Abstract
Ovarian hormones organize and activate neural circuits for reproduction and may also mediate cognition. Research has focused on estradiol’s mnemonic effects, albeit progesterone co-varies with estradiol and its mechanisms for cognition require attention. Studies tested the hypothesis that cognitive effects of progesterone occur subsequent to its metabolism to 5α-pregnan-3α-ol-20-one (3α,5α-THP), which does not bind progestin receptors. Cognitive performance and progestogen levels in plasma, hippocampus, and cortex, were determined in ovariectomized mice administered vehicle, or progestins that differentially form 3α,5α-THP and bind progestin receptors (progesterone, 3α,5α-THP, and/or medroxyprogesterone acetate). Only treatments that increased 3α,5α-THP levels during memory consolidation (progesterone, 3α,5α-THP, 3α-5α-THP+medroxyprogesterone acetate, but not progesterone+medroxyprogesterone acetate) improved cognitive performance. Thus, formation of 3α,5α-THP may be required for progesterone’s cognitive-enhancing effects.
Keywords: cognition, learning, memory, neurosteroids, hormone replacement therapy
Introduction
Ovarian hormones, such as estradiol (E2) and progesterone (P4), are known for their role in organizing and activating neural circuits related to reproduction, but there is emerging evidence for their role for cognitive processes across the lifespan. For example, women are twice as likely as are men to develop Alzheimer’s Disease [1]. Among younger individuals, differences in cognitive performance have been noted with variations in hormonal milieu (e.g. pregnancy) [2,3]. There has been considerable interest in the effects of aging-related decline in these hormones for cognition, and whether deficits can be ameliorated with their replacement. Despite evidence from animal models that ovarian hormones can benefit cognitive performance [4,5,6,7], the data on whether hormone therapies reduce risk of cognitive decline with aging are controversial. Synthetic E2/progestin (medroxyprogesterone acetate; MPA) therapies (e.g. PremPro®) may not be beneficial [8] and increase risk for Alzheimer’s Disease and stroke [9,10]. However, PremPro® improved memory and attention in a younger population of women in another study [11]. Women receiving synthetic E2 and micronized P4 had better encoding, consolidation and retrieval in verbal memory tasks [12]. It must be noted that the focus in these studies has been on E2, but P4 naturally co-varies with E2 and its mechanisms for cognition are largely unknown. Understanding how P4 influences cognitive performance is important for gaining insight into the neurobiology of learning and memory.
A question that needs to be addressed to further understand the role of hormones for cognition is why there is heterogeneity in the responses of individuals to progestins for cognitive performance. Differences in responses may be related to the divergent mechanisms of action of progestins. Although P4 and MPA bind progestin receptors, they differ in their actions at androgen [13] and glucocorticoid [14] receptors. The effects of P4 to improve cognitive performance may not rely upon actions at cognate progestin receptors. When administered P4, both progestin receptor-replete mice, and those with lifelong depletion of progestin receptors, have improved performance across several cognitive tasks, compared to that seen with vehicle/placebo [15]. P4 and MPA also differ in their ability to be metabolized to 5α-pregnan-3α-ol-20-one (3α,5α-THP), a progestin that does not bind progestin receptors. MPA inhibits the enzyme 3α-hydroxysteroid dehydrogenase, and decreases formation of 3α,5α-THP [16]. P4 is a precursor for 3α,5α-THP, and can augment E2’s effects to enhance formation of 3α5α-THP. Thus, some cognitive effects of P4 may occur subsequent to metabolism to 3α,5α-THP, and its actions independent of progestin receptors.
To address mechanisms by which progestins exert mnemonic effects, we investigated acute effects on cognitive performance of female mice administered progestins that differentially form 3α,5α-THP and bind progestin receptors. We compared the effects of P4, 3α,5α-THP, and/or MPA on cognitive performance of ovariectomized mice, and measured levels of progestins in plasma and putative brain targets (cortex, hippocampus). We hypothesized that mnemonic effects of P4 occur subsequent to its metabolism to 3α,5α-THP, which does not bind progestin receptors.
Methods
Methods were approved by the IACUC at University at Albany and carried out using measures to minimize pain or discomfort (NIH Guide for the Care and Use of Laboratory Animals, #80-23, 1996).
Animals and housing
Female mice (C57BL/6UA; N=71) were obtained from our breeding colony at University at Albany. All mice were ovariectomized under sodium pentobarbital anesthesia (80 mg/kg; intraperitoneal injection) as adults (8 weeks old) and started in the testing protocol one week later. Mice were group-housed (4–5/cage) on a reversed 12/12 h light/dark cycle (lights on 0800 h) with continuous access to rodent chow and tap water.
Hormone treatments
Mice were randomly-assigned to treatment conditions, and grouping was counter-balanced across cages and two cohorts. In Experiment 1, the role of 3α,5α-THP was determined by comparing effects of subcutaneous administration of vehicle (vegetable oil, 20% DMSO), P4 (4 mg/kg; Steraloids, Newport, RI), and 3α,5α-THP (4 mg/kg; Sigma, St. Louis, MO). In Experiment 2, the effects of MPA, and whether MPA can block effects of P4 and 3α,5α-THP, were determined by comparing vehicle, MPA (10 mg/kg; Sigma), P4+MPA, and 3α,5α-THP+MPA. The dosing utilized was based upon previous and pilot studies [4,17]. All treatments were administered immediately post-training, during consolidation [4], and levels of progestogens at this time were verified.
Procedure
Mice were screened for normative motor and species-typical behavior (grooming, nest building, huddling with cagemates) and sensory responses and visually inspected for good general health (fur, whiskers, posture, gait, muscle tone) before inclusion in the study [4]. Mice were trained and tested in each of the tasks described below once per week. Behavioral data was collected by an observer uninformed of the coded treatment conditions of mice, using automated tracking systems (Any-Maze for object recognition/placement, water maze; San Diego Instruments Freeze Monitor for conditioned fear).
Object Recognition and Object Placement
Mice were trained with two novel objects placed in an open field (46×57×35 cm) in a single 3-min trial [17]. Four hours later, mice were tested for object recognition and object placement memory. For testing, the percentage of time mice spent exploring a novel (object recognition), or displaced (object placement), object as a function of total time exploring both objects during the 3-min trial was calculated. Mice were tested in these tasks with different objects to minimize potential for test decay effects [17].
Water Maze
Mice were habituated to the water maze (San Diego Instruments; 97 cm diameter, 23–25 °C water made opaque with white tempera paint) for 60 s. Mice were given 12, 1-min trials, which were organized into three blocks of 4 trials. Mice were randomly placed in one of the 4 starting positions in the maze during these trial blocks. Mice were tested in a 60-sec probe trial 1 hour later, where the time spent in the quadrant where the platform had been located during training was recorded as an index of memory in this task [4]
Conditioned Contextual/Cued Fear
Mice were habituated to the conditioning chamber for 4 min. During training, a 10-sec tone was sounded, immediately followed by a footshock (2 s, 0.35 mA). Mice received tone-shock pairings in 3 consecutive training trials. Twenty-four hours later, mice were tested for contextual and cued conditioning as determined by time spent freezing t these stimuli over 8, 1-min trials [4].
Tissue collection/radioimmunoassay
Some mice were re-administered treatments and trunk blood and whole brains were collected after cervical subluxation/decapitation so that progestin levels during consolidation could be determined by radioimmunoassay of plasma and dissected out cortex and hippocampus [5,17].
Data Analyses
One-way analyses of variance (ANOVAs), with Fisher’s post hoc tests, were utilized, with an α level of p < 0.05 for significance, and p < 0.10 for a trend.
Results
Experiment 1: P4 and 3α,5α-THP enhance cognitive performance, and increase plasma, cortical, and hippocampal 3α,5α-THP levels
Post-training, subcutaneous administration of P4 or 3α,5α-THP, but not vehicle, significantly increased the percentage of time mice spent exploring the novel object in the object recognition task (F(2,52)=4.19, P<0.02; Figure 1, top left). However, in the object placement task, there were no differences across groups in the percentage of time spent exploring the displaced object (p=0.83; vehicle: 57.5±3.4, P4: 55.7±3.3, 3α5α-THP: 59.2±4.8). In the water maze, P4 or 3α,5α-THP, compared to vehicle, significantly decreased latencies to reach the platform quadrant (F(2,26)=4.93, P<0.01; Figure 1, bottom left). In the conditioned fear paradigm, P4 and 3α,5α-THP, compared to vehicle, tended to, and significantly increased, time spent freezing in the context and cued tests, respectively (F(2,26)=2.85, P<0.08; F(2,26)=4.52, P<0.02; Figure 1, right).
Figure 1.
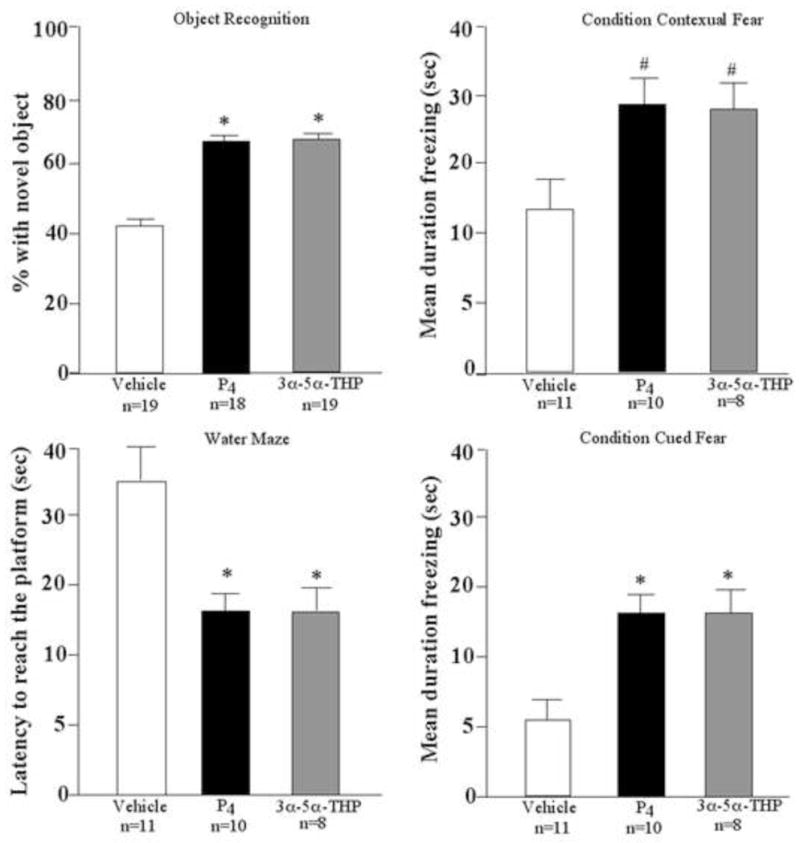
Effects of vehicle, progesterone (P4), and 3α,5α-THP for performance in the object recognition, water maze, conditioned contextual and cued fear tasks. * p < 0.05.
P4, but neither 3α,5α-THP, nor vehicle, significantly increased plasma (F(2,18)=15.37, P<0.01), hippocampus (F(2,20)=8.64, P<0.01), and cortical (F(2,20)=10.13, P<0.01) levels of P4. Plasma (F(2,18)=18.06, P<0.01), hippocampus, (F(2,20)=39.71, P<0.01), and cortex (F(2,20)=16.08, P<0.01) levels of 3α,5α-THP were significantly increased by P4 and 3α,5α-THP, compared to vehicle. See Table 1.
Table 1.
Progestogen levels in Experiments 1 and 2.
| Progesterone (P4) | 3α-5α-THP | |||||
|---|---|---|---|---|---|---|
| Plasma (ng/ml) | Hippo (ng/mg) | Cortex (ng/mg) | Plasma (ng/ml) | Hippo (ng/mg) | Cortex (ng/mg) | |
| Experiment 1 | ||||||
| Vehicle | 2.1 (±0.01) | 3.4 (±1.6) | 3.1 (±0.9) | 3.6 (±1.2) | 3.4 (±0.6) | 3.3 (±0.9) |
| P4 | 18.5 (±2.7)* | 18.6 (±3.6)* | 14.5 (±2.4)* | 17.6 (±2.1)* | 18.3 (±0.6)* | 18.8 (±1.1)* |
| 3α5α-THP | 4.2 (±1.2) | 7.4 (±1.2) | 5.8 (±1.1) | 20.6 (±1.0)* | 20.6 (±1.8)* | 16.0 (±2.4)* |
| Experiment 2 | ||||||
| Vehicle | 2.1 (±0.1) | 1.9 (±0.6) | 2.6 (±1.0) | 1.9 (±0.3) | 3.6 (±0.8) | 3.2 (±1.2) |
| MPA | 5.1 (±1.9) | 5.3 (±0.5) | 4.4 (±0.9) | 4.7 (±3.1) | 7.3 (±2.5) | 5.9 (±2.9) |
| MPA + P4 | 14.7 (±4.8)* | 16.3 (±3.3)* | 14.8 (±2.5)* | 13.0 (±2.9)# | 9.3 (±3.0) | 6.3 (±2.4) |
| MPA + 3α,5α-THP | 5.6 (±1.9) | 8.0 (±2.7) | 4.1 (±0.1) | 6.4 (±3.0) | 21.3 (±1.5)* | 18.1 (±1.7)* |
p < 0.05.
p < 0.10.
Experiment 2: MPA alone, or with P4, fails to enhance cognitive performance or increase plasma, cortical, and hippocampal 3α,5α-THP levels
Post-training 3α,5α-THP+MPA significantly increased the percentage of time spent exploring the novel object in the object recognition task, compared to vehicle, MPA, or P4+MPA (F(3,71)=2.66, P<0.05; Figure 2, top left). Yet, in the object placement task, there were no differences among groups in the percentage of time spent exploring the displaced object (p=0.55; vehicle: 49.8±4.8, MPA: 53.8±5.0, MPA+P4: 52.6±6.3, MPA+3α5α-THP: 61.6±7.3). In the water maze, post-training 3α,5α-THP+MPA significantly decreased latencies to reach the platform quadrant compared to vehicle or MPA (F(3,38)=4.02, P<0.01; Figure 2, bottom left). In the conditioned fear paradigm, 3α,5α-THP+MPA, compared to vehicle, MPA, or P4+MPA tended to, and significantly increased, time spent freezing in the context and cued tests, respectively, (F(3,38)=2.49, P<0.07; F(3,38)=3.32, P<0.03; Figure 2, right).
Figure 2.

Effects of vehicle, medroxyprogesterone acetate (MPA), MPA+progesterone (P4) and MPA+3α,5α-THP for performance in the object recognition, water maze, conditioned contextual and cued fear tasks. * p < 0.05. # p < 0.10.
Administration of P4 +MPA, but not vehicle, MPA, or 3α,5α-THP+MPA, increased P4 levels in plasma (F(3,21)=2.92, P<0.05), hippocampus (F(3,25)=5.29, P<0.01), and cortex (F(3,25)=12.60, P<0.01). Plasma (F(3,21)=2.67, P<0.07) levels of 3α,5α-THP tended to be increased, and hippocampus (F(3,25)=10.42, P<0.01) and cortex (F(3,25)=8.27, P<0.01) levels of 3α,5α-THP were significantly increased, only with 3α,5α-THP+MPA. See Table 1.
Discussion
Results support our hypothesis that P4’s cognitive-enhancing effects may be due to actions of 3α,5α-THP in the cortex and hippocampus. Treatments that increased 3α,5α-THP levels during memory consolidation (P4, 3α,5α-THP, or 3α-5α-THP+MPA) improved performance in the water maze, object recognition, and conditioned fear tasks, compared to vehicle. Thus, formation of 3α,5α-THP in the hippocampus and cortex may be essential for some of P4’s mnemonic effects.
The present results contribute to the growing literature on the influence of ovarian hormones for the neurobiology of learning and memory. These results show that P4, but not MPA, improve cognitive performance when administered post-training. These data suggest that different mechanisms of action of progestins, may underlie their mnemonic effects. P4 and MPA both bind progestin receptors; whereas, MPA also binds androgen and glucocorticoid receptors. Interestingly, P4, but not MPA, protects hippocampal neurons in culture against glutamate toxicity [18], and can prevent neuronal loss in the hilus of rats injected with kainic acid [19]. P4, but not MPA, reduces spatial learning deficits following cerebral edema of male rats [20]. P4 alone reduces brain inflammation and enhances functional recovery of mice following ischemia [21]. These data substantiate further investigation of whether there are actions at divergent receptor targets underlying the beneficial effects of progestins in the central nervous system.
The present results suggest that progestins may improve cognitive performance, in part, subsequent to conversion to 3α,5α-THP. First, similar effects were found in mice administered P4 and 3α,5α-THP (which both increased 3α,5α-THP levels), to enhance performance in the object recognition, water maze, and conditioned contextual/cued fear task. Second, MPA, alone or in conjunction with P4, neither improved cognitive performance nor increased 3α,5α-THP levels. We have previously demonstrated in other mouse strains that reduced production of 3α,5α-THP in the hippocampus negatively effects cognitive performance [5,17]. Age-related decrements in cognitive performance coincides with reduced hippocampal 3α,5α-THP levels among wildtype mice and those with Alzheimer’s Disease-related pathologies [17]. Third, in this study, P4, 3α,5α-THP, or 3α,5α-THP+MPA, enhanced cognitive performance and increased 3α,5α-THP levels in brain areas associated with cognitive tasks. Notably, P4 and MPA, can bind progestin receptors, but 3α,5α-THP does not. Indeed, regimen that enabled 3α,5α-THP formation enhanced cognitive performance, irrespective of their capacity to bind progestin receptors. 3α,5α-THP can have rapid actions at neurotransmitter targets, such as glutamate [22,23,24], that may underlie these effects. Thus, cognitive effects may be independent of progestogens’ acute actions at progestin receptors and, instead, involve formation of 3α,5α-THP, and rapid, downstream actions at neurotransmitter targets to initiate signal transduction cascades.
Short conclusion
Post-training administration of the synthetic progestin, MPA, unlike P4 or 3α,5α-THP, was ineffective in enhancing cognitive performance in young mice or increasing cortical and hippocampal 3α,5α-THP levels during memory consolidation. MPA and P4 bind progestin receptors, but 3α,5α-THP does not. The beneficial effects of progestins may be independent of intracellular progestin receptors, and subsequent to production of 3α,5α-THP, to improve cognitive function. Further investigation of the mechanisms of progestins in the cortex and hippocampus are important for elucidating neurobiological factors underlying sex/gender differences, and age-related decline, in cognitive processes.
Acknowledgments
The assistance of L. Avalone and D. Osborne is appreciated.
References
- 1.Gao S, Hendrie HC, Hall KS, Hui S. The relationship between age, sex, and the incidence of dementia and Alzheimer’s Disease: a meta-analysis. Arch Gen Psych. 1998;55:809–15. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JG, Buckwalter DK, Bluestein BW, Stanczyk FZ. Pregnancy and post partum: changes in cognition and mood. Prog Brain Res. 2001;133:303–19. doi: 10.1016/s0079-6123(01)33023-6. [DOI] [PubMed] [Google Scholar]
- 3.Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–15. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008;90:171–7. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harburger LL, Pechenino AS, Saadi A, Frick KM. Post-training progesterone dose-dependently enhances object, but not spatial, memory consolidation. Behav Brain Res. 2008;194:174–80. doi: 10.1016/j.bbr.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis MC, Orr PT, Frick KM. Differential effects of acute progesterone administration on spatial and object memory in middle-aged and aged female C57BL/6 mice. Horm Behav. 2008;54:455–62. doi: 10.1016/j.yhbeh.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walf AA, Koonce CJ, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–60. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. WHIMS Investigators, Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 9.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. WHIMS Investigators, Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in post-menopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 10.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, et al. WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 11.Reboussin BA, Greendale GA, Espeland MA. Effect of hormone replacement therapy on self-reported cognitive symptoms: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. Climacteric. 1998;1:172–9. doi: 10.3109/13697139809085538. [DOI] [PubMed] [Google Scholar]
- 12.Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psych. 2000;1158:227–33. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- 13.Bentel JM, Birrell SN, Pickering MA, Holds DJ, Horsfall DJ, Tilley WD. Androgen receptor agonist activity of the synthetic progestin, medroxyprogesterone acetate, in human breast cancer cells. Mol Cell Endocrinol. 1999;154:11–20. doi: 10.1016/s0303-7207(99)00109-4. [DOI] [PubMed] [Google Scholar]
- 14.Wiegratz I, Kuhl H. Progestogen therapies: differences in clinical effects? Trends in Endocrinology and Metabolism. 2004;15:277–85. doi: 10.1016/j.tem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Frye CA, Walf AA. Progesterone enhances learning and memory of aged wildtype and progestin receptor knockout mice. Neurosci Lett. 2010;472:38–42. doi: 10.1016/j.neulet.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belelli D, Herd MB. The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–20. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frye CA, Walf AA. Effects of progesterone administration and APPswe+PSEN1Deltae9 mutation for cognitive performance of mid-aged mice. Neurobiol Learn Mem. 2008;89:17–26. doi: 10.1016/j.nlm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinol. 2002;143:205–12. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 19.Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–28. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- 20.Wright DW, Hoffman SW, Virmani S, Stein DG. Effects of medroxyprogesterone acetate on cerebral oedema and spatial learning performance after traumatic brain injury in rats. Brain Inj. 2008;22:107–13. doi: 10.1080/02699050701867399. [DOI] [PubMed] [Google Scholar]
- 21.Gibson CL, Gray LJ, Bath PM, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131:318–28. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- 22.Frye CA. Neurosteroids-From Basic Research to Clinical Perspectives. In: Rubin Robert T, Pfaff Donald W., editors. Hormones/Behavior Relations of Clinical Importance. San Diego: Academic Press; 2009. pp. 395–416. [Google Scholar]
- 23.Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–13. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbs TT, Yaghoubi N, Weaver CE, Park-Chung M, Russek SJ, Farb DH. Modulation of ionotropic glutamate receptors by neuroactive steroids. In: Baulieu EE, Robel P, Schumacher M, editors. Contemporary Endocrinology: Neuosteroids: A New Regulating Function In the Nervous System. Totowa NJ: Humana Press; 1999. pp. 167–190. [Google Scholar]


