Abstract
Sequence variants at or near the leucine-rich repeat kinase 2 (LRRK2) locus have been associated with susceptibility to three human conditions: Parkinson disease (PD), Crohn’s disease and leprosy. Because all three disorders represent complex diseases with evidence of inflammation, we hypothesized a role for LRRK2 in immune cell functions.
Here, we report that full-length Lrrk2 is a relatively common constituent of human peripheral blood mononuclear cells (PBMC) including affinity-isolated, CD14+ monocytes, CD19+ B-cells, and CD4+ as well as CD8+ T-cells. Up to 25% of PBMC from healthy donors and up to 43% of CD14+ monocytes were stained by anti-Lrrk2 antibodies using cell sorting. PBMC lysates contained full-length (>260 kDa) and higher molecular weight Lrrk2 species. The expression of LRRK2 in circulating leukocytes was confirmed by microscopy of human blood smears and in sections from normal midbrain and distal ileum. Lrrk2 reactivity was also detected in mesenteric lymph nodes and spleen (including in dendritic cells), but was absent in splenic mononuclear cells from lrrk2-null mice, as expected. In cultured bone marrow-derived macrophages (BMDM) from mice we made three observations: (i) a predominance of higher molecular weight lrrk2; (ii) the reduction of autophagy marker LC3-II in R1441Clrrk2-mutant cells (≥31%); and (iii) a significant up-regulation of lrrk2 mRNA (>4-fold) and protein after exposure to microbial structures including bacterial lipopolysaccharide and to lentiviral particles.
We conclude that Lrrk2 is a constituent of many cell types in the immune system. Following the recognition of microbial structures, stimulated macrophages respond with increased lrrk2 gene expression. In the same cells, lrrk2 appears to co-regulate autophagy, which is reduced in R1441Clrrk2-mutant mice. A pattern recognition receptor-type function for LRRK2 could explain the locus association with Crohn’s disease and leprosy risk. We speculate that the role of Lrrk2 in immune cells may also be of relevance for the susceptibility to develop PD or its propagation.
Keywords: Parkinsonism, terminal ileitis, Hansen’s disease, lymphocyte, monocyte, macrophage, leucine-rich repeat protein, pattern recognition molecule, Toll-like receptor, autophagy, LC3, kinase, cytokine, interleukin
INTRODUCTION
Parkinson disease (PD) is a progressive, late-onset disorder of the nervous system of unclear pathogenesis. Several loci, multiple genotypes and select environmental triggers have been identified in the aetiology of this complex disorder (Klein and Schlossmacher 2007). Previously published autopsy studies of brain from PD patients have provided evidence for neuroinflammation (Hirsch and Hunot 2009; Simon-Sanchez et al. 2009; Reale et al. 2009), including activation of microglia and infiltration of the midbrain by CD4+ and CD8+ lymphocytes (Brochard et al. 2009).
Heterozygous missense mutations of the LRRK2 gene represent a common cause of sporadic and heritable PD (Mata et al. 2006; Greggio and Cookson 2009; Klein and Schlossmacher 2007; Marin 2006; Taylor et al. 2006). Intriguingly, the G2019S variant of Lrrk2 (the protein is also referred to as Dardarin) can be found in ~40% of all PD patients of Arab background and in ~20% of PD subjects with Ashkenazi Jewish heritage (Klein and Schlossmacher 2007). Depending on ethnicity and geography, the frequency of bona fide mutations in the LRRK2 gene can vary considerably (Grimes et al. 2007). In contrast to other genes linked to late-onset neurological disorders, the mean penetrance rate of pathogenic LRRK2 mutations is relatively low (~30%). When taken together, these results suggested that LRRK2 may act chiefly as a susceptibility gene, and that a “second hit” is required to express the PD phenotype in an autosomal dominant-type manner (Klein and Schlossmacher 2007).
In situ hybridization and Northern blotting results revealed surprisingly low signals for LRRK2 mRNA in mammalian brain with modest amounts found in the putamen and caudate, and very low levels recorded in critical PD sites including the Substantia nigra of human midbrain (Galter et al. 2006; Zimprich et al. 2004). In contrast, LRRK2 transcripts are more abundant in kidney, lung, spleen and testis of mammals (eg, Maekawa et al. 2010).
In their first report on the discovery of LRRK2, Zimprich et al. identified an unexpected heterogeneity of neuropathological findings in several mutation-carrying patients. The post mortem analysis of their initial cases and of subsequently examined material revealed three distinct phenotypes: (i) brainstem synucleinopathy (=typical PD); (ii) diffuse Lewy body pathology (=definite dementia with Lewy bodies); and (iii) nigral degeneration without a distinctive histopathology. Rarely, a tangle-bearing, progressive supranuclear palsy-type pathology was diagnosed (Zimprich et al. 2004; Marin 2006; Wider et al. 2010). This range of expressivity in neurodegeneration thus positioned LRRK2 mutations farther away from the dysregulation of α–synuclein (SNCA) metabolism than mutational events affecting the SNCA gene and the GBA1 gene (Neumann et al. 2009; Cullen et al. 2011).
The LRRK2 gene contains 51 exons that encode 2527 amino acid-long, >260 kDa-large protein (Klein and Schlossmacher 2007). Full-length (FL) Lrrk2 contains a leucine-rich repeat (LRR) domain, a Roc GTPase domain, a COR domain, a mitogen-activated protein kinase (MAPK) domain, and a C-terminal WD40 domain (Mata et al. 2006; Greggio and Cookson 2009; Klein and Schlossmacher 2007). Its LRR and WD40 elements are thought to be involved in interactions with negatively charged proteins, phospholipids or nucleic acids. Lrrk2 protein is associated with the cytoplasm, the plasma membrane, autophagic vacuoles and mitochondria (West et al. 2005; Alegre-Abarrategui et al. 2009; Melrose 2008). The physiological and pathological substrates of Lrrk2’s MAPK-type activity are under intense investigation (Mata et al. 2006; Greggio and Cookson 2009). Its GTPase function is thought to control MAPK activity including auto-phosphorylation (Mata et al. 2006; Greggio and Cookson 2009). Berger et al recently demonstrated that Lrrk2 multimers (eg, dimer and trimer) are more likely to be associated with membrane structures, to show more efficient binding of GTP, and to confer greater kinase activity (Berger et al. 2010).
Mutations in the GTPase domain (eg, R1441C) cause accumulation of autophagic vacuoles with increased levels of p62 (Alegre-Abarrategui et al. 2009). Intriguingly, Tong et al recently created knock-out mouse models of lrrk2, which –unexpectedly- revealed no neurological or neurochemical defects, but showed impaired protein degradation via the autophagy-lysosome pathway, in particular in both kidneys. Accumulation of lipofuscin, a rise in ubiquitylated proteins, dysregulation of LC3-II and p62, and up-regulation of pro-inflammatory as well as pro-apoptotic proteins were recorded in 20 month-old lrrk2−/− mice (Tong et al., 2010). These findings identified an essential role for lrrk2 in lysosomal autophagy outside the nervous system, and -if confirmed by other lrrk2-null models- raise further questions as to the precise mechanism(s) by which LRRK2 mutations confer neurological disease in humans.
In a recently published genome-wide association study, single nucleotide polymorphisms at the LRRK2/MUC19 locus were identified as modulators of Crohn’s disease (CD) susceptibility (Barrett et al. 2008). CD is classified as an autoimmune disease where the terminal ileum comes under attack by the host’s immune system in response to microbial colonization and/or invasion, thereby resulting in chronic inflammation. To date, 30 distinct loci have been involved in CD susceptibility including genes encoding innate pattern recognition molecules, lymphocyte differentiation factors, autophagy components, and regulators of secondary immune responses (van Limbergen et al. 2009). Among the group of ‘pattern recognition receptors’, mutations in the gene encoding nucleotide-binding domain-contacting-2 (Nod2) are strongly associated with CD risk (Stockton et al. 2004; Ogura et al. 2001; Maeda et al. 2005). Of note, the structure of Nod2 contains six LRRs. Nod2 is predominantly expressed in peripheral leukocytes and is involved in the activation of nuclear factor-κB (NF-κB) signaling following the recognition of bacterial lipopolysaccharides (LPS).
In an unrelated genome-wide association study of leprosy susceptibility, Zhang et al identified single-nucleotide polymorphisms at the LRRK2 locus as risk modifiers (Zhang et al. 2009). Leprosy (aka Hansen’s disease) is caused by infection with Mycobacterium leprae, which shows tropism for macrophages and Schwann cells (Schurr et al. 2006; Alcais et al. 2005). The authors proposed a gene-interaction network involving multiple loci including LRRK2 that when altered confers susceptibility to leprosy. Importantly, variations in several of the genes associated with leprosy risk were previously linked to inflammatory bowel disease, including CD (Schurr and Gros 2009; Buschman and Skamene 2004).
Given the evidence reviewed above, we speculated that the function of endogenous Lrrk2 may: (i) be similar to a LRR domain-carrying member of the pattern recognition receptor family (eg, Nod2); and (ii) play an important role in autophagy by cells of the innate or adaptive immune system, or both. To date, three studies have provided evidence for a role of lrrk2 in immune system function (Maekawa et al. 2010; Kubo et al. 2010). Maekawa et al found high lrrk2 expression in one specific subtype of murine B-lymphocytes (ie, B1-cells). The same group reported that lrrk2 mRNA and protein levels progressively decrease in mouse spleen during ageing. This decline was not observed in kidneys, lungs or brain (Maekawa et al. 2010; Kubo et al. 2010). More recently, Gardet et al identified several other leukocyte populations with relevance to CD pathogenesis that expressed lrrk2 and provided evidence that the gene’s transcription is up-regulated through an interferon-gamma (IFN-γ)-mediated signaling pathway (Gardet et al. 2010).
In this study, we extend these findings and show that full-length Lrrk2 is a constituent of human peripheral blood mononuclear cells (PBMC) including monocytes, T-cells and B-cells. We confirm the expression of LRRK2 in circulating leukocytes by microscopy of peripheral blood smears, in sections from normal midbrain and distal ileum, and by isolating cells from organ homogenates of mesenteric lymph nodes and spleen. We also find that cultured bone marrow-derived macrophages (BMDM) from mice show robust basal levels of higher molecular weight (HMW) lrrk2; there, we also provide evidence for autophagy dysregulation in R1441Clrrk2 mutant knock-in mice, and demonstrate the up-regulation of lrrk2 mRNA and protein in BMDM after exposure to microbial pathogens.
MATERIALS AND METHODS
Human blood and tissue collection
Venous blood was collected by routine phlebotomy from healthy adult donors (n=10) at The Ottawa Hospital using EDTA as an anticoagulant. Blood collection vacutainers were purchased from Becton, Dickinson and Company (Franklin Lakes, NJ). Epstein-Barr Virus (EBV)-transformed lymphoblasts generated from healthy control donors (n=4) and PD subjects (n=2) were obtained from the Harvard NeuroDiscovery Center and Dr. I. Irrcher, respectively (Aleyasin et al. 2010). Sections of formalin-fixed, paraffin-embedded human tissues (encompassing midbrain, distal ileum, spleen, mesenteric lymph nodes; n=2 each) were collected during routine autopsies from neurologically healthy subjects at The Ottawa Hospital and kindly provided by Dr. J. Woulfe. Human specimens were collected according to ethics board-approved institutional guidelines.
Animal models expressing mutant lrrk2 genotypes
The generation of lrrk2 knock-in mice carrying the R1441Clrrk2 variant and of germ line deleted lrrk2−/− mice (targeting the promoter and exon 1) were previously reported (Tong et al. 2009, 2010; Tong and Shen 2009). These mice as well as human wild-type LRRK2 cDNA-transgenic flies and their littermates (carrying the same regulatory domains but without LRRK2 cDNA) were used as positive and negative controls in microscopic and biochemical experiments. The generation and characterization of these flies were previously reported (Venderova et al. 2009).
Sorting of leukocytes
Collection of PBMC from human donors (20-60 ml), their separation from red blood cells and granulocytes using Ficoll gradients, and affinity-isolation of CD14+ monocytes, CD19+ B-cells, and CD4+ as well as CD8+ T-cells were carried out, as described. Leukocyte subtypes were magnetically labeled using Microbeads directed against cell surface markers and processed by MACS® Separator. Selected cells were eluted, fixed, permeabilized, stained with primary and secondary antibodies, and gated by FACS, as reported (Vranjkovic et al. 2011). In related experiments, homogenates of select wild-type (WT) mouse organs (age 13 weeks; C57BL/6 strain) were dissected; single cell suspensions were prepared by mechanically passing tissue lysates through a sterile cell strainer (70 μm nylon mesh) using a pestle. Cells were then seeded in 96-well plates, and stained with anti-lrrk2 antibody MJFF-1 (clone, C5-8) along with antibodies directed against various sub-populations of leukocytes in each organ. Anti-mouse antibodies CD11b (PE) (a marker of macrophages), CD45R (B220) APC-eFluor® 780 (a marker of B-cells ), TCRβ APC-eFluor® 780 (a marker of T-cells), Ly-6G (Gr-1) Cy5 (a marker of granulocytes) and CD11c PE-Cy7(a marker of dendritic cells) were used to sort different cell types. Detection of lrrk2 intracellular staining was carried out by applying secondary antibody (anti-rabbit IgG (H+L) Alexa Fluor ® 488) at optimized concentrations. Subsequent flow cytometry analysis determined the relative expression levels of proteins of interest (Le Bourhis et al. 2009).
Bone marrow-derived macrophage cultures
Protocols for obtaining BMDM from mice were adapted from those by Werts et al. 2007. Briefly, BMDM were cultured in RPMI 1640 medium, fetal calf serum (10% v/v), penicillin (100 U/ml), streptomycin (100 μg/ml), sodium pyruvate (1 mM), non-essential amino acids (0.1 mM), and supplemented with conditioned media of a macrophage colony-stimulating factor (M-CSF)-expressing cell line (L929, 30% v/v). BMDM were plated and maintained at 5% CO2 at 37°C, and stimulated on dayin-vitro (DIV) 7. A separate set of macrophages were transduced with lentivirus. Recombinant viral particles were produced by co-transfection of HEK293T cells with lentiviral vector (pHR-SINCSGWΔNotI-GFP-LC3, from C. Münz) and the helper plasmids pCMVΔR8.91 and pMDG. The supernatants of HEK293T cells that contained lentiviral particles were collected and added to BMDM. The transduction medium contained polybrene (8 mg/ml). Macrophages were centrifuged at 2,000g for 90 minutes. After overnight incubation at 37 °C in 5% CO2, fresh medium was added to the cells and transduction was repeated after 24 hrs, as described by Travassos et al. 2010.
Stimulants used to activate BMDM included C12-iE-DAP, L18-MDP (Nod1 and Nod2 ligands, respectively; 50 μg/ml each), MDP (muramyldipeptide; 10 μg/ml), FK156 [D-lactyl-L-alanyl-D-glutamyl-(L)-mesodiaminopimelyl-(L)-glycine; 10 μg/ml)], rapamycin (50 μg/ml); LPS (lipopolysaccharide; 100 ng/ml), ATP (adenosine triphosphate; 5mM), or nigericin (1-15 μM), heat-killed (HK) listeria (8×108 CFU/ml, MOI 25), CpG (synthetic oligonuceotides with unmethylated CpG dinucleotides serving as TLR9 ligand; 1 μM). All stimulants were purchased from Invivogen (San Diego, CA) except HK bacterial lysates, which were prepared, as described (Travassos et al. 2004). Cells supernatants were collected 16 hrs after stimulation and analyzed using ELISA kits from R&D (Minneapolis, MN).
For quantification of mouse lrrk2 mRNA, BMDM were harvested from femur of WT mice and differentiated in medium containing 10n/ml GM-CSF for 7 days. BMDM were stimulated for 5 hrs with different Toll-like receptors (TLR) agonists: Pam3CSK4 (P3S; 5 μg/ml) targeting TLR2; Poly (I:C; PIC; 10 μg/ml) for TLR3; LPS (1 μg/ml) for TLR4; R848 (4 μg/ml) for TLR7; and CpG (10μg/ml) for TLR9. Pam3Cys, poly(I:C), ultrapure LPS, imiquimod (R837), and mouse CpG-B (ODN 1826) were purchased from InvivoGen (San Diego, CA).
Antibodies, immunoblotting and microscopy
Rabbit polyclonal antibodies to Lrrk2 (#NB300-268) and LC3 (#NB100-2220) were from Novus Biol (Littleton, CO). Each of these two antibodies were used at a dilution of 1:3000 for Western blotting (WB). Five rabbit monoclonal antibodies to Lrrk2 (MJFF-1 to -5) were provided by the Michael J. Fox Foundation (New York, NY). For WB, these antibodies were used at a dilution of 1:1000; for FACS at 1:200. An affinity-purified rabbit polyclonal anti-human Lrrk2 antibody (“HL-2”) was prepared at OPEN Biosystems Inc (Huntsville, AL.) against residues 2508-2527 and used at a dilution of 1:3000 for WB and 1:300-1:1000 in immunohistochemistry (IHC). SDS/PAGE, WB and IHC protocols were previously described (Schlossmacher et al. 2002; Schlossmacher and Shimura 2005; Cullen et al. 2009). Antibodies to CD4+, CD8+, CD14+ and CD19+ conjugated to microbeads were purchased from Miltenyi Biotec (Auburn, CA) and used as per manufacturer’s protocol. Staining of peripheral blood smears was carried out as per our IHC protocol using the avidin-biotin-peroxidase (ABC) method and 3,3′-diaminobenzidine substrate for color (brown) visualization of antibody reactivity. The ABC kit was purchased from Vector Laboratories, Inc. (Burlingame, CA). During fixation of smears, methanol was substituted for formalin.
RT- and qPCR to quantify mRNA
Human LRRK2 mRNA was isolated from EBV-transformed lymphoblasts and analyzed by RT-PCR using three distinct pairs of primers: #1: AGGTGGGTTGGTCACTTCTG (forw.), ATGAGTGCATGGCATCAAAA (rev.); #2: AGATGCCAATCAAGCAAAGG (forw.), AAGGACCAAGCCAAGAAGGT (rev.); #3: TTCTTCCCCTGTGATTCTCG (forw.), GCTGTCCAACAACAAGCTGA (rev.). Mouse mRNA was purified from BMDM using RNeasy MiniKitCat# 74104 (Qiagen MA). Murine lrrk2 primers were: TCTGGCTGGAACCCTGCTAT (forw.); and AACTGGCCATCTTCA TCTCC (rev.). Quantification of mRNA was performed using the ΔΔCT method (relative to 18S RNA) and compared to unstimulated controls.
Cytokine and chemokine quantification by ELISA
Cyto-/chemokine antibody kits were purchased from R&D Systems (Minneapolis, MN). ELISA were used to measure the levels released into the conditioned media of BMDM from genotyped mice. BMDM were stimulated with inflammatory reagents for 16 hrs prior to collection of their supernatants. 96-well plates were coated with cyto-/chemokine antibodies including keratinocyte-derived chemokine (KC) and IL-6.
RESULTS
Detection of Lrrk2 reactivity in circulating leukocytes
To screen for evidence of LRRK2 expression in cells of the immune system, we first examined peripheral blood smears from healthy adult controls by immunocytochemistry using a panel of anti-Lrrk2 antibodies (Figure 1). There, affinity-purified polyclonal HL-2 reacted with cytoplasmic and membrane-associated structures in a subset of leukocytes that were 10-12 mm in size and showed morphological characteristics of monocytes (Fig.1A). Smaller cells that appeared lymphocytic in nature with a larger nucleus-to-cytoplasm ratio stained infrequently (see also below). Longer development of sister slides generated an occasional but faint HL-2 immunoreactivity in typical granulocytes, which we identified by counterstaining with hematoxylin (Fig.1B); in contrast, erythrocytes and platelets, which were readily detectable by anti-SNCA antibodies (Scherzer et al. 2008), were never stained by anti-Lrrk2.
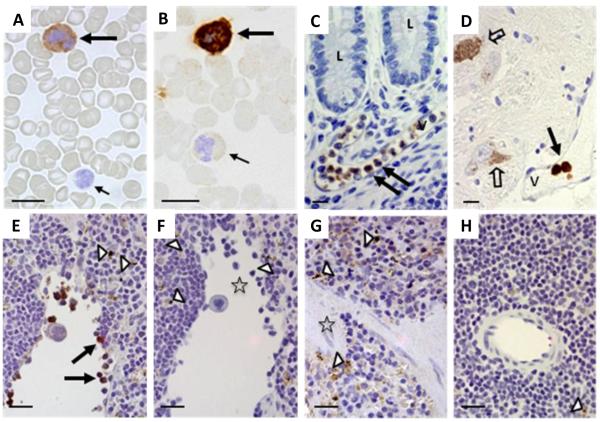
Figure 1. Lrrk2 reactivity in mammalian leukocytes using immunocytochemistry and immunohistochemistry.
(A-D) Lrrk2 reactivity was detected in select leukocytes of peripheral blood smears (A-B; healthy controls; n=5), and in intravascular leukocytes of the submucosa in the distal ileum (C; neurological controls; n=3) and human midbrain (D) from a Parkinson disease donor, as denoted by large arrows. Small arrows point to unstained and poorly stained leukocytes; open arrows identify autofluorescent neuromelanin in dopaminergic cells of the midbrain. V, indicates vessel; L, lumen of the gut. Bar length equals 12 μm; original magnification, 60x. Lrrk2 signal was detected by affinity-purified, polyclonal anti-Lrrk2 antibody [“HL-2” raised against a synthetic peptide comprising residues 2508-2527 of the human, wild-type Lrrk2 (Berger et al. 2010)].
(E-H) The specificity of the Lrrk2 antibody used in A-D was confirmed in spleen sections from age-matched, wild-type (E-F) and lrrk2 knock-out (G-H) mice (n=4 each). Parallel sections were developed with anti-Lrrk2 HL-2 as primary antibody (E; G) or without primary antibody (F; H). Arrows depict lrrk2-immunoreactive leukocytes in the spleen; open arrow heads show autofluorescent cytoplasmic granules seen irrespective of antibody use; open asterisks denote groups of unstained leukocytes (E-H). Bar length equals 20 μm; original magnification, 40x.
We next examined neural and gastrointestinal tissue sections for Lrrk2 reactivity by routine IHC with anti-Lrrk2 (HL-2). Examining sections of distal ileum (Fig.1C), ascending colon and human midbrain (Fig.1D) from neurologically healthy donors, we detected occasional intravascular leukocytes that stained for Lrrk2. Under these conditions, no HL-2 reactivity was seen in enteric neurons of the submucosal and myenteric plexi or in any neuromelanin-carrying cells of the S. nigra (Fig.1C-D). However, in parallel sections these ganglion cells and neurons were labelled by anti-α-synuclein and anti-tyrosine hydroxylase antibodies, respectively (not shown).
Given the controversy in the PD field regarding the specificity of many anti-Lrrk2 antibodies (Biskup et al. 2007), we validated the staining obtained by anti-Lrrk2 HL-2 using sections of genotyped mouse spleens, which were processed like human tissue (above) and by the same IHC protocol. There, HL-2 identified a subset of mononuclear splenic leukocytes in WT mice (n=4; Fig.1E,F), but not in lrrk2-null animals (n=4; Fig.1G,H). In sections from lrrk2-deficient mice, only autofluorescent intracellular granules (representing lipofuscin) were visible. Signals were also absent when the primary antibody was omitted, as expected (Fig.1F,H). We concluded from these results that antibody HL-2 was specific and that Lrrk2 reactivity is present in select populations of circulating leukocytes from human blood, including in capillaries of tissues that are affected by the pathogenesis of CD and PD.
Quantification of Lrrk2-expressing mononuclear cells from human blood
To better characterize the subpopulations of leukocytes that express Lrrk2, we first enriched PBMC by routine Ficoll preparation, and then carried out fluorescence-based automated cell sorting (FACS; see Materials and Methods) with anti-Lrrk2 antibodies (Figure 2). For the purpose of optimizing conditions for anti-Lrrk2 to bind and capture permeabilized cells, we also explored the use of several monoclonal rabbit antibodies, which recently became available (MJFF 1-5; see below). Panels in Fig.2A1-4 show the successful gating of PBMC from 0.58% (no anti-Lrrk2 antibody present) to a mean of 25.13% (range, 24.54-25.71%) by anti-Lrrk2 MJFF-1. Panels in Fig.2B1-4 show the successful gating of a pool of specifically isolated CD14+ monocytes from 0.81% (no anti-Lrrk2 antibody present) to a mean of 26.7% (range, 10.31-43.17%) using anti-Lrrk2 MJFF-1. From these results we concluded that ~25% of all PBMC were Lrrk2-reactive, and that within the pool of CD14+ monocytes a mean of 27% were Lrrk2-positive.
Figure 2. Lrrk2 reactivity in human peripheral blood mononuclear cells and isolated monocytes.
(A-B) Lrrk2 reactivity was detected in peripheral blood mononuclear cells (PBMC) (A) and anti-CD14+ monocytes (B) that were isolated by Ficoll separation and magnetic beads (carrying anti-CD14 antibody), respectively, from healthy adult controls (n=3) (Vranjkovic et al. 2011). FACS sorting of fixed and permeabilized cells was carried out using monoclonal anti-Lrrk2 antibody MJFF-1(C5-8). In each panel, a selection of cells is made by the forward and side scatter light properties (horizontal and vertical axis respectively in the left diagram) and a histogram (left diagram, horizontal and vertical axis represent the number of the cells counted and fluorescence respectively) of the fluorescence (IgG) profile of the Lrrk2 is generated to show the degree of the antibody binding. Red vertical lines show mean fluoresce of unstained cells. PBMC and monocytes were collected from two different individuals. Note, only 0.58% of PBMC (A) and 0.81% of monocytes (B) were gated in the absence of anti-Lrrk2 antibody. However, following the optimization of primary and secondary antibody concentrations the percentage of Lrrk2-positive cells measured 19.04 (A2), 24.54 (A3), and 25.71 (A4) for PBMC; and 1.8 (B2), 10.31 (B3), and 43.17 (B4). Thus, staining with anti-Lrrk2 revealed that <25.71% of all PBMC (A) and <43.17% of all monocytes (B) were successfully gated.
Detection of full-length Lrrk2 in monocytes, B-cells and T-cells
Detection of Lrrk2 in PBMC was further substantiated by SDS/PAGE and immunoblotting by probing lysates with a panel of antibodies (Figure 3; Suppl. Figure 1A-D). There, we specifically detected the presence of the FL-Lrrk2 monomer (>260 kDa) and of a 245-250 kDa variant in PBMC lysates, as detected by anti-Lrrk2, HL-2. No Lrrk2 signal was seen in red blood cell (and granulocyte) lysates (Fig.3A). Monoclonal MJFF-1 confirmed these findings (Fig.3B), and also clearly detected higher molecular weight (HMW) Lrrk2 species. The specificity of our antibodies was confirmed in all WB experiments using lysates of fly heads that were either transgenic for LRRK2 cDNA (tg) or from WT littermates (Fig.3A-F). Four additional monoclonal rabbit antibodies to Lrrk2 produced identical results to those shown in Fig.3A-B although with variable staining of HMW Lrrk2 (Suppl.Fig.1A-D).
Figure 3. Detection of full-length Lrrk2 protein in isolated monocytes, B-cells and T-cells.
(A-F) Peripheral blood mononuclear cells (PBMC; A-B) were obtained by Ficoll separation from healthy adult controls (n=5) and separated from erythrocytes (RBC) and granulocytes. PBMC were further processed using antibodies to CD14 (monocyte marker; in C), CD4 and CD8 (both T-cell markers; in panels D-E), and to CD19 (B-cell marker; E), which were conjugated to and immobilized by magnetic beads (see Materials and Methods) (Vranjkovic et al. 2011). Isolated subpopulations of leukocytes were lysed and then subjected to denaturing SDS/PAGE under reducing conditions, followed by Western blotting. Transgenic flies expressing full-length hLRRK2 cDNA (tg) and their wild-type, non-transgenic littermates (wt) were used as positive and negative controls, respectively. EBV-transformed, B-cell derived lymphoblasts from healthy control and Parkinson disease donors (n=5) were run in parallel as additional controls (panel F). Immunoblot in A was probed with affinity-purified, polyclonal anti-human Lrrk2 antibody (HL-2) raised against residues 2508-2527; probing with a monoclonal rabbit anti-Lrrk2 antibody [MJFF-1] is shown in B. Note, polyclonal anti-Lrrk2 antibody NB300-268 was used for membranes C to F. Anti-acid β-glucosidase was used as an independent marker of peripheral monocytes (see bottom panel in C). All membranes were stripped and reprobed with anti-β-actin antibody as loading control. Note, the robust and specific detection of endogenous, full-length Lrrk2 proteins (245-260 kDa) and higher molecular weight (HMW) species thereof in human PBMC, monocytes, B-cells and T-cells. There, CD19+ B-cells showed a slightly stronger signal for Lrrk2 when compared to isolated CD4+ T-cells enriched from the same donor (E).
Next, we confirmed the presence of endogenous Lrrk2 protein in peripheral monocytes, which was suggested by our analysis of blood smears and FACS data (above). As shown in Fig.3C, CD14+ monocytes isolated by magnetic beads contained FL-Lrrk2. The cellular identity of sorted cells was further confirmed by the presence of the ~62 kDa acid-β-glucosidase protein, a well established constituent of monocytes (lowest panel in Fig.3C) and tissue macrophages (Cullen et al. 2011).
Following affinity enrichment by magnetic beads, we also detected FL-Lrrk2 in CD8+ and CD4+ T-cells as well as in CD19+ B-cells (a pan-B-cell marker; Fig.3D-E). In comparing their adjusted signals, we found that members of the B-cell family consistently showed higher Lrrk2-to-β-actin ratios than CD4+ or CD8+ T-cells in peripheral blood specimens from healthy adults (examined under basal rates). The highest relative expression of endogenous LRRK2 in the myeloid lineage was recorded in EBV-transformed lymphoblasts (Fig.3F; Suppl.Fig.1A). The expression of lrrk2 in these different subpopulations of white blood cells (monocytes/macrophages; B-cells; T-cells) was confirmed in leukocytes isolated from tissue homogenates of WT mice, eg, mesenteric lymph nodes, spleen (Suppl.Fig.2A) and Peyer’s patches (not shown). Lrrk2 signals in granulocytes varied greatly (see below).
Lrrk2 is expressed in macrophages, induced by pathogens and involved in autophagy regulation
Given the robust expression of the LRRK2 gene in human monocytes, we next explored its presence in tissue macrophages (Figure 4); the latter are derived from circulating monocytes and play a key role in the pathogenesis of CD and leprosy. To this end, we isolated and cultured primary BMDM from femurs and tibias of both WT mice and R144Clrrk2-mutant animals (heterozygous and homozygous) using a published ‘macrophage colony stimulating factor’-based protocol (see Materials and Methods). When probing BMDM cultures, which were prepared in parallel from age-matched mice of three different genotypes, by SDS/PAGE/WB, we found comparable and robust amounts of HMW lrrk2 in all lysates examined (Fig.4A); when probing BMDM lysates from lrrk2-deficient mice we observed no HMW lrrk2 signal, as expected (not shown). These results suggested two things: similar expression and stability rates for the endogenous R1441Clrrk2 mutant protein when compared with WT lrrk2; and a predominance of HMW lrrk2 (>290 kDa) in cultured murine macrophages vs the FL Lrrk2 species seen in circulating human monocytes (>260 kDa) (Berger et al., 2010).
Figure 4. A likely role for Lrrk2 during autophagy and pathogen response in macrophages.
(A-B) SDS/PAGE under denaturing, reducing conditions followed by immunoblotting of murine bone marrow-derived macrophages (BMDM) that were cultured for 48 hrs without stimulation. Lysate of human EBV-transformed lymphoblasts from a control donor was loaded as control. Lanes 2-4 show lysates of BMDM prepared in parallel from age-matched mice of three distinct lrrk2 genotypes: wild type (WT); heterozygous (HET); and homozygous knock-in (KI) mice expressing mutant R1441Clrrk2. Note, the robust expression of HMW lrrk2 in murine BMDM, as detected by monoclonal anti-Lrrk2 antibody [MJFF-4] in A. Sister aliquots of the same lysates were probed with an antibody to autophagy markers LC3-I and LC3-II (B). All membranes were stripped and reprobed with anti-β-actin antibody as loading control. Note, the reduction of LC3-II levels in mutant R1441Clrrk2-expressing BMDM compared to WT cells (≥31%; see text for details).
(C) BMDM from WT mice were stimulated for 5 hrs with different Toll-like receptor (TLR) agonists: Pam3CSK4 (P3S) for TLR2; Poly I:C (PIC) for TLR3; LPS for TLR4; R837 for TLR7; and CpG for TLR9. Quantification of lrrk2 mRNA was performed using the ΔΔCT method (relative to 18S) and compared to levels in unstimulated BMDM cells. * denotes significant up-regulation (p<0.05 using ANOVA followed by Tukey test, n=3). For comparison, TNFα was upregulated in all samples >30-fold (not shown).
(D) BMDM cultures from homozygous (R1441C / R1441C) and heterozygous (R1441C / WT) mice were stimulated with different inflammatory stimulants. Macrophages were transduced with a lentivirus expressing eGFP 48 hours prior to stimulations. Stimulants were added 24hrs after plating of cells. Note, transduction by virus caused an upregulation in lrrk2 protein levels when compared to the untransduced BMDM (compares lane 10 with lanes 2). EBV-lymph and Rapa refer to EBV-transformed lymphoblast and treatment with rapamycin, respectively. See Result section for further details.
When we probed BMDM with autophagy markers, we observed variable results for LC3-I and p62 proteins in their lysates, but found a consistent reduction in LC3-II levels in all R1441Clrrk2-expressing macrophages (Fig.4B). Quantifying the ratio of LC3-II-to-β-actin signals, we recorded a ≥31% reduction in R1441Clrrk2 mutant vs. WT cells. The expression of endogenous lrrk2 in tissue macrophages was confirmed independently in cells isolated from homogenates of spleen, lymph nodes (Suppl.Fig.2A) and Peyer’s patches (not shown).
Given the robust expression of lrrk2 in isolated macrophages from multiple murine tissues, we next explored the possible induction of lrrk2 transcription following the exposure of BMDM to established inflammatory stimuli through ‘pattern recognition receptors’ (Fig.4C). Using rt-qPCR, we recorded specific down- (rather than up)-regulation of lrrk2 mRNA following the activation of Toll-like receptor (TLR)-2 by Pam3CSK4. However, in parallel we recorded the specific and significant up-regulation of lrrk2 mRNA in BMDM that were stimulated with ligands for TLR4 (ie, LPS); >4-fold], for TLR7 (with R837; >2-fold), and for TLR9 [with CpG molecules; >3-fold] (Fig.4C). These transcriptional changes were observed as early as 5 hours after the addition of stimulants signalling microbial invasion.
To determine whether viral transduction of BMDM also induced the transcription of lrrk2 and – if so - whether increased mRNA signals also translated into increased protein levels, we next exposed cultured macrophages to eGFP cDNA-carrying lentiviral preparations. When examining transduced BMDM vs virus-free sister cultures 16 hours later by SDS/PAGE/WB with anti-Lrrk2 (and anti-β-actin), we consistently saw a rise in lrrk2 following viral transduction (Fig.4D; compare lanes 2 and 10). The effect was the same for R1441Cmutant and WTlrrk2-expressing alleles; furthermore, the lrrk2-to-β-actin ratio remained elevated in all virally transduced BMDM irrespective of the nature of additional stimuli (Fig.4D; compare signals in lanes 2-9 vs. lane 10). We concluded from these results that our mRNA and protein data in BMDM were consistent; they revealed a modest but significant up-regulation of lrrk2 following the recognition of microbial structures.
Lrrk2 expression does not control IL-6 and KC cytokine release by stimulated macrophages
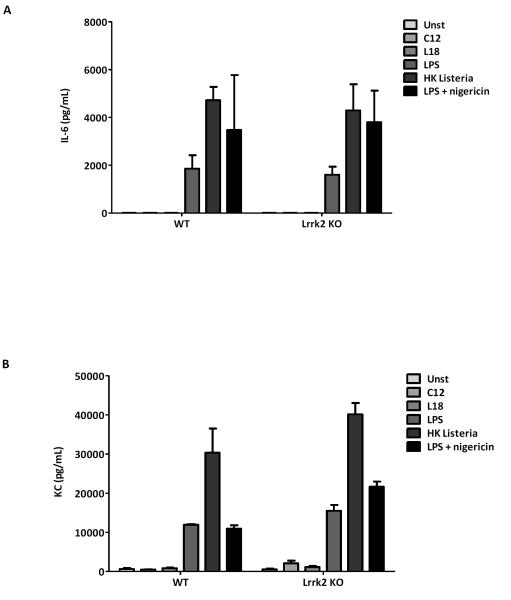
To investigate the involvement of Lrrk2 in cytokine production and/or release rates, we also screened conditioned media of BMDM culture following the addition of C12 (a Nod1 ligand), L18 (Nod2 ligand), LPS, and heat-killed (HK) listeria. There, we focused on interleukin-6 (IL-6) and keratinocyte chemokine (KC), two cytokines that have been previously linked to macrophage function (Figure 5; Suppl.Fig.2A,B). When we first compared the effect of R1441Clrrk2 mutant vs WTlrrk2-expressing cells, we saw some variability but no consistent changes in IL-6 and KC concentrations in BMDM supernatants (Suppl.Fig.2A,B). We next explored the effect of presence vs deficiency in lrrk2 (Fig.5). There, we recorded the significant and multi-fold rise in IL-6 and KC concentrations in the supernatants of stimulated BMDM 16 hours after exposure to LPS, HK listeria and LPS plus nigericin (an NLRP3 inflammasome stimulator), as expected (Said-Sadier et al. 2010). However, in parallel we did not detect a lrrk2 genotype-dependent difference in IL-6 and KC levls (eg, compare bars for WT vs KO mice in Fig.5A,B). We concluded from these cytokine screening experiments that the expression of WT (or mutant) lrrk2 did not measurably alter the release of IL-6 and KC in these cells.
Figure 5. Lrrk2 protein expression does not control IL-6 and KC cytokine signaling.
(A-B) Screening of lrrk2 genotype-dependent cytokine signaling by cultured BMDM following exposure to different stimulants was explored by probing the release of interleukin-6 (IL-6; pg/ml in A-B) and keratinocyte-derived chemokine (KC; pg/ml; Suppl. Fig. 2) by ELISA. BMDM were isolated from lrrk2 knock-out (KO), and from age-matched, wild-type (WT) littermates (12 weeks-old; n=2 for each genotype) and cultured for 16 hrs (each supernatant analyzed in quadruplicates). Similarly, stimulants were added 24 hrs after plating. No specific lrrk2 expression-associated differences were detected in the conditioned media M for the production and release rate of KC and IL-6 (for additional genotypes, see also Suppl. Fig.2).
DISCUSSION
The pathogenetic mechanisms leading to typical PD remain unknown. Prevailing concepts have favored either an environmental factor or genetic contributors as the primary event in its aetiology, but few models have explored the impact of both in in vivo models (Aleyasin et al. 2010). The discovery that late-onset PD could be caused by the inheritance of a mutation in the LRRK2 gene leading to familial as well as sporadic forms has provided us with an opportunity to explore the pathophysiological events underlying this complex disease. However, as summarized in the introduction, more questions have arisen than been answered surrounding the function(s) of LRRK2 (reviewed in: Cookson 2010); most poignantly, to date no model has adequately explained how a gene, which is expressed at exceedingly low levels in brain nuclei that are severely affected by the process that leads to PD (eg, S. nigra), can promote a neurological disorder when mutated.
Here, we provided experimental evidence to support the following two conclusions: one, Lrrk2 is a relatively abundant protein in mammalian leukocytes of circulating blood and organs of the immune system; and two, lrrk2 expression is up-regulated in cultured macrophages following their recognition of microbial structures. We arrived at these two conclusions based on several, complementary approaches; our findings are in agreement with the recently published, elegant study by Gardet et al. These authors demonstrated the involvement of LRRK2 in the IFN-γ response after leukocyte invasion and pointed out how it possibly relates to CD pathogenesis (Gardet et al. 2010). In their study, LRRK2 was up-regulated in intestinal tissues of CD patients with expression detected in macrophages, B-cells and CD103+ dendritic cells of inflamed intestines. Gardet et al further showed that exogenous expression of Lrrk2 promoted transcriptional activation of the NF-κB pathway, and that its down-regulation facilitated enhanced bacterial survival in a S. typhimurium cell infection assay (Gardet et al. 2010). However, we believe our data further expand on these findings by adding three additional observations regarding Lrrk2 biology: one, we demonstrated –to our knowledge for the first time- that the expression of R1441Cmutant lrrk2 in a primary, non-neural cell model (ie, BMDM) reveals an autophagy defect; two, we showed that the up-regulation of lrrk2 also occurs in response to viral transduction of a host cell by lentiviral particles (and likely, in B-lymphocytes transformed by EBV); and three, when screening for a lrrk2 genotype-dependent effect on IL-6 and KC cytokine release, we found that these two signaling molecules are not regulated by leukocytic lrrk2.
Below, we mention five specific limitations of this study followed by a discussion of five reasons why we consider our Lrrk2 findings relevant to the pathogenesis of PD. One, although we consistently demonstrated the expression of LRRK2 in CD19+ B-cells, CD4+ T-cells (in contrast to Gardet et al. 2010; Makaewa et al. 2010), CD8+ T-cells, CD14+ monocytes, macrophages and dendritic cells, we obtained highly variable -and thus, inconclusive- results regarding granulocytes, which were: mostly negative in staining of human blood smears; positive in cellular isolates from murine spleen and mesenteric lymph nodes analyzed by FACS; and negative in genotyped spleen sections examined by IHC. These differences may be due to low LRRK2 expression rates in healthy donors, species differences in accessible epitopes of granulocytic Lrrk2, or subtle changes in the binding strength of the antibodies used in our different protocols. In future work, we will revisit the degree of LRRK2 expression in granulocytes focusing on mRNA-based techniques (eg, rt-qPCR).
Two, in our IHC work of brain tissue, where we used a protocol that includes formalin-fixed, paraffin-embedded material that included two antigen retrieval steps, we have not yet identified staining of microglia (or of any other glia) with our panel of anti-Lrrk2 antibodies, in contrast to a report by Miklossy et al. 2006. As before, differences in antibody specificity and antigen accessibility could account for this mismatch in results. To convincingly answer the question of expression of lrrk2 in resting or activated microglia, which are derived from the monocytic lineage, and to exclude any false positive signals due to antibody cross-reactivity, future studies will employ the induction of microglial activation in WT lrrk2 versus lrrk2-deficient mice using established inflammatory paradigms (Mount et al. 2007).
Three, although we recorded a mean, ≥31% reduction in basal LC3-II (but not LC3-I) levels in BMDM from R1441Clrrk2-expressing mice when compared with age-matched WT littermates processed at the same time, more autophagy markers need to be examined in primary cells (and tissue) using a larger series of animals and comparing unstimulated vs stimulated conditions than we did here (mice, n=6). If our data were to be confirmed, ie, reduced autophagy rates can be seen in R1441Clrrk2-expressing BMDM and tissue homogenates as concluded from our LC3-II results, then the effect of this point mutation could be similar to the recently observed dysregulation of autophagy described by Tong et al in lrrk2-deficient mice (Tong et al. 2010). Such a reduction in autophagic efficiency due to the loss of dimeric WT Lrrk2 activity is principally consistent with the results of Gardet et al., who recorded increased bacterial survival rates in macrophages after lrrk2 knock-down (Gardet et al. 2010). Effective phagocytosis following pathogen opsonization requires intact autophagy. In parallel, future studies need to systematically screen additional cytokines (and signaling pathways) beyond those carried out so far to better define the full complement of cellular changes downstream of lrrk2 gene expression in activated immune cells.
Four, in our work we have not yet defined the post-translational nature of the HMW lrrk2 band(s) seen with multiple specific antibodies in PBMC and, even more strikingly, in BMDM. We performed our SDS/PAGE experiments under reducing and denaturing conditions, thereby precluding dimer (or higher order multimer) stability. We predict that the relative abundance of Lrrk2 protein in the monocyte/macrophage lineage will significantly aid its biochemical dissection in the future, including the delineation of post-translational modifications, of its still elusive binding partners, and of authentic substrates for its kinase activity (Cookson 2010).
Fifth, one could argue that the precise single nucleotide polymorphisms at or near the LRRK2 locus, which were associated with CD and leprosy risk in genome-wide association studies, have not yet been independently validated, thereby theoretically minimizing the general applicability of our cellular studies. Intriguingly, most recently LRRK2/MUC19 was identified as one of seven CD loci (out of 30 examined) that was positively associated with risk modulation for yet another human condition, ie, ankylosing spondylitis, which often occurs together with CD (Danoy et al. 2010). Importantly, the dysregulation of monocytes, macrophages, B-cells and T-cells plays a pivotal role in the pathogenesis of CD, ankylosing spondylitis and leprosy (Schurr and Gros 2009).
Importantly, five arguments can be made in favor of studying LRRK2 biology in leukocytes as a suitable cell model for PD and to functionally compare it with other CD- and leprosy-associated genes in suitable paradigms. One, in vivo studies and post-mortem analyses of PD subjects have generated robust evidence from multiple centers in favor of ongoing inflammation (Hirsch and Hunot 2009; Simon-Sanchez 2009; Reale et al. 2009). Furthermore, the infiltration by CD4+ and CD8+ lymphocytes and the activation of microglia in PD brain have been well documented (Brochard et al. 2009). In addition, the analysis of peripheral cytokine concentrations in PD subjects has revealed abnormal levels for MCP-1 (monocyte chemoattractant protein-1), MIP-1 (macrophage inflammatory protein-1), IFN-γ, IL-8, IL-1β, IL-6, and TNF-α (Reale et al. 2009; Chen et al. 2008) among others, thereby justifying the systematic exploration as to the possible reason(s) for their dysregulation in immune cells.
Two, the mammalian immune system undergoes significant changes during the process of ageing including for example in the rate of lrrk2 expression (Maekawa et al. 2010) and by inference, in WT lrrk2 activity, which could further influence the age-of-onset, the expressivity of the PD phenotype, or its progression rate.
Three, the two central themes in the aetiology of typical, late-onset PD, which Jellinger recently referred to as a “progressive multisystem (multiorgan) disorder” (Jellinger 2011), have not yet been successfully integrated. However, its environmental and genetic contributors are not mutually exclusive, but rather represent the foundation of many human disorders (Klein and Schlossmacher 2007; Klein et al. 2011). The immune system governs the host’s susceptibility to pathogen invasion (eg, viruses, bacteria, fungi etc.), controls its tolerance of possible colonization, and determines the response mounted by the host to eliminate any threat. Nucleotide exchange domain- and LRR domain-carrying proteins (“NLR”) encoded by the genome play an evolutionarily conserved role in immune surveillance from plants to mammals (reviewed in: Takken and Tameling 2009; Taxman et al. 2010). Together with TLR proteins, NLR molecules are functionally understood as pattern recognition receptors. NLR proteins oversee inflammasome assembly and control the pathogen-induced regulation of cytokine release (Lamkanfi and Dixit 2010). Based on the functional domain similarities between Nod2 and Lrrk2, we seek to further test the hypothesis of a NLR-type function for Lrrk2.
Fourth, if established successfully, such a LRRK2-dependent regulation of immune cell function during the interaction between host and environment would be expected to significantly vary based on geography. This scenario –together with other factors- could help explain several known, previously difficult to explain aspects of LRRK2-linked PD, eg, variable rates of penetrance in carriers of the same mutation in different regions of the world, differences in the phenolconversion rate among members of the same family, variable rates of disease progression, and possibly, even the pleomorphic pathology (Zimprich et al, 2004) that could have been triggered by distinct agents in the environment.
Lastly, and most compellingly, a primary role for Lrrk2 at the interface between host and environment, which we propose here, could provide a new platform to revisit Braak’s hypothesis as it relates to the pathogenesis of typical PD. Braak et al postulated that an elusive environmental pathogen (microbial or otherwise) is essential for pathology to ensue (Braak et al. 2003). At stage 0, these authors described the earliest signs of synucleinopathy in the submucosal plexus of the gut and in the olfactory bulb, two structures in close proximity to the environment. The convergence of several independent pieces of evidence from the fields of CD pathogenesis, leprosy genetics and LRRK2 research in leukocyte function promises to bring together five previously irreconcilable elements in the aetiology of PD: one, the importance of the environment; two, the evolution of its neuropathology; three, the significance of genetic susceptibility; four, the role of the immune system; and five, the progress of ageing in a long lived host.
Supplementary Material
Supplementary Figure 1: LRRK2 mRNA and protein detection in EBV-transformed lymphoblasts and peripheral blood mononuclear cells. (A-D) Reducing SDS/PAGE was carried out using lysates of Ficoll-separated peripheral blood mononuclear cells (PBMC) from one human control donor (lane 3), followed by Western blotting with four different monoclonal rabbit anti-Lrrk2 antibodies (provided by the Michael J Fox Foundation, MJFF; see Materials and Methods), as indicated. Transgenic flies expressing full-length human LRRK2 cDNA (tg; lane 1) and their wild-type littermates (wt; lane 2) were used as positive and negative controls, respectively. Note, the specific detection of full-length Lrrk2 (260 kDa), and higher molecular weight (HMW) species thereof in PBMC lysates, as well as of HMW Lrrk2 reactivity in tg fly homogenates. Membranes were stripped and redeveloped with anti-β-actin antibody (lower panels). (E) LRRK2, GAPDH transcripts and 18S RNA isolated from EBV-transformed lymphoblasts from a healthy donor were processed by reverse transcriptase and PCR to generate distinct amplification products, as indicated (for primer pairs, see Materials and Methods).
Supplementary Figure 2: Murine Lrrk2 expression in cells from immune organs and exploration of its role in cytokine release by genotyped macrophages. (A) FACS-based quantification of lrrk2-positive immune cells (in mean %) isolated from homogenates of spleen and mesenteric lymph nodes of wild-type mouse (n=3) as described (Werts et al. 2007). Note, among four distinct leukocyte populations sorted, neutrophils revealed the strongest signal for lrrk2 reactivity (see also Discussion). Lrrk2 expression was measured by FACS analysis using anti-Lrrk2 antibody MJFF-4 on B220+ B-cells, TCRβ+ T-cells, CD11b+/CD11c+ macrophages, Gr-1+ neutrophils, and CD11c+ dendritic cells. (B) Lrrk2-genotype dependent cytokine signaling following exposure to different stimulants was explored by probing KC and IL-6 release by ELISA (Werts et al. 2007) from cultured BMDM. Cells were isolated from R1441C lrrk2 knock-in (KI) mice, heterozygous (HET) animals, and age-matched wild-type (WT) littermates (12 weeks-old, n=2 each; supernatants were analyzed in quadruplicates). Note, no consistent lrrk2 genotype-associated effect (WT vs HET / KI) was detected in the conditioned media of BMDM cells.
ACKNOWLEDGMENT
This manuscript was contributed in honor of Dr. Kurt A. Jellinger’s 80th birthday and his distinguished career. We thank the editor for its solicitation. This work was supported by grants from the Government of Canada [Canada Research Chair Program (to M.G.S.)], the Michael J. Fox Foundation for Parkinson’s Research [LRRK2 Award; Supplementary LRRK2 Award (to D.S.P., J.S., M.G.S.)], and the Parkinson Research Consortium Ottawa (to D.S.P., M.G.S.). We are grateful for critical comments by Drs. D. Galter, W. Schulz-Schaeffer, S. Hayley, and J. Ngsee and for the assistance of Dr. I. Irrcher, Dr. J. Woulfe and E. Abdel-Messih.
REFERENCES
- Alcaïs A, Mira M, Casanova JL, Schurr E, Abel L. Genetic dissection of immunity in leprosy. Curr Opin Immunol. 2005;17:44–48. doi: 10.1016/j.coi.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleyasin H, Rousseaux MW, Marcogliese PC, Hewitt SJ, Irrcher I, Joselin AP, Parsanejad M, Kim RH, Rizzu P, Callaghan SM, Slack RS, Mak TW, Park DS. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci USA. 2010;107:3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, NIDDK IBD Genetics Consortium. Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Belgian-French IBD Consortium. Wellcome Trust Case Control Consortium. Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Smith KA, Lavoie MJ. Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry. 2010;49:5511–5523. doi: 10.1021/bi100157u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biskup S, Moore DJ, Rea A, Lorenz-Deperieux B, Coombes CE, Dawson VL, Dawson TM, West AB. Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 2007;8:102. doi: 10.1186/1471-2202-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman E, Skamene E. Linkage of leprosy susceptibility to Parkinson’s disease genes. Int J Lepr Other Mycobact Dis. 2004;72:169–170. doi: 10.1489/1544-581X(2004)072<0169:LOLSTP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Chen H, O’Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol. 2008;167:90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci. 2010;11:791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen V, Lindfors M, Ng J, Paetau A, Swinton E, Kolodziej P, Boston H, Saftig P, Woulfe J, Feany MB, Myllykangas L, Schlossmacher MG, Tyynelä J. Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Mol Brain. 2009 doi: 10.1186/1756-6606-2-5. doi: 10.1186/1756-6606-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen V, Sardi SP, Ng J, Xu YH, Sun Y, Tomlinson JJ, Kolodziej P, Kahn I, Saftig P, Woulfe J, Rochet JC, Glicksman MA, Cheng SH, Grabowski GA, Shihabuddin LS, Schlossmacher MG. Acid β-glucosidase mutants linked to gaucher disease, parkinson disease, and lewy body dementia alter α-synuclein processing. Ann Neurol. 2011;2011 doi: 10.1002/ana.22400. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- Danoy P, Pryce K, Hadler J, Bradbury LA, Farrar C, Pointon J, Australo-Anglo-American Spondyloarthritis Consortium. Ward M, Weisman M, Reveille JD, Wordsworth BP, Stone MA, Spondyloarthritis Research Consortium of Canada. Maksymowych WP, Rahman P, Gladman D, Inman RD, Brown MA. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn’s disease. PLoS Genet. 2010;6:e1001195. doi: 10.1371/journal.pgen.1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galter D, Westerlund M, Carmine A, Lindqvist E, Sydow O, Olson L. LRRK2 expression linked to dopamine-innervated areas. Ann Neurol. 2006;59:714–719. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- Gardet A, Benita Y, Li C, Sands BE, Ballester I, Stevens C, Korzenik JR, Rioux JD, Daly MJ, Xavier RJ, Podolsky DK. LRRK2 is involved in the IFN-gamma response and host response to pathogens. J Immunol. 2010;185:5577–5585. doi: 10.4049/jimmunol.1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio E, Cookson MR. Leucine-rich repeat kinase 2 mutations and Parkinson’s disease: three questions. ASN Neuro. 2009 doi: 10.1042/AN20090007. doi: 10.1042/AN20090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes DA, Racacho L, Han F, Panisset M, Bulman DE. LRRK2 screening in a Canadian Parkinson’s disease cohort. Can J Neurol Sci. 2007;34:336–338. doi: 10.1017/s0317167100006788. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Changing concepts in Parkinson’s disease. Lancet Neurol. 2011;10:307. [Google Scholar]
- Klein C, Krainc D, Schlossmacher MG, Lang AE. Translational Research in Neurology and Neuroscience 2011: Movement Disorders. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.11. doi:10.1001/archneurol.2011.11. [DOI] [PubMed] [Google Scholar]
- Klein C, Schlossmacher MG. Parkinson disease, 10 years after its genetic revolution: multiple clues to a complex disorder. Neurology. 2007;69:2093–2104. doi: 10.1212/01.wnl.0000271880.27321.a7. [DOI] [PubMed] [Google Scholar]
- Kubo M, Kamiya Y, Nagashima R, Maekawa T, Eshima K, Azuma S, Ohta E, Obata F. LRRK2 is expressed in B-2 but not in B-1 B cells, and downregulated by cellular activation. J Neuroimmunol. 2010;229:123–128. doi: 10.1016/j.jneuroim.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L, Magalhaes JG, Selvanantham T, Travassos LH, Geddes K, Fritz JH, Viala J, Tedin K, Girardin SE, Philpott DJ. Role of Nod1 in mucosal dendritic cells during Salmonella pathogenicity island 1-independent Salmonella enterica serovar Typhimurium infection. Infect Immun. 2009;77:4480–4486. doi: 10.1128/IAI.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. NOD2 mutation in Crohn’s disease potentiates NF-κB activity and IL-1ß processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Kubo M, Yokoyama I, Ohta E, Obata F. Age-dependent and cell-population-restricted LRRK2 expression in normal mouse spleen. Biochem Biophys Res Commun. 2010;392:431–435. doi: 10.1016/j.bbrc.2010.01.041. [DOI] [PubMed] [Google Scholar]
- Marín I. The Parkinson disease gene LRRK2: evolutionary and structural insights. Mol Biol Evol. 2006;23:2423–2433. doi: 10.1093/molbev/msl114. [DOI] [PubMed] [Google Scholar]
- Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, Gallo KA. LRRK2 in Parkinson’s disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–293. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Melrose H. Update on the functional biology of Lrrk2. Future Neurol. 2008;3:669–681. doi: 10.2217/14796708.3.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, Arai T, Guo JP, Klegeris A, Yu S, McGeer EG, McGeer PL. LRRK2 expression in normal and pathologic human brain and in human cell lines. J Neuropathol Exp Neurol. 2006;65:953–963. doi: 10.1097/01.jnen.0000235121.98052.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R, Anisman H, Hayley S, Park DS. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci. 2007;27:3328–3337. doi: 10.1523/JNEUROSCI.5321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Bras J, Deas E, O’Sullivan SS, Parkkinen L, Lachmann RH, Li A, Holton J, Guerreiro R, Paudel R, Segarane B, Singleton A, Lees A, Hardy J, Houlden H, Revesz T, Wood NW. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Reale M, Greig NH, Kamal MA. Peripheral chemo-cytokine profiles in Alzheimer’s and Parkinson’s diseases. Mini Rev Med Chem. 2009;9:1229–1241. doi: 10.2174/138955709789055199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saïd-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One. 2010;5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer TM, Staufer K, Novacek G, Steindl-Munda P, Schumacher S, Hofer H, Ferenci P, Vogelsang H. Efficacy and safety of antiviral therapy in patients with Crohn’s disease and chronic hepatitis C. Aliment Pharmacol Ther. 2008;28:742–748. doi: 10.1111/j.1365-2036.2008.03779.x. [DOI] [PubMed] [Google Scholar]
- Schlossmacher MG, Frosch MP, Gai WP, Medina M, Sharma N, Forno L, Ochiishi T, Shimura H, Sharon R, Hattori N, Langston JW, Mizuno Y, Hyman BT, Selkoe DJ, Kosik KS. Parkin localizes to the Lewy bodies of Parkinson disease and dementia with Lewy bodies. Am J Pathol. 2002;160:1655–1667. doi: 10.1016/S0002-9440(10)61113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmacher MG, Shimura H. Parkinson’s disease: assays for the ubiquitin ligase activity of neural Parkin. Methods Mol Biol. 2005;301:351–369. doi: 10.1385/1-59259-895-1:351. [DOI] [PubMed] [Google Scholar]
- Schurr E, Alcaïs A, de Léséleuc L, Abel L. Genetic predisposition to leprosy: a major gene reveals novel pathways of immunity to Mycobacterium leprae. Semin Immunol. 2006;18:404–410. doi: 10.1016/j.smim.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Schurr E, Gros P. A common genetic fingerprint in leprosy and Crohn’s disease. N Engl J Med. 2009;361:2666–2668. doi: 10.1056/NEJMe0910690. [DOI] [PubMed] [Google Scholar]
- Simón-Sánchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Krüger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton JC, Howson JM, Awomoyi AA, McAdam KP, Blackwell JM, Newport MJ. Polymorphism in NOD2, Crohn’s disease, and susceptibility to pulmonary tuberculosis. FEMS Immunol Med Microbiol. 2004;41:157–160. doi: 10.1016/j.femsim.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Takken FL, Tameling WI. To nibble at plant resistance proteins. Science. 2009;324:744–746. doi: 10.1126/science.1171666. [DOI] [PubMed] [Google Scholar]
- Taxman DJ, Huang MT, Ting JP. Inflammasome inhibition as a pathogenic stealth mechanism. Cell Host Microbe. 2010;8:7–11. doi: 10.1016/j.chom.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, Mata IF, Farrer MJ. LRRK2: a common pathway for parkinsonism, pathogenesis and prevention? Trends Mol Med. 2006;12:76–82. doi: 10.1016/j.molmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci USA. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Shen J. alpha-synuclein and LRRK2: partners in crime. Neuron. 2009;64:771–773. doi: 10.1016/j.neuron.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, 3rd, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci USA. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhães JG, Yuan L, Soares F, Chea E, Le Bourhis L, Boneca IG, Allaoui A, Jones NL, Nuñez G, Girardin SE, Philpott DJ. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- Travassos LH, Girardin SE, Philpott DJ, Blanot D, Nahori MA, Werts C, Boneca IG. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 2004;10:1000–1006. doi: 10.1038/sj.embor.7400248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Limbergen J, Wilson DC, Satsangi J. The genetics of Crohn’s disease. Annu Rev Genomics Hum Genet. 2009;10:89–116. doi: 10.1146/annurev-genom-082908-150013. [DOI] [PubMed] [Google Scholar]
- Venderova K, Kabbach G, Abdel-Messih E, Zhang Y, Parks RJ, Imai Y, Gehrke S, Ngsee J, Lavoie MJ, Slack RS, Rao Y, Zhang Z, Lu B, Haque ME, Park DS. Leucine-Rich Repeat Kinase 2 interacts with Parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson’s disease. Hum Mol Genet. 2009;18:4390–4404. doi: 10.1093/hmg/ddp394. [DOI] [PubMed] [Google Scholar]
- Vranjkovic A, Crawley AM, Patey A, Angel JB. IL-7-dependent STAT-5 activation and CD8+ T cell proliferation are impaired in HIV infection. J Leukoc Biol. 2011;89:499–506. doi: 10.1189/jlb.0710430. [DOI] [PubMed] [Google Scholar]
- Werts C, le Bourhis L, Liu J, Magalhaes JG, Carneiro LA, Fritz JH, Stockinger S, Balloy V, Chignard M, Decker T, Philpott DJ, Ma X, Girardin SE. Nod1 and Nod2 induce CCL5/RANTES through the NF-kappaB pathway. Eur J Immuno. 2007;37:2499–2508. doi: 10.1002/eji.200737069. [DOI] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wider C, Dickson DW, Wszolek ZK. Leucine-rich repeat kinase 2 gene-associated disease: redefining genotype-phenotype correlation. Neurodegener Dis. 2010;7:175–179. doi: 10.1159/000289232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FR, Huang W, Chen SM, Sun LD, Liu H, Li Y, Cui Y, Yan XX, Yang HT, Yang RD, Chu TS, Zhang C, Zhang L, Han JW, Yu GQ, Quan C, Yu YX, Zhang Z, Shi BQ, Zhang LH, Cheng H, Wang CY, Lin Y, Zheng HF, Fu XA, Zuo XB, Wang Q, Long H, Sun YP, Cheng YL, Tian HQ, Zhou FS, Liu HX, Lu WS, He SM, Du WL, Shen M, Jin QY, Wang Y, Low HQ, Erwin T, Yang NH, Li JY, Zhao X, Jiao YL, Mao LG, Yin G, Jiang ZX, Wang XD, Yu JP, Hu ZH, Gong CH, Liu YQ, Liu RY, Wang DM, Wei D, Liu JX, Cao WK, Cao HZ, Li YP, Yan WG, Wei SY, Wang KJ, Hibberd ML, Yang S, Zhang XJ, Liu JJ. Genomewide association study of leprosy. N Engl J Med. 2006;361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Müller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: LRRK2 mRNA and protein detection in EBV-transformed lymphoblasts and peripheral blood mononuclear cells. (A-D) Reducing SDS/PAGE was carried out using lysates of Ficoll-separated peripheral blood mononuclear cells (PBMC) from one human control donor (lane 3), followed by Western blotting with four different monoclonal rabbit anti-Lrrk2 antibodies (provided by the Michael J Fox Foundation, MJFF; see Materials and Methods), as indicated. Transgenic flies expressing full-length human LRRK2 cDNA (tg; lane 1) and their wild-type littermates (wt; lane 2) were used as positive and negative controls, respectively. Note, the specific detection of full-length Lrrk2 (260 kDa), and higher molecular weight (HMW) species thereof in PBMC lysates, as well as of HMW Lrrk2 reactivity in tg fly homogenates. Membranes were stripped and redeveloped with anti-β-actin antibody (lower panels). (E) LRRK2, GAPDH transcripts and 18S RNA isolated from EBV-transformed lymphoblasts from a healthy donor were processed by reverse transcriptase and PCR to generate distinct amplification products, as indicated (for primer pairs, see Materials and Methods).
Supplementary Figure 2: Murine Lrrk2 expression in cells from immune organs and exploration of its role in cytokine release by genotyped macrophages. (A) FACS-based quantification of lrrk2-positive immune cells (in mean %) isolated from homogenates of spleen and mesenteric lymph nodes of wild-type mouse (n=3) as described (Werts et al. 2007). Note, among four distinct leukocyte populations sorted, neutrophils revealed the strongest signal for lrrk2 reactivity (see also Discussion). Lrrk2 expression was measured by FACS analysis using anti-Lrrk2 antibody MJFF-4 on B220+ B-cells, TCRβ+ T-cells, CD11b+/CD11c+ macrophages, Gr-1+ neutrophils, and CD11c+ dendritic cells. (B) Lrrk2-genotype dependent cytokine signaling following exposure to different stimulants was explored by probing KC and IL-6 release by ELISA (Werts et al. 2007) from cultured BMDM. Cells were isolated from R1441C lrrk2 knock-in (KI) mice, heterozygous (HET) animals, and age-matched wild-type (WT) littermates (12 weeks-old, n=2 each; supernatants were analyzed in quadruplicates). Note, no consistent lrrk2 genotype-associated effect (WT vs HET / KI) was detected in the conditioned media of BMDM cells.