Abstract
Soft-rot bacteria Pectobacterium and Dickeya use N-acyl homoserine lactones (NAHSLs) as diffusible signals for coordinating quorum sensing communication. The production of NAHSLs was investigated in a set of reference strains and recently-collected isolates, which belong to six species and share the ability to infect the potato host plant. All the pathogens produced different NAHSLs, among which the 3-oxo-hexanoyl- and the 3-oxo-octanoyl-l-homoserine lactones represent at least 90% of total produced NAHSL-amounts. The level of NAHSLs varied from 0.6 to 2 pg/cfu. The involvement of NAHSLs in tuber maceration was investigated by electroporating a quorum quenching vector in each of the bacterial pathogen strains. All the NAHSL-lactonase expressing strains produced a lower amount of NAHSLs as compared to those harboring the empty vector. Moreover, all except Dickeya dadantii 3937 induced a lower level of symptoms in potato tuber assay. Noticeably, aggressiveness appeared to be independent of both nature and amount of produced signals. This work highlights that quorum sensing similarly contributed to virulence in most of the tested Pectobacterium and Dickeya, even the strains had been isolated recently or during the past decades. Thus, these key regulatory-molecules appear as credible targets for developing anti-virulence strategies against these plant pathogens.
Keywords: soft-rot bacteria, Solanum tuberosum L., N-acyl homoserine lactones, quorum sensing, AttM lactonase, quorum quenching
1. Introduction
Several Proteobacteria synthesize N-acyl homoserine lactones (NAHSLs) that allow synchronizing the expression of several collective behaviors (for a review see [1]), a mechanism called quorum sensing (QS) by Fuqua et al. [2]. Among them, numerous plant-pathogenic bacteria produce NAHSLs, which are involved in aggressiveness and colonization of plant-host [3]. The pectinolytic phytopathogens belonging to Dickeya and Pectobacterium genera, formerly grouped in the same Erwinia genus, are responsible for similar pathologies (i.e., blackleg, soft-rot) compromising plant survival as well as integrity of harvested vegetal products. In Pectobacterium genus, QS belongs to a complex regulatory cascade controlling the production and exportation of carbapenem antibiotic and numerous lytic enzymes [4,5]. In Dickeya, the contribution of QS to virulence is less documented and needs to be clarified [6–8].
The involvement of QS in virulence was intensely investigated in Pectobacterium atrosepticum, a particular virulent potato pathogen (for review see [9]). Initially, bacteria are present in infection areas such as plant wounds and accumulate a low level of NAHSLs. Then, they multiply actively to reach the quorum cell density and the associated critical concentration of NAHSL, which trigger the production of the macerating enzymes [10–12]. As a result of these observations, NAHSL-based QS system had been consequently proposed as the target for a biocontrol approach using NAHSL-degrading bacterial strains, which aims at reducing the expression of virulence in Pectobacterium atrosepticum [13,14].
In addition to the classical Pectobacterium atrosepticum, which causes blackleg in temperate regions, several soft-rot Pectobacterium and Dickeya bacterial species, including numerous recent isolates and the emerging species “Dickeya solani”, are able to cause damage to the potato Solanum tuberosum L. and other important crops for which protecting methods are lacking [15–22]. The question rose about the relevance of NAHSL-targeting strategies for protecting crops against these pathogens. To address this question, the nature and relative abundance of NAHSL signaling molecules which were synthesized by potato soft-rot bacteria were compared in a set of Dickeya and Pectobacterium isolates, including recent isolates found in potato fields throughout Western Europe. In a second step, involvement of NAHSLs in tuber soft-rot was evaluated using a quorum quenching approach. This work revealed the key role of NAHSLs and QS-regulation in soft-rot induction by a wide range of pectinolytic bacteria.
2. Experimental Section
2.1. Bacterial Strains and Plasmids
The characteristics of the bacterial strains and plasmids used in this work are presented in Table 1. Two panels of six strains each were compared for signal production. One is composed of reference strains, the other of soft-rot bacteria recently isolated from potato plants affected by blackleg. Field potato strains were characterized by both biochemical tests [16,23] and molecular methods using specific primers set for P. atrosepticum, Pectobacterium carotovorum and Dickeya spp. [24]. Rep-PCR genomic fingerprinting characterization was performed to identify “D. solani” and D. dianthicola [19].
Table 1.
Strains and plasmids used in the present study.
| Strains and plasmids | Relevant characteristic(s) or origin (year of plant host isolation) | Reference or source |
|---|---|---|
| Reference strains | ||
| Pectobacterium atrosepticum | ||
| P. atrosepticum CFBP 1526T | Solanum tuberosum (1957) | * CFBP, species type strain |
| P. atrosepticum CFBP 6276 | Solanum tuberosum (1999) | [25] |
| Pectobacterium carotovorum | ||
| P. carotovorum CFBP 2046T | Solanum tuberosum (1952) | CFBP, species type strain |
| P. carotovorum EC153 | Capsicum annuum (1951) | [26] |
| Dickeya spp. | ||
| D.chrysanthemi CFBP 2048T | Chrysanthemum morifolium (1956) | CFBP, type strain |
| D. dadantii 3937 | Saintpaulia ionantha (1981) | [27] |
| Strains recently isolated from fields | ||
| Pectobacterium atrosepticum | ||
| P. atrosepticum 100T | Solanum tuberosum (2003) | [21] |
| P. atrosepticum RNS 08.30.1A | Solanum tuberosum (2008) | V.Hélias/#FN3PT’ collection |
| Pectobacterium carotovorum | ||
| P. carotovorum 98.1 | Solanum tuberosum (1998) | [21] |
| P. carotovorum RNS 08.42.1A | Solanum tuberosum (2008) | V. Hélias/FN3PT’ collection |
| Dickeya spp. | ||
| D. dianthicola RNS 04.9 | Solanum tuberosum (2004) | V. Hélias/FN3PT’ collection |
| “D. solani” RNS 08.23.3.1A | Solanum tuberosum (2008) | [21] |
| Plasmids | ||
| pME6010 | Broad host-range vector, pVS1 derivative, low copy number, Tcr | [28] |
| pMIR102 | pME6010 expressing attM | [29] |
CFBP: French Collection of Plant associated Bacteria;
FN3PT: Fédération Nationale des Producteurs de Plants de Pomme de Terre.
The plasmid pME6010 [28] and its derivative expressing the lactonase-encoding gene attM, pMIR102 [29], were introduced into Pectobacterium and Dickeya strains by electroporation.
2.2. Growth Media and Conditions
Pectobacterium and Dickeya strains were cultivated at 25 °C in polygalacturonic acid (PGA) mineral salt medium [25] the composition of which was modified as follows: K2HPO4, 16.266 g/L; KH2PO4, 899 mg/L; (NH4)2SO4, 1.2 g/L; MgSO4.6H2O, 818 mg/L; CaCl2, 75 mg/L (pH 8.0, Merck, Fontenay-sous-bois, France) and polygalacturonic acid 4.0 g/L (potassium salt, Sigma-Aldrich, St. Quentin Fallavier, France). Batch cultures were performed under gyratory agitation (180 rpm) in Erlenmeyer flasks in which the liquid medium is 10% of the total flask volume. Batch precultures and cultures were made in the same experimental conditions. Bacterial growth was monitored by measuring optical density (OD) at 580 nm. The initial OD580 of the cultures was usually 0.05. For each strain, at least three independent cultures were made. When necessary, growth media were supplemented with tetracycline (10 mg/L, Sigma-Aldrich).
2.3. NAHSL Standards, Extraction of Supernatants and NAHSL Assays
NAHSLs were extracted and analyzed as described previously [30,31]. Briefly, the synthetic standards (Sigma-Aldrich) and stock solutions prepared in high performance liquid chromatography (HPLC)-grade ethyl acetate (Fisher Scientific, Courtaboeuf, France) were stored at −20 °C. The supernatants (1 mL) were extracted twice with equal volumes of ethyl acetate. The combined extracts were dried over anhydrous magnesium sulphate (MgSO4, Merck), evaporated to dryness, dissolved in 500 μL of HPLC-grade ethyl acetate and stored at −20 °C until analysis.
Concentrated extracts were analyzed by on-line liquid chromatography mass spectrometry (LC-MS-MS). They were applied to a C18 reverse-phase HPLC column (Agilent Hypersyl ODS, 250 4.6 mm, particle size 5 μm, Interchim, Montluçon, France) using an Agilent Technologies Series 1100 vacuum degasser, LC pump and autosampler (Hewlett Packard, Boeblingen, Germany). The elution procedure consisted of an isocratic profile of methanol-water (50:50, v/v) for 10 min at a flow rate of 0.4 mL/min, followed by a linear gradient from 50% to 90% methanol in water over 15 min, and an isocratic profile over 25 min. The post-column flow was split (1/10) by a micro-splitter valve (Upchurch Scientific, Oak Harbor, WA, USA) and a mixture of 5 mM ammonium acetate (Merck) and 0.05% trifluoroacetic acid (Sigma-Aldrich) in methanol-water (50:50, v/v, 150 μL/h) was added using a Cole-Parmer syringe pump. Detection was made by electrospray ionisation-ion trap mass spectrometry using a Bruker Esquire-LC spectrometer (Bruker Daltonic, Wissembourg, France) under positive-ion conditions. The identification of NAHSLs from supernatant extracts was carried out by comparison with synthetic standards, based on three criteria: HPLC retention times, MS-MS fragment ions of the molecular [M+H]+ ions (four product ions: the lactone ring m/z 102, [M+H–101]+ ion corresponding to the acyl chain, [M+H–H2O]+ and [M+H–CO]+ ions) and on their relative intensities. The chromatographic peak area of the m/z 102 ion was measured for quantification [30].
2.4. Aggressiveness Test on Potato Tubers
Overnight cultures of bacterial strain harboring pME6010 or pMIR102 were washed twice in 0.8% NaCl. Each tuber of S. tuberosum var. Allians was inoculated with 10 μL of the cell suspension, which was adjusted to 1.0 at OD580nm. At least 8 tubers were inoculated and then incubated at 24 °C. Five days post-infection, tubers were cut in half and the observed symptoms were categorized into four classes according to diameter (D) of the maceration zone: 1, no maceration; 2, low maceration (D < 2 mm); 3, moderate maceration (2 < D < 5 mm); 4, strong maceration (D > 5 mm). The Mann and Whitney test (α = 0.05) was used to analyze the maceration categories.
3. Results and Discussion
Two sets of Pectobacterium and Dickeya strains (Table 1) were compared for NAHSL production and virulence assays. The first panel was composed of three type strains used as international taxonomic references of P. atrosepticum, Pectobacterium carotovorum and Dickeya chrysanthemi species, isolated in the 1950s. Three other strains which are commonly used for virulence studies were included in this panel: (i) the psychrotroph strain P. atrosepticum CFBP 6276 known both for its virulence on the potato and for the unusual ability among this species to induce a hypersensitive reaction in non host plant [10,25]; (ii) the strain P. carotovorum EC153, isolated from rotting bell peppers fruit shipped from Mexico and which NAHSL production and virulence were exceptionally enabled at elevated temperatures above 34 °C [26]; and (iii) Dickeya dadantii 3937, a mesophile strain isolated from the African violet, widely used as a strain model for research in molecular biology [32], found to be highly virulent on various host plants (V. Hélias, personnal communication), and from which the entire genome has recently been sequenced [33].
The second set of strains was composed of six recent isolates, between 1998 to 2008, from potato blackleg wounds. They represent the six soft-rot potato species currently encountered in European soils, including a representative member of the emerging Dickeya clade, provisionally called “D. solani” [17,21,22].
3.1. Characterization of NAHSL Signaling Molecules Produced by Potato Soft-Rot Bacteria
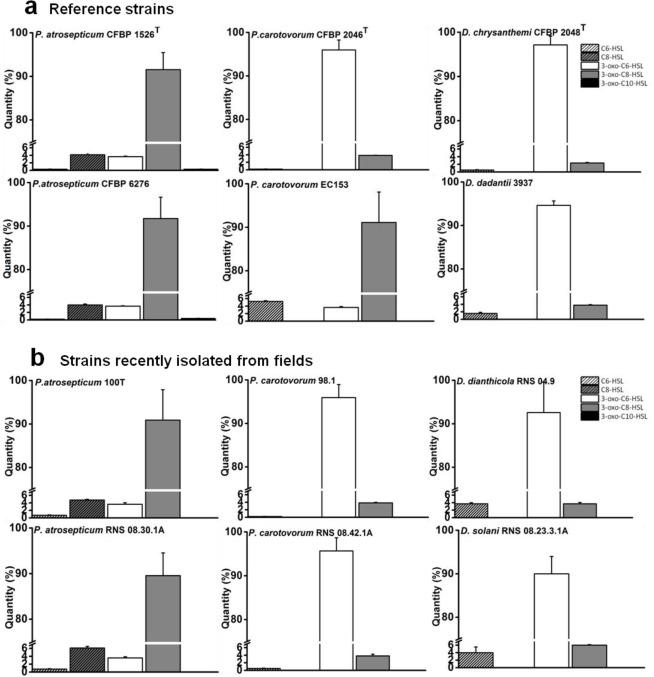
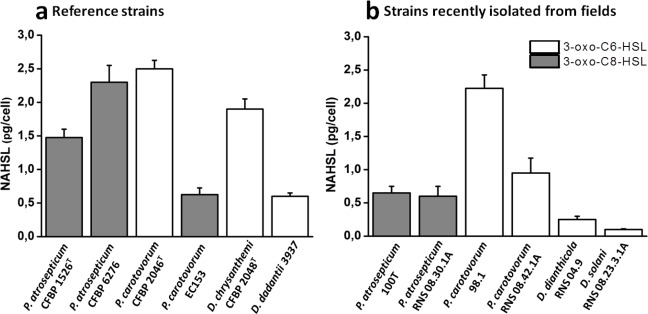
The characterization of NAHSL signal molecules was carried out on a mineral salt medium supplemented with PGA, a plant cell-wall compound which contributes to induce the synthesis of signaling molecules and virulence factors as in situ conditions [10]. Culture supernatants were recovered during the transition from exponential-phase to stationary-phase growth, when the cell density reached the quorum and the NAHSL concentration is the highest. Produced NAHSLs were identified and quantified using HPLC coupled with mass spectrometry. In these experimental conditions, all P. atrosepticum strains produced mainly (90%) N-3-oxo-octanoyl-l-HSL (3-oxo-C8-HSL) and a low percent of N-octanoyl-l-HSL (C8-HSL) and N-3-oxo-hexanoyl-l-HSL (3-oxo-C6-HSL). They also synthesized traces (<1%) of N-hexanoyl-l-HSL (C6-HSL) and N-3-oxo-decanoyl-l-HSL (3-oxo-C10-HSL) (Figure 1). Among these strains, P. atrosepticum CFBP 6276 and 1526T were the largest producers of 3-oxo-C8-HSL with amounts greater than 1 pg/cfu (Figure 2). The other P. atrosepticum strains displayed lower production of the major NAHSL with about 0.6 pg/cfu. All Dickeya spp. strains and three of the four studied P. carotovorum strains produced mainly 3-oxo-C6-HSL and minor percentages of 3-oxo-C8-HSL (Figure 1).
Figure 1.
Abundance and diversity of N-acyl homoserine Lactone molecules produced by Pectobacterium and Dickeya strains.
NAHSLs were extracted from PGA medium culture supernatants of reference strains (a) and recent strains isolated from fields (b) during the transition from exponential to stationary phases. On-line liquid chromatography mass spectrometry was used to identify and quantify N-hexanoyl-l-homoserine lactone (slashed white bar), N-octanoyl-l-homoserine lactone (slashed gray bar), N-3-oxo-hexanoyl-l-homoserine lactone (white bar), N-3-oxo-l-octanoyl-homoserine lactone (gray bar) and N-3-oxo-decanoyl-l-homoserine lactone (black bar). Values are the average of three independent experiments.
Figure 2.
Concentrations of the main N-acyl homoserine lactones produced by Pectobacterium and Dickeya strains.
NAHSLs were extracted from PGA medium culture supernatants of reference strains (a) and recent strains isolated from fields (b) during the transition from exponential to stationary phases and quantified by HPLC coupled to mass spectrometry. For each point, at least 3 independent cultures were analyzed, with standard deviation shown. Legend: white bars, N-3-oxo-hexanoyl-l-homoserine lactone; gray bars, N-3-oxo-octanoyl-l-homoserine lactone.
The exception concerns the P. carotovorum EC153 strain which demonstrated the same NAHSL production patterns as P. atrosepticum strains with the production of about 0.6 pg/cfu of 3-oxo-C8-HSL (Figure 2). The largest producers of 3-oxo-C6-HSL were represented by P. carotovorum 2046 and 98.1 strains and D. chrysanthemi 2048T strain with amounts greater than 2 pg/cfu (Figure 2). Comparisons between Dickeya and Pectobacterium genera revealed that the NAHSL production is lower in the former than in the latter.
The characterization of NAHSL molecules produced by the twelve strains belonging to Pectobacterium and Dickeya genera revealed an apparent intraspecies homogeneity in NAHSL production pattern rather than differences between earlier and recent isolates. This is strengthened by the observation of the same NAHSL production profile of both strains from the current taxonomically described species and emerging isolates. Each NAHSL production pattern contained a major NAHSL, 3-oxo-C8-HSL for P. atrosepticum strains and 3-oxo-C6-HSL for Dickeya strains, the two molecules identified as true QS signals [29,34,35]. These traits are also observable in the literature for some other soft-rot strains [8,36–38]. There is an exception for P. atrosepticum SCRI1043, which is the only P. atrosepticum studied strain producing 3-oxo-C6-HSL as a signal instead of 3-oxo-C8-HSL [38,39]. 3-oxo-C6-HSL or 3-oxo-C8-HSL production have always coexisted with minor species of NAHSLs, likely to be less specific products of the NAHSL synthase or catabolites resulting from NAHSL turnover [29,34,35]. Contrary to the nature of NAHSL, the amount of each NAHSL species was variable within P. atrosepticum strains and the genus Dickeya. The intraspecies homogeneity in NAHSL production pattern was not clearly marked in P. carotovorum strains, as already observed by Hasegawa et al. [26] and Jafra et al. [40]. Although a majority of strains produce 3-oxo-C6-HSL, namely three out of the four studied strains, the P. carotovorum EC153 strain used in this study and at least another P. carotovorum strain (SCC3193) produce 3-oxo-C8-HSL as signaling molecules [26]. The strong heterogeneity of the P. carotovorum species and the diversity of their hosts and environmental niches [41,42] could justify these differences in NAHSL production.
3.2. Involvement of NAHSL-Based QS in Potato Soft-Rot Virulence
The contribution of QS using NAHSL in disease establishment and development is now well established for P. atrosepticum [9]. Indeed, just an insertional mutation in the gene encoding the NAHSL synthase or only the break down of signaling molecules before their release in the microenvironment are sufficient to remove all symptoms on potato tubers [10,31]. These observations led us to consider NAHSL-QS system as a key target for the development of a novel biocontrol strategy based on the catabolism of signal molecules using natural NAHSL degrading bacteria isolated from the potato rhizosphere [14]. The quorum quenching activity exerted by these bacteria can be attributed to at least three enzymatic activities: a lactonase that opens the γ-butyrolactone-ring of NAHSL [43,44]; an acylase that releases a homoserine lactone and a fatty acid, and an oxido-reductase that reduces the 3-oxo-substitution to a hydroxyl on some NAHSL [44,45].
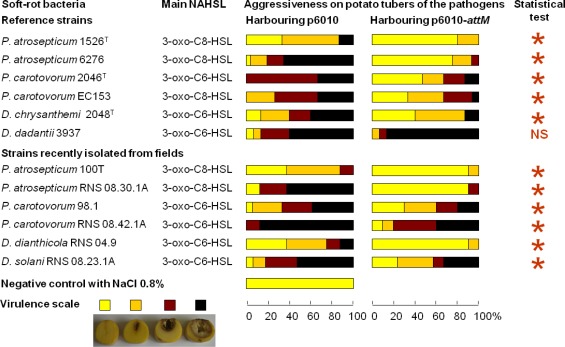
We used a quorum quenching approach to evaluate the involvement of NAHSLs in virulence of the Pectobacterium and Dickeya strains. The occlusion of QS signals in the pathogens was based on the introduction of a plasmid carrying a NAHSL-lactonase gene attM into each of the twelve studied phytopathogens. The attM gene belongs to the attKLM operon located on the At plasmid from Agrobacterium tumefaciens, which is involved in degradation of γ-butyrolactone, γ-hydroxybutyrate and NAHSLs [29,46,47]. All strains harboring the empty vector p6010 were able to induce tissue maceration five days after potato tuber inoculation (Figure 3). Overall, recent isolates were not more virulent than the reference strains isolated several years before. Symptom severity was variable between Pectobacterium and Dickeya genera and within Pectobacterium species and Dickeya genus. For P. atrosepticum species, moderate and strong macerations were detected in 10, 80, 10 and 90% of tubers infected by P. atrosepticum 1526, 6276, 100T and RNS 08.30.1A, respectively. Interestingly, the two P. atrosepticum 100T and RNS 08.30.1A shared the same NAHSL production profile with similar amounts for each NAHSL molecule. Moreover, P. atrosepticum RNS 08.30.1A exhibited a similar aggressiveness profile to D. dadantii 3937, even if their NAHSL production patterns were different. These observations revealed that symptom severity does not seem to be related to both nature and amount of NAHSL species.
Figure 3.
Involvement of NAHSLs in the virulence symptoms on potato tubers.
Each tuber of Solanum tuberosum var. Allians was inoculated with pathogens harboring the empty vector p6010 or its lactonase AttM-encoding derivative pMIR102. Symptoms were observed five days post-infection and compared using the Mann and Whitney test (α = 0.05). Asterisks indicate statistical differences between symptoms in bacterial derivatives harboring empty and lactonase-expressing vectors. Legend: NS, non-significant.
The ectopic expression of the lactonase AttM triggered a decrease about of 100 fold in NAHSL quantity produced by each strain, confirming the capacity of this enzyme in degrading a wide range of NAHSLs (Figure 3). More interestingly, the NAHSL decrease correlated a lower level of symptoms in all pathogen derivatives expressing lactonase AttM as compared those harboring the empty vector. A unique exception was the strain D. dadantii 3937 for which no significant symptom attenuation was observed, suggesting no major NAHSL-based QS-dependent expression of the virulence factors [6,48–51].
The AttM expression quenched virulence in the P. atrosepticum strains, which produced mainly the 3-oxo-C8-HSL but also in the P. carotovorum and Dickeya spp. strains, for which the majority produced 3-oxo-C6-HSL as a signal.
This work points out that involvement of NAHSLs in the virulence was conserved in most of the tested Pectobacterium and Dickeya, which were isolated over the last decades. These key regulatory-molecules appear to be credible targets for developing anti-virulence strategies against the Pectobacterium and Dickeya plant pathogens. These anti-QS strategies encompass the application of natural or synthetic anti-QS compounds which may target NAHSL-sensors, the construction transgenic plants expressing NAHSL-degrading lactonase, or the application of NAHSL-degrading biocontrol agent, such as the Rhodococcus erythropolis (a review by Faure and Dessaux [52]). In the rhizosphere of potato plants, the growth of the NAHSL-degrading R. erythropolis may be stimulated by adding γ-caprolactone, a structural analog of NAHSL [13,53].
4. Conclusions
By comparing several type strains, well-studied strains from different laboratories [5,36,38,40] and recently isolated strains from fields, this work highlights the key role of QS signals in plant virulence due to soft-rot bacteria. A noticeable exception is the strain D. dadantii 3937, in which NAHSLs may not be strongly involved in the expression of virulence factors. These findings reinforce the interest in developing quorum quenching strategies, including biological control based on signal-break down by rhizospheric microbial populations [44,54,55].
Acknowledgments
This work was supported by grants from Conseil Régional de Haute-Normandie, Ministère délégué à l’Enseignement Supérieur et à la Recherche, Ministère de l’Écologie, du Développement durable, des Transports et du Logement (PESTICIDES), Ministère de l’Agriculture de la Pêche (CAS-DAR AAP N°7124), Association Nationale de la Recherche et de le Technologie (ANRT-CIFRE n°698/2009), and GRR VATA & FEDER (European Union), Works are related to COST631 action-Understanding and Modelling Plant-Soil Interactions in the Rhizosphere Environment.
The authors thank Arun Chatterjee for kindly providing P. carotovorum EC153 strain and the French Collection of Plant associated Bacteria (CFBP) for the maintenance of a reference strains collection on French territory. They thank also Laure Taupin (Laboratoire de Biotechnologie et Chimie Marines, EA 3884, Université de Bretagne-Sud, France) for her technical assistance.
Abbreviations
- QS
Quorum sensing
- NAHSL
N-acyl homoserine lactone
- 3-oxo-C6-HSL
N-3-oxo-hexanoyl-L-HSL
- 3-oxo-C8-HSL
N-3-oxo-octanoyl-L-HSL
- HPLC
High performance liquid chromatography
- LC-MS
Liquid chromatography mass spectrometry
References
- 1.Ryan R.P., Dow J.M. Diffusible signals and interspecies communication in bacteria. Microbiology. 2008;154:1845–1858. doi: 10.1099/mic.0.2008/017871-0. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua W.C., Winans S.C., Greenberg E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Bodman S.B., Bauer W.D., Coplin D.L. Quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 2003;41:455–482. doi: 10.1146/annurev.phyto.41.052002.095652. [DOI] [PubMed] [Google Scholar]
- 4.Barras F., van Gijsegem F., Chatterjee A.K. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Ann. Rev. Phytopathol. 1994;32:201–234. [Google Scholar]
- 5.Barnard A.M., Salmond G.P. Quorum sensing in Erwinia species. Anal. Bioanal. Chem. 2007;387:415–423. doi: 10.1007/s00216-006-0701-1. [DOI] [PubMed] [Google Scholar]
- 6.Nasser W., Bouillant M.L., Salmond G.P.C., Reverchon S. Characterization of the Erwinia chrysanthemi expI-expR locus directing the synthesis of two N-acyl-homoserine lactone signal molecules. Mol. Microbiol. 1998;29:1391–1405. doi: 10.1046/j.1365-2958.1998.01022.x. [DOI] [PubMed] [Google Scholar]
- 7.Charkowsky A.O. The Soft Rot Erwinia. In: Gnanamanickam S., editor. Plant-Associated Bacteria. Springer; Dordrecht, The Netherlands: 2006. pp. 423–505. Part 3, [Google Scholar]
- 8.Hussain M.B.B., Zhang H.B., Xu J.L., Liu Q., Jiang Z., Zhang L.H. The acyl-homoserine lactone-type quorum sensing system modulates cell motility and virulence of Erwinia chrysanthemi pv. zeae. J. Bacteriol. 2008;190:1045–1053. doi: 10.1128/JB.01472-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H., Coulthurst S.J., Pritchard L., Hedley P.E., Ravensdale M., Humphris S., Burr T., Takle G., Brurberg M.B., Birch P.R., et al. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog. 2008;4:e1000093. doi: 10.1371/journal.ppat.1000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smadja B., Latour X., Faure D., Chevalier S., Dessaux Y., Orange N. Involvement of N-acylhomoserine lactones throughout the plant infection by Erwinia carotovora subsp. atroseptica (Pectobacterium atrosepticum) Mol. Plant Microbe Interact. 2004;17:1269–1278. doi: 10.1094/MPMI.2004.17.11.1269. [DOI] [PubMed] [Google Scholar]
- 11.Toth I.K., Newton J.A., Hyman L.J., Lees A.K., Daykin M., Ortori C., Williams P., Fray R.G. Potato plants genetically modified to produce N-acylhomoserine lactones increases susceptibility to soft rot Erwiniae. Mol. Plant Microbe Interact. 2004;17:880–887. doi: 10.1094/MPMI.2004.17.8.880. [DOI] [PubMed] [Google Scholar]
- 12.Maë A., Montesano M., Koiv V., Palva E.T. Transgenic plants producing the bacterial pheromone N-acylhomoserine lactone exhibit enhanced resistance to the bacterial phyto-pathogen Erwinia carotovora. Mol. Plant Microbe Interact. 2001;14:1035–1042. doi: 10.1094/MPMI.2001.14.9.1035. [DOI] [PubMed] [Google Scholar]
- 13.Cirou A., Diallo S., Kurt C., Latour X., Faure D. Growth promotion of quorum-quenching bacteria in the rhizosphere of Solanum tuberosum. Environ. Microbiol. 2007;9:1511–1522. doi: 10.1111/j.1462-2920.2007.01270.x. [DOI] [PubMed] [Google Scholar]
- 14.Crépin A., Barbey C., Cirou A., Tannières M., Orange N., Feuilloley M., Dessaux Y., Burini J.F., Faure D., Latour X. Biological control of pathogen communication in the rhizosphere: A novel approach applied to potato soft rot due to Pectobacterium atrosepticum. Plant Soil. 2011 doi: 10.1007/s11104-011-1030-5. [DOI] [Google Scholar]
- 15.Ma B., Hibbing M.E., Kim H.S., Reedy R.M., Yedidia I., Breuer J., Glasner D., Perna N.T., Kelman A., Charkowski A.O. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology. 2007;97:1150–1163. doi: 10.1094/PHYTO-97-9-1150. [DOI] [PubMed] [Google Scholar]
- 16.Laurila J., Ahola V., Lehtinen A., Joutsjoki T., Hannukkala A., Rahkohnen A., Pirhonen M. Characterization of Dickeya strains isolated from potato and river water samples in Finland. Eur. J. Plant Pathol. 2008;122:213–225. [Google Scholar]
- 17.Czajkowski R., Grabe G.J., van der Wolf J.M. Distribution of Dickeya spp. and Pectobacterium carotovorum subsp. carotovorum in naturally infected seed potatoes. Eur. J. Plant Pathol. 2009;125:263–275. [Google Scholar]
- 18.Slawiak M., van Beckhoven J.R.C.M., Specksnijder A.G.C.L., Czajkowski R., Grabe G., van der Wolf J.M. Biochemical and genetical analysis reveal a new clade of biovar 3 Dickeya spp. strains isolated from potato in Europe. Eur. J. Plant Pathol. 2009;125:245–261. [Google Scholar]
- 19.Tsor (Lahkim) L., Erlich O., Lebiush S., Hazanovsky M., Zig U., Slawiak M., Grabe G., van der Wolf J.M., van de Haar J.J. Assessment of recent outbreaks of Dickeya sp. (syn. Erwinia chrysanthemi) slow wilt in potato crops in Israel. Eur. J. Plant Pathol. 2009;123:311–320. [Google Scholar]
- 20.Pitman A.R., Harrow S.A., Visnovsky S.B. Genetic characterization of Pectobacterium wasabiae causing soft rot disease of potato in New Zealand. Eur. J. Plant Pathol. 2010;126:423–435. [Google Scholar]
- 21.Hélias V., Hamon P., Huchet E., van der Wolf J., Andrivon D. Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathol. 2011 doi: 10.1111/j.1365-3059.2011.02508.x. [DOI] [Google Scholar]
- 22.Toth I.K., van der Wolf J.M., Saddler G., Lojkowska E., Hélias V., Pirhonen M., Tsror (Lahkim) L., Elphinstone J.G. Dickeya species: An emerging problem for potato production in Europe. Plant Pathol. 2011;60:385–399. [Google Scholar]
- 23.Samson R., Legendre J.B., Christen R., Fisher-Le Saux M., Achouak W., Gardan L. Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. as Dickeya chrysanthemi comb. nov. and Dickeya paradisiaca comb. nov. and delineation of four novel species: Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov., Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. Int. J. Syst. Evol. Microbiol. 2005;55:1415–1427. doi: 10.1099/ijs.0.02791-0. [DOI] [PubMed] [Google Scholar]
- 24.Diallo S., Latour X., Groboillot A., Copin P., Smadja B., Orange N., Chevalier S. Simultaneous and selective detection of two major soft rot pathogens of potato: Pectobacterium atrosepticum (Erwinia carotovora subsp. atrosepticum) and Dickeya spp. (Erwinia chrysanthemi) Eur. J. Plant Pathol. 2009;125:349–354. [Google Scholar]
- 25.Smadja B., Latour X., Trigui S., Burini J.F., Chevalier S., Orange N. Thermodependence of growth and enzymatic activities implicated in pathogenicity of two Erwinia carotovora subspecies (Pectobacterium spp.) Can. J. Microbiol. 2004;50:19–27. doi: 10.1139/w03-099. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa H., Chatterjee A., Cui Y., Chatterjee A.K. Elevated temperature enhances virulence of Erwinia carotovora subsp. carotovora strain EC153 to plants and stimulates production of the quorum-sensing signal, N-acyl homoserine lactone, and extracellular proteins. Appl. Environ. Microbiol. 2005;71:4655–4663. doi: 10.1128/AEM.71.8.4655-4663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotoujansky A., Lemattre M., Boistard P. Utilization of a thermosensitive episome bearing transposon Tn10 to isolate Hfr donor strains of Erwinia carotovora subsp. chrysanthemi. J. Bacteriol. 1982;150:122–131. doi: 10.1128/jb.150.1.122-131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heeb S., Itoh Y., Nishijyo T., Schnider U., Keel C., Wade J., Walsh U., O’Gara F., Haas D. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol. Plant Microbe Interact. 2000;13:232–237. doi: 10.1094/MPMI.2000.13.2.232. [DOI] [PubMed] [Google Scholar]
- 29.Carlier A., Uroz S., Smadja B., Latour X., Fray R., Dessaux Y., Faure D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-acyl homoserine lactonase activity. Appl. Environ. Microbiol. 2003;69:4989–4993. doi: 10.1128/AEM.69.8.4989-4993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morin D., Grasland B., Vallée-Réhel K., Dufau C., Haras D. On-line high-performance liquid chromatography-mass spectrometric detection and quantification of N-acyl homoserine lactones, quorum-sensing signal molecules, in the presence of biological matrices. J. Chromatogr. A. 2003;1002:79–92. doi: 10.1016/s0021-9673(03)00730-1. [DOI] [PubMed] [Google Scholar]
- 31.Latour X., Diallo S., Chevalier S., Morin D., Smadja B., Burini J.F., Haras D., Orange N. Thermoregulation of N-acyl homoserine lactones-based quorum sensing in the soft rot bacterium Pectobacterium atrosepticum. Appl. Environ. Microbiol. 2007;73:4078–4081. doi: 10.1128/AEM.02681-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hugouvieux-Cotte-Pattat N., Condemine G., Nasser W., Reverchon S. Regulation of pectinolysis in Erwinia chrysanthemi. Ann. Rev. Microbiol. 1996;50:213–257. doi: 10.1146/annurev.micro.50.1.213. [DOI] [PubMed] [Google Scholar]
- 33.Glasner J.D., Yang C.H., Reverchon S., Hugouvieux-Cotte-Pattat N., Condemine G., Bohin J.P., van Gijsegem F., Yang S., Franza T., Expert D., et al. Genome sequence of the plant-pathogenic bacterium Dickeya dadantii 3937. J. Bacteriol. 2011;193:2076–2077. doi: 10.1128/JB.01513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brader G., Sjöblom S., Hyytiäinen H., Sims-Huopaniemi K., Palva E.T. Altering substrate chain length specificity of an acylhomoserine lactone synthase in bacterial communication. J. Biol. Chem. 2005;280:10403–10409. doi: 10.1074/jbc.M408603200. [DOI] [PubMed] [Google Scholar]
- 35.Welch M., Dutton J.M., Glansdorp F.G., Thomas G.L., Smith D.S., Coulthurst S.J., Barnard A.M.L., Salmond G.P.C., Spring D.R. Structure-activity relationships of Erwinia carotovora quorum sensing signaling molecules. Bioorg. Med. Chem. Lett. 2005;15:4235–4238. doi: 10.1016/j.bmcl.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 36.Cha C., Gao P., Chen Y.C., Shaw P., Farrand S.K. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol. Plant Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 37.Ham J.H., Cui Y., Alfano J.R., Rodriguez-Palenzuela P., Rojas C.M., Chatterjee A.K., Collmer A. Analysis of Erwinia chrysanthemi EC16 pelE::uidA, pel::uidA, and hrpN::uidA mutants reveals strain-specific atypical regulation of the Hrp type III secretion system. Mol. Plant Microbe Interact. 2004;17:184–194. doi: 10.1094/MPMI.2004.17.2.184. [DOI] [PubMed] [Google Scholar]
- 38.Chatterjee A., Cui Y., Hasegawa H., Leigh N., Dixit V., Chatterjee A.K. Comparative analysis of two classes of quorum-sensing signaling systems that control production of extracellular proteins and secondary metabolites in Erwinia carotovora subspecies. J. Bacteriol. 2005;187:8026–8038. doi: 10.1128/JB.187.23.8026-8038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toth I.K., Pritchard L., Birch P.R.J. Comparative genomics reveals what makes an enterobacterial plant pathogen. Annu. Rev. Phytopathol. 2006;44:305–336. doi: 10.1146/annurev.phyto.44.070505.143444. [DOI] [PubMed] [Google Scholar]
- 40.Jafra S., Jalink H., van der Schoor R., van der Wolf J.M. Pectobacterium carotovorum subsp. carotovorum strains show diversity in production of response to N-acyl homoserine lactones. J. Phytopathol. 2006;154:729–739. [Google Scholar]
- 41.Gardan L., Gouy C., Christen R., Samson R. Elevation of three subspecies of Pectobacterium carotovorum to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. Int. J. Syst. Evol. Microbiol. 2003;53:381–391. doi: 10.1099/ijs.0.02423-0. [DOI] [PubMed] [Google Scholar]
- 42.Glasner J.D., Marquez-Villavicencio M., Kim H.S., Jahn C.E., Ma B., Biehl B.S., Rissman A.I., Mole B., Yi X., Yang C.H., et al. Niche-specificity and the variable fraction of the Pectobacterium Pan-genome. Mol. Plant Microbe Interact. 2008;21:1549–1560. doi: 10.1094/MPMI-21-12-1549. [DOI] [PubMed] [Google Scholar]
- 43.Park S.Y., Hwang B.J., Shin M.H., Kim J.A., Kim H.K., Lee J.K. N-Acylhomoserine lactonase producing Rhodococcus spp. with different AHL-degrading activities. FEMS Microbiol. Lett. 2006;261:102–108. doi: 10.1111/j.1574-6968.2006.00336.x. [DOI] [PubMed] [Google Scholar]
- 44.Uroz S., Oger P.M., Chapelle E., Adeline M.T., Faure D., Dessaux Y. A Rhodococcus qsdA-encoded enzyme defines a novel class of large-spectrum quorum-quenching lactonases. Appl. Environ. Microbiol. 2008;74:1357–1366. doi: 10.1128/AEM.02014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uroz S., Chhabra S.R., Camara M., Williams P., Oger P., Dessaux Y. N-Acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology. 2005;151:3313–3322. doi: 10.1099/mic.0.27961-0. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H.B., Wang L.H., Zhang L.H. Genetic control of quorum sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA. 2002;99:4638–4643. doi: 10.1073/pnas.022056699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlier A., Chevrot R., Dessaux Y., Faure D. In Agrobacterium tumefaciens strain C58, the assimilation of gamma-butyrolactone interferes with the accumulation of the N-acyl-homoserine lactone signal. Mol. Plant Microbe Interact. 2004;17:951–957. doi: 10.1094/MPMI.2004.17.9.951. [DOI] [PubMed] [Google Scholar]
- 48.Yang S., Zhang Q., Guo J., Charkowski A.O., Glick B.R., Ibekwe A.M., Cooksey D.A., Yang C.H. Global effect of indole-3-acetic acid biosynthesis on multiple virulence factors of Erwinia chrysanthemi 3937. Appl. Environ. Microbiol. 2007;73:1079–1088. doi: 10.1128/AEM.01770-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S., Peng Q., San Francisco M., Wang Y., Zeng Q., Yang C.H. Type III secretion system genes of Dickeya dadantii 3937 are induced by plant phenolic acids. PLoS One. 2008;3:e2973. doi: 10.1371/journal.pone.0002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Effantin G., Rivasseau C., Gromova M., Bligny R., Hugouvieux-Cotte-Pattat N. Massive production of butanediol during plant infection by phytopathogenic bacteria of the genera Dickeya and Pectobacterium. Mol. Microbiol. 2011;82:988–997. doi: 10.1111/j.1365-2958.2011.07881.x. [DOI] [PubMed] [Google Scholar]
- 51.Mhedbi-Hajri N., Malfatti P., Pédron J., Gaubert S., Reverchon S., van Gijsegem F. PecS is an important player in the regulatory network governing the coordinated expression of virulence genes during the interaction between Dickeya dadantii 3937 and plants. Environ. Microbiol. 2011;13:2901–2914. doi: 10.1111/j.1462-2920.2011.02566.x. [DOI] [PubMed] [Google Scholar]
- 52.Faure D., Dessaux Y. Quorum sensing as a target for developing control strategies for the plant pathogen Pectobacterium. Eur. J. Plant Pathol. 2007;119:353–365. [Google Scholar]
- 53.Cirou A., Raffoux A., Diallo S., Latour X., Dessaux Y., Faure D. Gamma-caprolactone stimulates the growth of quorum-quenching Rhodococcus populations in a large-scale hydroponic system for culturing Solanum tuberosum. Res. Microbiol. 2011;162:945–950. doi: 10.1016/j.resmic.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Cirou A., Mondy S., An S., Charrier A., Sarrazin A., Thoison O., DuBow M., Faure D. Efficient biostimulation of the native and introduced quorum-quenching Rhodococcus erythropolis is revealed by a combination of analytical chemistry, microbiology and pyrosequencing. Appl. Environ. Microbiol. 2012;78:481–492. doi: 10.1128/AEM.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbey C., Crépin A., Cirou A., Budin-Verneuil A., Orange N., Feuilloley M., Faure D., Dessaux Y., Burini J.F., Latour X. Catabolic pathway of gamma-caprolactone in the biocontrol agent Rhodococcus erythropolis. J. Proteome Res. 2012;11:206–216. doi: 10.1021/pr200936q. [DOI] [PubMed] [Google Scholar]