Abstract
Background
Black patients with myocardial infarction (MI) have worse outcomes than white patients, including higher mortality, more angina, and worse quality of life. The TRIUMPH study was designed to examine whether racial differences in socioeconomic, clinical, genetic, metabolic, biomarker or treatment characteristics mediate observed disparities in outcomes.
Methods and Results
Between June 1, 2005 and December 31, 2008, 31567 patients with MI were prospectively screened, 6152 had an eligible MI, and 4340 (71%) were enrolled from 24 US centers. Consenting patients had detailed chart abstractions of their medical history and processes of inpatient care, supplemented with a detailed baseline interview. Detailed genetic and metabolic data were obtained at hospital discharge in 2979 (69%) and 3013 patients (69%), respectively. In a subset of patients, blood and urine samples were obtained at 1-month (obtained in 27% of survivors) and blood samples at 6-months (obtained in 19% of survivors). Centralized follow-up interviews sought to quantify patients’ post-discharge care and outcomes, with a focus on their health status (symptoms, function, and quality of life). At 1, 6 and 12 months, 23%, 27% and 24% were lost to follow-up. Vital status was available for 99% of patients at 12-months.
Conclusions
TRIUMPH is a novel MI registry with detailed information on patients’ socio-demographic, clinical, treatment, health status, metabolic, and genetic characteristics. The wealth of patient data collected in TRIUMPH will provide unique opportunities to examine factors that may mediate racial differences in mortality and health status after MI and the complex interactions between genetic and environmental determinants of post-MI outcomes.
Keywords: myocardial infarction, angina, outcomes research, health status, registries
Although minority patients bear a disproportionate share of death and disability from cardiovascular disease,1–4 understanding the reasons why racial differences in outcomes exist is complex.5, 6 Recognizing and rectifying such racial disparities is a national priority7 and a primary goal of the Department of Health and Human Services’ Healthy People 2010 agenda.8 However, achieving racial equity in outcomes (both mortality and health status—patients’ symptoms, function and quality of life) requires research to illuminate the root causes of observed disparities. This is particularly challenging when investigating race, since black and white patients differ substantially in economic, social, clinical and treatment characteristics. In a previous analysis of the Prospective Registry Evaluating Myocardial Infarction: Event and Recovery (PREMIER) registry that identified significant racial differences in a broad spectrum of outcomes 1 year after a myocardial infarction (MI), these differences did not persist after adjustment for patient factors that also differed by race and site of care.6
Further underscoring the need to define mediators of racial disparities in post-MI outcomes, the evolving epidemic of obesity, metabolic syndrome and diabetes disproportionately affects minorities, and these metabolic disturbances both increase the incidence of MI and the adverse outcomes after an MI.1, 9, 10 Data from the 2003 Behavioral Risk Factor Surveillance System survey of >250,000 adults revealed that 49% of blacks had ≥2 major risk factors for cardiovascular disease, as compared with 36% of whites.11 Given these trends for worsening metabolic risk factors and the disproportionate burden of these factors in blacks, further insights into the association between diabetes and glucose metabolism with adverse outcomes are needed to better understand and, eventually, minimize racial disparities in post-MI outcomes.
In addition to clinical, socioeconomic and metabolic factors, racial differences have been reported for both acute12, 13 and post-discharge14, 15 treatment. However, studies that examined the outcomes associated with different treatment patterns found no effect of these variations in care on mortality after adjustment for patient factors.16, 17 Finally, there are racial differences in genetics,18 but the extent to which these explain differences in clinical presentation and response to medical therapy is poorly understood. Because race is a complex social construct, more detailed information is needed on socioeconomic, treatment, metabolic and genetic characteristics to better clarify the contribution of each to racial differences in MI outcomes so that a rational strategy to eliminate racial disparities can be designed and implemented.
To address these existing gaps in knowledge, a collaboration of outcomes-oriented researchers from the Cardiovascular Outcomes Research Consortium partnered with genetic researchers to conduct the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH) study. This NIH-funded observational study was designed to quantify differences in health status outcomes between black and white patients 1 year after MI and to identify potential determinants of these outcomes. In addition, by collecting detailed social, economic, clinical, laboratory, genetic, and health status information, TRIUMPH has the broader goal of identifying novel genetic and metabolic mediators of mortality and health status after MI for all patients, regardless of race. The purpose of this article is to describe the design of TRIUMPH and how it differs from other registries, to summarize the clinical characteristics of the study sample, and to describe how these data will be used to illuminate new opportunities to improve the quality of care and outcomes of patients with MI.
Background
Over the past 30 years, a number of prospective MI registries have been conducted. Many of them, including the NRMI19 and CRUSADE20 registries, have focused on the presentation and acute management of MI patients, along with their in-hospital outcomes. The GRACE registry,21 launched in 1999, was one of the first to gather 6-month clinical outcomes data (mortality, hospitalization, cardiac procedures and use of certain medications). Although these studies have provided important insights into the in-hospital treatment of MI patients, their outcome assessments were generally limited to short-term mortality, and none of them provided insights into patient-centered outcomes, including symptoms, function, and/or quality of life. They also did not include baseline assessments of patients’ psychosocial or health status, which are known to be prognostically important.22–24
The PREMIER study,25 which enrolled ~2500 patients from 19 US hospitals in 2003–2004, addressed this gap in knowledge by collecting information on patients’ health status (their symptoms, function, and quality of life) at baseline and 1 year after MI. The TRIUMPH study, designed to build upon the infrastructure of PREMIER, collected not only extensive information on patients’ socioeconomics, clinical factors, and health status, but also detailed laboratory and genetic data not available in PREMIER. In addition, TRIUMPH sought to adjudicate all hospitalizations after the index MI so as to provide much more detailed information on resource utilization. Similarly, while prior studies have examined the association between various biomarkers or genetic variants with mortality, TRIUMPH sought to expand the existing knowledge base by evaluating possible genetic and metabolic determinants of both short- and long-term mortality and patient-centered outcomes, such as angina and quality of life. Thus, the purpose of TRIUMPH is to further illuminate the markers and mediators of racial disparities across a broad spectrum of 1-year outcomes after MI. This paper seeks to explain the rationale, methods and selection biases of TRIUMPH and serve as a resource for better understanding subsequent studies from this unique registry.
Methods
The first step in designing TRIUMPH was to consider the broad range of potential mediators of patient-centered outcomes, so that all relevant determinants could be prospectively captured. Given the focus on racial disparities, it was important to recognize that race is a complex social construct (not limited to skin color) and thus economic, social, genetic and metabolic characteristics are needed to identify which patient characteristics are most associated with outcomes. Once the desired domains were identified, it was necessary to ensure the generalizability of the study results by developing a representative approach to patient enrollment and to include a broad spectrum of institutions across the United States. Finally, methods for tracking detailed, patient-centered outcomes and for analyzing these data were designed.
Conceptualizing the determinants of patient-centered outcomes
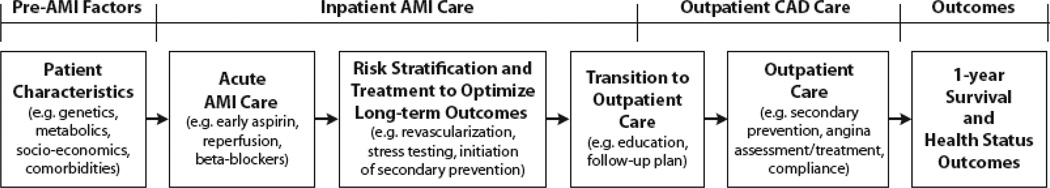
Although all MI patients are at risk for health status deficits after discharge, a broad range of factors, involving several phases of MI care, may influence patients’ subsequent outcomes. The fundamental goal of TRIUMPH was to independently quantify as many of these potential determinants of outcome as possible, so that those factors most associated with racial disparities in outcomes could be identified. Conceptually, we first defined the processes of MI care and then considered what data elements were needed to best quantify patients’ status at each phase of care. Figure 1 provides a conceptual model for the phases of care that can influence 1-year outcomes.
Figure 1.
Potential mediators of clinical outcomes: overview of TRIUMPH data collection. AMI indicates acute myocardial infraction; CAD, coronary artery disease.
Phases of MI care and relevant patient characteristics at each phase
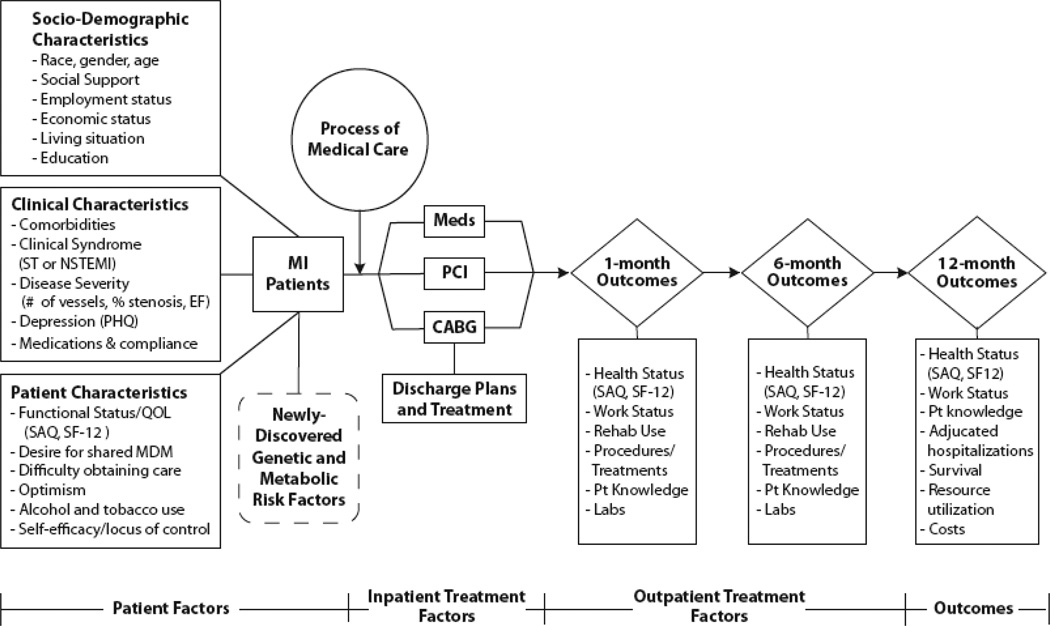
MI care consists of an initial presentation, an acute treatment phase, additional care throughout hospitalization, discharge treatment and planning, and follow-up care. Through this process, patients experience different rates of post-discharge outcomes, including mortality, readmission, health status, psychological status and costs. Because the focus of TRIUMPH was on the entire range of these post-discharge outcomes, patients were recruited during the final phase of their hospitalization to identify a cohort that was likely to survive hospitalization and experience post-discharge outcomes. Thus, many of the analyses emanating from TRIUMPH are relevant to the care and risk-stratification of MI patients preparing for discharge. The most important characteristics for modeling the impact of each phase of MI care differ, and TRIUMPH intentionally sought to quantify factors throughout multiple phases of the treatment process. A more detailed description of TRIUMPH data collection is shown in Figure 2.
Figure 2.
Detailed overview of potential mediators of post-MI outcomes. NSTEMI indicates non–st-elevation–MI; EF, ejection fraction; QOL, quality of life; SAQ, Seattle Angina Questionnaire; PCI, percutaneous coronary intervention; MDM, medical decision making; and CABG, coronary artery bypass graft.
Selecting measures to collect in the TRIUMPH study
Whenever possible, ACC/AHA Clinical Data Standards for Acute Coronary Syndromes was used to define clinical characteristics of patients’ comorbidities, presentation, complications and disposition.26 For categorization of race, patients were first asked to self-report their ethnicity and race, with an explicit opportunity to select multiple racial groups. A 2-generation pedigree was also collected to better evaluate patients’ race, ethnicity and recent ancestral country of origin, all critical components in genetic epidemiology.27 Procedural characteristics were captured using definitions from the American College of Cardiology’s National Cardiovascular Database Registry28 and the Society of Thoracic Surgeons database.29 These clinical data were supplemented with established instruments to quantify other domains likely to influence patients’ 1-year health status outcomes (Figure 2). Whenever possible, measures were selected from the existing literature so that valid, reproducible, and sensitive quantification of each concept could be acquired. Table 1 lists the instruments used to assess these psychosocial and health status factors.
Table 1.
Health Status and Psychosocial Instruments Used in TRIUMPH
| Instrument | Factors Assessed |
|---|---|
| Seattle Angina Questionnaire30, 31 | Angina; disease-specific health status |
| Short Form-1232 | General health status |
| EuroQol-5D33 | Utility estimate |
| 9-item Patient Health Questionnaire50 | Depressive symptoms |
| 4-item Perceived Stress Scale51 | Chronic stress |
| INTERHEART Short Stress Questions52 | Work, home, financial stress |
| 7-item ENRICHD Social Support Inventory53 | Social support |
| Deber instrument54 | Shared decision making |
| Rose dyspnea scale55 | Dyspnea |
| Rapid Estimate of Adult Literacy in Medicine | Health literacy |
| Telephone Interview for Cognitive Status35 | Elderly cognition |
A broad range of outcomes were measured in TRIUMPH, each requiring its own method of collection. The primary health status outcome of the TRIUMPH study was patients’ disease-specific symptoms, function and quality of life, as measured by the Seattle Angina Questionnaire (SAQ). The SAQ is a valid, reliable, responsive, and prognostic measure of patients’ perspectives of how their coronary disease impacts their symptoms, function, satisfaction with treatment, and quality of life.30, 31 In addition to disease-specific health status, generic health status was assessed with the Short Form-12,32 which quantifies patients’ overall physical and mental health, and the EQ-5D,33 which can also provide societal-based utilities and patient-reported assessments of overall health. All patient-centered data were collected through a detailed interview conducted by trained data collectors during patients’ presenting hospitalization and at each follow-up assessment. Copies of the surveys are available from the corresponding author.
Selecting patients for enrollment
The goal of TRIUMPH was to recruit a consecutive cohort of MI patients from each enrolling center. Because an important component of the study was to perform a detailed patient interview, patients needed to be prospectively identified as early as possible during their hospitalization. All patients with a positive troponin, as established by the norms of the recruiting center, were screened for possible inclusion. For sites with large volumes of MI patients, a systematic sampling strategy (e.g. screening every second or every third MI case based on the time of the first positive cardiac enzyme blood test and not convenience) was performed. Because the timing of consecutive positive laboratory tests is not influenced by patient characteristics or disease severity, no selection biases should have been introduced.
Once a patient was identified, a brief screening form was completed to establish eligibility. Only patients with a Type 1 acute MI34 (i.e. spontaneous MI related to ischemia due to a primary coronary event) were eligible for enrollment. Patients had to fulfill the following criteria for eligibility: (1) ≥18 years, (2) elevated troponin level (cardiac enzyme elevation as a complication of elective coronary revascularization did not qualify), (3) clinical features of ischemia (e.g. prolonged ischemic signs/symptoms, electrocardiographic ST changes in ≥2 consecutive leads), and (4) initial presentation to the enrolling institution or transfer within the first 24 hours of original presentation. This latter criterion ensured that the primary clinical decision making was conducted at the enrolling site. Incarcerated patients were not eligible, and all patients signed an informed consent that was approved by each institution.
Baseline data collection
Four discrete sources of data contributed to patients’ baseline data collection. First, a chart abstraction of patients’ presentation, clinical comorbidities, admission medications, presenting electrocardiogram, and treatments during the first 24 hours was done. Second, a detailed baseline interview of up to 250 questions was administered, taking from 30 to 50 minutes to complete. Third, all patients were asked to donate blood specimens at the time of enrollment in TRIUMPH for detailed metabolic and genetic analyses. They were also asked to sign a medical records release form so that the records from subsequent hospitalizations could be obtained and adjudicated. Finally, at the time of discharge, patients’ diagnostic data (including angiography and electrocardiography), in-hospital treatment, in-hospital complications, discharge recommendations, discharge medications, follow-up, and final diagnoses (including ICD-9 codes) were collected. Electrocardiograms and angiographic reports were abstracted by the principal investigator or their designee at each site. Approximately 800 baseline variables were collected for each patient. All data were entered into a Web-based data collection program that allowed front-end range and logic checks to ensure the accuracy of collected data (Velos, Freemont CA). In addition, a broad range of additional logic checks were performed by the data-coordinating center on an ongoing basis. Data queries were routinely sent and resolved by the study sites.
Blood Specimen Procurement and Processing
Because TRIUMPH was designed to investigate 1-year (as opposed to in-hospital) outcomes, fasting blood specimens were acquired as close to discharge as possible for laboratory and genetic analyses. This minimized artifactual alterations in patients’ lipoprotein profiles due to transiently heightened adrenergic states at the time of their MI and was felt to be most reflective of patients’ chronic metabolic state. However, given both the dynamic adrenergic state and use acute lipid-lowering therapy, patients’ lipid status prior to admission may have been underestimated using these measurements. For technical reasons (e.g., discharge before labs could be drawn, insufficient sample, etc.), some patients who agreed to collection of genetic material did not have blood drawn prior to hospital discharge. In order to maximize the number of genetic samples available for analyses, these patients were mailed a saliva-based genetics kit.. There were 124 patients (4% of genetic samples) whose genetic data was obtained through this method and an additional 89 patients (3% of genetic samples) whose saliva supplemented genetic data obtained through primary blood collection.
While genotyping is expected to vary by the type and purpose of subsequent genetic analyses, it is hoped that the analysis of TRIUMPH genetic samples will permit a careful evaluation of the magnitude of outcome differences attributable to genetic factors, as opposed to other patient and healthcare characteristics. Congruent with the intent if the NIH to support data sharing, future collaborators can apply to use these genetic samples by conducting the corresponding author.
Follow-up Interviews and Laboratories
Follow-up was attempted on all survivors at 1, 6, and 12 months. For patients who did not agree to blood collection and for all patients’ 12-month follow-ups, the interviews were performed by telephone from a single specialized center. For patients who agreed to additional blood collection, an in-home visit was performed by trained medical personnel at 1- and 6-months. Patients were assessed for weight, waist circumference, blood pressure, and pulse. Elderly patients (age ≥65 years) were also assessed for relevant domains for that population, including mobility, strength, balance, cognition, nutrition, endurance and physical activity—including 15-foot walk speed, chair stands and hand grip strength. Table 2 details the laboratory tests that were obtained at baseline and follow-up (see Appendix 1 for more detail on the processing of the baseline and follow-up laboratory samples, including the processing of genetic samples).
Table 2.
Laboratory tests assessed in TRIUMPH
| Assay | Baseline | 1-month | 6-month |
|---|---|---|---|
| Pro-BNP, troponin T, hs-CRP | √ | √ | √ |
| Expanded lipid panel* | √ | √ | √ |
| ApoA1 | √ | ||
| 25(OH) Vitamin D, parathyroid hormone, phosphate, calcium | √ | √ | √ |
| Hemoglobin A1c | √ | √ | |
| Insulin, plasma glucose | √ | √ | √ |
| Complete blood count | √ | √ | |
| Blood urea nitrogen, creatinine | √ | √ | |
| Total protein, albumin | √ | ||
| Urine microalbumin, creatinine | √ |
Abbreviations: BNP=brain naturetic peptide; hs-CRP=high sensitivity C-reactive protein
HDL, HDL-2, HDL-2a, HDL-2b, HDL-2c, HDL-3, HDL-3a, HDL-3b, HDL-3c, HDL-3d, LDL, LDL-1, LDL-2, LDL-3, LDL-4, LDL-R, LDL Pattern, Time To Peak LDL, VLDL, VLDL-1+2, VLDL-3, VLDL-3a, VLDL-3b, IDL, IDL-1, IDL-2, Lp(a), Lp(a)-1, Lp(a)-2, Lp(a)-3, Lp(a)-4, Lp(a)-5, Total Cholesterol, Remnant Lipoprotein, Triglycerides
Follow-up interviews included assessment of the primary health status outcomes of this study in addition to current medications, lifestyle and secondary risk factor management practices, use of cardiac rehabilitation, and many of the patient-centered domains assessed at baseline. Elderly patients were also assessed with the modified Telephone Interview for Cognitive Status35 and asked to self-report memory difficult, unintentional weight loss, and exhaustion. All patients were asked to report interval events (e.g. procedures, diagnostic tests, hospitalizations, and outpatient visits) since their last study contact. If a patient reported being hospitalized since the previous interview, records of that hospitalization were requested to adjudicate cardiovascular events, including myocardial infarction, heart failure, or revascularization procedures. Chart abstractions were sent to 2 cardiologists who independently classified the reason for hospitalization. If there was disagreement between the 2 cardiologists, the record was adjudicated by a third senior cardiologist, and if disagreement persisted, up to 5 cardiologists independently reviewed the charts until consensus was obtained.
Selecting sites for TRIUMPH
The goal of site selection was to capture a broad spectrum of hospitals, while also ensuring that compliance with the complex data collection procedures would be maintained. Figure 3 shows the final 24 sites for TRIUMPH enrollment which include several academic centers (Tufts-New England Medical Center, Yale-New Haven Medical Center, Duke University, Washington University in St. Louis, Virginia Commonwealth University, and the Universities of Iowa, Michigan, Wisconsin and Colorado), inner city hospitals (Truman Medical Center, Cook County, Montefiore Medical Center, Parkland Hospital, Grady Memorial, Henry Ford and Denver Health Hospitals), single-payer systems (Veterans Affairs Iowa City), and non-university hospitals (Chabert Medical Center, Presbyterian Health System, Christiana Care, Bridgeport Hospital, Sentara Leigh Hospital, MeritCare, and Saint Luke’s Mid America Heart Institute) (Appendix 1). Three centers initially participated in the TRIUMPH study but were excluded from analyses due to the small numbers of patients enrolled at these sites. Rural institutions and hospitals providing less specialty care were underrepresented.
Figure 3.
Participating sites in the TRIUMPH registry.
Among the 24 centers, 21 were not-for-profit institutions, 1 was for-profit, and 2 were governmental. Ten centers were university-owned, 10 were university-affiliated, and 4 were self-classified as nonacademic. Sixteen centers were in urban areas, and 7 primarily cared for indigent patients. The size of the institutions ranges from 66 to 1000 beds with a mean of 598 beds. All centers had onsite angiography, 21 performed PCI, and 17 performed CABG.
Results
The TRIUMPH study began on June 1, 2005, and ended on December 31, 2008. Enrolling patients into TRIUMPH required approximately 5 hours of data collection for each patient (~15 minutes for screening, ~2 hours of chart abstraction, ~45 minutes for interviews, ~1 hour of data entry, ~1 hour for blood procurement and site processing, and ~15 minutes of a cardiologist’s time to interpret electrocardiograms and angiograms). Over the course of the study, 31567 patients with elevated troponin levels were screened, 6152 were determined to be eligible and 4563 (74%) were enrolled, of which 223 either did not meet inclusion criteria (204 patients) or were from the 3 excluded sites with low enrollment (19 patients). Thus, the final number of patients eligible for analysis is 4340.
Although detailed information was not collected about patients who were eligible but not enrolled in TRIUMPH, Table 3 compares a few demographic and clinical characteristics between these two groups among the 10 top-enrolling centers (where this information was reliably collected and representing 81% of the final cohort). Eligible patients not enrolled were more likely to be older and white than those who agreed to participate. Table 4 describes in more detail the sociodemographic and clinical data for selected patient characteristics of enrolled patients, along with a comparison between white and non-white participants. It is important to note that TRIUMPH was designed to investigate long-term outcomes after an MI, and patients were required to be alive 24–48 hours after admission to participate in the enrollment interview. Thus, the low in-hospital mortality rate of 0.6% (24 patients) is not unexpected, and those who died will be excluded for many of the planned analyses of post-MI outcomes.
Table 3.
Baseline characteristics of patients eligible for and enrolled in TRIUMPH
| Characteristic | Not Enrolled n=1466 |
Enrolled n=3537* |
p-value | Follow-up n=3632† |
No Follow-up n=631† |
p-value |
|---|---|---|---|---|---|---|
| Age (years) | 62.2±14.5 | 58.6±12.4 | <0.001 | 59.3±12.0 | 57.1±13.7 | <0.001 |
| Male | 65.7% | 66.9% | 0.40 | 66.9% | 65.6% | 0.54 |
| White | 77.1% | 64.4% | <0.001 | 72.5% | 55.7% | <0.001 |
| No insurance | 24.5% | 23.4% | 0.42 | 21.4% | 28.9% | <0.001 |
| Diabetes mellitus | 31.0% | 31.7% | 0.63 | 29.7% | 35.0% | 0.007 |
| Hypertension | 65.9% | 69.3% | 0.104 | |||
| Prior myocardial infarction | 20.2% | 24.6% | 0.012 | |||
| Prior heart failure | 7.4% | 14.3% | <0.001 | |||
| ST-elevations on admission | 45.5% | 40.3% | 0.019 | |||
| Peak troponin (ng/mL) | 29.3±75.2 | 23.7±55.3 | 0.075 | |||
| Ejection fraction (%) | 49.0±12.9 | 47.2±13.8 | 0.004 | |||
| Coronary angiography | 93.5% | 86.2% | <0.001 | |||
| GRACE score (6-month mortality)60 | 100.1±29.2 | 100.8±34.3 | 0.554 | |||
| Length of stay (days) | 5.5±6.5 | 6.5±8.5 | <0.001 |
From the top 10 enrolling hospitals; represents 81% of the total study population
Includes only patients who survived to 1-month (i.e. had the opportunity to participate in follow-up)
Table 4.
Baseline characteristics of TRIUMPH patients
| Characteristics | All n=4340 |
White n=3022 |
Non-White n=1305 |
p-value |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age (years) | 59.1±12.3 | 59.9±12.3 | 57.1±12.2 | <0.001 |
| Male | 67% | 71% | 58% | <0.001 |
| White | 70% | |||
| Married | 51% | 59% | 33% | <0.001 |
| No insurance | 21% | 18% | 34% | <0.001 |
| High school education | 79% | 85% | 67% | <0.001 |
| Clinical factors | ||||
| ST-elevations on admission | 43% | 48% | 32% | <0.001 |
| Hypertension | 67% | 62% | 78% | <0.001 |
| Prior MI | 21% | 20% | 24% | 0.005 |
| Prior PCI | 20% | 20% | 18% | 0.062 |
| Prior heart failure | 9% | 6% | 15% | <0.001 |
| Diabetes | 31% | 27% | 40% | <0.001 |
| Family history of heart disease | 74% | 79% | 63% | <0.001 |
| Dyslipidemia | 49% | 51% | 44% | <0.001 |
| Chronic kidney disease | 7% | 5% | 13% | <0.001 |
| Chronic lung disease | 7% | 7% | 8% | 0.73 |
| History of smoking | 59% | 59% | 60% | 0.93 |
| Body mass index (kg/m2) | 29.5±6.5 | 29.4±6.1 | 29.8±7.3 | 0.12 |
| Disease severity | ||||
| Systolic blood pressure (mmHg) | 143±30 | 141±30 | 148±31 | <0.001 |
| Diastolic blood pressure (mmHg) | 83±19 | 82±19 | 86±19 | <0.001 |
| Heart rate on admission (bpm) | 83±22 | 82±23 | 85±21 | <0.001 |
| Ejection fraction (%) | 49±13 | 49±12 | 48±15 | 0.018 |
| Peak troponin level (ng/mL) | 29±73 | 33±73 | 19±65 | <0.001 |
| Initial creatinine level (mg/dL) | 1.2±1.1 | 1.2±0.8 | 1.5±1.6 | <0.001 |
| Initial hemoglobin level (g/dL) | 14.0±2.1 | 14.3±2 | 13.3±2.3 | <0.001 |
| Hs-CRP level (mg/L) | 3.7±5.0 | 3.7±4.9 | 3.6±5.3 | 0.51 |
| LDL level (mg/dL) | 95±32 | 95±31 | 96±35 | 0.51 |
| GRACE score for 6 -month mortality56 | 101±30 | 101±30 | 100±30 | 0.39 |
| Hospital procedures | ||||
| Coronary angiography | 92% | 95% | 85% | <0.001 |
| Primary PCI | 34% | 38% | 26% | <0.001 |
| Nonprimary PCI | 29% | 32% | 24% | <0.001 |
| Coronary bypass surgery | 9% | 10% | 8% | 0.007 |
| Quality performance measures* | ||||
| Aspirin within 24 hours | 97% | 98% | 97% | 0.47 |
| Aspirin at discharge | 95% | 96% | 94% | <0.001 |
| ACE-I/ARB for left ventricular systolic dysfunction | 85% | 87% | 83% | 0.17 |
| Smoking cessation counseling | 75% | 80% | 66% | <0.001 |
| Beta-blocker at discharge | 93% | 95% | 90% | <0.001 |
| Fibrinolytics by 30 min or primary PCI by 90 min | 52% | 58% | 40% | <0.001 |
| In-hospital outcomes | ||||
| Mortality | 0.6% | 0.5% | 0.7% | 0.43 |
| Length of stay (days) | 5.7±7.0 | 5.5±7.2 | 6.1±6.3 | 0.007 |
Abbreviations: MI=myocardial infarction; PCI=percutaneous coronary intervention; Hs-CRP=high sensitivity C-reactive protein; LDL=low density lipoprotein; ACE-I=angiotensin converting enzyme-inhibitor; ARB=angiotensin receptor blocker
For those eligible
Adherence to process measures of quality care was generally high among the population, with the majority of patients being prescribed aspirin (95%) and beta blockers (93%) at discharge, concordant with national averages.13 Among patients with left ventricular systolic dysfunction, 85% were prescribed ACE inhibitors. Eighty-three percent of STEMI patients received an attempt at primary reperfusion, with 52% of eligible patients receiving thrombolytics within 30 minutes or primary percutaneous coronary intervention within 90 minutes upon hospital arrival. White patients were more likely than non-white patients to undergo invasive management of their MI and were more likely to receive processes of care considered quality metrics.
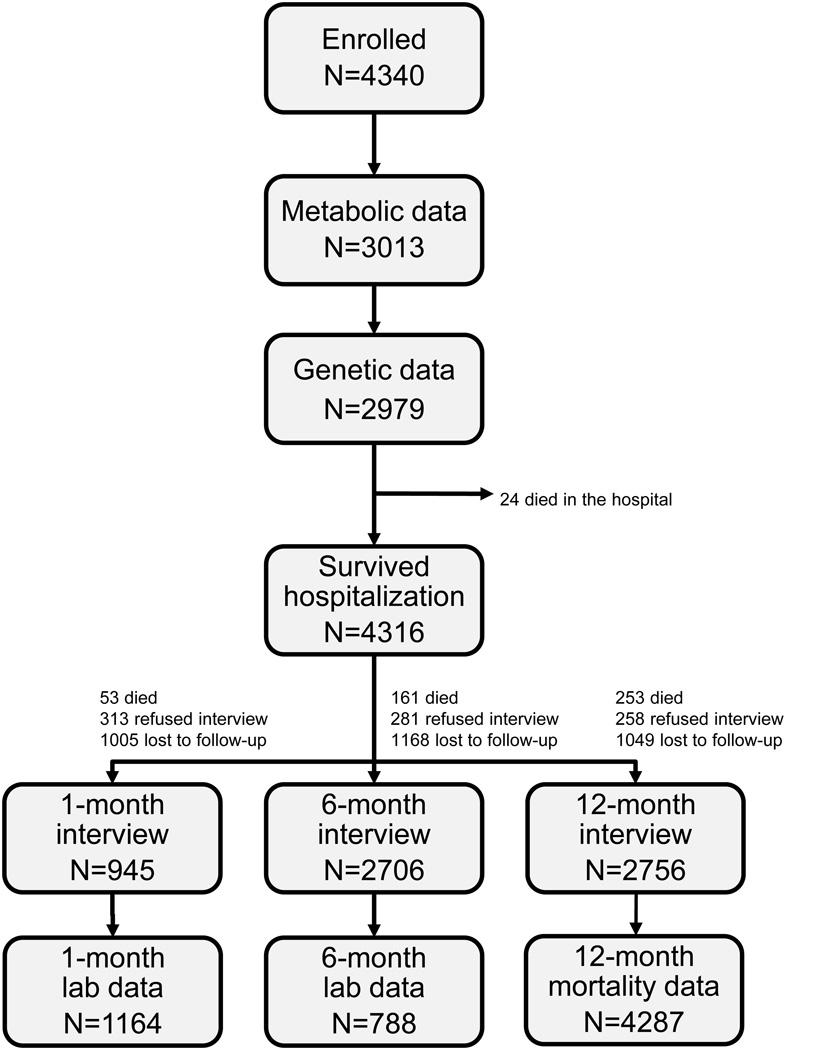
As of January 2010, all enrolled patients reached their 1-year follow-up time point. Figure 4 shows the summary of follow-up on the patients enrolled in TRIUMPH. Detailed genetic and metabolic data was obtained during baseline hospitalization in 69% of patients. Vital status was available for 99% of patients at 12-months, at which time 6.5% of patients had died. At the 1-, 6-, and 12-month follow-up time points, 23%, 27%, and 24% of patients were lost to follow-up. The demographic and clinical characteristics of those who participated and those who did not participate in follow-up are shown in Table 3. Patients who were younger and non-white were less likely to follow-up. Patients who were sicker were also less likely to follow-up, with heart failure, diabetes, depressed ejection fraction, and longer hospital stay being associated with a lower likelihood of follow-up.
Figure 4.
Summary of follow-up on the patients enrolled in TRIUMPH.
Discussion
Although improving the quality of American healthcare is a national priority,36 significant barriers remain. Among these is the need to better understand where the greatest opportunities lie in optimizing patient outcomes and how to ensure that all components of our society are able to receive the best treatment. While one of the Institute of Medicine’s (IOM) six aims for improving the healthcare of the American population includes making it more evidence-based, in many instances, certain patient characteristics will likely alter the ‘average’ benefit observed in clinical trials. Therefore, to achieve another of the IOM’s goals—patient-centeredness—a better understanding of the association of patients characteristics with treatment and outcomes is needed. Furthermore, achieving the IOM’s goal of equity in healthcare requires illuminating patient characteristics and treatments that vary by race so that proactive steps can be developed to minimize differences in care and eliminate disparities in outcomes.
Against this backdrop, TRIUMPH offers a unique opportunity to evaluate racial disparities in outcomes among patients with MI. By prospectively enrolling a multi-center cohort of MI patients, a broad spectrum of patients can be studied with the goal of illuminating novel mediators and moderators of outcomes. Unfortunately, as a result of conscious efforts to enroll a representative MI patient population with regards to race and socioeconomic status independent of likelihood to participate in follow-up (as is often done in clinical trials), non-participation in follow-up interviews was slightly greater than the anticipated 20% rate, with lower participation in non-white patients. Analyses using follow-up data will need to address this selection bias with advanced statistical techniques, such as assigning an inversely-weighted propensity score to each patient to emphasize the scores for those most likely to miss data.24, 37, 38 Towards that end, the collection of detailed socioeconomic, clinical, treatment, metabolic, and genetic information, will also enable investigators to richly characterize each patient and define the prognostic importance of clinical and biological parameters on outcomes. Finally, by investing in the collection of a broad spectrum of outcomes, with a focus on health status but also including economic and clinical outcomes, the associations of different patient and treatment characteristics with outcomes can be identified.
TRIUMPH was built upon the structure of PREMIER,25 which was unique among existing observational MI studies at the time. Enrolling ~2500 patients from 19 US hospitals from 2003–2004, PREMIER was the first MI registry to focus on patients’ health status outcomes and also provide greater clinical detail of patients’ presentations and treatment than most previous cardiovascular registries. While prior registries were able to include more patients by collecting less data, the richer data collection of TRIUMPH and PREMIER is able to identify new predictors of outcomes, such as patients’ ability to afford healthcare,39, 40 insurance status,41 medication adherence,15, 42 psychosocial and health status,43, 44 and social support45 and their association with long-term survival, hospitalization and health status.46–49 It is through this collection of extensive data on patients’ social, economic, and psychological status (with reliable and valid instruments) that analyses of PREMIER have been able to identify how important these aspects are in influencing the long-term outcomes of patients after an MI. By supplementing the rich collection of socioeconomic, clinical, treatment and health status variables previously collected in PREMIER with genetic and metabolic information, TRIUMPH will allow for even greater insights into the mediators of outcomes after MI and potentially discern some of the complex interactions between genetic and clinical (including treatment) factors. In addition, given the similar structure of the data collection in PREMIER and TRIUMPH (identical inclusion and exclusion criteria, time points of assessment, psychosocial, socioeconomic, health status, and quality of life assessments), the data from the two registries will be able to be combined for additional unique analyses, such as time trend analyses, model creation and validation, and analyses of rarer events that require increased power.
In summary, TRIUMPH is a prospective study of the outcomes of patients recovering from MI with detailed information about patients’ socio-demographic, clinical, treatment, health status, metabolic, and genetic characteristics. It has been designed to explicitly describe patient-centered health status outcomes 1 year after discharge and to document the determinants and trajectory of that recovery. TRIUMPH should provide unique insights into factors that mediate racial differences in mortality and health status outcomes after MI and, ultimately, these insights will allow for the creation of rational strategies to reduce racial disparities in outcomes.
Supplementary Material
Acknowledgments
Funding Sources: TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, Blood Institute): Washington University School of Medicine SCCOR Grant #P50HL077113-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: Dr. Spertus owns the copyright to the Seattle Angina Questionnaire.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: A report from the american heart association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Henry J. Racial/ethnic differences in cardiac care: The weight of the evidence. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 2002. Kaiser Family Foundation ACoCF. [Google Scholar]
- 3.Nelson AR. Unequal treatment: Report of the institute of medicine on racial and ethnic disparities in healthcare. Annals of Thoracic Surgery. 2003;76:S1377–S1381. doi: 10.1016/s0003-4975(03)01205-0. [DOI] [PubMed] [Google Scholar]
- 4.Services USDoHaH. Healthy people 2010: Understanding and improving health. 2000 [Google Scholar]
- 5.Rathore SS, Krumholz HM. Differences, disparities, and biases: Clarifying racial variations in health care use. Ann Intern Med. 2004;141:635–638. doi: 10.7326/0003-4819-141-8-200410190-00011. [DOI] [PubMed] [Google Scholar]
- 6.Spertus JA, Jones PG, Masoudi FA, Rumsfeld JS, Krumholz HM. Factors associated with racial differences in myocardial infarction outcomes. Ann Intern Med. 2009;150:314–324. doi: 10.7326/0003-4819-150-5-200903030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rumsfeld JS. Health status and clinical practice: When will they meet? Circulation. 2002;106:5–7. doi: 10.1161/01.cir.0000020805.31531.48. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of health and human services: Office of disease prevention and health promotion--healthy people 2010. Nasnewsletter. 2000;15:3. [PubMed] [Google Scholar]
- 9.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 10.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the united states. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 11.Racial/ethnic and socioeconomic disparities in multiple risk factors for heart disease and stroke--united states, 2003. MMWR Morb Mortal Wkly Rep. 2005;54:113–117. [PubMed] [Google Scholar]
- 12.Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: Review of the literature and prescription for future research. Ann Intern Med. 2001;135:352–366. doi: 10.7326/0003-4819-135-5-200109040-00012. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MG, Fonarow GC, Peterson ED, Moscucci M, Dai D, Hernandez AF, Bonow RO, Smith SC., Jr Racial and ethnic differences in the treatment of acute myocardial infarction: Findings from the get with the guidelines-coronary artery disease program. Circulation. 2010;121:2294–2301. doi: 10.1161/CIRCULATIONAHA.109.922286. [DOI] [PubMed] [Google Scholar]
- 14.Gregory PC, LaVeist TA, Simpson C. Racial disparities in access to cardiac rehabilitation. Am J Phys Med Rehabil. 2006;85:705–710. doi: 10.1097/01.phm.0000233181.34999.3d. [DOI] [PubMed] [Google Scholar]
- 15.Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 16.Petersen LA, Wright SM, Peterson ED, Daley J. Impact of race on cardiac care and outcomes in veterans with acute myocardial infarction. Med Care. 2002;40:I86–I96. doi: 10.1097/00005650-200201001-00010. [DOI] [PubMed] [Google Scholar]
- 17.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the department of veterans affairs. JAMA. 1994;271:1175–1180. [PubMed] [Google Scholar]
- 18.Lanfear DE, Marsh S, Cresci S, Shannon WD, Spertus JA, McLeod HL. Genotypes associated with myocardial infarction risk are more common in african americans than in european americans. J Am Coll Cardiol. 2004;44:165–167. doi: 10.1016/j.jacc.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 19.French WJ. Trends in acute myocardial infarction management: Use of the national registry of myocardial infarction in quality improvement. Am J Cardiol. 2000;85:5B–9B. doi: 10.1016/s0002-9149(00)00752-9. discussion 10B–12B. [DOI] [PubMed] [Google Scholar]
- 20.Hoekstra JW, Pollack CV, Jr, Roe MT, Peterson ED, Brindis R, Harrington RA, Christenson RH, Smith SC, Ohman EM, Gibler WB. Improving the care of patients with non-st-elevation acute coronary syndromes in the emergency department: The crusade initiative. Acad Emerg Med. 2002;9:1146–1155. doi: 10.1111/j.1553-2712.2002.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 21.Fox KA, Eagle KA, Gore JM, Steg PG, Anderson FA. The global registry of acute coronary events, 1999 to 2009-grace. Heart. 2010;96:1095–1101. doi: 10.1136/hrt.2009.190827. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–1022. doi: 10.1016/S0002-8703(03)00436-8. [DOI] [PubMed] [Google Scholar]
- 23.Frasure-Smith N, Lesperance F, Gravel G, Masson A, Juneau M, Talajic M, Bourassa MG. Social support, depression, and mortality during the first year after myocardial infarction. Circulation. 2000;101:1919–1924. doi: 10.1161/01.cir.101.16.1919. [DOI] [PubMed] [Google Scholar]
- 24.Smolderen KG, Spertus JA, Reid KJ, Buchanan DM, Krumholz HM, Denollet J, Vaccarino V, Chan PS. The association of cognitive and somatic depressive symptoms with depression recognition and outcomes after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:328–337. doi: 10.1161/CIRCOUTCOMES.109.868588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The prospective registry evaluating myocardial infarction: Events and recovery (premier)--evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–597. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, Weintraub WS, Mitchell KR, Morrisson SL, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins-Nakai RL, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, McKay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American college of cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the american college of cardiology task force on clinical data standards (acute coronary syndromes writing committee) J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 27.Risch NJ. Searching for genetic determinants in the new millennium. Nature. 2000;405:847–856. doi: 10.1038/35015718. [DOI] [PubMed] [Google Scholar]
- 28.Weintraub WS, McKay CR, Riner RN, Ellis SG, Frommer PL, Carmichael DB, Hammermeister KE, Effros MN, Bost JE, Bodycombe DP. The american college of cardiology national database: Progress and challenges. American college of cardiology database committee. J Am Coll Cardiol. 1997;29:459–465. doi: 10.1016/s0735-1097(96)00545-1. [DOI] [PubMed] [Google Scholar]
- 29.Edwards FH, Clark RE, Schwartz M. Coronary artery bypass grafting: The society of thoracic surgeons national database experience. Ann Thorac Surg. 1994;57:12–19. doi: 10.1016/0003-4975(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 30.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 31.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the seattle angina questionnaire: A new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 32.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Euroqol--a new facility for the measurement of health-related quality of life. The euroqol group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 34.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 35.Brandt J, Welsh KA, Breitner JC, Folstein MF, Helms M, Christian JC. Hereditary influences on cognitive functioning in older men. A study of 4000 twin pairs. Arch Neurol. 1993;50:599–603. doi: 10.1001/archneur.1993.00540060039014. [DOI] [PubMed] [Google Scholar]
- 36.Institute of Medicine. Crossing the quality chasm: A new health system for the twenty-first century. Washington: National Academy Press; 2001. [Google Scholar]
- 37.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 38.Little RA, Rubin DB. Statistical analysis with missing data. New York: John Wiley; 2002. [Google Scholar]
- 39.Bernheim SM, Spertus JA, Reid KJ, Bradley EH, Desai RA, Peterson ED, Rathore SS, Normand SL, Jones PG, Rahimi A, Krumholz HM. Socioeconomic disparities in outcomes after acute myocardial infarction. Am Heart J. 2007;153:313–319. doi: 10.1016/j.ahj.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 40.Rahimi AR, Spertus JA, Reid KJ, Bernheim SM, Krumholz HM. Financial barriers to health care and outcomes after acute myocardial infarction. JAMA. 2007;297:1063–1072. doi: 10.1001/jama.297.10.1063. [DOI] [PubMed] [Google Scholar]
- 41.Smolderen KG, Spertus JA, Nallamothu BK, Krumholz HM, Tang F, Ross JS, Ting HH, Alexander KP, Rathore SS, Chan PS. Health care insurance, financial concerns in accessing care, and delays to hospital presentation in acute myocardial infarction. JAMA. 2010;303:1392–1400. doi: 10.1001/jama.2010.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, Messenger JC, Khanal S, Peterson ED, Bach RG, Krumholz HM, Cohen DJ. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: Results from the premier registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 43.Parashar S, Rumsfeld JS, Spertus JA, Reid KJ, Wenger NK, Krumholz HM, Amin A, Weintraub WS, Lichtman J, Dawood N, Vaccarino V. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006;166:2035–2043. doi: 10.1001/archinte.166.18.2035. [DOI] [PubMed] [Google Scholar]
- 44.Plomondon ME, Magid DJ, Masoudi FA, Jones PG, Barry LC, Havranek E, Peterson ED, Krumholz HM, Spertus JA, Rumsfeld JS. Association between angina and treatment satisfaction after myocardial infarction. J Gen Intern Med. 2008;23:1–6. doi: 10.1007/s11606-007-0430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leifheit-Limson EC, Reid KJ, Kasl SV, Lin H, Jones PG, Buchanan DM, Parashar S, Peterson PN, Spertus JA, Lichtman JH. The role of social support in health status and depressive symptoms after acute myocardial infarction: Evidence for a stronger relationship among women. Circ Cardiovasc Qual Outcomes. 2010;3:143–150. doi: 10.1161/CIRCOUTCOMES.109.899815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spertus JA, Dawson J, Masoudi FA, Krumholz HM, Reid KJ, Peterson ED, Rumsfeld JS. Prevalence and predictors of angina pectoris one month after myocardial infarction. Am J Cardiol. 2006;98:282–288. doi: 10.1016/j.amjcard.2006.01.099. [DOI] [PubMed] [Google Scholar]
- 47.Ho PM, Eng MH, Rumsfeld JS, Spertus JA, Peterson PN, Jones PG, Peterson ED, Alexander KP, Havranek EP, Krumholz HM, Masoudi FA. The influence of age on health status outcomes after acute myocardial infarction. Am Heart J. 2008;155:855–861. doi: 10.1016/j.ahj.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 48.Maddox TM, Reid KJ, Spertus JA, Mittleman M, Krumholz HM, Parashar S, Ho PM, Rumsfeld JS. Angina at 1 year after myocardial infarction: Prevalence and associated findings. Arch Intern Med. 2008;168:1310–1316. doi: 10.1001/archinte.168.12.1310. [DOI] [PubMed] [Google Scholar]
- 49.Arnold SV, Alexander KP, Masoudi FA, Ho PM, Xiao L, Spertus JA. The effect of age on functional and mortality outcomes after acute myocardial infarction. J Am Geriatr Soc. 2009;57:209–217. doi: 10.1111/j.1532-5415.2008.02106.x. [DOI] [PubMed] [Google Scholar]
- 50.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen S, Kamarck T, Mermelstein R. A global measure of psychological stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 52.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11 119 cases and 13 648 controls from 52 countries (the interheart study): Case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 53.Vaglio J, Jr, Conard M, Poston WS, O'Keefe J, Haddock CK, House J, Spertus JA. Testing the performance of the enrichd social support instrument in cardiac patients. Health Qual Life Outcomes. 2004;2:24. doi: 10.1186/1477-7525-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deber RB, Kraetschmer N, Irvine J. What role do patients wish to play in treatment decision making? Arch Intern Med. 1996;156:1414–1420. [PubMed] [Google Scholar]
- 55.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 56.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.