Abstract
Objective
Previous findings from our laboratory demonstrated that neo-vascularization was impaired in osteopontin (OPN) knockout animals. However, the mechanisms responsible for regulation of OPN expression in the setting of ischemia remain undefined. Therefore, we sought to determine if OPN is upregulated in response to ischemia and hypothesized that H2O2 is a critical component of the signaling mechanism by which OPN expression is upregulated in response to ischemia in vivo.
Methods and Results
To determine if ischemic injury upregulates OPN, we used a murine model of hind limb ischemia. Femoral artery ligation in C57Bl/6 mice significantly increased OPN expression and H2O2 production. Infusion of C57Bl/6 mice with PEG-catalase (10,000 U/kg/day) or the use of transgenic mice with smooth muscle cell specific catalase overexpression blunted ischemia-induced OPN, suggesting ischemia-induced OPN expression is H2O2-dependent. Decreased H2O2-mediated OPN blunted reperfusion and collateral formation in vivo. In contrast, the overexpression of OPN using lentivirus restored neovascularization.
Conclusions
Scavenging H2O2 blocks ischemia-induced OPN expression, providing evidence that ischemia-induced OPN expression is H2O2-dependent. Decreased OPN expression impaired neo-vascularization, whereas overexpression of OPN increased angiogenesis, supporting our hypothesis that OPN is a critical mediator of post-ischemic neo-vascularization and a potential novel therapeutic target for inducing new vessel growth.
Keywords: Osteopontin, Reactive Oxygen Species, Ischemia, Angiogenesis, Collateral Circulation
The occlusion of blood vessels ultimately leads to ischemia, initiating multiple processes that promote neo-vascularization as a compensatory mechanism to restore blood flow and preserve tissue function. The ability to develop new collaterals is strongly associated with reduced long-term cardiac mortality in patients with acute myocardial infarction and stable coronary artery disease.1 The formation of new collaterals is a multi-factorial process that requires cytokines, the infiltration of inflammatory cells, cell proliferation, cell migration, and matrix remodeling. Several of these processes are modulated by the presence of osteopontin (OPN),2, 3 a secreted, phosphorylated matricellular protein critical for neo-vascularization.4, 5
OPN is important for normal arterial physiology6 and is expressed by multiple cell types including monocytes/macrophages, endothelial cells, and smooth muscle cells (SMCs),7 all of which play a role in the neo-vascularization process. OPN mediates several processes relevant to collateral formation, including cell survival,8 cell adhesion,9, 10 and migration.9, 11 Additionally, wound healing, a process that requires angiogenesis, is significantly impaired in OPN-/- mice,4, 12 strongly supporting a role for OPN in angiogenesis. Moreover, our group previously demonstrated a direct role for OPN in post-ischemic neo-vascularization by showing dramatically impaired collateral formation in OPN-/- mice compared to wild-type controls in a murine model of hind limb ischemia (HLI).5
OPN is a non-collagenous, phosphorylated glycoprotein thought to mediate cellular functions by providing a link between cell surface receptors and structural extra-cellular matrix molecules.13 OPN functions in a variety of biological processes and signals by binding to cell surface integrins through a conserved RGD domain or the SVVYGLR binding domain, which is exposed when OPN is cleaved by thrombin or MMPs.13 OPN is also a ligand for the CD44 receptor.14 Additionally, OPN promotes the migration of multiple cell types, including macrophages,15 endothelial cells,9, 10 and vascular smooth muscle cells.9, 10, 16 An alternative translation start site in the OPN messenger RNA generates two OPN isoforms: a secreted form and an intracellular form (iOPN).17 Both secreted and intracellular OPN are linked to cell migration, where iOPN localizes to the cell membrane and associates with CD4418 and secreted OPN regulates cell responses through association with integrin receptors and the CD44 receptor.
Reactive oxygen species (ROS), such as superoxide (O2•-) and hydrogen peroxide (H2O2), are involved in physiological and pathophysiological responses. When produced in excess, ROS promote cell injury and disease pathologies. In contrast, at physiologic levels, ROS function as second messengers and regulate redox signaling pathways that modulate cell migration, proliferation, and matrix remodeling, all of which are necessary events for neovascularization.19-22 A recent study by Tojo, et. al. provided a direct link between NADPH oxidases and post-ischemic neo-vascularization in a murine model of HLI. The study demonstrated that animals lacking gp91phox, a subunit of the Nox2 NADPH oxidase, produce less ROS and form fewer collaterals compared to wild-type animals.23
While several recent publications link ROS to OPN expression in vitro,24-26 little is known regarding the role of ROS in regulating OPN expression in vivo. Furthermore, the precise molecular identity of the relevant ROS remains unclear. The goals of this study were to determine if OPN is upregulated in response to ischemia, if increased ROS production is the mechanism by which OPN expression is increased, and to define the molecular identity of the relevant ROS involved in OPN expression in vivo. In this study, we demonstrate that OPN is upregulated in response to ischemia using a murine model of hind limb ischemia. Femoral artery ligation in C57Bl/6 mice significantly increased OPN expression and H2O2 production in the ischemic leg (IL), compared to the non-ischemic leg (NIL). Using pharmacologic and transgenic approaches to scavenge H2O2, we demonstrate that ischemia-induced OPN expression is H2O2-dependent. Infusion of mice with PEG-catalase (10,000 U/kg/day) or the use of transgenic mice with SMC-specific catalase overexpression (TgSMC-Cat) significantly blunted ischemia-induced OPN expression, resulting in delayed reperfusion and impaired collateral formation in response to ischemia. In addition, using the same model of TgSMC-Cat mice, when we utilized lentiviral delivery to overexpress OPN in the ischemic limb, we were able to completely restore neo-vascularization in the ischemic limb. Altogether, the results presented herein define a novel mechanism for the regulation of OPN expression in vivo, establish an absolute requirement for H2O2-dependent OPN for effective collateral formation, and support the concept that OPN is a critical mediator of neo-vascularization. Therefore, OPN may be a novel therapeutic target for promoting collateral vessel formation.
MATERIALS and METHODS
A detailed, expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Animals
Male C57BL/6 mice were purchased from Jackson Laboratories (Maine, USA). TgSMC-Cat mice were bred in house in the Department of Animal Resources at Emory University. TgSMC-Cat mice have increased expression of human catalase through the SMC-specific smooth muscle myosin heavy chain (MHC) promoter and were characterized previously.27 For all experiments TgSMC-Cat mice were compared to wild-type littermates. In some experiments, PEG-Catalase (10,000 U/kg/day) dissolved in saline was delivered by intravenous infusion via osmotic mini-pump. PEG-catalase was infused continuously from time of HLI surgery until the designated time points. All animals used were male and between 8 and 10 weeks of age. The animals were housed and cared for according to the guidelines approved by the Emory University Institutional Animal Care and Use Committee.
Osmotic Mini-pump Implantation
Mice were anesthetized using 3% isofluorane (oxygen delivered at 0.5L/min with 3% isofluorane for induction and 2.0% isofluorane for maintenance). A catheter attached to a primed osmotic mini-pump (Alzet osmotic mini-pump, model 1007D, Durect Corporation, Cupertino, CA) was inserted into the jugular vein and the pump inserted subcutaneously. Mice were administered buprenex (0.01-0.1mg/kg, SC) as needed.
Hind Limb Ischemia Surgery
Mice were anesthetized with 3% isoflurane in a chamber and then anesthetized with 2% isoflurane through a nose cone. Ligation and excision of the left superficial femoral artery was performed as described previously.5, 28
LASER Doppler Perfusion Imaging
LASER Doppler perfusion imaging (LDPI) was performed as described previously.5, 28 Briefly, mice were anesthetized by inhalation of 2% isofluorane and scanned with the LDPI system (PIM II LASER Doppler Perfusion Imager). Perfusion of the proximal region was quantified and normalized to the non-surgical limb.
Micro-CT Imaging
Quantitative micro-CT was used for evaluation of collateral vessel formation in the ischemic limb at postoperative day 5 as described previously.5, 28. Briefly, mice were euthanized (n=6-9 for each group) and sequentially perfused with saline containing 4 mg/mL of papaverine, 10% formalin, followed by a lead chromate-based contrast agent (Flow Tech, Inc, South Windsor, CT). Bone was de-mineralized in a formic acid based solution (Cal-Ex II, Fisher Scientific, Pittsburgh, PA) for 48 hours. Samples were then imaged at a 30μm voxel size, and the tomograms were used to render binarized 3-D images. Stereological algorithms were used to quantify vascular volume to tissue volume ratio, connectivity, and vascular density, which were then normalized to the contralateral control limb.
Lentivirus
The lentiviral vector was derived from the HIV-based lentivirus backbone pLV-CMVGFP-U3Nhe, as described previously.29-31 pLV-CMV-GFP-U3Nhe control vector will hereafter be referred to as LV-GFP. We generated a dual-tagged human osteopontin construct with an HA-tag at the N-terminus and myc-tag at the C-terminus. We inserted a wild-type internal ribosome entry site (IRES),32 followed by dual-tagged OPN, downstream of GFP to generate a lentivirus that expresses both GFP and tagged OPN, hereafter referred to as LV-OPN. The wild-type IRES used allows high translation of the downstream compared to the upstream coding sequence in the same mRNA,32 thereby allowing the final construct to express high OPN and relatively lower GFP protein.
Viral production procedures have been described in detail previously.29, 33, 34 For in vivo use, both lentiviruses were used at a final concentration of ~1×109 infectious particles/mL. All animals received HLI, after which the IL adductor was injected with 20 μL of LV-GFP or LV-OPN lentivirus using a similar approach as that described previously.29
Detection of ROS
The production of O2•- was evaluated by measuring the conversion of DHE to 2-hydroxyethidium using HPLC, as described previously.35 Superoxide production was normalized to protein concentration. H2O2 production was measured using the Amplex Red Assay (Invitrogen, Carslbad, CA), as described previously.35 H2O2 production was normalized to tissue wet weight.
Immunofluoresence
Mice were sacrificed and tissues were perfused with saline and fixed with 10% buffered formalin. Sections from paraffin embedded hind limbs were cut in 5 μm increments. Antigen retrieval was performed in citrate buffer, pH 6.0 (Invitrogen, Carlsbad, CA), prior to incubation with OPN antibody (1:200 in 3% BSA), followed by incubation with anti-mouse or anti-goat secondary antibody (1:400 in 3% BSA; Vector Labs, Burlingame, CA), and incubation with Streptavadin QDot 655 (1:200 in 3% BSA; Invitrogen, Carlsbad, CA). Images were acquired on a Zeiss Axioskop microscope equipped with an AxioCam camera.
Immunoblotting
Adductor muscle tissues and cultured SMCs were lysed in Hunter’s buffer.35 Briefly, tissues were homogenized with glass mortar and pestle and cell samples were sonicated at 10 watts for 10 seconds. Whole tissue or cell lysates were used for immunoblotting. The osteopontin antibodies used were from Santa Cruz Biotechnology (Santa Cruz, CA) and R&D Systems (Minneapolis, MN). GAPDH antibody was also obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The β-actin antibody was from Cell Signaling (Danvers, MA). Band intensity was quantified by densitometry using ImageJ 1.38 software and expressed/normalized to β-actin or GAPDH.
Cell Culture
Murine aortic SMCs (passages 4-10) were cultured in low-glucose Dulbecco’s Modified Eagle’s Media (DMEM; Sigma Aldrich, Saint Louis, MO) supplemented with 10% fetal bovine serum (FBS; Sigma Aldrich, Saint Louis, MO), 2 mM L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin, all acquired from Mediatech, Inc. (Manassas, VA). Cells were stimulated with H2O2 (Sigma Aldrich, Saint Louis, MO) after 48 hours of quiescence in serum-free DMEM for all experiments.
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted from muscle tissue or cells using the RNeasy kit (Qiagen, Valencia, CA). OPN and 18S rRNA, were measured by amplification of cDNA using the thermocycler (Applied Biosystems) and SYBR green dye. Copy number was calculated by the instrument software from standard curves of genuine templates. OPN copy number was normalized to 18S rRNA.
Statistical Analysis
Results are expressed as mean ± S.E.M. from at least three independent experiments. Statistical significance for quantitative results was assessed using analysis of variance (ANOVA), followed by Bonferroni’s Multiple Comparison post-hoc test. In some cases, a Students’ t-test was used to assess significance. A value of p<0.05 was considered statistically significant.
RESULTS
Effects of Hind Limb Ischemia on Osteopontin Expression
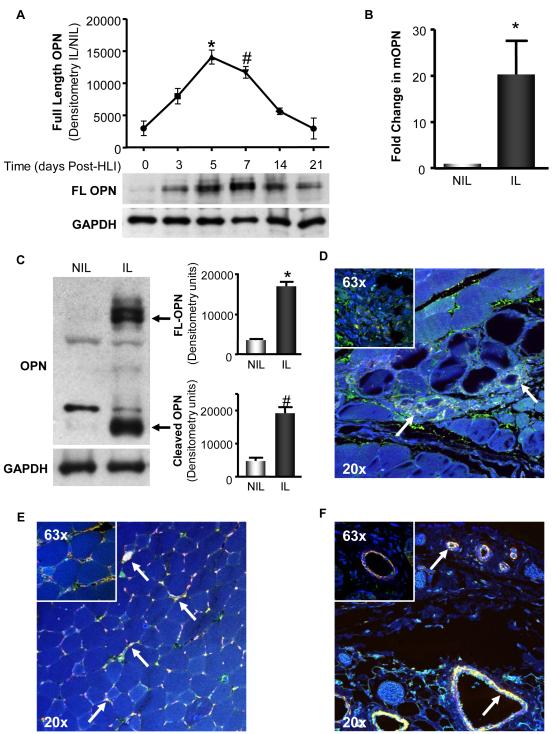
Our previous study demonstrated that OPN-/- mice exhibit impaired collateral formation.5 Therefore, we set out to determine if OPN is upregulated in response to ischemia by utilizing amurine model of HLI in which the femoral artery was ligated and excised, thus providing a stimulus for collateral formation. We measured OPN protein expression in the adductor muscles of the IL and NIL at 0, 3, 5, 7, 14, and 21 days post-HLI. As shown in Figure 1A, full-length (FL) OPN protein expression in the IL peaked between 5 and 7 days post-HLI, followed by a significant decline thereafter. Therefore, we investigated the effects of HLI on OPN expression at 5 days post-surgery. To evaluate if ischemia increases OPN mRNA levels, we used qRT-PCR to measure OPN message in the adductor muscles of the IL and NIL after 5 days. OPN mRNA levels were increased 20-fold in the IL compared to NIL (Figure 1B). OPN protein expression in the IL was also significantly increased compared to NIL, as measured by Western blot (WB) (Figure 1C) and immunofluoresence (IF) (Figure 1D). The ischemia-induced increases in OPN protein appeared as multiple bands by WB, presumably due to post-translational modification. Full-length OPN is a complex matricellular protein that is glycosylated, phosphorylated, and cleaved by other proteins after secretion into the extracellular space.36 We detected an increase in both full-length, modified OPN (upper bands in 1C), as well as cleaved OPN (lower band in 1C).
Figure 1. Hind Limb Ischemia Increases OPN expression in the Proximal Region of the Ischemic Limb – Contributing Cell Types Include Macrophages, Endothelial Cells, and Smooth Muscle Cells.
Femoral artery ligation was performed on C57Bl/6 mice and OPN expression was assessed in the adductor muscles of the non-ischemic (NIL) and ischemic limbs (IL) at the time points indicated. A. Full-length (FL) OPN protein expression in the IL was measured by Western blot (WB) at 0, 3, 5, 7, 14, and 21 days post-surgery. (*p<0.05 vs. day 0, 3, 14, & 21; #p<0.05 vs. day 0, 14, & 21; n=3-4). GAPDH demonstrates equal loading. B. OPN mRNA was assessed by qRT-PCR (*p<0.05, n=3). C. FL and cleaved OPN protein expression at 5d was measured by WB. (FL, *p<0.001; cleaved, #p<0.05; n=4). GAPDH demonstrates equal loading. D-F. OPN expression and distribution was assessed in the adductor muscles of the IL at 5 days in C57Bl/6 mice. In all panels, DAPI appears blue and stains the nuclei, OPN appears green, the designated cell marker appears as red, and co-localization is yellow and denoted with arrows. All panels include an image taken at 20x, to show anatomical structure, and 63x, to show co-localization detail. D. OPN (green) co-localizes with Mac3 (red), a marker for macrophages. E. OPN (green) co-localizes with lectin (red), a marker for endothelial cells. F. OPN (green) co-localizes with smooth muscle α-actin (red), a marker for SMCs. Bars are means ± S.E.M.
Multiple cells types involved in the process of collateral formation are reported to express OPN in other systems. To determine the specific cell types that contribute ischemia-induced increases in OPN expression at 5 days post-ischemia, we performed a series of immunofluorescence co-staining experiments. As shown in Figure 1D-E, multiple cell types express OPN in response to ischemia. OPN clearly co-localizes with Mac3 (Figure 1D), a marker of macrophages, with lectin (Figure 1E), a marker of endothelial cells, and with smooth muscle α-Actin (Figure 1F), a marker of SMCs. Taken together, these data demonstrate that ischemia increases OPN expression at the mRNA and protein levels, and that multiple cell types, including macrophages, endothelial cells, and smooth muscle cells, contribute to ischemia-induced increases in OPN expression.
Effects of Hind Limb Ischemia on Reactive Oxygen Species Production
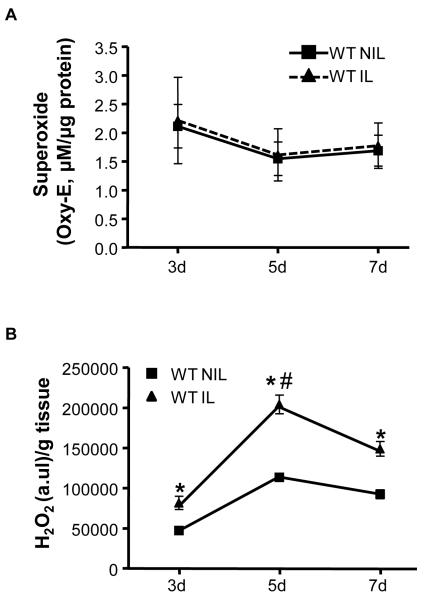
To determine what ROS, if any, are increased in response to ischemic injury and to establish a time course for ROS production in this model, we used dihydroethidium (DHE)-HPLC and the Amplex Red Assay to measure O2•- and H2O2 production, respectively. ROS were measured in the adductor muscles of the NIL and IL of C57Bl/6 mice at postoperative days 3, 5, and 7. There were no detectable differences in O2•- between the IL and NIL at any time point (Figure 2A). In contrast, H2O2 production was significantly increased in response to ischemia at all time points (Figure 2B), with a peak in H2O2 at 5 days.
Figure 2. Hind Limb Ischemia Increases ROS in the Proximal Region of the Ischemic Hind Limb.
ROS production was assessed in the adductor muscles of the non-ischemic (NIL) and ischemic limbs (IL) of C57Bl/6 mice at postoperative day 3, 5, and 7. A. O2•- production was measured using DHE-HPLC. (ns; n=5-6) B. The Amplex Red Assay was used to measure H2O2 levels. H2O2 measurements were normalized to tissue wet weight. H2O2 was increased in the IL compared to the NIL at 3, 5, and 7 days (*p<0.001), with a peak at 5d (#p<0.05 vs. 3d). n=3-6. Bars are means ± S.E.M.
H2O2 Stimulates Osteopontin Expression In Vivo
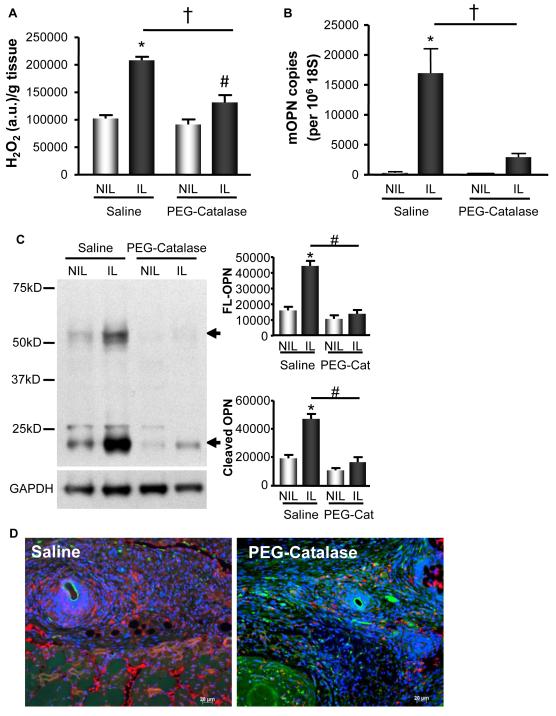
Since we have demonstrated that in response to ischemia there is a significant increase in H2O2 and that OPN levels are increased in response to ischemia, we set out to determine if the increases in OPN are H2O2-dependent. We performed HLI on C57Bl/6 mice infused with saline or PEG-catalase (10,000 U/kg/day), which converts H2O2 to water and other metabolites, thus diminishing H2O2. PEG-catalase blunted ischemia-induced H2O2 production compared to saline infused animals (Figure 3A), as measured by Amplex Red Assay. This decrease in H2O2 production in the IL substantially blocked ischemia-induced OPN expression at the mRNA (Figure 3B). In addition, we found a similar corroborating result at the protein level when measured by WB and IF (Figure 3C & 3D). Taken together, these data support our hypothesis that the increase in OPN expression is response to ischemia is H2O2-dependent.
Figure 3. OPN Expression in the Proximal Region of the Ischemic Limb is H2O2-dependent.
H2O2 production and OPN expression were measured in the adductor muscles of the non-ischemic (NIL) and ischemic limbs (IL) at postoperative day 5 in C57Bl/6 mice infused with Saline or 10,000 U/kg/day PEG-Catalase (PEG-Cat). A. H2O2 was measured by Amplex Red Assay. H2O2 was increased in the IL of both treatment groups (*p<0.001 vs. Saline NIL; #p<0.05 vs. PEG-Catalase NIL); however, PEG-catalase blunted ischemia-induced H2O2 compared to control (†p<0.001). n=5. B. OPN mRNA levels were measured by qRT-PCR. OPN was increased in the IL of saline controls (*p<0.001 vs. Saline NIL), but not animals infused with PEG-catalase. PEG-catalase treatment blocked increased OPN mRNA in the IL (†p<0.01). n=5. C. Full-length (FL) and cleaved OPN protein expression in the IL of saline infused animals were increased at 5d by WB analysis (FL, *p<0.001; cleaved, *p<0.001; n=8). PEG-catalase (PEG-Cat) treatment blunted this increase (FL, #p<0.0001; cleaved, #p<0.0001; n=8). GAPDH demonstrates equal loading. D. PEG-catalase decreased OPN protein expression in the IL compared to control at 5d, as detected by immunofluorescence. Blue=DAPI-nuclei, Red=OPN, Green=autofluorescence. Bars are means ± S.E.M.
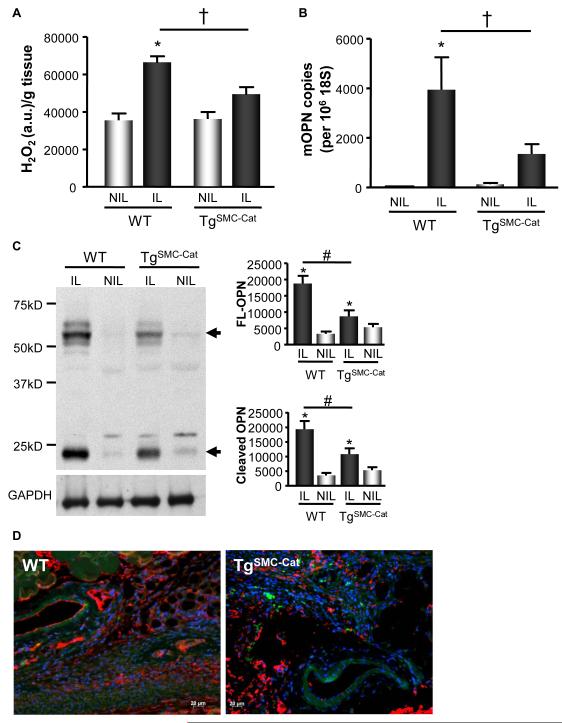
Multiple cells types involved in ischemia-induced collateral formation express OPN in response to ischemia, including SMCs (Figure 1F). SMCs are also known to generate ROS and play a vital role in arteriogenesis. To confirm and further substantiate that ischemia-induced OPN expression is H2O2-dependent, and to verify that SMCs may be a substantial contributor to H2O2-dependent OPN expression in the ischemic hind limb, we utilized transgenic mice that overexpress catalase specifically in their SMCs (TgSMC-Cat). We then measured ROS and OPN expression in these mice. We found that TgSMC-Cat mice have significantly diminishedischemia-induced H2O2 production compared to wild-type controls (Figure 4A). This decrease in H2O2 production mediated by SMC-specific catalase overexpression blunted ischemia-induced increases in OPN mRNA (Figure 4B). Additionally, OPN protein levels were substantially blunted in TgSMC-Cat animals compared to wild-type controls, as measured by Western blot and IF (Figures 4C & 4D). Taken together, these data support that ischemia-induced OPN expression is H2O2-dependent and that SMC-derived H2O2 significantly contributes to ROS-dependent OPN expression.
Figure 4. OPN Expression in the Proximal Region of the Ischemic Limb is Decreased by SMC-specific Catalase Overexpression.
H2O2 production and OPN expression were measured at postoperative day 5 days in TgSMC-Cat or wild-type (WT) littermates. A. H2O2 production was increased in the IL of WT and TgSMC-Cat animals, as measured by Amplex Red (*p<0.001); however, TgSMC-Cat animals exhibit decreased H2O2 production in the IL compared to WT (†p<0.0001). H2O2 measurements were normalized to tissue wet weight. n=5. B. OPN mRNA levels were increased in the WT IL (*p<0.001 vs. NIL); however, OPN mRNA levels in the TgSMC-Cat IL was not increased compared to the NIL. TgSMC-Cat animals had decreased OPN mRNA in the IL compared to WT (†p<0.05). n=6. C. Full-length (FL) and cleaved OPN protein expression in the WT IL was increased at 5d by WB analysis (FL, *p<0.001; cleaved, *p<0.001; n=5). This increase was blunted in TgSMC-Cat animals (FL, #p<0.01; cleaved, #p<0.01; n=5). GAPDH demonstrates equal loading. D. OPN expression in the TgSMC-Cat IL is decreased compared to WT IL at 5d by immunofluorescence. Blue=DAPI - nuclei, Red=OPN, Green=autofluorescence. Bars are means ± S.E.M.
H2O2-dependent OPN Mediates Collateral Formation
To determine the functional importance H2O2 and OPN on collateral formation, we used LDPI to evaluate reperfusion and Micro-CT to quantify collateral formation in the IL. LDPI images acquired at postoperative day 5 clearly illustrate that PEG-catalase infusion, which decreased ischemia-induced OPN expression and H2O2 levels, impaired reperfusion (Supplemental Figure 2A). Quantitative analysis illustrates a significant lag in perfusion recovery in the IL of PEG-catalase infused animals (Supplemental Figure 2B). Quantitative analysis of Micro-CT images (Supplemental Figure 2C) revealed PEG-catalase animals have a 19.4% reduction in vascular volume (saline 1.3±0.1 vs. PEG-catalase 1.0±0.1; normalized to tissue volume), 8.1% decrease in vascular density (saline 1.2±0.03 vs. PEG-catalase 1.0±0.02), and a 41.0% reduction in connectivity (saline 2.6±0.40 vs. PEG-catalase 1.5±0.2) compared to controls. Together, these data demonstrate that H2O2 increases OPN expression, which has a direct effect in the formation of collateral vessels after HLI injury.
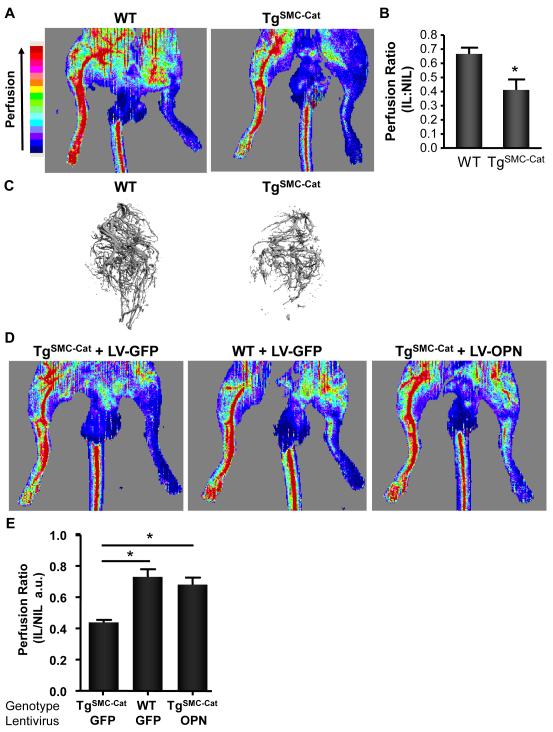
To determine the functional importance SMC-derived H2O2 and OPN on collateral formation, we used LDPI to evaluate reperfusion and Micro-CT to quantify collateral formation in the IL of TgSMC-Cat animals using LDPI. TgSMC-Cat animals showed significantly decreased reperfusion compared to wild-type animals (Figure 5A-B). Quantitative analysis of Micro-CT images (Figure 5C) revealed TgSMC-Cat mice have a 34.6% reduction in vascular volume (WT 1.23±0.16 vs. TgSMC-Cat 0.81±0.06), 14.2% decrease in vascular density (WT 1.04±0.04 vs. TgSMC-Cat 0.89±0.04), and a 40.9% reduction in connectivity (WT 1.45±0.20 vs. TgSMC-Cat 0.85±0.09) compared to controls. Altogether, these data suggest that SMC-derived H2O2 significantly contributes to ROS-dependent OPN expression and neo-vascularization.
Figure 5. H2O2-dependent OPN Mediates Collateral Formation.
LASER Doppler Perfusion Imaging (LDPI) was used to evaluate perfusion and Micro-CT to quantify collateral formation in the IL. Perfusion and collateral formation was assessed in the proximal regions of the IL and normalized to the NIL. All measurements were performed at 5d post-surgery. Ischemic legs were compared between treatment groups. A. Representative LDPI tracings from WT and TgSMC-Cat animals at day 5. B. Quantitative analysis of WT and TgSMC-Cat LDPI tracings (*p<0.05, n=5). C. Representative Micro-CT angiographs from WT and TgSMC-Cat mice at postoperative day 5. To determine the relative contribution of OPN to collateral formation compared to other H2O2-dependent pathways, we performed an OPN add-back experiment. TgSMC-Cat+LV-GFP treatment group had less reperfusion than WT+LV-GFP mice. Add-back of OPN to TgSMC-Cat mice using LV-OPN (TgSMC-Cat+LV-OPN) restored collateral formation and perfusion to the level of WT animals. D. Representative LDPI tracings from WT+LV-GFP control, TgSMC-Cat+LV-GFP, and TgSMC-Cat+LV-OPN treated animals. E. Quantitative analysis of WT+LV-GFP control, TgSMCCat+LV-GFP, and TgSMC-Cat+LV-OPN LDPI tracings. (*p<0.001 vs. TgSMC-Cat+LV-GFP, n=6). Bars are means ± S.E.M.
ROS influence the expression of several inflammatory factors in this model, including monocyte chemoattractant protein (MCP)-1 (Supplemental Figure 1), tumor necrosis factor (TNF)-α (Supplemental Figure 1), and OPN (Figures 3 & 4). Therefore, to determine the relative contribution of OPN to collateral formation compared to other targets downstream of H2O2, we designed an experiment to add OPN back to the ischemic limb. To do this, we developed a lentivirus to overexpress Myc-tagged OPN (LV-OPN). We then used this lentivirus to perform an add-back experiment to determine what effects observed in our TgSMC-Cat model were due specifically to the loss of H2O2-dependent OPN expression versus the loss of other H2O2-dependent proteins. We directly injected the adductor muscles of the IL with either control lentivirus (LV-GFP) or LV-OPN. We verified successful transduction of cells in the adductor muscle by immunofluoresence staining for Myc or GFP (Supplemental Figure 3).29 As expected, the TgSMC-Cat+LV-GFP treatment group had less reperfusion than the WT+LV-GFP group, such as that observed in Figure 5A. Interestingly, the add-back of OPN to TgSMC-Cat animals using LV-OPN (TgSMC-Cat+LV-OPN) restored collateral formation and reperfusion to a level similar to that seen in WT+LV-GFP control animals (Figure 5D-E). These data strongly support OPN as a critical mediator of post-ischemic neo-vascularization.
H2O2 Increases OPN Expression In Vitro
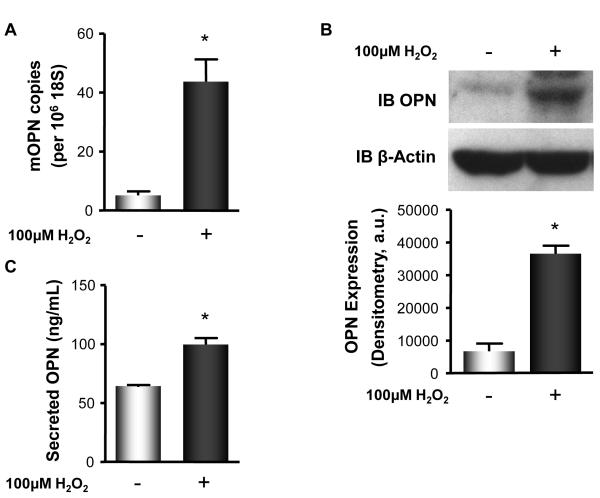
Our data demonstrate that ischemia-induced OPN expression in a murine model of HLI is H2O2-dependent and that SMC-derived H2O2 promotes increased OPN expression in the IL. To determine if H2O2 directly mediates an increase in OPN expression, we used an in vitro system in which MASMs were quiesced for 48 hours prior to stimulation with 100μM H2O2 for 4 hours. Stimulation of MASMs with H2O2 significantly increased OPN mRNA expression (Figure 6A), cellular OPN protein expression (Figure 6B), and secreted OPN protein expression (Figure 6C) compared to control. Taken together, these data demonstrate that OPN expression can be directly induced by H2O2 in vitro.
Figure 6. H2O2 Increases OPN Expression in Mouse Aortic Smooth Muscle Cells.
Murine SMCs were used as an in vitro system. SMCs were quiesced for 48 hours prior to no treatment ( − ) or stimulation ( + ) with 100 μM H2O2 for 4 hours. Cells were harvested for mRNA and protein and DMEM collected to assess secreted OPN by ELISA. A. H2O2 stimulation increased OPN mRNA expression (*p<0.01), B. cellular OPN protein expression (*p<0.0001), measured by WB, and C. secreted OPN protein expression, measured by ELISA (*p<0.001). Bars are means ± S.E.M., n=4.
DISCUSSION
In this study, we demonstrate that ischemia-induced OPN is H2O2-dependent and is necessary to promote neo-vascularization. We not only demonstrate that OPN expression in the IL is increased, but that the removal of H2O2 via pharmacologic or transgenic approaches significantly blunts H2O2-dependent OPN expression in vivo and results in substantially impaired reperfusion and collateral formation. Our results define a novel mechanism for the regulation of OPN expression by H2O2, demonstrate that H2O2-dependent OPN expression is a critical mediator of post-natal neo-vascularization and implicate OPN as a potential therapeutic target for modulating collateral vessel formation.
A recent study provided a direct link between NADPH oxidases, which produce ROS, and post-ischemic neo-vascularization in a murine model of HLI.23 However, the role of specific ROS species, as well as the major cellular sources of ROS, remains unclear. Superoxide has been implicated in the process of neo-vascularization,23, 37-39 where the contributions of O2•- as a positive or negative contributor to the neo-vascularization process depends on the overall level of oxidative stress. The role of H2O2 in angiogenesis has been studied in vitro, where it induces the formation of tube-like structures by endothelial cells19 and promotes the proliferation and migration of endothelial cells and SMCs.13-16 However, a role for H2O2 in the process of collateral vessel formation had yet to be established. Our data show that at 5 days post-HLI, H2O2 production in the IL of C57Bl/6 animals was significantly increased (Fig. 2B). In contrast, no significant increases in O2•- were detected in response to ischemia (Fig. 2A). Our data clearly implicate a central role for H2O2 in the neo-vascularization process, where both PEG-catalase infused mice and TgSMC-Cat animals, which have decreased H2O2 production (Fig. 3A & 4A), exhibit impaired blood flow and neo-vascularization in response to ischemia (Sup.Fig. 2A and Fig. 5A). A recent study from our laboratory established myeloid lineage cells as one source of H2O2 in the collateral formation process.28 The new data presented herein provide evidence that SMC-derived H2O2 promotes neo-vascularization, as demonstrated by the impaired reperfusion and collateral formation seen in TgSMC-Cat mice (Fig. 5A-C). These data provide strong evidence that neo-vascularization in response to ischemia is attributable to the effects of H2O2.
OPN is expressed by multiple cell types including monocytes and macrophages, ECs, and SMCs,7 all of which play a role in the neo-vascularization process. OPN mediates several processes relevant to collateral formation, including cell survival,8 cell adhesion,9, 10 and migration.9, 11 Additionally, our lab has previously established OPN as a critical component of the neo-vascularization process, where OPN-/- mice exhibit dramatically impaired collateral formation in response to ischemia.5 Our new data show a strong up-regulation of OPN in response to ischemia (Fig. 1). The increase in OPN mRNA levels in response to ischemia at 5 days was >20-fold. Increased OPN mRNA also translated into substantial increases in OPN protein (Fig. 1). Additionally, we present evidence that multiple cells types contribute to ischemia-induced increases in OPN expression (Fig. 1D-F), including macrophages, endothelial cells, and smooth muscle cells. This strong up-regulation in OPN expression in response to ischemia, paired with the lack of collateral formation in OPN-/- mice, define OPN as a critical mediator of the processes required for neo-vascularization. Therefore, it becomes important to fully understand how OPN expression is regulated in this setting and how OPN may function to promote collateral formation.
How OPN functions to increase collateral formation remains largely unknown. One mechanism by which OPN signals is through its RGD domain, which is exposed after OPN cleavage by thrombin or MMPs, and allows OPN to bind multiple integrin subtypes. Another potential mechanism by which OPN may increase collateral formation is through its ability to bind CD44, which does not require OPN cleavage. As shown in Figure 1C, we observe substantial increases in both full-length and cleaved OPN in response to ischemia. However, it remains unclear what functional domains of OPN are required to increase neo-vascularization. Future studies will investigate the precise mechanisms by which OPN increases collateral formation, what protein domains are necessary, and how post-translational modifications alter the ability of OPN to stimulate neo-vascularization.
Little is understood regarding the identity of the specific ROS, O2•- or H2O2, that regulate OPN expression in vivo in response to ischemia. Previous findings suggest a link between ROS and OPN, where in settings in which ROS are increased, such as type 2 diabetes40 and atherosclerosis,41 there is a correlative increase in OPN expression. Similarly, our data show that when H2O2 is increased (Fig. 2), there is a correlative increase in OPN in response to ischemia (Fig. 1). To determine if the increase in OPN in response to ischemia was mediated directly by H2O2, we used both pharmacologic and transgenic approaches with our in vivo ischemia model. Catalase, an enzyme that converts H2O2 to water and other secondary metabolites, diminishes H2O2 levels. To decrease H2O2 levels in vivo, we infused animals with PEG-Catalase, since the addition of polyethylene glycol has been shown to significantly increase the circulating half-life of the enzyme without impairing enzymatic function.42, 43 When H2O2 was decreased pharmacologically by PEG-catalase infusion, there were significant decreases in ischemia-induced OPN mRNA and protein expression (Fig. 3). Additionally, when H2O2 levels were decreased using SMC catalase overexpressing mice, there was a similar decrease in OPN mRNA and protein expression in response to ischemia (Fig. 4). Several recent publications link ROS to OPN expression in different in vitro cell systems, including renal epithelial and vascular smooth muscle cells.24-26 Consistent with this, our in vitro data also provide evidence that H2O2 can directly induce increased OPN expression (Fig. 6). The precise mechanism by which H2O2 increases OPN in vascular smooth muscle cells requires further investigation.
Altogether, these data strongly support a direct relationship between H2O2 and OPN expression in vivo, specifically that H2O2 produced in response to ischemia increases OPN expression and that SMCs are one of the contributing sources of H2O2 in this model. As evidenced by IF (Fig. 1 D-F), SMCs are but one cell type that contributes to increased OPN production in the IL. The dramatic effects observed in TgSMC-Cat animals may be due, in part, to SMC-catalase overexpressing cells serving as a sink for H2O2 generated by other cell types in response to ischemia, since H2O2 is freely diffusible. In both scenarios where H2O2-dependent OPN expression is decreased, there is substantially impaired reperfusion and collateral formation in response to ischemia compared to controls, where OPN expression is maintained and collateral formation is preserved. Furthermore, when OPN was overexpressed in the ischemic limb of TgSMC-Cat animals using lentivirus, collateral formation and reperfusion was restored to levels similar to those seen in WT+LV-GFP control animals (Fig. 5D-E), further substantiating OPN as a critical mediator of post-ischemic neo-vascularization.
The experiments performed in this study establish that H2O2-dependent OPN is an important mediator of post-ischemic neo-vascularization. One mechanism by which H2O2 stimulates collateral vessel formation is through the H2O2-dependent up-regulation of OPN expression, which functions to promote neo-vascularization. Future studies will focus on determining how H2O2 regulates OPN expression and defining the pathways activated by H2O2 and upstream of OPN. Fully understanding how OPN functions to increase neo-vascularization may ultimately allow for the development of new therapeutics to increase collateral formation in ischemic tissues to restore blood flow and preserve tissue function.
Supplementary Material
Acknowledgements
We thank Dr. Kerry J. Ressler (Emory University) and Dr. Bernard Lassegue (Emory University) for assistance with lentivirus construct design; Viral Vector Core of the Emory Neuroscience NINDS Core Facilities, funded by grant P30NS055077, for providing the lentivirus vector plasmid; and Drs. Yury A. Bochkov and Ann C. Palmenberg (University of Wisconsin – Madison) for providing the pF/R-wt plasmid containing the WT IRES.
Sources of Funding This work was supported by NIH PO1 HL095070, NIH RO1 HL09058, NIH RO1HL062820 and a post-doctoral grant to Alicia N. Lyle from the American Heart Association.
Non-Standard Abbreviations and Acronyms
- DHE
Dihydroethidium
- GFP
Green Fluorescent Protein
- H2O2
Hydrogen Peroxide
- HLI
Hind Limb Ischemia
- HPLC
High-performance Liquid Chromatography
- IF
Immunofluorescence
- IL
Ischemic Limb
- IRES
Internal Ribosomal Entry Site
- LDPI
LASER Doppler Perfusion Imaging
- LV
Lentivirus
- NIL
Non-ischemic Limb
- O2•-
Superoxide
- OPN
Osteopontin
- PEG
Polyethylene Glycol
- RGD
Arginine-Glycine-Aspartate
- ROS
Reactive Oxygen Species
- SMCs
Smooth Muscle Cells
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sabia PJ, Powers ER, Jayaweera AR, Ragosta M, Kaul S. Functional significance of collateral blood flow in patients with recent acute myocardial infarction. A study using myocardial contrast echocardiography. Circulation. 1992;85:2080–2089. doi: 10.1161/01.cir.85.6.2080. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto J, Kon S, Matsui Y, Uede T. Osteopontin; as a target molecule for the treatment of inflammatory diseases. Curr Drug Targets. 11:494–505. doi: 10.2174/138945010790980321. [DOI] [PubMed] [Google Scholar]
- 3.Wang KX, Denhardt DT. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;22:286–297. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- 5.Duvall CL, Weiss D, Robinson ST, Alameddine FM, Guldberg RE, Taylor WR. The role of osteopontin in recovery from hind limb ischemia. Arterioscler Thromb Vasc Biol. 2008;28:290–295. doi: 10.1161/ATVBAHA.107.158485. [DOI] [PubMed] [Google Scholar]
- 6.Myers DL, Harmon KJ, Lindner V, Liaw L. Alterations of arterial physiology in osteopontin-null mice. Arterioscler Thromb Vasc Biol. 2003;23:1021–1028. doi: 10.1161/01.ATV.0000073312.34450.16. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien ER, Garvin MR, Stewart DK, Hinohara T, Simpson JB, Schwartz SM, Giachelli CM. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb. 1994;14:1648–1656. doi: 10.1161/01.atv.14.10.1648. [DOI] [PubMed] [Google Scholar]
- 8.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. Nf-kappab mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liaw L, Almeida M, Hart CE, Schwartz SM, Giachelli CM. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ Res. 1994;74:214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- 10.Liaw L, Skinner MP, Raines EW, Ross R, Cheresh DA, Schwartz SM, Giachelli CM. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Invest. 1995;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- 12.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scatena M, Liaw L, Giachelli CM. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 14.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between cd44 and osteopontin (eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 15.Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol. 2002;72:752–761. [PubMed] [Google Scholar]
- 16.Liaw L, Lombardi DM, Almeida MM, Schwartz SM, deBlois D, Giachelli CM. Neutralizing antibodies directed against osteopontin inhibit rat carotid neointimal thickening after endothelial denudation. Arterioscler Thromb Vasc Biol. 1997;17:188–193. doi: 10.1161/01.atv.17.1.188. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci U S A. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, Sodek J. Intracellular osteopontin is an integral component of the cd44-erm complex involved in cell migration. J Cell Physiol. 2000;184:118–130. doi: 10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med. 2002;33:1047–1060. doi: 10.1016/s0891-5849(02)01005-5. [DOI] [PubMed] [Google Scholar]
- 20.Martin AS, Griendling KK. Redox control of vascular smooth muscle migration. Antioxid Redox Signal. doi: 10.1089/ars.2009.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ushio-Fukai M. Redox signaling in angiogenesis: Role of nadph oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Griendling KK, Ushio-Fukai M. Redox control of vascular smooth muscle proliferation. J Lab Clin Med. 1998;132:9–15. doi: 10.1016/s0022-2143(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 23.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (nox2)-containing nad(p)h oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 24.Hu T, Luan R, Zhang H, Lau WB, Wang Q, Zhang Y, Wang HC, Tao L. Hydrogen peroxide enhances osteopontin expression and matrix metalloproteinase activity in aortic vascular smooth muscle cells. Clin Exp Pharmacol Physiol. 2009;36:626–630. doi: 10.1111/j.1440-1681.2008.05124.x. [DOI] [PubMed] [Google Scholar]
- 25.Maziere C, Gomila C, Maziere JC. Oxidized low-density lipoprotein increases osteopontin expression by generation of oxidative stress. Free Radic Biol Med. 2010;48:1382–1387. doi: 10.1016/j.freeradbiomed.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Umekawa T, Tsuji H, Uemura H, Khan SR. Superoxide from nadph oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int. 2009;104:115–120. doi: 10.1111/j.1464-410X.2009.08374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Griendling KK, Dikalova A, Owens GK, Taylor WR. Vascular hypertrophy in angiotensin ii-induced hypertension is mediated by vascular smooth muscle cell-derived h2o2. Hypertension. 2005;46:732–737. doi: 10.1161/01.HYP.0000182660.74266.6d. [DOI] [PubMed] [Google Scholar]
- 28.Hodara R, Weiss D, Joseph G, Velasquez-Castano JC, Landazuri N, Han JW, Yoon YS, Taylor WR. Overexpression of catalase in myeloid cells causes impaired postischemic neovascularization. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.233247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of bdnf in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering rna. Proc Natl Acad Sci U S A. 2003;100:1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bochkov YA, Palmenberg AC. Translational efficiency of emcv ires in bicistronic vectors is dependent upon ires sequence and gene location. Biotechniques. 2006;41:283–284. 286, 288 passim. doi: 10.2144/000112243. [DOI] [PubMed] [Google Scholar]
- 33.Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM. Delivery of the cre recombinase by a self-deleting lentiviral vector: Efficient gene targeting in vivo. Proc Natl Acad Sci U S A. 2001;98:11450–11455. doi: 10.1073/pnas.201415498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 2007;102:912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- 37.Haddad P, Dussault S, Groleau J, Turgeon J, Michaud SE, Menard C, Perez G, Maingrette F, Rivard A. Nox2-containing nadph oxidase deficiency confers protection from hindlimb ischemia in conditions of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1522–1528. doi: 10.1161/ATVBAHA.109.191437. [DOI] [PubMed] [Google Scholar]
- 38.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular sod in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 39.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing nad(p)h oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 40.San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 41.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin ii-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252:3582–3586. [PubMed] [Google Scholar]
- 43.Beckman JS, Minor RL, Jr., White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.