Abstract
Objective
The bidirectional Glenn procedure (BDG) is a routine intermediary step in single-ventricle palliation. In this study, we examined risk factors for death or transplant and failure to reach Fontan completion after BDG in patients, who had previously undergone stage one palliation (S1P).
Methods
All patients at our institution, who underwent BDG following S1P between 2002 and 2009 (n = 194), were included in the analysis.
Results
Transplant-free survival through 18 months post BDG was 91%. Univariable competing risk analyses showed atrioventricular valve regurgitation (AVVR) >mild, age ≤3 months at BDG, ventricular dysfunction >mild, and prolonged hospital stay after S1P to be associated with increased risk of death or orthotopic heart transplant. Multivariable competing risk analysis through 5 years of follow-up showed >mild AVVR (hazard ratio (HR) 7.5, 95% confidence interval (CI) 3.0–18.8), prolonged hospitalization after S1P (HR 4.5, 95% CI 1.8–11.5), and age ≤3 months at BDG (HR 6.8, 95% CI 2.3–20.0) to be independent risk factors for death or transplant. Concomitantly, >mild AVVR and age ≤3 months were independently associated with an overall decreased rate of Fontan completion.
Conclusions
Pre-BDG AVVR, age ≤3 months at time of BDG, and prolonged hospitalization after S1P are independently associated with decreased successful progression of staged palliation in midterm follow-up after BDG.
Keywords: Congenital heart disease, CHD, Hypoplastic left heart syndrome, Outcomes, Pediatric
1. Introduction
The bidirectional Glenn procedure (BDG) is a standard intermediate step in a staged approach to palliation of hypoplastic left heart syndrome (HLHS) and other single-ventricle heart disease [1–3]. Most single-center series report survival rates over 90% after BDG [1–5]. Despite improved overall survival, there are subsets of patients, who may be at increased risk. Patients with unfavorable interstage hemodynamics, often manifested as multiple interstage re-admissions, prolonged hospitalization after stage one palliation (S1P), and/or poor interstage weight gain, are likely at high risk for poor outcome after BDG [6–8]. Currently, options for these patients are limited. To improve outcomes in high-risk patients, some authors have proposed repair of the tricuspid valve at BDG in those with significant tricuspid regurgitation [8,9]. Others have advocated performing BDG at younger ages to shorten the relatively high-risk interstage period, volume unload the single ventricle, and remove shunt-dependent circulation [10–12]. Still others argue for earlier transplant evaluation in this population [13].
In previous studies, atrioventricular valve regurgitation (AVVR) and ventricular dysfunction have frequently been identified as risk factors for poor outcome during staged palliation [14–17]. Bilateral superior vena cavae, heterotaxy syndrome, non-left ventricular morphology, S1P with a Blalock–Taussig shunt, and lower weight at BDG have also been associated with worse outcomes after BDG in some studies [18–21]. The goal of this study is to identify risk factors for death or orthotopic heart transplant (OHT) and failure to achieve Fontan completion after BDG in the current era.
2. Patients and methods
2.1. Patient population
The records of all patients under 9 months of age undergoing BDG at our institution between January 2002 and May 2009 were reviewed. Patients were excluded, if they did not undergo a comprehensive S1P (Norwood procedure) or were initially palliated at an outside institution. Data from 194 patients are included in the analysis. Patient demographic characteristics, cardiac morphology, preoperative variables, operative variables, and hospital course were reviewed for each patient.
Duration of hospital stay after S1P was assessed for all patients. Patients in the highest decile of length of stay and those who never left the hospital after S1P were classified as having a prolonged S1P hospital stay and were compared to the remainder of the cohort. Weight and weight z-score at BDG were assessed for all patients. Patients in the lowest decile in weight for age z-score were compared with the remainder of the cohort. Patients who underwent BDG at ≤3 months of age, which equated to the lowest decile for age at BDG, were compared with those who underwent BDG at >3 months of age. For the patients undergoing BDG at ≤3 months of age, we classified the primary indication for earlier BDG as cyanosis, unfavorable S1P hemodynamics, or unknown/elective, based on retrospective review of operative reports, preoperative catheterization reports, and clinic notes.
All patients were followed until death, transplant, Fontan completion, or loss to follow-up through 1 May 2010. The Children’s Hospital Boston Institutional Review Board approved the study protocol.
2.2. Study design
Complete two-dimensional, pulse-wave Doppler and color Doppler echocardiograms and complete hemodynamic catheterizations are routinely performed prior to BDG at our institution. All available echocardiographic and catheterization data were retrospectively reviewed. All 194 patients had preoperative echocardiograms included in the analysis. Catheterization data on 153 patients were included in the analysis. As only 50 patients in the cohort had magnetic resonance imaging (MRI), these data were excluded from the analyses.
2.3. Echocardiograms
The most recent complete echocardiogram prior to BDG for each patient was included in the analysis. All patients had grades for both AVVR and systemic semilunar valve regurgitation, reported on an ordinal scale from 0 (none) to 4 (severe) in half-unit increments. Ventricular function of the systemic ventricle was graded on an ordinal scale from 0 (normal function) to 4 (severe dysfunction) in half-unit increments.
2.4. Catheterizations
Hemodynamic data were reviewed retrospectively from reports produced at the time of the catheterization. Interventions performed at preoperative catheterization, including atrial septostomy and balloon dilation of aortic coarctation, were recorded. For cases where interventions were performed, post-intervention hemodynamic data were included in the analysis.
2.5. Statistical analysis
Preliminary analyses focused on transplant-free survival from BDG up to the period of typical Fontan completion (18 months post BDG). The rationale for limiting the primary analysis to 18 months post BDG was to limit the confounding impact of Fontan or biventricular repair. At 18 months post BDG, <10% of the cohort (n = 18) had undergone Fontan.
Median and range or interquartile range were used to express measures of central tendency and dispersion due to observed skewness in the distribution of descriptive and predictor variables. Associations between demographic or clinical risk factors and transplant-free survival at 18 months after BDG were assessed with the Mann–Whitney test and Fisher’s exact test for continuous and categorical variables, respectively. The Armitage test for trend was used to compare ordinal variables.
To examine risk factors, competing risks analyses were used with the competing events defined as death or OHT and Fontan. The median time of follow-up for these analyses was 3.8 years (interquartile range (IQR) 2.0–5.9 years). Although death and transplantation are themselves competing outcomes, they were modeled together, as the number of each of these events was small. Patients with biventricular repair (n = 7) were reclassified into one of the following three outcomes: Fontan, if the patient had a Fontan before biventricular repair (n = 1); death or OHT, if the patient experienced death or OHT after biventricular repair (n = 2); and censored at time of biventricular repair, if the patients did not experience either of the two competing events (n = 4). Using this approach, the cumulative incidence of each event (death or OHT and Fontan) was compared over time. The cumulative incidence of an event at time t is the proportion of patients experiencing the event by time t. Competing risk models were used to perform univariable and multivariable analyses of preoperative risk factors on the cumulative incidence of death or OHT and Fontan. The number of hazard regression model covariates was limited by the number of deaths and OHTs through 5 years of follow-up after BDG (n = 23). Only preoperative variables that were statistically significant in univariable analysis or characterized as primary covariates of interest were considered for multivariable modeling. All statistical analyses were two-sided and type I error was controlled at a level of 0.05. Analyses were performed with Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc, Chicago, IL, USA) and R version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Patient outcomes
Of the 194 patients in our cohort, 23 patients died or underwent transplantation through 5 years after BDG (Fig. 1). Nineteen of these 23 patients died or underwent OHT prior to Fontan. One patient died at the time of BDG. Twelve patients died between BDG discharge and Fontan, while six patients underwent OHT during the same period. A total of 139 patients have completed Fontan, while 22 are currently awaiting the procedure. Median age at Fontan (n = 139) in this cohort was 30.1 months (range 17.1–63.7). Median duration between BDG and Fontan was 25.4 months (range 12.9–57.3). Seven patients had biventricular repair after BDG: one patient has subsequently died, while one other patient has undergone OHT. In those who have undergone Fontan, there have been two deaths, while one patient has undergone biventricular repair.
Fig. 1.
Outcome of 194 patients after BDG following a comprehensive S1P from 2002 through 2009. BDG, bidirectional Glenn procedure; OHT, orthotopic heart transplant.
3.2. Demographic, morphologic, and preoperative variables
Demographic, anatomic, and hemodynamic variables were compared between patients who died or underwent OHT and those in the transplant-free survival group through 18 months after BDG (Table 1). Patients who experienced death or transplant were younger and had lower weight and weight z-score than those in the transplant-free survival group. Bilateral superior vena cavae, HLHS morphology, and prolonged S1P hospitalization were all associated with death or transplant in univariable analyses.
Table 1.
Characteristics of patients prior to BDG: Alive versus dead or transplanted at 18 months after BDG.
| Alive (n = 177) | Dead or OHT (n = 17) | p value | |
|---|---|---|---|
| Demographic variables | |||
| Age (days), median (range) | 157 (61–267) | 109 (65–254) | 0.008 |
| Weight (kg) | 5.6 (3.0–9.5) | 4.3 (2.4–7.0) | <0.001 |
| Weight z-score | −2.3 (35.8 to 2.2) | −3.4 (36.9 to 1.3) 0.03 | |
| Male sex, n (%) | 111 (63%) | 9 (53%) | 0.44 |
| Morphology | |||
| HLHS morphology | 141 (80%) | 9 (47%) | 0.03 |
| MS/AA subtype of HLHS | 25 (18%) | 1 (11%) | 1.00 |
| Systemic left ventricle | 24 (14%) | 1 (6%) | 0.70 |
| Heterotaxy syndrome | 4 (2%) | 0 | 1.00 |
| Bilateral SVCs | 15 (8%) | 5 (29%) | 0.02 |
| S1P and interstage variables | |||
| RV-PA shunt at S1P | 96 (54%) | 10 (59%) | 0.80 |
| Prolonged S1P hospital stay | 15 (8%) | 6 (35%) | 0.004 |
| Pre-BDG PA dilation | 13 (7%) | 3 (18%) | 0.15 |
| Echocardiogram | |||
| AVVR, median (range) | 2 (0–3.5) | 2.5 (0–4) | <0.001 |
| Ventricular function | 0 (0–4) | 2 (0–4) | <0.001 |
| Semilunar valve regurgitation | 0 (0–2.5) | 1 (0–2) | 0.12 |
| Catheterization | (n = 139) | (n = 14) | |
| Cardiac index (l/min/m2) 3.2 (1.5–6.1) | 2.8 (1.7–6.0) | 0.55 | |
| Transpulmonary gradient (mmHg) | 7 (1–18) | 4 (4–11) | 0.25 |
| PVR (indexed WU) | 1.9 (0.5–7.5) | 2.1 (0.8–4.5) | 0.97 |
| Qp:Qs | 1.0 (0.3–3.1) | 1.3 (0.5–5.0) | 0.10 |
| Arterial oxygen saturation (%) | 76 (59–91) | 80 (66–90) | 0.02 |
| Systemic ventricle EDP (mmHg) | 10 (3–20) | 10 (6–20) | 0.87 |
Prolonged S1P hospital stay defined as hospitalization length of stay in the highest decile after S1P (>60 days). BDG, bidirectional Glenn procedure; OHT, orthotopic heart transplant; HLHS, hypoplastic left heart syndrome; MS/AA, mitral stenosis aortic atresia; SVC, superior vena cavae; S1P, stage one palliation; RV-PA, right ventricle to pulmonary artery; PA, pulmonary artery; AVVR, atrioventricular valve regurgitation; PVR, pulmonary vascular resistance; WU, Woods Units; Qp:Qs, ratio of pulmonary to systemic blood flow; and EDP, end-diastolic pressure.
Analysis of preoperative echocardiograms showed more AVVR and more ventricular dysfunction in those who died or underwent transplant at 18 months (Table 1). Preoperative cardiac catheterization data were similar between groups, except for higher arterial oxygen saturations in the death/OHT group.
Patients in the highest decile for hospital LOS after S1P had a median hospital stay of 75 days with a range of 62–100 days. Seven of these 19 patients were not discharged to home between S1P and BDG. Eight of the 19 (42%) died or underwent transplant after BDG.
Nineteen patients underwent BDG at <3 months of age. Eight events, six deaths, and two transplants occurred in this subgroup. Subgroup analysis showed that patients undergoing BDG at ≤3 months weighed less (p = 0.004), had lower weight z-score (p = 0.03), more AVVR (p = 0.008), worse ventricular function (p = 0.001), and were more likely to have prolonged stay after S1P (p = 0.03) than those undergoing BDG at >3 months of age. The rationale for earlier BDG was cyanosis in 9 and unstable S1P hemodynamics in 10 patients. Within the cyanotic group, there was a predominance of obstructed right ventricle to pulmonary artery (RV-PA) shunts, a factor removed with completion of the BDG. Interstage clinical course in the group undergoing early BDG secondary to failing S1P physiology was characterized by inability to be discharged to home (n = 5), interstage readmissions (n = 2), concern for coronary ischemia (n = 2), necrotizing enterocolitis (n = 2), and cardiac arrest or near arrest (n = 3). In those undergoing early BDG secondary to cyanosis, there has been one death and no transplantations, while 7 of the 10 patients undergoing BDG at <3 months for reasons other than cyanosis have died or undergone transplant.
3.3. Operative and postoperative variables
Univariate evaluation of operative variables between the death or OHT group and the transplant-free survival group at 18 months after BDG revealed no differences between groups in perfusion variables at the time of BDG. However, AV valvuloplasty at the time of BDG was associated with worse outcome through 18 months (Table 2). As only those with >mild AVVR underwent AV valvuloplasty in this cohort, a subgroup analysis was performed to evaluate the impact of AV valvuloplasty in those with significant AVVR. Thirty-one patients (16% of the entire cohort) had >mild AVVR preoperatively, of whom 14 (45%) underwent AV valvuloplasty at BDG. Of the 14 patients with >mild AVVR undergoing AV valvuloplasty at BDG, four have died, and one underwent OHT (36%). Among the 17 patients with >mild AVVR, who did not undergo AV valvuloplasty at BDG, five have died, and two underwent OHT (41%, p = 0.99). Thus, for those with > mild AVVR at the time of BDG, outcomes at 18 months were similar between those who did and did not have a concomitant AV valvuloplasty.
Table 2.
Operative and postoperative data: Alive versus dead or transplanted at 18 months after BDG.
| Alive (n = 177) | Dead or OHT (n = 17) | p value | |
|---|---|---|---|
| Operative data | |||
| Perfusion variables | |||
| Total pump time (min), median (range) | 73 (36–257) | 87 (57–166) | 0.14 |
| Cross-clamp time (min) | 0 (0–162) | 0 (0–59) | 0.95 |
| Use of circulatory arrest, n (%) | 31 (18%) | 3 (18%) | 1.00 |
| Additional procedures | |||
| AV valvuloplasty, n (%) | 9 (5%) | 4 (24%) | 0.01 |
| Atrial septal resection | 15 (8%) | 1 (6%) | 1.00 |
| Aortic arch intervention | 15 (8%) | 4 (24%) | 0.07 |
| Postoperative data | |||
| Ventilator time (h), median (range) | 18 (6–528) | 36 (14–552) | 0.002 |
| Duration of pleural drains (days) | 2 (1–13) | 3 (2–12) | 0.003 |
| O2 saturation in initial 12 h (%) | 79 (58–90) | 73 (63–84) | 0.007 |
| Discharge O2 saturation (%) | 83 (69–93) | 79 (69–90) | 0.002 |
| LOS after BDG (days) | 6 (1–110) | 13 (4–61) | 0.006 |
BDG, bidirectional Glenn procedure; OHT, orthotopic heart transplant; AV, atrioventricular; and LOS, length of hospital stay.
Hospital LOS, ventilator time, and duration of pleural drainage were all significantly longer in those who died or underwent transplantation. In addition, oxygen saturation in the first 12 postoperative hours and at discharge was significantly lower in those who died or underwent transplant by 18 months after BDG (Table 2).
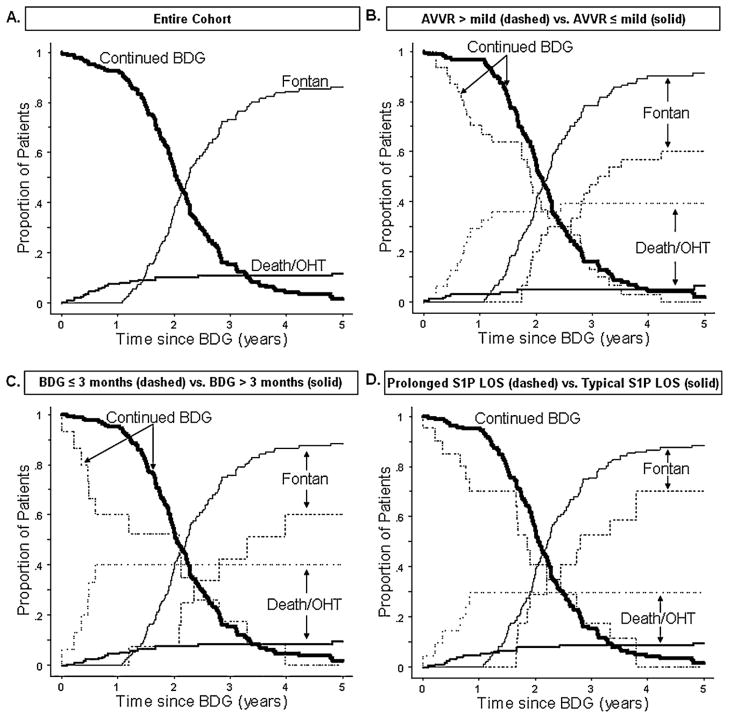
3.4. Cumulative incidence graphs for competing risk outcomes after BDG
The competing risk outcomes after BDG for the entire cohort are shown in Fig. 2(A). The proportion of patients experiencing death or OHT rises gradually in the first 9 months after BDG, reaching 7%. From 1 through 5 years post BDG, the proportion of patients who have died or undergone transplant rises slowly and plateaus at 12%. The proportion of patients undergoing Fontan begins to rise at 13 months post BDG and reaches 73% of the cohort by 3 years post BDG.
Fig. 2.
A–D: Competing risk analysis. Panel A: Competing time-dependent outcomes for the entire cohort. Panel B: Competing outcomes stratified by the degree of preoperative AVVR (≤mild vs >mild). Panel C: Competing outcomes comparing those with age ≤3 months vs age >3 months at BDG. Panel D: Competing outcomes comparing prolonged S1P hospital stay versus typical S1P hospital stay. BDG, bidirectional Glenn procedure; CI, confidence interval; S1P, stage one palliation; AVVR, atrioventricular valve regurgitation; and OHT, orthotopic heart transplant. Prolonged S1P hospital stay defined as hospitalization length of stay in the highest decile.
Fig. 2(B) shows competing outcomes after BDG stratified by preoperative AVVR. The proportion of patients with >mild AVVR who died or had undergone transplant has an initial rapid rise, reaching 30% 1 year after BDG and plateauing at 36% 2 years post BDG. Nearly 90% of patients with mild or less AVVR undergo Fontan by 4 years post BDG, while <60% of patients with >mild AVVR undergo Fontan by 4 years post BDG. The time to Fontan completion is delayed by approximately 8 months in the cohort with >mild AVVR. The proportions of patients remaining at BDG appear similar between groups in the later periods of follow-up.
Fig. 2(C) shows competing outcomes after BDG stratified by age at BDG. The proportion of patients undergoing BDG at ≤3 months of age, who died or have undergone transplant, increases rapidly in the first 6 months after BDG, reaching 40%. By contrast, in patients >3 months of age at BDG, the proportion, who died or have undergone transplant, remains below 10% for the 5-year period of follow-up. At 4 years post BDG, 87% of patients at >3 months of age at time of BDG have undergone Fontan, while only 60% of those ≤3 months of age at BDG have undergone Fontan. Similar to those with greater than mild AVVR, those who underwent BDG at 3 months of age or less typically undergo Fontan completion later than those undergoing BDG at an older age. Further, similar to AVVR, the proportions of patients remaining at BDG appear similar between groups in the later periods of follow-up.
Fig. 2(D) shows competing outcomes after BDG stratified by LOS after S1P. Among those with a prolonged hospitalization after S1P, there is an initial rise in the proportion of patients, who have died or undergone transplant, reaching 35% by 9 months post BDG, while in those with shorter LOS after S1P, the proportion of patients, who have died or undergone transplant, rises slowly over the first 30 months post BDG, to a maximum of 9%. At 4 years post BDG, only 70% of patients who had prolonged stay after S1P have undergone Fontan, while 88% of those with shorter LOS after S1P have undergone Fontan. The time to Fontan procedure and proportions of patients remaining at BDG in later follow-up appear similar between groups.
3.5. Regression models for competing risk outcomes after BDG
In univariable analysis, AVVR >mild (hazard ratio (HR) 7.9, 95% confidence interval (CI) 3.3–18.8), age ≤3 months at BDG (6.4, 2.4–17.2), prolonged hospital stay after S1P (4.0, 1.5–10.3), and ventricular dysfunction >mild (4.5, 1.9–10.5) were all associated with increased risk of death or OHT after BDG. Conversely, each of these patient characteristics was associated with decreased risk of Fontan completion (HR 0.42, 95% CI 0.26–0.67); (0.39, 0.19–0.79); (0.51, 0.28–0.93); and (0.50, 0.32–0.78); respectively. However, weight z-score in the lowest decile at BDG was not significantly associated with an increased risk of death or transplant (1.6, 0.5–4.8) or a decreased risk of Fontan completion (0.80, 0.51–1.27) after BDG.
Multivariable analysis of preoperative variables showed greater than mild AVVR, age <3 months at BDG, and prolonged hospitalization after S1P to be independent risk factors for death or transplant after BDG (Table 3). Conversely, AVVR >mild, age, ≤3 months at BDG, and prolonged hospitalization were associated with decreased rate of Fontan completion.
Table 3.
Multivariable regression analysis of risk factors for competing outcomes death or OHT and Fontan.
| BDG variables† | Death or OHT
|
p value | Fontan
|
p value | ||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard Ratio | 95% CI | |||
| AVVR >mild prior to BDG | 7.7 | 3.1–19.4 | <0.001 | 0.45 | 0.28–0.71 | <0.001 |
| Age ≤3 months at BDG | 6.9 | 2.3–20.5 | <0.001 | 0.44 | 0.23–0.85 | 0.02 |
| Prolonged S1P hospital stay | 4.5 | 1.7–11.4 | 0.002 | 0.58 | 0.33–0.996 | 0.048 |
n = 194. Total number of death or transplant events = 21, total number of Fontan procedures = 139, continued BDG status = 34 censored observations.
BDG, bidirectional Glenn procedure; OHT, orthotopic heart transplant; CI, confidence interval; AVVR, atrioventricular valve regurgitation; and S1P, stage one palliation. Prolonged S1P hospital stay defined as hospitalization length of stay in the highest decile.
4. Discussion
In this single-center study examining a large cohort undergoing BDG as an intermediate step in staged palliation after S1P, we found greater than mild AVVR, age ≤3 months at BDG, and prolonged hospitalization after S1P to be risk factors for failure to progress through intended staged palliation to a Fontan procedure.
AVVR is an important prognosticator of survival after BDG. Moreover, AVVR and ventricular dysfunction have been shown to be risk factors for worse outcome at all stages of single-ventricle palliation [9,16–19]. In this study, significant AVVR was associated with increased risk of death or transplant, a decreased rate of Fontan completion, and an older age of Fontan, if indeed that outcome was reached. Increased risk of death or transplant was noted in the interstage period prior to typical Fontan completion as well as into the early period of Fontan completion in this cohort. It is interesting, however, that the proportion of patients remaining at the BDG stage later in follow-up (through the period of typical Fontan completion) was not different from those with significant AVVR. This suggests that there is not a significant proportion of patients with significant AVVR kept at BDG physiology secondary to being poor Fontan candidates: these patients have already died, been transplanted, or have proceeded to successful Fontan.
Previous studies have suggested that AV valvuloplasty as part of a staged palliation for single-ventricle physiology improves short-term outcome in some higher-risk patients [8,9]. In our cohort, only 14 patients underwent AV valvuloplasty at the time of BDG, making it difficult to determine the utility of AV valvuloplasty in improving outcomes for high-risk patients. However, there was no difference in transplant-free survival between those patients with moderate or severe AVVR, who did or did not undergo AV valvuloplasty. Further work to determine the effect of AV valvuloplasty during staged palliation on long-term outcomes is warranted.
In this cohort, age ≤3 months at BDG was a risk factor for death or transplant, decreased occurrence of Fontan, and older age at Fontan. At our institution, we generally perform elective BDG between 4 and 6 months, rather than at younger ages, as others have suggested [12,15]. In this study, earlier BDG was typically used as a means to either alleviate cyanosis or to attempt to alter failing hemodynamics. Patients at our institution undergoing BDG at <3 months of age had more AVVR and longer hospital stays after S1P, but, when controlling for these factors, younger age remained a significant risk factor for death or transplant. Given our institutional predilection to proceed with early BDG only in higher-risk patients, we are unable with these data to determine whether an age-based strategy for BDG is associated with improved outcomes (i.e., whether planned earlier or later BDG is associated with improved clinical outcomes in patients with significant AVVR and/or ventricular dysfunction). Previous studies have yielded conflicting results on patients undergoing early BDG, with more recent studies indicating increased morbidity but no increase in mortality in patients undergoing earlier BDG [11,20–22].
Despite the limitations of the data set, the outcomes for those undergoing early BDG due to cyanosis are notably discrepant from those undergoing early BDG secondary to a hemodynamic indication. The rate of death or transplantation for those undergoing early BDG due to poor hemodynamics was 70%, while midterm outcomes for patients undergoing early BDG secondary to cyanosis were comparable to those undergoing BDG after 3 months of age. Petrucci et al. have recently published a report on a series of patients in which they find similar survival between infants undergoing BDG at <3 months and older infants [21]. The majority of patients in Petrucci’s series (65%) did not undergo an S1P prior to BDG, making it likely that most were selected for earlier BDG secondary to cyanosis. Similar to these findings, our results suggest that proceeding with earlier BDG may be associated with favorable outcomes in some, but not all, patients. Evaluation of age-based strategies for higher- and lower-risk infants would be best determined by larger multi-institutional databases in which risk factors could be more equally stratified between age groups.
In addition to younger age, the size of the patient at the time of BDG may impact outcome; decreased survival and increased resource use in patients with lower weight z-score at the time of BDG has recently been reported [10]. Lower body mass in infancy may be correlated to diminished pulmonary vascular cross-sectional area [20,21]. While lower pulmonary vascular cross-sectional area could be hypothesized to be associated with decreased total pulmonary blood flow and cyanosis after the BDG operation, lower weight z-score at BDG was not an independent risk factor for death or transplant. However, our cohort size and characteristics did not allow us to determine whether there is an optimal age and weight threshold for successful BDG.
Although this study was not designed to evaluate the effect of S1P variables on longitudinal outcomes, in this cohort, there was no difference in transplant-free survival prior to Fontan between patients with BT shunts compared with those with RV-PA conduits at S1P. Recent results have suggested that patients with RV-PA conduits have more ventricular dysfunction and a higher number require OHT [23]. Our sample size and limited number of events did not allow us to separately evaluate the competing risks of death and OHT; thus, we are unable to determine the independent effects of shunt type on each of the outcomes. The influence of surgical shunt type at S1P on longitudinal outcome may be clarified with longer follow-up on the Single Ventricle Reconstruction Trial cohort [24].
Limitations of this study include the retrospective design, which may not allow for identification of all risk factors that could significantly impact outcomes. Particularly, our cohort of patients undergoing BDG at ≤3 months was relatively small, which limits the ability of the study to detect all factors that might be independently associated with the longitudinal outcome measure in this group. The generalizability of this study may be limited based on our institutional bias against performing elective early BDG and a tendency to proceed to early BDG in high-risk patients.
5. Conclusions
AVVR greater than mild, age ≤3 months at BDG, and prolonged hospital stay after S1P are risk factors for poor outcome after BDG following S1P in the current era. Defining a population of infants with failing staged palliation in which early transplantation should be considered is an ongoing challenge for the field [13,25].
Footnotes
The Rochelle Rose Fund of Children’s Hospital Boston Cardiovascular Program supported this work.
References
- 1.Bridges ND, Jonas RA, Mayer JE, Flanagan MF, Keane JF, Castaneda AR. Bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation. 1990;82:IV170–6. [PubMed] [Google Scholar]
- 2.Hawkins JA, Shaddy RE, Day RW, Sturtevant JE, Orsmond GS, McGough EC. Mid-term results after bidirectional cavopulmonary shunts. Ann Thorac Surg. 1993;56:833–7. doi: 10.1016/0003-4975(93)90340-n. [DOI] [PubMed] [Google Scholar]
- 3.Jonas RA. Indications and timing for the bidirectional Glenn shunt versus the fenestrated Fontan circulation. J Thorac Cardiovasc Surg. 1994;108:522–4. [PubMed] [Google Scholar]
- 4.Lai L, Laussen PC, Cua CL, Wessel DL, Costello JM, del Nido PJ, Mayer JE, Thiagarajan RR. Outcomes after bidirectional Glenn operation: Blalock-Taussig shunt versus right ventricle-to-pulmonary artery conduit. Ann Thorac Surg. 2007;83:1768–73. doi: 10.1016/j.athoracsur.2006.11.076. [DOI] [PubMed] [Google Scholar]
- 5.Scheurer MA, Hill EG, Vasuki N, Maurer S, Graham EM, Bandisode V, Shirali GS, Atz AM, Bradley SM. Survival after bidirectional cavopulmonary anastomosis: analysis of preoperative risk factors. J Thorac Cardiovasc Surg. 2007;134:82–9. doi: 10.1016/j.jtcvs.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JB, Beekman RH, III, Border WL, Kalkwarf HJ, Khoury PR, Uzark K, Eghtesady P, Marino BS. Lower weight-for-age z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. 2009;138:397–404. doi: 10.1016/j.jtcvs.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Sano S, Huang SC, Kasahara S, Yoshizumi K, Kotani Y, Ishino K. Risk factors for mortality after the Norwood procedure using right ventricle to pulmonary artery shunt. Ann Thorac Surg. 2009;87:178–85. doi: 10.1016/j.athoracsur.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Ohye RG, Gomez CA, Goldberg CS, Graves HL, Devaney EJ, Bove EL. Repair of the tricuspid valve in hypoplastic left heart syndrome. Cardiol Young. 2006;16:21–6. doi: 10.1017/s1047951106000722. [DOI] [PubMed] [Google Scholar]
- 9.Reyes A, Bove EL, Mosca RS, Kulik TJ, Ludomirsky A. Tricuspid valve repair in children with hypoplastic left heart syndrome during staged surgical reconstruction. Circulation. 1997;96:II-344–5. [PubMed] [Google Scholar]
- 10.Ghanayem NS, Tweddell JS, Hoffman GM, Mussatto K, Jaquiss RD. Optimal timing of the second stage of palliation for hypoplastic left heart syndrome facilitated through home monitoring, and the results of early cavopulmonary anastomosis. Cardiol Young. 2006;16:61–6. doi: 10.1017/S1047951105002349. [DOI] [PubMed] [Google Scholar]
- 11.Jaquiss RD, Ghanayem NS, Hoffman GM, Fedderly RT, Cava JR, Mussatto KA, Tweddell JS. Early cavopulmonary anastomosis in very young infants after the Norwood procedure: impact on oxygenation, resource utilization, and mortality. J Thorac Cardiovasc Surg. 2004;127:982–9. doi: 10.1016/j.jtcvs.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Jaquiss RD, Siehr SL, Ghanayem NS, Hoffman GM, Fedderly RT, Cava JR, Mussatto KA, Tweddell JS. Early cavopulmonary anastomosis after Nor-wood procedure results in excellent Fontan outcome. Ann Thorac Surg. 2006;82:1260–5. doi: 10.1016/j.athoracsur.2006.04.095. [DOI] [PubMed] [Google Scholar]
- 13.Michielon G, Parisi F, Di Carlo D, Squitieri C, Carotti A, Buratta M, Di Donato RM. Orthotopic heart transplantation for failing single ventricle physiology. Eur J Cardiothorac Surg. 2003;24:502–10. doi: 10.1016/s1010-7940(03)00342-7. [DOI] [PubMed] [Google Scholar]
- 14.Kotani Y, Kasahara S, Fujii Y, Yoshizumi K, Oshima Y, Otsuki S, Akagi T, Sano S. Clinical outcome of the Fontan operation in patients with impaired ventricular function. Eur J Cardiothorac Surg. 2009;36:683–7. doi: 10.1016/j.ejcts.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 15.Mcguirk SP, Stickley J, Griselli M, Stumper OF, Laker SJ, Barron DJ, Brawn WJ. Risk assessment and early outcome following the Norwood procedure for hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2006;29:675–81. doi: 10.1016/j.ejcts.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 16.Ota N, Ikai A, Hirose K, Sakamoto K. Retrospective analysis of stage I Norwood procedures with tricuspid valve insufficiency in the past 5 years. Interact Cardiovasc Thorac Surg. 2007;6:121–3. doi: 10.1510/icvts.2006.142596. [DOI] [PubMed] [Google Scholar]
- 17.Alejos JC, Williams RG, Jarmakani JM, Galindo AJ, Isabel-Jones JB, Drinkwater D, Laks H, Kaplan S. Factors influencing survival in patients undergoing the bidirectional Glenn anastomosis. Am J Cardiol. 1995;75:1048–50. doi: 10.1016/s0002-9149(99)80722-x. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury UK, Airan B, Kothari SS, Talwar S, Saxena A, Singh R, Subramaniam GK, Pradeep KK, Patel CD, Venugopal P. Specific issues after extracardiac Fontan operation: ventricular function, growth potential, arrhythmia, and thromboembolism. Ann Thorac Surg. 2005;80:665–72. doi: 10.1016/j.athoracsur.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Kogon BE, Plattner C, Leong T, Simsic J, Kirshborn PM, Kanter KR. The bidirectional Glenn operation: a risk factor analysis for morbidity and mortality. J Thorac Cardiovasc Surg. 2008;136:1237–42. doi: 10.1016/j.jtcvs.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Chang AC, Hanley FL, Wernovsky G, Rosenfeld HM, Wessel DL, Jonas RA, Mayer JE, Lock JE, Castaneda AR. Early bidirectional cavopulmonary shunt in young infants. Postoperative course and early results. Circulation. 1993;88:II149–58. [PubMed] [Google Scholar]
- 21.Petrucci O, Khoury PR, Manning PB, Eghtesady P. Outcomes of the bidirectional Glenn procedure in patients less than 3 months of age. J Thorac Cardiovasc Surg. 2010;139:562–8. doi: 10.1016/j.jtcvs.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Slavik Z, Lamb RK, Webber SA, Devlin AM, Keeton BR, Monro JL, Salmon AP. Bidirectional superior cavopulmonary anastomosis: how young is too young? Heart. 1996;75:78–82. doi: 10.1136/hrt.75.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham EM, Zyblewski SC, Phillips JW, Shirali GS, Bradley SM, Forbus GA, Bandisode VM, Atz AM. Comparison of Norwood shunt types: do the outcomes differ 6 years later? Ann Thorac Surg. 2010;90:31–5. doi: 10.1016/j.athoracsur.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 24.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michielon G, Parisi F, Squitieri C, Carotti A, Gagliardi G, Pasquini L, Di Donato RM. Orthotopic heart transplantation for congenital heart disease: an alternative for high-risk Fontan candidates? Circulation. 2003;108:II140–9. doi: 10.1161/01.cir.0000087442.82569.51. [DOI] [PubMed] [Google Scholar]