Abstract
Choroidal blood flow (ChBF) compensates for changes in arterial blood pressure (ABP) and thereby remains relatively stable within a ±40 mmHg range of basal ABP in rabbits, humans and pigeons. In the present study, we investigated if ChBF can compensate for increases and decreases in ABP in rats. ChBF was continuously monitored using laser Doppler flowmetry in anesthetized rats, and ABP measured via the femoral artery. At multiple intervals over a 2-4 hour period during which ABP varied freely, ChBF and ABP were sampled and the results compiled across rats. We found that ChBF remained near baseline over an ABP range from 40 mmHg above basal ABP (90-100 mmHg) to 40 mmHg below basal ABP, but largely followed ABP linearly below 60 mmHg. Choroidal vascular resistance increased linearly as BP increased above 100 mmHg, and decreased linearly as BP declined from basal to 60 mmHg, but resistance declined no further below 60 mmHg. Inhibition of nitric oxide (NO) formation by either a selective inhibitor of neuronal nitric oxide synthase (NOS) (Nω-propyl-L-arginine) or a nonselective inhibitor of both neuronal NOS and endothelial NOS (Nω-nitro-L-arginine methyl ester) did not affect compensation above 100 mmHg ABP, but did cause ChBF to linearly follow declines in BP below 90 mmHg. In NOS-inhibited rats, vascular resistance increased linearly with BP above 100 mmHg, but remained at baseline below 90 mmHg. These findings reveal that ChBF in rats, as in rabbits, humans and pigeons, compensates for rises and/or declines in arterial blood pressure so as to remain relatively stable within a physiological range of ABPs. The ChBF compensation for low ABP in rats is dependent on choroidal vasodilation caused by neuronal NO formation but not the compensation for elevated BP, implicating parasympathetic nervous system vasodilation in the ChBF compensation to low ABP.
Keywords: Rats; Choroidal blood flow; Blood Pressure; Baroregulation, Autoregulation; Nitric oxide
Introduction
Cerebral blood flow remains relatively stable, despite variations in systemic blood pressure, over a 60-150 mmHg range (Paulson et al., 1990; Wahl and Schilling, 1993). The vascular compensation (mediated by vasodilation) for the low perfusion pressure resulting from low systemic blood pressure prevents tissue ischemia, while the compensation for the high perfusion pressure (mediated by vasoconstriction) resulting from high systemic blood pressure prevents tissue edema (Johnson, 1980; Kiel, 1994). The phenomenon of blood flow stability during systemic blood pressure (BP) variation has typically been termed autoregulation, because of the view that the compensation is mediated at the level of the organ or tissue itself. For the cerebral vasculature, however, both an intrinsic vascular myogenic mechanism (which acts to maintain vessel wall stretch within a preferred range) and a neurally mediated parasympathetic mechanism have been proposed to contribute to blood flow compensation during low systemic BP (Pavlov et al., 1983, 1987; Gotoh and Tanaka, 1988; Paulson et al., 1990; Morita et al., 1995; Ishitsuka et al., 1996). Because of the latter, we will use the term baroregulation to refer to the phenomenon of blood flow stability despite BP variation, since it is more descriptive and avoids the possibly erroneous mechanistic implications connoted by the term “autoregulation”.
It was once thought that, in contrast to the cerebral vasculature, choroidal blood flow did not show stability when perfusion pressure into the eye was manipulated - that is, choroidal blood flow (ChBF) was reported to increase linearly with increasing ocular perfusion pressure and decrease linearly with decreasing ocular perfusion pressure (Friedman, 1970; Bill, 1985). More recent studies in rabbits, humans and pigeons, however, have shown, that when choroidal perfusion pressure is adjusted by manipulating BP rather than intraocular pressure, prominent choroidal blood flow compensation for BP fluctuations over a range of ±40-50% of basal BP is observed (Kiel and Shepherd, 1992; Kiel and van Heuven, 1995; Riva et al., 1997a; Kiel, 1999; Lovasik et al., 2003; Reiner et al., 2003). A myogenic mechanism has been proposed to play a role in choroidal compensation for variation in systemic BP (Kiel and Shepherd, 1992; Kiel, 1994), but there is evidence for a neurogenic contribution as well (Kiel, 1999; Hardy et al., 2001). For example, pharmacological studies implicate formation of nitric oxide in the compensation to low BP, and anatomical studies show that parasympathetic circuitry controlling ChBF in rodents receives input from BP-sensitive brain regions (Cuthbertson et al., 2003).
In the present study, we sought to determine whether choroidal blood flow in rats also compensates for variation in systemic BP. Kiel and van Heuven (1995) have noted that choroidal blood flow regulation in response to a change in perfusion pressure is most effective when ocular perfusion pressure is altered by a change in ABP, and intraocular pressure (which affects perfusion pressure into the eye) is not controlled. This circumstance simulates the natural condition of ocular perfusion pressure variation due to the state of activity, and is most likely to engage baroreceptive control of ChBF. Thus, we examined ChBF responses to spontaneously occurring variation in mean arterial BP in normal rats, and in rats in which nitric oxide (NO) formation was pharmacologically inhibited. The results presented here indicate that the choroidal vasculature in rats shows significant compensation for both spontaneous rises and declines in BP, and the compensation for declines requires neuronal NO formation, presumably by peripheral parasympathetic neurons that exert a vasodilatory effect on choroidal blood flow.
Methods
General Procedures
Adult male Sprague-Dawley rats (300-400g) were anesthetized with ketamine (0.66 mg/kg i.p.; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (0.33 mg/kg i.p., The Butler Company, Columbus, OH). Supplemental doses of ketamine/xylazine were administered every 20-30min to maintain deep anesthesia. Artificial respiration was not used, and pulse oximetry in a sample of 5 rats showed that blood oxygenation remained within 5% of the initial 84.2% oxygenation over about a 3 hour mean duration, even with a 25% drop in ABP. Choroidal blood flow (ChBF) was continuously monitored using transcleral laser Doppler flowmetry, and systemic arterial BP (ABP) was continuously monitored via a femoral artery catheter and a Blood Pressure Analyzer (BPA-100 Micro-Med Inc., Louisville, KY), using methods as in our prior studies with pigeons and rats (Fitzgerald et al., 1990, 1996; Zagvazdin et al., 1996a,b, 2000). Subsequent to catheterization, rats were positioned in a stereotaxic device, and body temperature maintained at 37°C with a Harvard heating blanket and rectal thermoprobe. Skin and fascia were removed to expose the sclera of the superior aspect of the right eye. The probe tip (1mm diameter) of a laser Doppler flowmetry instrument (LASERFLO blood perfusion monitor, Model BPM 403 A, Vasamedics, St. Paul MN) was positioned using a micromanipulator over the sclera between the superior and medial rectus muscles, and the site kept moist with ultrasound gel or 33% glycerol. Conjunctival or superficial scleral vessels were avoided in targeting the probe tip. For reasons explained previously (Fitzgerald et al., 1996, 2001; Zagvazdin et al., 2000), the relative blood flow values provided by the LASERFLO blood flow monitor are referred to as blood flow units (BFUs). A MacLab or PowerLab data acquisition system, and Macintosh computers were used to view, store and analyze the data.
In one set of studies, we sought to determine if ChBF baroregulation occurred during spontaneous BP fluctuation over a 50 mmHg range above and 75 mmHg range below basal ABP of about 95 mmHg. Spontaneously occurring changes in systemic blood pressure have been used by others to study the dynamics of cerebral blood flow regulation (Blaber et al., 1997; Panerai et al., 1998; Zhang et al., 1998), and we have previously found it effective for revealing choroidal baroregulation in pigeons (Reiner et al., 2003). For our studies, a population of 32 rats was analyzed, with continuous ChBF and ABP measurements made in each. Simultaneous 10-second samples of ChBF and ABP were taken at approximately 1-2 minute intervals over the course of a 2-4 hour recording session. The ABP and ChBF samples for the current study of choroidal baroregulation were taken during intervals when both were relatively stable and neither was changing rapidly, since different mechanisms may be involved in dynamic and static phases of baroregulation (Blaber et al., 1997; Panerai et al., 1998; Zhang et al., 1998; Reiner et al., 2003). The basis of the spontaneous fluctuation in ABP in the present study is uncertain. The regular anesthetic supplements maintained deep anesthesia, and thus fluctuation in level of anesthesia was rarely a factor in the ABP fluctuation. The average ABP range sampled for these 32 rats was 32.2mmHg ± 3.2 (SEM). In many rats, ABP declined toward the end of the session, which may have been the consequence of the cumulative anesthetic doses and/or blood loss.
In a second set of studies in 12 of the same rats, additional simultaneous samples of ABP and ChBF were collected >10 minutes after administration of the nitric oxide synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester (LNAME, 30-60mg/kg) in eight rats, or Nω -propyl-L-arginine (NPA, 3-5mg/kg) in four rats. These studies were conducted because of the anatomical and pharmacological evidence for a role of NO-mediated vasodilation in the parasympathetic control of ChBF (Nilsson, 1996, 2000; Cuthbertson et al., 2003), and because NOS inhibition has been shown to impair cerebral baroregulation (Preckel et al., 1996; Kobari et al., 1994), and choroidal baroregulation (Kiel, 1999). Since LNAME acts on endothelial and neuronal NO production, it elevates ABP (Zagvazdin et al., 1996a,b). By contrast, NPA is selective for neuronal NOS (Zhang et al., 2004), and it does not elevate ABP. We did, in fact, find this to be the case in our rats. LNAME caused a 51% increase in ABP, while NPA yielded no change in ABP. The average ABP range sampled for these 12 rats was 29.2mmHg ± 4.7 (SEM).
For rats in both groups, the data were grouped into 10 mmHg bins over the 140 to 90 range, and 5 mmHg bins over the 90 to 20 range. Because ABP ranged over 140 in few rats, the data above 140 mmHg were grouped in a 160 to a 140 bin. For each animal, all ABP values within a bin were then averaged to calculate the mean ABP for that bin, as were all ChBF values within that same bin. The basal mean ABP for the 32 normal rats was defined as the mean ABP for the 90-100 mmHg range for the normal rats, based on our prior observations in rats (Zagvazdin et al., 1996b), and basal ChBF was defined as the mean ChBF for this same bin. Basal ABP and ChBF were similarly defined as the mean values in the 90-100 mmHg range for the 12 NOS inhibition rats. The results for the LNAME and NPA NOS inhibition rats were calculated and are presented separately, as well as combined. Note that while 32 normal rats and 12 NOS inhibition rats were analyzed by this approach, not all rats yielded mean ABP values that fell within each bin, given the limited range of the spontaneous BP fluctuation in any given rat (about 30 mmHg). For the purposes of Table 1 (normal rats) and Table 2 (NOS inhibition rats), the mean ABP and ChBF for each bin was based on those animals whose ABP had included values within that ABP range. The number of animals whose values fell within each bin are shown in Tables 1 and 2, as are the mean number of samples per animal for each ABP range. The efficacy of choroidal compensation for BP change is indicated by its stability at ABPs above and below basal ABP. For ease of visualization, both ABP and ChBF for each range are shown in Tables 1 and 2 in mmHg and BFUs, as well as a percent of basal.
Table 1. Baroregulation in Normal Rats (N=32).
Tabulation of choroidal blood flow during fluctuation in arterial blood pressure in the 32 normal rats analyzed. The mean ABP for each 5, 10 or 20 mmHg step in blood pressure range was determined, as was the mean ChBF at each ABP step. The first column shows the ABP ranges into which data samples were grouped. The subsequent columns show the number of animals per range, the number of data samples per animal for each range, the mean ABP (± SEM) per ABP range (in mmHg), the mean ChBF (± SEM) per ABP range (in BFUs), the mean ABP per range expressed as a percent of basal ABP (taken as the mean ABP in the 90-100mmHg bin), the mean ChBF per range expressed as a percent of basal ChBF (taken as the mean ChBF during ABP in the 90-100 mmHg range), and the mean choroidal resistance per ABP range expressed as a percent of basal resistance (taken as resistance during ABP in the 90-100 mmHg range). As can be seen, ChBF values were consistently higher than would be predicted if ChBF passively followed ABP in the low ABP range and consistently lower than expected if ChBF passively followed ABP in the high ABP range. In general, ChBF remained near basal levels over the 135 to 60 mmHg ABP range, and choroidal resistance declined linearly over this range.
| Normal ABP Range | Rats per ABP Range | Mean BP-ChBF Samples per Rat | Mean ABP ± SEM per ABP Range | Mean ChBF ± SEM per ABP Range | Mean ABP per ABP Range as % of basal ABP | Mean ChBF per ABP Range as % of basal ChBF | ChBF Resistance per ABP Range as % of basal |
|---|---|---|---|---|---|---|---|
| 160-140 | 0 | 0 | |||||
| 130-140 | 2 | 1.5 | 135.68±2.02 | 13.69±3.98 | 143.4% | 88.4% | 162.3% |
| 120-130 | 7 | 4.9 | 124.24±1.10 | 15.08±1.45 | 131.3% | 97.3% | 134.9% |
| 110-120 | 11 | 3.8 | 115.02±0.55 | 15.60±1.50 | 121.6% | 100.7% | 120.7% |
| 100-109 | 19 | 10.3 | 104.91±0.74 | 15.65±1.26 | 110.9% | 101.0% | 110.8% |
| 90-100 | 23 | 12.5 | 94.62±0.44 | 15.50±0.85 | 100.0% | 100.0% | 100.0% |
| 85-90 | 21 | 10.8 | 87.34±0.34 | 16.24±0.94 | 92.3% | 104.8% | 88.1% |
| 80-85 | 22 | 8.9 | 82.55±0.25 | 15.44±1.24 | 87.2% | 99.6% | 87.6% |
| 75-80 | 22 | 5.0 | 77.20±0.23 | 16.04±1.41 | 81.6% | 103.5% | 78.8% |
| 70-75 | 15 | 4.6 | 72.72±0.24 | 16.49±2.27 | 76.9% | 106.4% | 72.2% |
| 65-70 | 8 | 6.8 | 67.44±0.43 | 16.93±3.17 | 71.3% | 109.2% | 65.3% |
| 60-65 | 8 | 4.1 | 63.24±0.30 | 16.22±3.52 | 66.8% | 104.7% | 63.8% |
| 55-60 | 5 | 2.0 | 56.54±0.86 | 9.83±2.35 | 59.8% | 63.4% | 94.2% |
| 50-55 | 4 | 3.5 | 52.00±0.39 | 10.68±5.05 | 55.0% | 68.9% | 79.8% |
| 45-50 | 5 | 2.6 | 48.21±0.58 | 10.80±4.26 | 51.0% | 69.7% | 73.1% |
| 40-45 | 2 | 3.0 | 42.99±0.02 | 8.11±1.21 | 45.4% | 52.3% | 86.8% |
| 35-40 | 3 | 3.0 | 37.13±0.86 | 7.78±1.48 | 39.2% | 50.2% | 78.2% |
| 30-35 | 2 | 2.0 | 33.30±0.91 | 6.80±0.02 | 35.2% | 43.9% | 80.3% |
| 25-30 | 3 | 2.3 | 27.68±0.62 | 5.33±0.27 | 29.3% | 34.0% | 85.0% |
| 20-25 | 2 | 2.0 | 23.76±0.28 | 5.91±2.28 | 25.1% | 38.2% | 66.3% |
Table 2. Baroregulation in NOS Inhibition Rats (N=14).
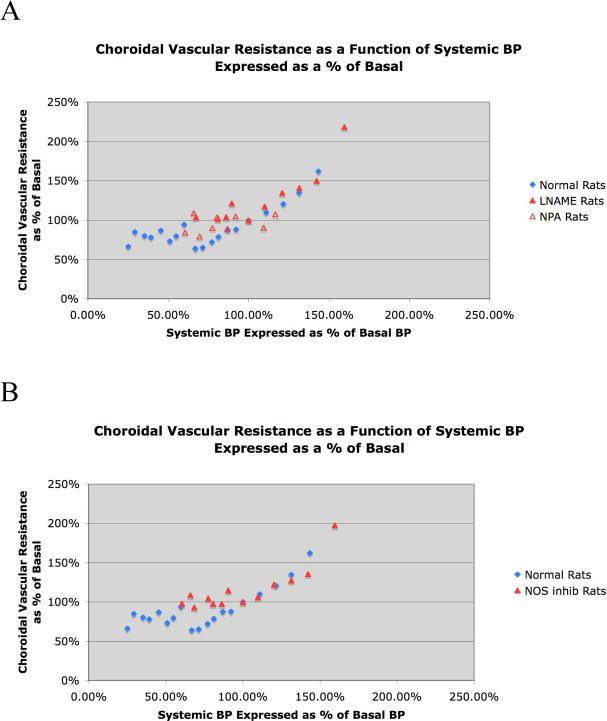
Tabulation of choroidal blood flow during spontaneous fluctuation in arterial blood pressure in the 12 rats analyzed that had been treated with the NOS inhibitors LNAME or NPA. The mean ABP for each 5, 10 or 20 mmHg step in pressure was determined, as was the mean ChBF at each ABP step. The first column shows the ABP ranges into which data samples were grouped. The subsequent columns show the number of animals per range, the number of data samples per animal for each range, the mean ABP (± SEM) per range (in mmHg), the mean ChBF (± SEM) per range (in BFUs), the mean ABP per range expressed as a percent of basal ABP (taken as the mean ABP in the 90-100 mmHg bin), the mean ChBF per range expressed as a percent of basal ChBF (taken as the mean ChBF during ABP in the 90-100 mmHg range), and the mean choroidal resistance per ABP range expressed as a percent of basal resistance (taken as resistance during ABP in the 90-100mmHg range). As can be seen for the NOS inhibition rats, ChBF values were as would be predicted if ChBF passively followed ABP in the low ABP range, but consistently lower than expected (and around 100% of basal) if ChBF passively followed ABP in the high ABP range. Resistance increased linearly above 100 mmHg, but remained near basal resistance (expressed as 100%) below 90 mmHg.
| Normal ABP Range | Rats per ABP Range | Mean BP-ChBF Samples per Rat | Mean ABP ± SEM per ABP Range | Mean ChBF ± SEM per ABP Range | Mean ABP per ABP Range as % of basal ABP | Mean ChBF per ABP Range as % of basal ChBF | ChBF Resistance per ABP Range as % of basal |
|---|---|---|---|---|---|---|---|
| 160-140 | 2 | 15.5 | 151.89±2.04 | 11.43±2.85 | 159.47% | 80.63% | 204.48% |
| 130-140 | 4 | 4.8 | 135.50±1.30 | 14.84±2.79 | 142.26% | 104.73% | 148.34% |
| 120-130 | 6 | 3.1 | 125.11±1.09 | 14.60±1.11 | 131.35% | 103.03% | 130.53% |
| 110-120 | 6 | 4.2 | 114.42±1.11 | 13.90±0.87 | 120.13% | 98.08% | 114.61% |
| 100-109 | 10 | 6.1 | 104.45±0.62 | 14.68±1.68 | 109.66% | 103.60% | 116.37% |
| 90-100 | 7 | 8.3 | 95.24±0.64 | 14.17±1.62 | 100.00% | 100.00% | 106.75% |
| 85-90 | 5 | 11.0 | 86.16±1.29 | 11.18±1.09 | 90.46% | 78.89% | 119.05% |
| 80-85 | 5 | 6.8 | 82.19±0.35 | 12.51±1.28 | 86.29% | 88.25% | 101.20% |
| 75-80 | 4 | 5.6 | 76.64±0.28 | 12.51±1.72 | 80.47% | 88.26% | 102.44% |
| 70-75 | 1 | 4.0 | 73.80 | 10.48 | 77.48% | 73.98% | 104.72% |
| 65-70 | 2 | 6.5 | 65.12±1.61 | 10.40±0.37 | 68.37% | 73.38% | 93.18% |
| 60-65 | 1 | 28.0 | 62.72 | 10.69 | 65.86% | 75.46% | 87.27% |
| 55-60 | 1 | 1.0 | 57.56 | 8.73 | 60.43% | 61.60% | 98.10% |
| 50-55 | 0 | ||||||
| 45-50 | 0 | ||||||
| 40-45 | 0 | ||||||
| 35-40 | 0 | ||||||
| 30-35 | 0 | ||||||
| 25-30 | 0 | ||||||
| 20-25 | 0 |
Additionally, the choroidal resistance was calculated for each ABP range, by dividing ChBF into ABP. For ease of interpretation, the resistance is shown in Tables 1 and 2 and in Figure 4 expressed as a percent of basal resistance, where 100% represents basal resistance for the 90-100 mmHg range. Note that in using ABP to estimate choroidal perfusion pressure, we did not measure or take into account the effect of intraocular pressure (IOP) on choroidal perfusion pressure for two reasons. First, any impact of IOP changes on choroidal perfusion pressure during ABP changes is likely to have been small, given the magnitude of any IOP changes is likely to be small (IOP in rats is about 20mmHg) (Pang et al., 2005). Secondly, we do not know the extent to which changes in extraocular vessels versus choroidal vessels contribute to changes in choroidal blood flow during ABP variation, and perfusion pressure in extraocular vessels would not be affected directly by IOP. To assess the statistical significance of the ChBF response to ABP variation, regression analysis was carried out for the individual animal values for the ABP range ≥ 95 mmHg, for the ≥ 60 mmHg but < 95 mmHg range, and for the < 60 mmHg range, in both normal and NOS inhibited rats. For the latter, the LNAME and NPA values were pooled. The analysis was carried out on the ABP data expressed in mmHg and the ChBF data expressed in BFUs.
4.
Graphic depiction of choroidal vascular resistance over an ABP range of 20 mmHg to 140 mmHg in 32 normal rats and 12 rats that received either of two inhibitors of neuronal NOS, with the results for the two inhibitors (LNAME and NPA) shown separately in A and averaged together in B. The mean choroidal vascular resistance for each ABP range is graphed as a function of the corresponding ABP, with choroidal resistance expressed as a percent of the resistance at basal ABP (about 95 mmHg), and ABP expressed as a percent of that basal ABP. In both graphs, the blue diamonds show the choroidal resistance per ABP bin for normal rats, while the red triangles show the choroidal resistance for the NOS inhibition rats. As can be seen, choroidal resistance decreased linearly as ABP declined from 40% above to 40% below basal ABP. Inhibition of neuronal NOS for both LNAME rats (closed red triangles) and NPA rats (open red triangles) prevented the decrease in resistance with ABPs below basal, as shown in image A. Combining the NOS rats in B (closed red triangles) shows that choroidal vascular resistance remained at basal levels (100%) below the basal ABP of about 95 mmHg.
Results
Normal Rats
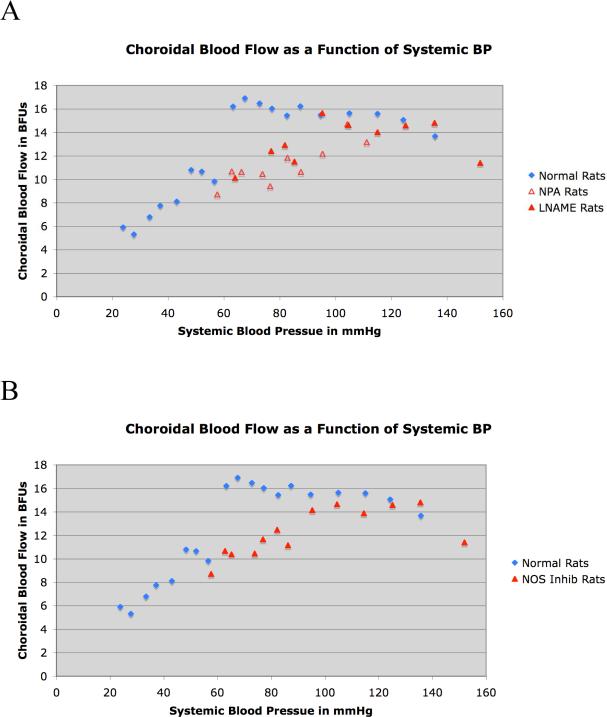
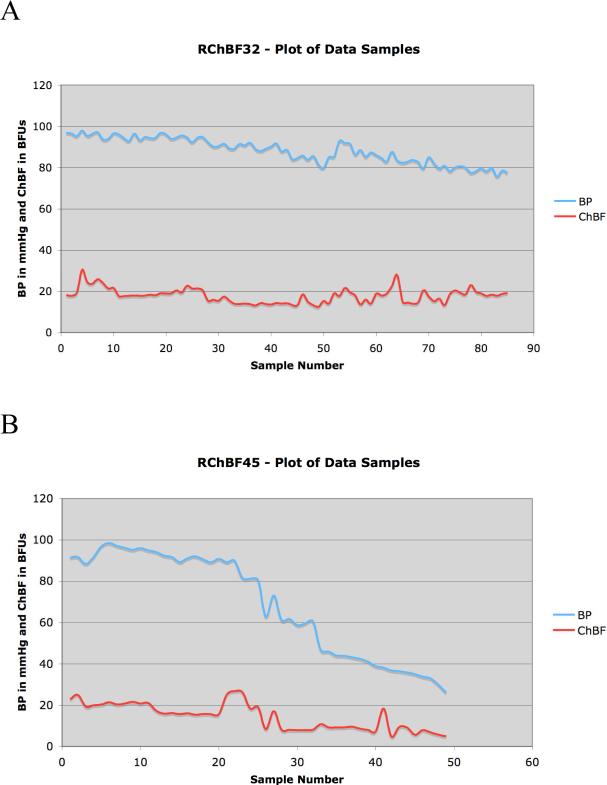
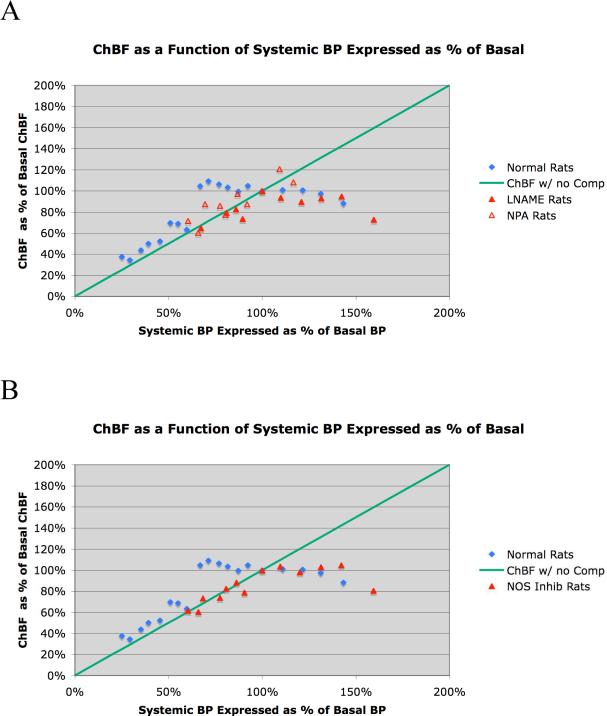
The findings from our studies on the effects of spontaneous ABP fluctuation on ChBF in normal rats are summarized in Table 1 and shown in Figures 1-4. In brief, the graphed data for all cases (Fig. 1) and the two illustrative cases (Fig. 2) indicate that ChBF was maintained near basal over an ABP range from 135 mmHg to 60 mmHg. Below 60 mmHg, ChBF fell well below basal ChBF. While perhaps some ChBF compensation was still present below 60 mmHg, the compensation for ABP decline was at best poor between 45 mmHg and 60 mmHg, and was negligible below 45 mmHg. This pattern of results is also evident with ChBF expressed as a percent of basal ChBF (Table 1, Fig. 3). For example, while the mean ABP showed about a 30% decline below basal levels over the 100 mmHg to 60 mmHg range, the ChBF consistently remained around 100% of the basal level (Table 1). With ABP fallen to below 60% of baseline (i.e. to 53.8 mmHg), however, the ChBF as a percent of basal tended to be near the ABP as a percent of basal. Statistical analysis confirms these observations, since ChBF was uncorrelated with ABPs for ≥ 95 mmHg (r = 0.05, N=43), as well as for ABPs ≥ 60 mmHg but < 95 mmHg (r = -0.03, N=114). By contrast, for ABPs < 60 mmHg, the ChBF was significantly correlated with ABP (r = 0.36, N=26). The calculated choroidal resistance showed a similar pattern (Table 1, Fig. 4). Above 100 mmHg, the resistance increased linearly with ABP, resulting in effective ChBF compensation, while from 100 mmHg to 60 mmHg, resistance decreased linearly with ABP, again yielding ChBF compensation. Below 60 mmHg, the resistance did not decrease further and ChBF was thus unable to compensate for ABP increasingly below 60 mmHg.
1.
Graphic depiction of ChBF over an ABP range of 20 mmHg to 140 mmHg in 32 normal rats and 12 rats that received either of two inhibitors of neuronal NOS, with the results for the two inhibitors (LNAME and NPA) shown separately in A and averaged together in B. The mean ChBF is graphed as a function of the corresponding ABP, with choroidal blood flow expressed in BFUs and ABP in mmHg. SEMs for the graphed data are shown in Tables 1 and 2. In both graphs, the blue diamonds show the actual ChBF per ABP bin for normal rats, while the red triangles show the ChBF for the NOS inhibition rats. As can be seen, ChBF remained near basal levels over an ABP range of 40 mmHg above and below basal ABP. Inhibition of neuronal NOS eliminated baroregulation in the low ABP range for both LNAME rats (closed red triangles) and NPA rats (open red triangles), as shown in image A. Combining the NOS rats in B (closed red triangles) shows that ChBF failed to compensate and linearly followed ABP below the basal ABP of about 95 mmHg.
2.
Graphs showing BP and ChBF for the sampled parts of the recording session for two rats. In both rats, BP declines toward the end of the session, in the second to below 30mmHg. In both records, ChBF remains constant at BP above 60mmHg. Note that in both records, spontaneous BP fluctuations occur throughout, superimposed on the general downward trend in BP.
3.
Graphic depiction of ChBF over an ABP range of 20 mmHg to 140 mmHg in 32 normal rats and 12 rats that received either of two inhibitors of neuronal NOS, with the results for the two inhibitors (LNAME and NPA) shown separately in A and averaged together in B. The mean ChBF is graphed as a function of the corresponding ABP, with choroidal blood flow expressed as a percent of basal ChBF (about 15 BFUs) and ABP expressed as a percent of basal ABP (about 95 mmHg). In both graphs, the blue diamonds show the ChBF for normal rats, while the red triangles show the ChBF for the NOS inhibition rats. The green line shows ChBF as it would be if it linearly followed ABP, that is, with no compensation. As can be seen, ChBF remained near basal levels (100%) over an ABP range from 40 mmHg above to 40 mmHg below basal ABP. Inhibition of neuronal NOS eliminated baroregulation in the low ABP range for both LNAME rats (closed red triangles) and NPA rats (open red triangles), as shown in image A. Combining the NOS rats in B (closed red triangles) shows that ChBF failed to compensate and linearly followed ABP below the basal ABP of about 95 mmHg.
NOS Inhibition Studies
We found that NOS inhibition impaired the ChBF baroregulation with ABP fluctuation below basal but not above basal ABP (Table 2, Figs. 1, 3, 4). For both the rats with LNAME inhibition of NOS and those with NPA inhibition of NOS, a similar result was observed. Below 100% of basal ABP, ChBF mirrored the ABP value (expressed as a percent of basal) in both drug treatment groups, and there was no statistically significant difference between the two drug treatment groups by independent t-test per range (Figs. 1A, 3A, 4A). Accordingly, the data for the 12 rats are tabulated together in Table 2, and additionally graphed together in Figures 1B, 3B, 4B). Note that the animals with high ABP tended to be those that received LNAME, reflecting our observation that LNAME but not NPA increased systemic BP. The results show that despite NOS inhibition, ChBF remained at about 100% of basal levels with ABP from 100 to 150 mmHg. Statistical analysis confirms these observations, since ChBF was uncorrelated with ABPs for ≥ 95 mmHg (r = -0.08, N=29) in the pooled NOS inhibition rats. By contrast, below 95 mmHg in the pooled NOS inhibition rats, the ChBF was significantly correlated with ABP (r = 0.43, N=24). A similar pattern is evident for the choroidal resistance measurements in the NOS inhibition rats, with resistance increasing linearly above basal ABP, but remaining at basal levels below basal ABPs (Table 2, Fig. 4).
It should be noted that in the NOS-inhibition rats, ChBF was actually reduced to about 80% of basal at 50% above basal ABP. We also observed an overreaction of ChBF in LNAME-treated pigeons to ABP reduction (Reiner et al., 2003). These findings are consistent with the observation of Kiel (1999) of interplay between vasodilatory NO-mediated mechanisms and vasoconstrictory mechanisms during ChBF compensation for ABP changes. In our LNAME-treated rats and birds, the NO-mediated mechanism was blocked, possibly leaving unopposed by vasodilation a mechanism that yields extreme choroidal vasoconstriction at very high ABPs.
Discussion
In the present study, we investigated whether the choroidal vasculature in rats can compensate for decreases and increases in systemic BP so as to maintain ChBF near baseline during periods of diminished or elevated perfusion pressure. We observed that ChBF during BP fluctuation remained stable over a 60-140 mmHg ABP range, as has also been reported for the cerebral vasculature (Paulson et al., 1990; Wahl and Schilling, 1993). The ChBF compensation above basal ABP was associated with a linear increase in choroidal resistance, while the compensation below basal ABP was associated with a linear decline in choroidal resistance. The ChBF compensation was poor or absent below 60 mmHg, and associated with no further reductions in choroidal resistance. Our results suggest that choroidal vasodilation may be maximal by an ABP of 60 mmHg, and thus the choroid is unable to compensate for BP declines below 60 mmHg. Note that the choroidal baroregulation could not have been the spurious byproduct of compensation in the retinal vasculature for the ABP decline, since retinal blood flow contributes relatively little to ocular blood flow (Bill, 1984), especially as measured by laser Doppler from the scleral side. Moreover, baroregulation to low systemic BP by the retinal vasculature in mammals is not NO-mediated (Gidday and Zhu, 1995), and we observed that the combined endothelial NOS - neuronal NOS inhibitor LNAME and the selective neuronal NOS inhibitor NPA blocked baroregulation in the low ABP range, suggesting that the mechanism of ChBF compensation for ABP decline involves NO production by neurons.
Note that in the very high and very low ABP ranges, our data is based on only a few rats. Thus, while the general ChBF trends in the high and low ABP ranges seem clear, we cannot (for example) be certain at what ABP baroregulation fails entirely in normal rats. For the same reason, we cannot be certain of the pattern of choroidal resistance changes at low ABP. For example, it is evident that the resistance changes did not compensate for <60 mmHg ABP, but it is uncertain if the resistance remained as it was at 60 mmHg or reverted to the basal level seen at an ABP of 95 mmHg. Additionally, we cannot rule out an impact of possible blood gas abnormalities at low ABPs – for example high CO2 and/or low O2. While we did use pulse oximetry to monitor blood oxygenation in a sample of five rats, and found that it remained stable even with a 25% drop in ABP, it may be that blood oxygenation was reduced and/or CO2 elevated at very low pressures. Prior studies have not, however, found low blood O2 concentration to affect ChBF (Milley et al., 1984; Kergoat et al., 2005). While high CO2 has been reported to increase ChBF (Alm and Bill, 1972; Wilson et al. 1977; Milley et al., 1984; Stiris et al., 1991; Geiser et al 2000), we found that ChBF was linear with ABP below 60 mmHg. Thus, even if there was high blood CO2 in our rats at low ABP that promoted choroidal vasodilation, our results still show a failure of baroregulation with low ABP. Moreover, for the cerebral vasculature at least, the vasodilatory effect of high CO2 is lost at low ABP (Paulson et al., 1990).
The earliest studies on the responses of the choroidal circulation to changes in systemic blood pressure had reported that ChBF changes linearly with choroidal perfusion pressure, and led to the view that choroidal blood flow did not show the phenomenon commonly called autoregulation (Alm and Bill, 1970, 1972, 1973; Friedman, 1970; Bill, 1985; Yu et al., 1987; Gherezghiher et al., 1991; Hardy et al., 1996). The explanation offered was that choroidal blood flow was so high that putative fluctuations in it caused by variations in ocular perfusion pressure were unimportant and of no consequence for the role of the choroid in supporting the retina (Bill, 1984, 1985). It is now known, however, that even modest reductions in choroidal blood flow adversely affect retinal metabolism and function (Steinberg, 1987; Yancey and Linsenmeier, 1988, 1989; Yu and Cringle, 2001), while increases cause overperfusion of the retina (Bill, 1984). Moreover, more recent studies in rabbits and humans, and ours in pigeons, have shown that when choroidal perfusion pressure is experimentally adjusted by manipulating BP rather than by manipulating IOP (as many prior studies had done to alter choroidal perfusion pressure), choroidal blood flow shows prominent compensation for BP fluctuations over a range of ±40-50% of basal ABP (Kiel and Shepherd, 1992; Kiel and van Heuven, 1995; Riva et al., 1997a; Lovasik et al., 2003; Reiner et al., 2003). The work of Riva et al. (1997b) in humans, however, has shown that even when ocular perfusion pressure is reduced by increasing IOP, significant compensation by ChBF for the reduced perfusion pressure is evident. Experimental considerations that may have led to the failure to observe or report choroidal baroregulation in prior studies have been discussed by others (Kiel and Shepherd, 1992; Kiel, 1994; Riva et al., 1997b).
For the cerebral vasculature, both an intrinsic vascular smooth muscle myogenic mechanism (which acts to maintain vessel wall stretch within a preferred range) and a neurally mediated parasympathetic mechanism have been suggested to contribute to baroregulation during systemic hypotension (Paulson et al., 1990; Gotoh and Tanaka, 1988; Koketsu et a., 1992; Morita et al., 1995; Ishitsuka et al., 1996). Consistent with a specific role of nitrergic parasympathetic innervation in cerebral baroregulation, NOS inhibition is known to impair cerebral blood flow compensation during low systemic BP (Preckel et al., 1996; Kobari et al., 1994). A myogenic mechanism has also been proposed to play a role in choroidal baroregulation as well (Kiel and Shepherd, 1992; Kiel, 1994), and a neurogenic contribution has been suggested as well (Kiel, 1999; Hardy et al., 2001). Given the evidence for NO-mediated parasympathetic control of ChBF by the autonomic subdivision of the facial nucleus (Nilsson, 1996, 2000; Cuthbertson et al., 2003), our current data for rats that NOS inhibition with either LNAME or NPA eliminates ChBF baroregulation in response to low BP, and the similar data of Kiel for rabbit (1999), suggest a role of nitrergic parasympathetic innervation to the choroid in the hypotensive compensatory process. The finding that the baroreceptive region of the nucleus of the solitary tract, which receives information about ABP via the aortic depressor nerve (Ciriello, 1983; Rogers et al., 1993), projects to the autonomic part of the facial motor complex that contains the preganglionic neurons (i.e. the superior salivatory nucleus) that project to neurons of the pterygopalatine ganglion that mediate neural NO-dependent parasympathetic vasodilation in the choroid and cerebral vascular bed, is also consistent with this possibility (Steinle et al., 2000; Agassandian et al., 2003; Cuthbertson et al., 2003). Our observation that baroregulation failed below 60 mmHg and the evidence that baroreceptors unload at this ABP (Brown, 1980) is also consistent with a role of neural mechanisms in the choroidal baroregulation to low BP that we observed here in rats. Although we did not specifically investigate the basis of the choroidal baroregulation to high BP, this baroregulation was associated with vasoconstriction, suggesting a possible role of the sympathetic nervous system. This interpretation is consistent with the observation that the sympathetic nervous system prevents choroidal overperfusion during high systemic blood pressure in cats (Bill, 1985), and with the finding that the sympathetic nervous system plays a major role in cerebral blood flow compensation for high systemic blood pressure (Gotoh and Tanaka, 1988; Morita et al., 1995). In this view then, the more prominent choroidal compensation in response to BP than IOP fluctuation can be explained by the fact that BP but not IOP fluctuation calls into action baroreceptor-mediated autonomic responses.
Other mechanisms may also contribute to choroidal baroregulation. For example, Kiel (1994) showed that declines in BP result in a decrease in choroidal vascular volume, resulting in slightly reduced IOP (2-3 mmHg over a 40-80 mmHg ABP range). It is possible that such IOP declines, which increase choroidal perfusion pressure by decreasing choroidal vascular resistance, could help stabilize ChBF during systemic hypotension. Any possible IOP declines, however, could not be great enough to account for the baroregulation observed here in rats, and it should be noted Kiel (1994) did not interpret such IOP declines as the basis of choroidal baroregulation during systemic hypotension. In any event, our studies in conjunction with prior studies on pigeon, rabbits, piglets and humans suggest that choroidal compensation for ChBF declines and increases may be a common ocular mechanism among warm-blooded vertebrates. Such ChBF baroregulation would serve to prevent overperfusion-related edema and oxidative injury during high ABP, and underperfusion-related ischemia in the retina during low ABP (Johnson, 1980; Kiel and Shepherd, 1992; Kiel, 1994), and thus seemingly be an important ocular homeostatic mechanism. Age-related declines in facial parasympathetic innervation of choroid occur in humans (Jablonski et al., 2007), which might impair baroregulation in the low ABP range, and thereby contribute to age-related injury to the retina (Feigl, 2009).
Acknowledgements
We thank Seth V. Jones, Toya Kimble, Sherry L. Cuthbertson, Julia Jones, Rebeca-Ann Weinstock, Amanda Taylor and Aminah Henderson for their invaluable assistance, and Dr. H. Peacher-Ryan for his statistical assistance. This study was supported by NIH-EY-05298 (AR), the Department of Ophthalmology at UTHSC (MECF), and the University of Tennessee Neuroscience Institute (CL). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agassandian K, Fazan VPS, Margaryan N, Dragon DN, Riley J, Talman WT. A novel central catecholaminergic pathway links arterial baroreceptors and pontine parasympathetic neurons in cerebrovascular control. Cell. Mol. Neurobiol. 2003;23:463–478. doi: 10.1023/A:1025059710382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm A, Bill A. Blood flow and oxygen extraction in the cat uvea at normal and high intraocular pressures. Acta Physiol. Scand. 1970;80:19–28. doi: 10.1111/j.1748-1716.1970.tb04765.x. [DOI] [PubMed] [Google Scholar]
- Alm A, Bill A. The oxygen supply to the retina. II. Effects of high intraocular pressure and of increased arterial carbon dioxide tension on uveal and retinal blood flows in cats: A study with radioactively labeled microspheres including flow determination in brain and some other tissues. Acta Physiol. Scand. 1972;84:306–319. doi: 10.1111/j.1748-1716.1972.tb05182.x. [DOI] [PubMed] [Google Scholar]
- Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressure in monkeys (Macaca irus): A study with radioactively labeled microspheres including flow determination in brain and some other tissues. Exp. Eye Res. 1973;15:15–29. doi: 10.1016/0014-4835(73)90185-1. [DOI] [PubMed] [Google Scholar]
- Bill A. The circulation in the eye. In: Renkin EM, Michel CC, editors. Handbook of Physiology. The Cardiovascular System: Microcirculation. The American Physiological Society; Baltimore, MD, U.S.A.: 1984. pp. 1001–1034. [Google Scholar]
- Bill A. Some aspects of the ocular circulation. Invest. Ophthalmol. Vis. Sci. 1985;26:403–424. [PubMed] [Google Scholar]
- Blaber AP, Bondar, Stein F, Dunphy PT, Moradshahi P, Kassam MS, Freeman R. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke. 1997;28:1686–1692. doi: 10.1161/01.str.28.9.1686. [DOI] [PubMed] [Google Scholar]
- Brown AM. Receptors under pressure. An update on baroreceptors. Circulation Res. 1980;46:1–10. doi: 10.1161/01.res.46.1.1. [DOI] [PubMed] [Google Scholar]
- Ciriello J. Brainstem projections of aortic baroreceptors afferent fibers in the rat. Neurosci Lett. 1983;36:37–42. doi: 10.1016/0304-3940(83)90482-2. [DOI] [PubMed] [Google Scholar]
- Cuthbertson S, LeDoux MS, Jones S, Jones J, Zhou Q, Gong S, Ryan P, Reiner A. Localization of preganglionic neurons that innervate choroidal neurons of the pterygopalatine ganglion. Invest. Ophthalmol. Vis. Sci. 2003;44:3713–3724. doi: 10.1167/iovs.02-1207. [DOI] [PubMed] [Google Scholar]
- Feigl B. Age-related maculopathy – Linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog. Retinal Eye Res. 2009;28:63–86. doi: 10.1016/j.preteyeres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MEC, Vana B, Reiner A. Control of choroidal blood flow by the nucleus of Edinger-Westphal: A laser-Doppler study. Invest. Ophthalmol. Vis. Sci. 1990;31:2483–2492. [PubMed] [Google Scholar]
- Fitzgerald MEC, Gamlin PDR, Zagvazdin Y, Reiner A. Central neural circuits for the light-mediated reflexive control of choroidal blood flow in the pigeon eye: A laser Doppler study. Vis. Neurosci. 1996;13:655–669. doi: 10.1017/s0952523800008555. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MEC, Tolley E, Frase S, Zagvazdin Y, Miller RF, Hodos W, Reiner A. Functional and morphological assessment of age-related changes in the choroid and outer retina in pigeons. Vis. Neurosci. 2001;18:299–317. doi: 10.1017/s0952523801182143. [DOI] [PubMed] [Google Scholar]
- Friedman E. Choroidal blood flow: pressure-flow relationships. Arch. Ophthalmol. 1970;83:95–99. doi: 10.1001/archopht.1970.00990030097018. [DOI] [PubMed] [Google Scholar]
- Gherezghiher T, Okubo H, Koss MC. Choroidal and ciliary body blood flow analysis: application of laser Doppler flowmetry in experimental animals. Exp. Eye Res. 1991;53:151–156. doi: 10.1016/0014-4835(91)90068-p. [DOI] [PubMed] [Google Scholar]
- Geiser MH, Riva CE, Dorner GT, Diermann U, Luksch A, Schmetterer L. Response of choroidal blood flow in the foveal region to hyperoxia and hyperoxia-hypercapnia. Curr. Eye Res. 2000;21:669–676. [PubMed] [Google Scholar]
- Gidday JM, Zhu Y. Nitric oxide does not mediate autoregulation of retinal blood flow in newborn pig. Am. J. Physiol. 1995;38:H1065–1072. doi: 10.1152/ajpheart.1995.269.3.H1065. [DOI] [PubMed] [Google Scholar]
- Gotoh F, Tanaka K. Handbook of Clinical Neurology. Vol. 53. 1988. Regulation of cerebral blood flow. pp. 47–77. [Google Scholar]
- Hardy P, Nyut AM, Abran D, St-Louis J, Varma DR, Chemtob S. Nitric oxide in retinal and choroidal blood flow autoregulation in newborn pigs: Interactions with prostaglandins. Pediatric Research. 1996;39:487–493. doi: 10.1203/00006450-199603000-00017. [DOI] [PubMed] [Google Scholar]
- Hardy P, Lamireau D, Hou X, Dumont I, Abran D, Nuyt AM, Varma DR, Chemtob S. Major role for neuronal nitric oxide synthase in curtailing choroidal blood flow autoregulation in newborn pigs. J. Appl. Physiol. 2001;91:1655–1662. doi: 10.1152/jappl.2001.91.4.1655. [DOI] [PubMed] [Google Scholar]
- Ishitsuka T, Iadecola C, Underwood MD, Reis DJ. Lesions of nucleus tractus solitarii globally impair cerebrovascular autoregulation. Am. J. Physiol. 1996;251:H269–H281. doi: 10.1152/ajpheart.1986.251.2.H269. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Iannaccone A, Reynolds DH, Gallaher P, Allen S, Wang XF, Reiner A. Age-related decline in VIP-positive parasympathetic nerve fibers in the human submacular choroid. Invest. Ophthalmol. Vis. Sci. 2007;48:479–485. doi: 10.1167/iovs.06-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PC. The myogenic response. In: Bohr DF, Somlyo AP, Sparks HV Jr., editors. Handbook of Physiology. The Cardiovascular System: Vascular Smooth Muscle. Am. Physiol. Society; Bethesda, MD: 1980. pp. 409–442. [Google Scholar]
- Kergoat H, Marinier JA, Lovasik JV. Effects of mild systemic hypoxia on the pulsatile choroidal blood flow in healthy young human adults. Curr. Eye Res. 2005;30:465–440. doi: 10.1080/02713680590956739. [DOI] [PubMed] [Google Scholar]
- Kiel JW. Choroidal myogenic autoregulation and intraocular pressure. Exp. Eye Res. 1994;58:529–544. doi: 10.1006/exer.1994.1047. [DOI] [PubMed] [Google Scholar]
- Kiel JW. Modulation of choroidal autoregulation in the rabbit. Exp. Eye Res. 1999;69:413–429. doi: 10.1006/exer.1999.0717. [DOI] [PubMed] [Google Scholar]
- Kiel JW, Shepherd AP. Autoregulation of choroidal blood flow in the rabbit. Invest. Ophthalmol. Vis. Sci. 1992;33:2399–2410. [PubMed] [Google Scholar]
- Kiel JW, van Heuven WAJ. Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest. Ophthalmol. Vis. Sci. 1995;36:579–585. [PubMed] [Google Scholar]
- Kobari M, Fukuuchi Y, Tomita M, Tanahashi N, Takeda H. Role of nitric oxide in regulation of cerebral microvascular tone and autoregulation of cerebral blood flow in cats. Brain Res. 1994;667:255–262. doi: 10.1016/0006-8993(94)91503-2. [DOI] [PubMed] [Google Scholar]
- Koketsu N, Moskowitz MA, Kontos HA, Yokota M, Shimizu T. Chronic parasympathetic sectioning decreases regional cerebral blood flow during hemorrhagic hypotension and increases infarct size after middle cerebral artery occlusion in spontaneously hypertensive rats. J. Cereb. Blood Flow Metab. 1992;12:613–620. doi: 10.1038/jcbfm.1992.85. [DOI] [PubMed] [Google Scholar]
- Lovasik JV, Kergoat H, Riva CE, Perig BL, Geiser M. Choroidal blood flow during exercise-induced changes in ocular perfusion pressure. Invest. Ophthalmol. Vis. Sci. 2003;44:2126–2132. doi: 10.1167/iovs.02-0825. [DOI] [PubMed] [Google Scholar]
- Milley JR, Rosenberg AA, Jones MD., Jr. Retinal and choroidal blood flows in hypoxic and hypercarbic newborn lambs. Pediatr. Res. 1984;18:410–414. doi: 10.1203/00006450-198405000-00003. [DOI] [PubMed] [Google Scholar]
- Morita Y, Hardebo JE, Bouskel E. Influence of cerebrovascular sympathetic, parasympathetic, and sensory nerves on autoregulation and spontaneous vasomotion. Acta Physiol. Scand. 1995;154:121–130. doi: 10.1111/j.1748-1716.1995.tb09894.x. [DOI] [PubMed] [Google Scholar]
- Nilsson SF. Nitric oxide as a mediator of parasympathetic vasodilation in ocular and extraocular tissues in the rabbit. Invest. Ophthalmol. Vis. Sci. 1996;37:2110–2119. [PubMed] [Google Scholar]
- Nilsson SF. The significance of nitric oxide for parasympathetic vasodilation in eye and other orbital tissues in the cat. Exp. Eye Res. 2000;70:61–72. doi: 10.1006/exer.1999.0752. [DOI] [PubMed] [Google Scholar]
- Panerai RB, White RP, Markus HS, Evans DH. Grading of cerebral dynamic autoregulation from spontaneous fluctuations in arterial blood pressure. Stroke. 1998;29:2341–2346. doi: 10.1161/01.str.29.11.2341. [DOI] [PubMed] [Google Scholar]
- Pang IH, Wang WH, Clark AF. Acute effects of glaucoma medications on rat intraocular pressure. Exp. Eye Res. 2005;80:207–214. doi: 10.1016/j.exer.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Pavlov NA, Krivchenko AI. Cerebral blood flow during changes of the systemic arterial blood pressure in the pigeon, Columba livia. J. Evol. Biochem. Physiol. 1983;19:245–250. [Google Scholar]
- Pavlov NA, Krivchenko AI, Cherepivskaia EN, Zagvazdin IS, Zaiats ND. Vascular reactivity of the brain in the pigeon Columba livia. J. Evol. Biochem. Physiol. 1987;23:624–628. [PubMed] [Google Scholar]
- Preckel MP, Leftheriotis G, Ferber C, Degoute CS, Bansillon V, Saumet JL. Effects of nitric oxide blockade on the lower limit of cerebral autoregulation in pentobarbital-anaesthetized rats. Microcirculation. 1996;16:277–283. doi: 10.1159/000179186. [DOI] [PubMed] [Google Scholar]
- Reiner A, Zagvazdin Y, Fitzgerald MEC. Choroidal blood flow in pigeons compensates for decreases in arterial blood pressure. Exp. Eye Res. 2003;76:273–282. doi: 10.1016/s0014-4835(02)00316-0. [DOI] [PubMed] [Google Scholar]
- Riva CE, Titze P, Hero M, Movaffaghy A, Petrig BL. Choroidal blood flow during isometric exercises. Invest. Ophthalmol. Vis. Sci. 1997a;38:2338–2343. [PubMed] [Google Scholar]
- Riva CE, Titze P, Hero M, Petrig BL. Effect of acute decrease of perfusion pressure on choroidal blood flow in humans. Invest. Ophthalmol. Vis. Sci. 1997b;38:1752–1760. [PubMed] [Google Scholar]
- Rogers RF, Patton JFR, Schwaber JS. NTS neuronal responses to arterial pressure and pressure changes in rat. Am. J. Physiol. 1993;265:R1355–R1368. doi: 10.1152/ajpregu.1993.265.6.R1355. [DOI] [PubMed] [Google Scholar]
- Steinberg RH. Monitoring communications between photoreceptors and pigment epithelial cells: Effects of “mild” systemic hypoxia. Invest. Ophthalmol. Vis. Sci. 1987;28:1888–1904. [PubMed] [Google Scholar]
- Steinle JJ, Krizsan-Agbas D, Smith PG. Regional regulation of choroidal blood flow by autonomic innervation in the rat. Am. J. Physiol. 2000;279:R202–209. doi: 10.1152/ajpregu.2000.279.1.R202. [DOI] [PubMed] [Google Scholar]
- Stiris T, Suguihara C, Hehre D, Goldberg RN, Flynn J, Bancalari E. Effect of cyclooxygenase inhibition on retinal and choroidal blood flow during hypercarbia in newborn piglets. Pediatr. Res. 1992;31:127–130. doi: 10.1203/00006450-199202000-00007. [DOI] [PubMed] [Google Scholar]
- Wahl M, Schilling L. Regulation of cerebral blood flow - A brief review. Acta Neurochir. 1993;59:3–10. doi: 10.1007/978-3-7091-9302-0_1. [DOI] [PubMed] [Google Scholar]
- Wilson TM, Strang R, MacKenzie ET. The response of the choroidal and cerebral circulations to changing arterial PCO2 and acetazolamide in the baboon. Invest. Ophthalmol. Vis. Sci. 1977;16:576–580. [PubMed] [Google Scholar]
- Yancey CM, Linsenmeier RA. The ERG and choroidal PO2 in the cat during elevated IOP. Invest. Ophthalmol. Vis. Sci. 1988;29:700–707. [PubMed] [Google Scholar]
- Yancey CM, Linsenmeier RA. Oxygen distribution and consumption in the cat retina at increased IOP. Invest. Ophthalmol. Vis. Sci. 1989;30:600–611. [PubMed] [Google Scholar]
- Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularized and avascular retinas and in animal models of retinal disease. Prog. Retinal Eye Res. 2001;20:175–208. doi: 10.1016/s1350-9462(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Yu DY, Alder VA, Cringle SJ, Brown MJ. Choroidal blood flow measured in the dog eye in vivo and in vitro by local hydrogen clearance polography: validation of a technique and response to raised intraocular pressure. Exp. Eye Res. 1987;46:289–303. doi: 10.1016/s0014-4835(88)80021-6. [DOI] [PubMed] [Google Scholar]
- Zagvazdin YS, Fitzgerald MEC, Sancesario G, Reiner A. Neural nitric oxide (NO) mediates Edinger-Westphal nucleus evoked increase in choroidal blood flow in the pigeon. Invest. Ophthalmol. Vis. Sci. 1996a;37:666–672. [PubMed] [Google Scholar]
- Zagvazdin Y, Sancesario G, Wang YX, Share L, Fitzgerald MEC, Reiner A. Evidence from its cardiovascular effects that 7-nitroindazole may inhibit endothelial nitric oxide synthase in vivo. Europ. J. Pharmacol. 1996b;303:61–69. doi: 10.1016/0014-2999(96)00106-9. [DOI] [PubMed] [Google Scholar]
- Zagvazdin Y, Fitzgerald MEC, Reiner A. Role of muscarinic cholinergic transmission in Edinger-Westphal nucleus-induced choroidal vasodilation in pigeon. Exp. Eye Res. 2000;70:315–327. doi: 10.1006/exer.1999.0791. [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by Nω-propyl-L-arginine. J. Med. Chem. 2004;40:3869–3870. doi: 10.1021/jm970550g. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am. J. Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]