for 24 h. For formalin-killed F. columnare (FKC) preparation, formalin was added to the culture at a final concentration of 0.3%. After a 24 h incubation at 26°C, bacteria were washed three times with PBS and resuspended in PBS to a bacterial concentration of 1 × 108 cells/mL. A viability test of 1% of the resulting volume was conducted on Shieh solid medium and determined to be culture negative. FKC preparation was frozen at −20°C until use.
2.6.3. Injection Immunization
Grass carp were divided randomly into three treatment groups, each group with one hundred and sixty fish. Fish in two vaccinated groups were injected intraperitoneally (ip) with 100 μL of FCG or FKC at 1 × 107 cells/fish using 27-gauge needles. Fish in the control group were injected ip with 100 μL of PBS as a mock immunization. At 4 weeks postprimary immunization, identical booster immunizations were administered in the vaccinated groups and fish in the control group were injected ip with 100 μL of PBS. Before vaccination, blood was collected by caudal venipuncture and pooled from a random sample of ten fish per group to confirm that the fish were free from F. columnare antibody.
2.6.4. Serum Agglutination Reaction and Bactericidal Activity Assay
At each time point of postimmunization, serum was collected from 30 randomly selected fish (ten pools of 3 fish) in each group. Serum agglutination reaction was applied to reflect the titres of anti-F. columnare antibody produced by grass carp [26]. F. columnare G4 was incubated in Shieh broth to midlogarithmic phase and resuspended in PBS. Serial twofold dilution of test serum in PBS and equal volume of F. columnare G4 suspension was added to each well of 96-well microtitre plates. Doubling dilutions of positive and negative sera were included on every plate as controls. The plates were covered and incubated in humidified air at 25°C for 18 h. The highest serum dilution that showed a circular diffuse button with fuzzy edges at the bottom of the well was considered a positive reaction, and a circular compact cell button was considered to be a negative reaction. Serum endpoint titre was defined as the reciprocal of the highest dilution.
Serum bactericidal activity analysis was performed as described [27]. Briefly, Two microliters of F. columnare G4 suspension were mixed with 20 μL of serum, and the mixture was incubated at 26°C for 1 h, followed by plating on Shieh agar plates supplemented with tobramycin and incubating at 26°C for 48 h. In the bacterial control group, the serum was replaced by PBS. The number of colonies on the plates was counted and verified to be F. columnare by specific PCR [23]. Bactericidal rate was calculated as follows: (1 − the number of viable bacteria after serum treatment/the number of viable bacteria after PBS treatment) × 100%.
2.6.5. Challenge Infection and Immune Protection
Before challenge, all fish were clinically healthy, and there was no evidence of F. columnare infection. A random sample of 120 fish was selected from each group (control group and both vaccinated groups) and divided into three replicate of 40 fish at time of challenge. The fish were challenged at day 50 postprimary immunization, via ip injection with F. columnare G4 at 2 × 106 cells/fish (5 × LD50 based on preliminary work). Mortality was monitored twice daily for 14 days following challenge, and the cumulative percent mortality (CPM) was calculated for each treatment group. Relative percent survival (RPS) was determined as previously described [28], RPS = (1 − immunized group mortality/control group mortality) × 100. Dead or moribund fish exhibited gross pathological changes, and routine bacteriological examination was performed on freshly dead fish.
2.7. Statistical Analysis
All data were presented as mean ± SD. Statistical analyses were performed using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). Serum agglutination titres and bactericidal activities were analyzed by two-way ANOVA followed by pairwise comparisons (Tukey's test). Challenge test data were analyzed by Chi-square test. Differences were considered significant at P < 0.05.
3. Results
3.1. Generation and Characterization of F. columnare G4cpN22 Ghost
Since F. columnare G4cpN22 was high sensitive to chloramphenicol, but not to ampicillin, a specific lysis vector with the cat gene had to be constructed (Figure 1). The lysis plasmid pBV E cat was successfully electroporated into F. columnare G4cpN22 which growth characteristics were similar to F. columnare G4. Generation of F. columnare ghosts (FCGs) was performed by shifting the incubation temperature to 42°C to inactivate the repressor protein and activate lysis gene E. Onset of the lysis occurred 1 h after temperature elevation because the numbers of viable cells began to decrease at 1 h after induction, and the lysis process was completed 10 h after induction (Figure 2). The results of three replicate experiments showed the efficiency of FCGs induction was 99.99% at the end of the lysis process. The lyophilized FCGs were analyzed for survivors by inoculating 100 mL of growth media with a tenfold immunization dose at 26°C, but no bacterial growth was detected. The lyophilized FCG preparation was used for vaccination studies in fish.
Figure 2.
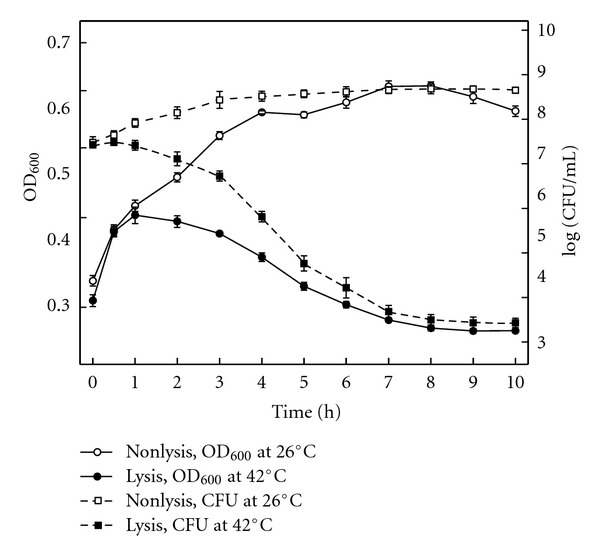
Growth and lysis kinetics of F. columnare G4cpN22 harboring plasmid pBV E cat by temperature induction of gene E expression. At time zero, the growth temperature of three bottles of the cultures was shifted from 26°C to 42°C (filled symbols), whereas the others served as noninduced control at 26°C (open symbols). Growth and lysis were monitored by the measurement of the OD600 (open circles and filled circles) and the determination of the number of CFU (open squares and filled squares and discontinuous lines).
Electron microscopic analysis of FCG revealed no gross alterations in cellular morphology compared to unlysed cells (Figures 3(a) and 3(b)) except for the lysis pore (arrow, Figure 3(a)). Pores ranging from 100 to 300 nm in diameter were observed in FCG by scanning electron microscopy. The structural integrity and the loss of cytoplasmic materials were observed in FCG by transmission electron microscopy (Figure 3(b)).
Figure 3.
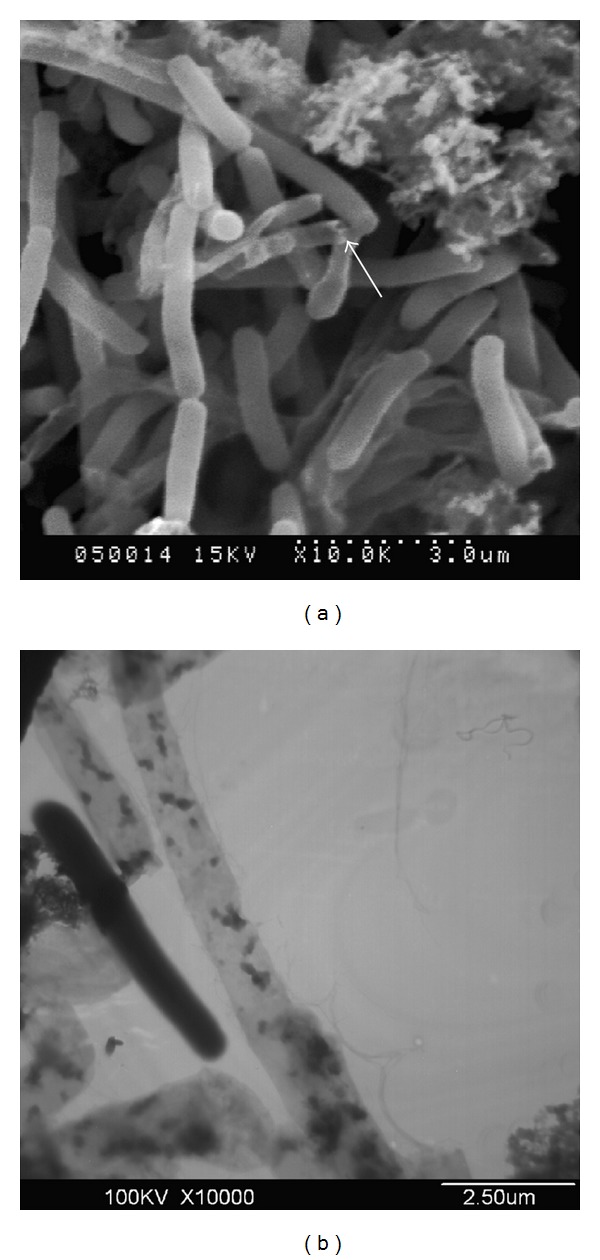
Electron microscopy analyses of F. columnare G4cpN22 ghosts. (a) Arrow showed the transmembrane lysis tunnel located mainly at the cell poles via SEM. (b) Loss of cytoplasmic materials of F. columnare G4cpN22 ghosts was shown by TEM. The lysed cells showed uneven and low electron density and retained the basic cell morphology of the bacterial cells, while the unlysed cells showed even and high electron density in an integral cell structure.
3.2. Serum Agglutination Reaction and Bactericidal Activity Assay
The agglutination reaction against F. columnare G4 was detected in grass carp immunized ip with bacterial ghosts, formalin-killed cells, or PBS. Fish immunized with FCG or FKC showed significantly higher agglutination titer than control fish in which no agglutination reaction was detected, while fish immunized with FCG showed significantly higher titers than fish immunized with FKC at all the examined time points (P < 0.01) (Figure 4). The agglutination reaction in FCG group initially peaked at 2 weeks postprimary immunization, subsided by week 4 and then more than doubled (mean geometric titre 478) 2 weeks after booster immunization (6 weeks). The high titer of FCG group was 3.5-fold higher than FKC group and persisted longer than FKC group.
Figure 4.
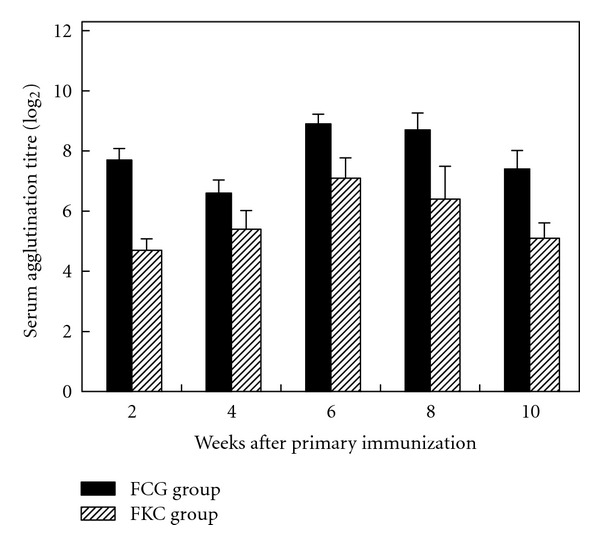
Serum agglutination titres of fish vaccinated with FCG and FKC at different time points postprimary immunization. Grass carp were vaccinated with FCG or FKC via intraperitoneal injection with a boost at 4 weeks after primary immunization. Serum agglutination titres represent the highest dilutions that gave rise to positive reaction. The titres of FCG group were significantly higher than FKC group at all the examined time points (P < 0.01).
Serum bactericidal activity was the lowest in PBS group and the highest in FCG group. Serum bactericidal activity of FCG group was elevated significantly above FKC group and PBS control group at all times postimmunization (P < 0.01) (Figure 5). The viable bacterial counts (41%) were closely 2-fold lower in FCG group compared with FKC group (80%) at 2 weeks after booster immunization (6 weeks).
Figure 5.
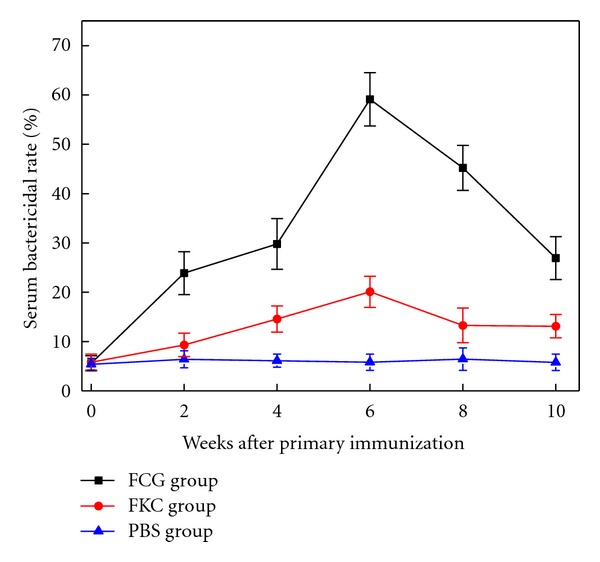
Serum bactericidal activities of grass carp vaccinated with FCG or FKC. The grass carp were vaccinated with FCG or FKC via intraperitoneal injection with a boost at 4 weeks after primary immunization. Serum bactericidal activities of FCG group were significantly higher than FKC group at the examined time points of postimmunization (P < 0.01).
3.3. Induction of Protective Immunity following Immunization with FCG Vaccine
A protective immune response against virulent F. columnare G4 was conferred to grass carp following immunization by ip injection with FCG preparation (Table 1). Fish given mock immunizations with PBS sustained high CPM (nearly 100%) upon challenge with the F. columnare G4 strain, while fish immunized with FCG or FKC exhibited a significantly decreased CPM at 7 weeks postprimary immunization (P < 0.05). The RPS of FCG group was 70.9%, significantly higher than the RPS of FKC group (41.9%). Challenge mortalities exhibited typical signs of F. columnare infection. Bacteria with phenotypic characteristics of F. columnare G4 were recovered from all dead fish.
Table 1.
Cumulative percent mortality (CPM) and relative percent survival (RPS) of grass carp following F. columnare G4 strain challenge at 50 days after primary immunization.
| group | Number of fish challenged | Mean CPM ± SD (%) | RPS (%) |
|---|---|---|---|
| FCG | 120 | 28.3 ± 5.0a | 70.9 |
| FKC | 120 | 56.7 ± 8.0b | 41.9 |
| PBS | 120 | 97.5 ± 2.5c |
FCG, F. columnare G4cpN22 ghosts; FKC, formalin-killed cells. Values with different superscripts indicate a significant difference at P < 0.05.
4. Discussion
Bacterial ghosts represent a potential new concept in genetically inactivated vaccines. In the present study, F. columnare ghosts were generated for the first time by the controlled expression of the lysis gene E. Gene E-mediated lysis of bacteria resulting in empty cell envelopes is suggested as an alternative approach for the inactivation of bacteria without chemical or physical stress, which have frequently caused the reduction of antigenicity [8–10]. Since the outer membrane proteins (OMPs) of pathogenic bacteria play important roles in protective antigenicity [29–31], the minimized conformational changes of OMPs in bacterial ghosts would provide a promising avenue for enhancing vaccine efficacy.
However, the lysis process of F. columnare G4cpN22 harboring plasmid pBV-E-cat was slower than many other Gram-negative bacteria such as E. coli, Salmonella enteritidis, and Vibrio cholerae, in which lysis was completed 2–4 h after lysis induction [32, 33]. Previous studies proved that the promoters which drive gene expression in other gram-negative bacteria generally do not function well in the Bacteroidetes including Flavobacterium species [34, 35]. It was speculated that the slower lysis behavior observed in F. columnare might result from reduced power of the lambda PR/PL promoter in F. columnare, reduced susceptibility of the F. columnare cell wall to the E protein, or lower copy of plasmid pBV-E-cat in F. columnare. Further study to quantify the gene E expression during lysis induction would be valuable to find the factor responsible for the weakened lysis kinetics.
The immune response and protection induced after immunization of grass carp with FCG vaccine were first tested. It was found that fish immunized with FCG through ip injection showed significantly higher serum agglutination titers and serum bactericidal activity than fish immunized with FKC, which indicated that FCG was more favorable to induce specific humoral immune responses. An elevated level of protection in FCG vaccinated fish to the ip injection challenge was also observed, which is consistent with previous findings [18, 36]. The low level of response to FKC preparations could be related to the inactivation of major immunogenic proteins. Bader et al. [9] reported that formalin inactivation resulted in masking of at least one potentially important F. columnare antigen in channel catfish. The effect of formalin on the antigenic properties of F. columnare was partially addressed here by killing the bacteria with a low formalin concentration (0.3%) and washing three times in PBS. Nevertheless, the protein cross-linking properties of formalin may have resulted in reduced antigenicity. Compared to formalin-killed bacterial vaccines, the adjuvant effect of ghost bacterial vaccines has been reported in many pathogenic bacteria [20]. Bacterial ghosts carry immunostimulatory compounds that have adjuvant properties, such as lipopolysaccharides (LPS), lipids, peptidoglycan, or flagella. These intrinsic adjuvant properties of bacterial ghosts were shown to activate the innate immune system as well as the acquired immune response [37, 38]. Moreover, some reports showed effective induction of cell-mediated immunity, which would play a key role in protection against intracellular pathogens, by bacterial ghost vaccines [39–41].
In conclusion, FCGs have been shown to induce stronger protective immunity than FKC in grass carp. Given its safety and high level of immunoprotective efficacy, FCG may provide an ideal alternative to pathogen-based vaccines against columnaris in aquaculture.
Authors' Contribution
W. Zhu and G. Yang contributed equally to this work.
Acknowledgment
This work was supported by a fund of New Fishery Genetic Engineering Vaccine from the University Innovation Plan of Jinan City (no. 200906020).
References
- 1.Durborow RM, Thune RL, Hawke JP, Camus AC. Publication. 479. Stoneville, Mich, USA: Aquaculture Center; 1998. Columnaris disease: a bacterial infection caused by Flavobacterium columnare. [Google Scholar]
- 2.Austin B, Austin DA. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish. Edinburgh, UK: Heriot-Watt University; 1999. [Google Scholar]
- 3.Gaikowski MP, Larson WJ, Gingerich WH. Survival of cool and warm freshwater fish following chloramine-T exposure. Aquaculture. 2008;275(1–4):20–25. [Google Scholar]
- 4.Darwish AM, Mitchell AJ, Straus DL. Evaluation of potassium permanganate against an experimental subacute infection of Flavobacterium columnare in channel catfish, Ictalurus punctatus (Rafinesque) Journal of Fish Diseases. 2009;32(2):193–199. doi: 10.1111/j.1365-2761.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 5.Darwish AM, Mitchell AJ. Evaluation of diquat against an acute experimental infection of Flavobacterium columnare in channel catfish, Ictalurus punctatus (Rafinesque) Journal of Fish Diseases. 2009;32(5):401–408. doi: 10.1111/j.1365-2761.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- 6.Gaunt PS, Gao D, Sun F, Endris R. Efficacy of florfenicol for control of mortality caused by Flavobacterium columnare infection in channel catfish. Journal of Aquatic Animal Health. 2010;22(2):115–122. doi: 10.1577/H09-057.1. [DOI] [PubMed] [Google Scholar]
- 7.Gudding R, Lillehaug A, Evensen O. Recent developments in fish vaccinology. Veterinary Immunology and Immunopathology. 1999;72(1-2):203–212. doi: 10.1016/s0165-2427(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 8.Moore AA, Eimers ME, Cardella NA. Attempts to control Flexibacter columnaris epizootics in pond-reared channel catfish by vaccination. Journal of Aquatic Animal Health. 1990;2(2):109–111. [Google Scholar]
- 9.Bader JA, Klesius PH, Vinitnantharat S. Comparison of whole-cell antigens of pressure- and formalin-killed Flexibacter columnaris from channel catfish (Ictalurus punctatus) American Journal of Veterinary Research. 1997;58(9):985–988. [PubMed] [Google Scholar]
- 10.Grabowski LD, LaPatra SE, Cain KD. Systemic and mucosal antibody response in tilapia, Oreochromis niloticus (L.), following immunization with Flavobacterium columnare. Journal of Fish Diseases. 2004;27(10):573–581. doi: 10.1111/j.1365-2761.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 11.Shoemaker CA, Klesius PH, Evans JJ. Modified live Flavobacterium columnare against columnaris disease in fish. United States Patent. 2005;(6,881,412 B1)
- 12.Shoemaker CA, Klesius PH, Evans JJ. Immunization of eyed channel catfish, Ictalurus punctatus, eggs with monovalent Flavobacterium columnare vaccine and bivalent F. columnare and Edwardsiella ictaluri vaccine. Vaccine. 2007;25(6):1126–1131. doi: 10.1016/j.vaccine.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 13.Shoemaker CA, Klesius PH, Drennan JD, Evans JJ. Efficacy of a modified live Flavobacterium columnare vaccine in fish. Fish and Shellfish Immunology. 2011;30(1):304–308. doi: 10.1016/j.fsi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Eko FO, Szostak MP, Wanner G, Lubitz W. Production of Vibrio cholerae ghosts (VCG) by expression of a cloned phage lysis gene: potential for vaccine development. Vaccine. 1994;12(13):1231–1237. doi: 10.1016/0264-410x(94)90249-6. [DOI] [PubMed] [Google Scholar]
- 15.Marchart J, Dropmann G, Lechleitner S, et al. Pasteurella multocida- and Pasteurella haemolytica-ghosts: new vaccine candidates. Vaccine. 2003;21(25-26):3988–3997. doi: 10.1016/s0264-410x(03)00383-9. [DOI] [PubMed] [Google Scholar]
- 16.Panthel K, Jechlinger W, Matis A, et al. Generation of Helicobacter pylori ghosts by PhiX protein E-mediated inactivation and their evaluation as vaccine candidates. Infection and Immunity. 2003;71(1):109–116. doi: 10.1128/IAI.71.1.109-116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon SR, Yoon KN, Sung KK, Dong SK, Kim KH. Generation of Edwardsiella tarda ghosts by bacteriophage PhiX174 lysis gene E. Aquaculture. 2005;250(1-2):16–21. [Google Scholar]
- 18.Kwon SR, Lee EH, Nam YK, Kim SK, Kim KH. Efficacy of oral immunization with Edwardsiella tarda ghosts against edwardsiellosis in olive flounder (Paralichthys olivaceus) Aquaculture. 2007;269(1–4):84–88. [Google Scholar]
- 19.Witte A, Wanner G, Blasi U, Halfmann G, Szostak M, Lubitz W. Endogenous transmembrane tunnel formation mediated by ΦX174 lysis protein E. Journal of Bacteriology. 1990;172(7):4109–4114. doi: 10.1128/jb.172.7.4109-4114.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. The bacterial ghost platform system: production and applications. Bioengineered Bugs. 2010;1(5):326–336. doi: 10.4161/bbug.1.5.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tu FP, Chu WH, Zhuang XY, Lu CP. Effect of oral immunization with Aeromonas hydrophila ghosts on protection against experimental fish infection. Letters in Applied Microbiology. 2010;50(1):13–17. doi: 10.1111/j.1472-765X.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- 22.Griffin BR. A simple procedure for identification of Cytophaga columnaris. Journal of Aquatic Animal Health. 1992;4(1):63–66. [Google Scholar]
- 23.Darwish AM, Ismaiel AA, Newton JC, Tang J. Identification of Flavobacterium columnare by a species-specific polymerase chain reaction and renaming of ATCC43622 strain to Flavobacterium johnsoniae. Molecular and Cellular Probes. 2004;18(6):421–427. doi: 10.1016/j.mcp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Decostere A, Haesebrouck F, Devriese LA. Shieh medium supplemented with tobramycin for selective isolation of Flavobacterium columnare (Flexibacter columnaris) from diseased fish. Journal of Clinical Microbiology. 1997;35(1):322–324. doi: 10.1128/jcm.35.1.322-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CY, Nace GW, Irwin PL. A 6x6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. Journal of Microbiological Methods. 2003;55(2):475–479. doi: 10.1016/s0167-7012(03)00194-5. [DOI] [PubMed] [Google Scholar]
- 26.Klesius PH, Shoemaker CA, Evans JJ. Efficacy of single and combined Streptococcus iniae isolate vaccine administered by intraperitoneal and intramuscular routes in tilapia (Oreochromis niloticus) Aquaculture. 2000;188(3-4):237–246. [Google Scholar]
- 27.Rao YV, Das BK, Jyotyrmayee P, Chakrabarti R. Effect of Achyranthes aspera on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. Fish and Shellfish Immunology. 2006;20(3):263–273. doi: 10.1016/j.fsi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Amend DF. Potency testing of fish vaccines. Developments in Biological Standardization. 1981;49:447–454. [Google Scholar]
- 29.Sengupta DK, Sengupta TK, Ghose AC. Major outer membrane proteins of Vibrio cholerae and their role in induction of protective immunity through inhibition of intestinal colonization. Infection and Immunity. 1992;60(11):4848–4855. doi: 10.1128/iai.60.11.4848-4855.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loosmore SM, Yang YP, Coleman DC, Shortreed JM, England DM, Klein MH. Outer membrane protein D15 is conserved among Haemophilus influenzae species and may represent a universal protective antigen against invasive disease. Infection and Immunity. 1997;65(9):3701–3707. doi: 10.1128/iai.65.9.3701-3707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordwell SJ, Nouwens AS, Walsh BJ. Comparative proteomics of bacterial pathogens. Proteomics. 2001;1(4):461–472. doi: 10.1002/1615-9861(200104)1:4<461::AID-PROT461>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 32.Eko FO, Szostak MP, Wanner G, Lubitz W. Production of Vibrio cholerae ghosts (VCG) by expression of a cloned phage lysis gene: potential for vaccine development. Vaccine. 1994;12(13):1231–1237. doi: 10.1016/0264-410x(94)90249-6. [DOI] [PubMed] [Google Scholar]
- 33.Yu SY, Peng W, Si W, et al. Enhancement of bacteriolysis of Shuffled phage PhiX174 gene e. Virology Journal. 2011;8, article no. 206 doi: 10.1186/1743-422X-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith CJ, Rogers MB, McKee ML. Heterologous gene expression in Bacteroides fragilis. Plasmid. 1992;27(2):141–154. doi: 10.1016/0147-619x(92)90014-2. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Bagdasarian M, Kaufman MG, Walker ED. Characterization of strong promoters from an environmental Flavobactenum hibernum strain by using a green fluorescent protein-based reporter system. Applied and Environmental Microbiology. 2007;73(4):1089–1100. doi: 10.1128/AEM.01577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon SR, Yoon KN, Sung KK, Kim KH. Protection of tilapia (Oreochromis mosambicus) from edwardsiellosis by vaccination with Edwardsiella tarda ghosts. Fish and Shellfish Immunology. 2006;20(4):621–626. doi: 10.1016/j.fsi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Jalava K, Eko FO, Riedmann E, Lubitz W. Bacterial ghosts as carrier and targeting systems for mucosal antigen delivery. Expert Review of Vaccines. 2003;2(1):45–51. doi: 10.1586/14760584.2.1.45. [DOI] [PubMed] [Google Scholar]
- 38.Riedmann EM, Kyd JM, Cripps AW, Lubitz W. Bacterial ghosts as adjuvant particles. Expert Review of Vaccines. 2007;6(2):241–253. doi: 10.1586/14760584.6.2.241. [DOI] [PubMed] [Google Scholar]
- 39.Haslberger AG, Kohl G, Felnerova D, Mayr UB, Fürst-Ladani S, Lubitz W. Activation, stimulation and uptake of bacterial ghosts in antigen presenting cells. Journal of Biotechnology. 2000;83(1-2):57–66. doi: 10.1016/s0168-1656(00)00298-4. [DOI] [PubMed] [Google Scholar]
- 40.Mayr UB, Haller C, Haidinger W, et al. Bacterial ghosts as an oral vaccine: a single dose of Escherichia coli O157:H7 bacterial ghosts protects mice against lethal challenge. Infection and Immunity. 2005;73(8):4810–4817. doi: 10.1128/IAI.73.8.4810-4817.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Lu C. Mice orally vaccinated with Edwardsiella tarda ghosts are significantly protected against infection. Vaccine. 2009;27(10):1571–1578. doi: 10.1016/j.vaccine.2009.01.002. [DOI] [PubMed] [Google Scholar]


