Abstract
Background
Patients with ESRD requiring renal replacement have impaired quality of life (HRQoL), and there is general consensus that HRQoL improves with successful transplant and evidence of improvement with frequent hemodialysis. This study reports changes in HRQoL associated with changes in treatment modality to daily hemodialysis (DHD) and transplant among patients requiring renal replacement.
Methods
This cohort study had assessments at baseline and 6-months following modality change. Subjects were non-diabetic individuals receiving conventional hemodialysis who a) remained on conventional hemodialysis (n=13), b) changed to daily hemodialysis (DHD) (n=10), or c) received a living donor transplant (n=20). Thirty-four healthy controls were assessed once for comparison. HRQoL was measured using the Kidney Disease Quality of Life Instrument. The Physical Functioning and Physical Composite Scale Scores were primary outcomes.
Results
Transplantation resulted in significant improvements in six of eight generic scales and the physical composite scale (PCS). Those changing to DHD had significant improvements in Physical Function and PCS scales. Those remaining on dialysis remained lower than controls on all scales except for Vitality; the transplant group remained lower than controls only on the Vitality and General Health scales. Transplant resulted in significant improvements in 4 of the 7 disease-specific scales (symptoms, effects and burden of kidney disease, work). DHD resulted in improvements in the effects of kidney disease.
Conclusions
Modality change to transplant results in significant improvement in HRQoL, achieving levels similar to controls. Change to daily hemodialysis improves only select HRQoL domains, and remains low in disease-specific domains.
Keywords: Quality of Life, Dialysis, Transplant, Daily Dialysis
Introduction
More options are becoming available for renal replacement therapies with growing interest in frequent hemodialysis. Similarly, interest in health-related quality of life (HRQoL) among the various treatments has grown with the increasing experience with frequent hemodialysis. It is well documented that patients with end stage renal disease (ESRD) needing renal replacement therapy have impaired HRQoL (1), and there is a general consensus that HRQoL improves with successful transplantation (1-4). However, the comparisons of HRQoL in hemodialysis and transplant are often complicated by patient selection bias for the different treatments since many of the differences in scores between dialysis and transplant patients are explained by age, clinical and sociodemographic characteristics and comorbidities (diabetes in particular) (4, 5). When these confounding factors are adjusted, most differences in quality of life between dialysis and transplant patients are in kidney disease-targeted domains rather than generic measures (5).
Frequent hemodialysis, in the form of daily hemodialysis (DHD) (six times/week for 1.5-3 h), has been shown to be more effective than conventional hemodialysis (CHD) (3-4 hours thrice weekly) in terms of fewer vascular access problems, better hypertension control with reduction of antihypertension medication, lower incidence of ventricular hypertrophy, better anemia control and reduction in the use of phosphate binders (6). HRQoL is also reported to improve when patients are converted from CHD to DHD, with consistent improvements in self-reported physical function, social function and energy, as well as dialysis related symptoms (6, 7). Most of these studies have limitations, with small numbers, cross-sectional design and often no control groups and short follow-up periods (6) and, as with comparisons with transplant, there is also concern for treatment selection bias for patients converting to the DHD (6, 7).
The Frequent Hemodialysis study (FHN) was a trial that randomized patients to 6 days/week hemodialysis and conventional hemodialysis (8). The two primary outcomes in the FHN was LV mass, and the physical component summary score of the RAND 36-item health survey. The FHN found that frequent dialysis resulted in reduction in left ventricular (LV) mass and improved self-reported physical health. They also found improved hypertension control with reduction of antihypertensive medications, fewer hypotensive events during dialysis, and improved hyperphosphatemia.
There have been no studies that have compared HRQoL of patients receiving DHD with that of transplant recipients. The purpose of this paper is to report changes in HRQoL associated with changes in treatment modality among patients requiring renal replacement therapy. We also compared patients following treatment change to normal controls. HRQoL was a secondary outcome of a study to determine the physiologic responses to exercise with changes in modality20, and patients were excluded if they had comorbidities that would affect exercise capacity, such as diabetes, cardiac comorbidiities, many of which also affect HRQoL. This selection design allows evaluation of changes in HRQoL without the confounding effects of age and comorbidities that are known to affect HRQoL, and which have confounded comparisons between hemodialysis and transplant groups in other studies.
MATERIALS and METHODS
Subjects
Four groups of patients with ESRD were studied in a pre-post design 6 months apart (baseline and visit 2): group 1 included patients who were treated with conventional hemodialysis (3-4 hr 3x/week) and did not change modality (CHD-CHD); group 2 (CHD-DHD) changed from conventional hemodialysis to daily hemodialysis (3 hours 5-6 days/week, performed at home); group 3 (transplant) received a living donor kidney transplant. Transplant recipients were tested within the 2 weeks before living donor transplant and again 6 months after the procedure. Those changing to daily dialysis were tested within a week of starting training for daily dialysis and again 6 months after beginning daily treatments. A control group comprised of sedentary individuals was recruited from a list of kidney donors and was tested once for comparison.
Primary outcome measures were the Physical composite scale score and the Physical Function scale on the SF-36 questionnaire, a generic measure of self-reported, or perceived health status. Analysis also included the other 8 generic scales from the SF-36 and 7 disease-specific scales from the Kidney Disease Quality of Life Questionnaire (KDQOL).
The study was designed and powered to evaluate the effects of reduction of uremia on the physiologic responses to exercise (22). Thus, patients with comorbidities/ conditions that would have independent effects on exercise capacity (i.e. diabetes mellitus, pulmonary disease, cardiac disease, etc) were excluded. Inclusion criteria were: ESRD requiring kidney replacement therapy; either stable on conventional hemodialysis for at least 3 months or scheduled for transplant from a living donor; able to provide informed consent; 18 years of age or greater; and English speaking. Exclusion criteria were: orthopedic or musculoskeletal factors that limit or could be exacerbated by exercise; hematocrit <33%; recent (within 1 year) cardiovascular event; pulmonary disease; peripheral vascular disease or progressive degenerative muscular disease; diabetes; and contraindications to maximal exercise testing as defined by the American College of Sports Medicine (9). All subjects on CHD were either qualified for or were on the transplant waiting list. Demographic and clinical information was obtained from the medical record or dialysis chart or from self-report.
Conventional hemodialysis patients were recruited from five centers, CHD-DHD from 3 centers and transplant recipients from the University of California San Francisco (UCSF) and the University of Minnesota (see acknowledgements). Dialysis patients responded to posted fliers or were referred by dialysis staff; transplant recipients gave permission to nurse coordinators or physicians to be referred. Patients scheduled to change treatment (to DHD or to transplant) were identified by the dialysis or transplant nursing staff and provided information about the study. Those interested in participating in the study were referred to the study either by the nurses, or by contacting the study staff. Patients were not randomized to treatment and referrals were fully dependent on nursing staff. Weekly contact was made to nursing staff to remind them to provide informational fliers to all patients scheduled to start DHD or who were scheduled for transplant. Sedentary healthy control subjects were recruited from kidney donors (>1 year post donation with normal kidney function as indicated by estimated GFR) at the University of Minnesota. A letter was sent to 420 kidney donors within a 100-mile radius of the medical center, from which 180 interested individuals responded. Study subjects were selected in an attempt to match the group of control subjects to the patient group by percentage of women, general physical activity levels and age decades. Because the donors were selected to match the patient groups, they were not necessarily representative of the general population of kidney donors as described by Ibrahim, et al (10). All subjects provided informed consent, and the study was approved by the Committee on Human Subjects at UCSF and the Institutional Review Board at the University of Minnesota.
HRQOL Assessment
The Kidney Disease Quality of Life (KDQOL) (version 1.2) (11) was used for assessment of HRQoL. The KDQOL contains the SF-36 Health Status Questionnaire (version 1.2), which includes 8 scales (physical function (PF), role limitations due to physical problems (RP), body pain (BP), general health perceptions (GH), vitality (VT), social function (SF), mental health (MH) and role limitations due to emotional problems (RE). The questionnaire also allows for calculation of normalized scores representing overall physical functioning (Physical Component Summary score (PCS)) and overall mental functioning (Mental Component Summary Score (MCS)). The KDQOL also includes several disease –targeted scales: symptoms, effects of kidney disease, burden of kidney disease, work, cognitive function, sleep and sexual functioning. Satisfaction with care, social interaction and patient satisfaction were not included because they were not relevant to patients not on dialysis following transplant so were not included in this analysis. The KDQOL questionnaire is considered the most appropriate diseases-specific instrument for assessing quality of life in end-stage renal disease patients (12).
Scoring
All responses were transformed and scaled into T-scores for the SF-36 generic scales where a mean of 50 is that of for the normal population (13) and linearly scaled for the disease-targeted scales from 0 to 100. Higher scale scores represent better functioning and well-being or more positive (less burden). Internal consistency reliability estimates calculated by Hays et al (11) for the 19 multi-item scales on the KDQOL exceed 0.75 for every measure except one.
Procedures
All testing was performed in the General Clinical Research Center at UCSF or the University of Minnesota. Testing was performed on a mid-week non-dialysis day. The KDQOL was completed by each subject independently.
Laboratory Measures
Fasting blood samples were used to assess blood chemistries and hematology. The most recent value for single pool Kt/V was obtained from the dialysis chart to assess dialysis adequacy. For the daily dialysis group, the single pool Kt/V value reported in the monthly labs was multiplied by the number of days dialyzed per week.
Statistical Analysis
Basic descriptive data was calculated for all groups. Scale scores for the patient groups were compared at visit 1 and to sedentary controls at visit 1 and at visit 2 using analysis of variance with post-hoc analysis to determine specific differences between groups. A change score was calculated for each scale (visit 2 minus visit 1). This change score was used for the primary analysis of differences in change among the groups. Data were pooled and analysis of covariance was performed with the change score as the dependent variable, adjusted for visit 1 values and group (dummy variables for each group assigned). Visit 1 scores were used as a covariate to minimize effects of differences in scores at baseline. Post hoc analysis (Bonferroni) compared each of the 2 groups that changed modalities to the change scores of the group that stayed on CHD (CHD-CHD). Significance for all tests was set at p=0.05. Statistical analysis was performed using SPSS v17.
RESULTS
Subjects
A total of 61 patients and 36 sedentary controls were recruited into the study and tested. Of the total recruited (n=97), 77 subjects are included in the analyses (43 patients and 34 controls). Figure 1 shows the reasons for loss to analysis in all groups. No follow-up questionnaires were obtained for those who dropped from the study.
Figure 1.
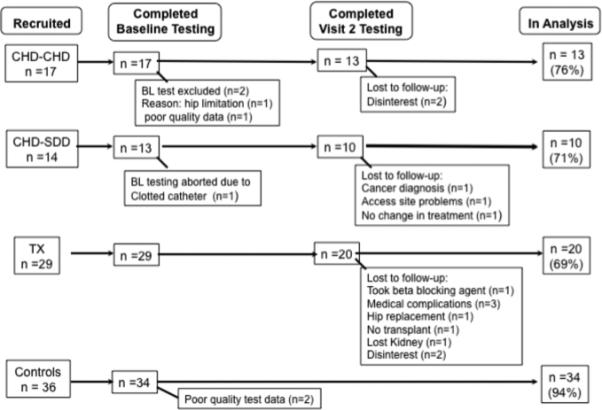
Recruitment and loss to analysis. Abbreviations: CHD-CHD, conventional hemodialysis to conventional hemodialysis; CHD-DHD, conventional hemodialysis to daily hemodialysis; TRANSPLANT, living donor transplant; conventional hemodialysis to transplant; BL, Baseline.
The patient groups consisted of 18% women (15% in the groups that remained on dialysis and 22% in the transplant group), and 17% of the controls were women (Table 1). The age within patient groups was similar; however control subjects were significantly older than the patient groups. There was no difference among groups in BMI. The transplant group was on dialysis less time than those in the CHD-CHD and the CHD-DHD groups (p<.001). There was no difference in the total number of antihypertensive medications among patient groups. Most patients treated with dialysis were taking erythropoietin stimulating agents at baseline. All patients were either on the transplant wait list or would qualify for transplant.
Table 1.
Demographic and Clinical Characteristics at Visit 1
| Variable | CHD-CHD (n=13) | CHD-DHD (n=10) | TRANSPLANT (n=20) | Controls (n=34) |
|---|---|---|---|---|
| Gender (male/female) | 11/2 | 9/1 | 17/3 | 28/6 |
| Age | 45.5 (10.4)* | 42.6 (12.4)* | 43.5 (10.9)* | 47.7 (8.5) |
| BMI | 27.4 (4.0) | 28.3 (4.4) | 28.1 (5.0) | 27.1 (4.7) |
| Time on dialysis (mo) | 28.5 (21.2) | 33.8 (44.3) | 5.3 (13.7) | - |
| Cause of Renal Failure | - | |||
| Hypertension | 23.5% | 21.4% | 6.9% | |
| GN/FSGN | 17.6% | 7.1% | 17.2% | |
| IgA Nephropathy | 11.8% | - | 24.1% | |
| PCKD | 5.9% | 14.3% | 24.1% | |
| Other/Unknown | 41.4% | 58.1% | 38.4% | |
| % on ESA at visit 1 | 82.4% | 78.6% | 41.4% | - |
| Serum Creatinine (mg/dL) | 8.9 (1.9)* | 10.2 (2.2)* | 6.8 (2.4)* | 1.2 (0.2) |
| Blood Urea Nitrogen (mg/dL) | 36.2 (9.2)* | 45.1 (23.8)* | 58.3 (25.0)* | 17.9 (5.1) |
| Hemoglobin (g/dL) | 12.6 (0.9)* | 12.2 (1.4)* | 12.5 (1.4)* | 13.4 (1.4) |
Data presented as mean (SD)
Abbreviations: GN/ FSGN: Glomerulonephritis/Focal Sclerosing Glomerulonephritis; PCKD polycystic Kidney Disease; CHD-CHD, remaining on conventional hemodialysis therapy; CHD-DHD, conventional hemodialysis to daily hemodialysis therapy; transplant, received kidney transplant
p<0.05 compared to controls
Five patients in the transplant group were on dialysis at baseline and 15 were scheduled for pre-emptive transplant. Following transplant there were significant improvements in serum BUN (to 21.6 ± 5.1 mg/dL) and creatinine (to 1.7 ± 0.5 mg/dL), but eGFR remained significantly lower than that of the control subjects (49.2 ± 2.4 vs. 69.1 ± 13.1 ml/min/1.73m2; p=0.01). Hemoglobin increased slightly (to 13.2± 1.9 g/L; p = 0.05); however, the hemoglobin remained low in the patient groups compared to the controls. Immunosuppression was primarily tacrolimus, sirolimus and mycophenolate mofetil; 12 patients were taking prednisone. Following transplant, 10 were taking beta-blocking agents and 2 remained on non-dihydropyridine calcium channel blockers.
Patients in the CHD-DHD group changed to the NxStage® dialysis machine, increasing the frequency of treatments from 3 days/week (3.4 ± 0.3 hours/session) to an average of 5.6 ± 0.5 days/week (2.8 ± 0.2 hours/session), which resulted in a weekly Kt/V to 2.77 ± 0.48. Those who remained on conventional hemodialysis dialyzed 3 days/week for 3.4 ± 0.5 hours/session and Kt/V averaged 1.44 ± 0.21; this did not change at visit 2. At visit 2, three patients in the CHD-DHD group had discontinued beta-blocking agents. There were no changes in blood pressure medications in the CHD-CHD group.
Primary Outcomes
At visit 1 all three patient groups scored significantly lower than the controls on the PF and PCS scales (p<0.05). There was no significant difference between the patient groups at visit 1 on the PF and PCS scales. Change scores from visit 1 to visit 2 were calculated and analysis of covariance was performed with the change score as the dependent variable, adjusting for visit 1 values and group. There were significant differences in the change scores from visit 1 to visit 2 among the patient groups on the PF and PCS scales (p<0.03) (table 2) , with both transplant and CHD-DHD showing positive change in scores and those remaining on CHD showing no change. Despite significant increases in PF and PCS in those patients who changed to DHD, the scores at visit 2 were not significantly different than those who remained on CHD, whereas those who received transplant were higher at visit 2 than both dialysis groups and were similar to controls (table 2).
Table 2.
SF-36 Scale Scores (t-scores) at visit 1 and visit 2 - Data presented is mean (SD)
| Scale | CHD-CHD (n=13) | CHD-DHD (n=10) | TRANSPLANT N=20) | Controls (n=34) | P for change among groups |
|---|---|---|---|---|---|
| Physical Function | |||||
| Baseline | 47.8 (12.9)a | 47.5 (9.9)a | 48.0 (8.4)a | 55.0 (3.1) | |
| 6 months | 48.9 (11.4)c,d | 52.5 (6.1) | 54.7 (3.5) | ||
| adjusted mean change* (95% CI) | 1.1 (-1.3, 3.5) | 4.7 (1.8, 7.6) | 6.7 (4.8, 8.7) | 0.02 | |
| Role Physical | |||||
| Baseline | 45.9 (12.8)a | 41.2 (12.8)a | 40.3 (10.5)a | 55.2 (3.0) | |
| 6 months | 46.3 (12.4)c,d | 43.8 (12.4)c,d | 51.9 (8.1) | ||
| adjusted mean change* (95% CI) | 2.5 (-2.8, 7.9) | 2.0 (-4.7, 8.8) | 10.5 (6.1, 14.8) | 0.09 | |
| Body Pain | |||||
| Baseline | 56§ (6.7) | 50.7 (8.3)a | 55.8 (9.9) | 56.8 (6.3) | |
| 6 months | 58.1 (7.0) | 55.9 (6.9) | 59.1 (6.0) | ||
| adjusted mean change* (95% CI) | 2.2 (-0.6, 5.1) | 3.0 (-0.5, 6.5) | 2.7 (0.5, 5.1) | 0.75 | |
| General Health | |||||
| Baseline | 47.4 (11.6)ab | 43.4 (10.1)a | 40.9 (9.2)a | 55.3 (6.8) | |
| 6 months | 46.0 (9.6)c | 48.1 (10.4)c | 47.0 (8.8)c | ||
| adjusted mean change* (95% CI) | 0.3 (-4.0, 4.5) | 4.6 (-0.3, 0.9) | 5.0 (1.6, 8.4) | 0.15 | |
| Vitality | |||||
| Baseline | 51.6 (10.7)ab | 48.5 (11.1)ab | 40.4 (11.2)a | 54.6 (8.2) | |
| 6 months | 48.1 (6.0)c | 51.1 (12.7)c | 50.8 (10.4)c | ||
| adjusted mean change* (95% CI) | 0.2 (-5.1, 5.5) | 4.4 (-1.7, 10.9) | 7.1 (2.8, 11.5) | 0.16 | |
| Role Emotional | |||||
| Baseline | 44.8 (12.9)a | 44.8 (15.8)a | 49.1 (11.5) | 52.9 (6.8) | |
| 6 months | 50.5 (10.2)cd | 44.8 (12.9)cd | 51.6 (9.8) | ||
| adjusted mean change* (95% CI) | 4.9 (1.3, 8.6) | -0.6 (-5.0, 3.6) | 3.4 (-0.4, 6.3) | 0.21 | |
| Mental Health | |||||
| Baseline | 55.8 (6.3) | 49.1 (12.0) | 51.4 (8.8) | 54.0 (7.2) | |
| 6 months | 54.2 (7.9) | 50.9 (9.9) | 51.9(8.1) | ||
| adjusted mean change* (95% CI) | -0.03 (-4.0, 3.9) | 0.4 (-4.4, 5.1) | -0.04 (-3.1, 3.2) | 0.94 | |
| Social Function | |||||
| Baseline | 81.7 ± 21.4a | 72.2 ± 22.3a | 71.4 ± 26.5a | 93.3 ± 12.7 | |
| 6 months | 82.3 ± 17.2 | 80.0 ± 23.7c | 90.6 ± 24.2 | ||
| adjusted mean change* (95% CI) | 1.9 (-8.8,12.8) | 3.0 (-9.1, 15.1) | 15.0 (6.8, 23.3) | 0.10 | |
| Physical Component summary score (PCS) | |||||
| Baseline | 48.8 ± 10.0a | 45.3 ± 11.3a | 45.1 ± 8.4a | 56.2 (3.0) | |
| 6 months | 48.4 ± 7.4c,d | 49.6 ± 9.1c,d | 53.8 ± 3.9 | ||
| adjusted mean change* (95% CI) | 1.0 (-2.5,4.5) | 5.3 (1.0, 9.5) | 7.1 (4.4, 9.8) | 0.03 | |
| Mental Component summary score (MCS) | |||||
| Baseline | 51.1 ± 9.1 | 48.1 ± 14.6 | 47.4 ± 10.2 | 52.5 (7.0) | |
| 6 months | 51.7 ± 9.6 | 48.9 ± 12.6 | 50.6 ± 9.5 | ||
| adjusted mean change* (95% CI) | 0.8 (-3.6, 5.3) | -0.9 (-6.3, 4.3) | 1.6 (-1.8, 5.0) | 0.77 | |
Unless otherwise indicated, values are given as mean (standard deviation).
Abbreviations:
CHD-CHD, remaining on conventional hemodialysis therapy; CHD-DHD, conventional hemodialysis to daily hemodialysis therapy; transplant, received kidney transplant
adjusted mean change for baseline values (95% confidence intervals)
p<0.01 compared to donors at visit 1
p<0.01 compared to transplant at visit 1
p<0.01 compared to donors at visit 2
p<.01 compared to transplant at visit 2
SF-36 Generic Scales
At visit 1, all three patient groups scored significantly lower than the controls on the following secondary outcome generic scale scores on the SF-36: RP, GH, VT,RE, and SF (p<.05) (table 2). The only difference among the patient groups on these scores at visit 1 was that transplant group was significantly lower on the vitality scales compared to the dialysis groups (p<.03).
The CHD-CHD group did not change from visit 1 to visit 2 on any scales. Those who received transplant had significant positive change in the following scales: RP,GH, VT, SF, with trends of positive change in BP. Although the CHD-DHD and transplant groups had change in GH, the difference in change among the groups was not statistically significant (p=0.15). There was a trend to difference in change of RP among the groups (p=0.09), with both transplant and CHDSDD showing positive change in scores and those remaining on CHD showing no change.
At visit 2, both groups that remained on dialysis were lower than transplant on the RP scale and they remained lower than controls in all the SF-36 generic scores except for VT. Those who received transplants were similar to the controls on all generic scales except for GH.
KDQOL Disease-Specific Scales
The scale scores and changes in scale scores for the KDQoL disease-specific scales for all groups at each time point are shown in table 3. There were no differences among the patient groups on any of the disease specific scales at visit 1 and no differences at visit 2 in cognitive function, sleep or sexual function. There were significant differences in the changes from visit 1 to visit 2 among the groups for symptoms (p=0.01), effects of kidney disease (p<0.001) and burden of kidney disease (p=0.001), and a trend for change in work (p=0.07). These differences in change from visit 1 to visit 2 were primarily due to improved scores in the transplant group. The CHD-DHD group had significant positive change in the effects of kidney disease, however they remained lower than transplant at visit 2 in symptoms, burden of kidney disease and work.
Table 3.
Kidney Disease-Targeted Scale Scores
| Scale | CHD-CHD (n=13) | CHD-DHD (n=10) | TRANSPLANT (n=20) | P for change among groups |
|---|---|---|---|---|
| Symptoms | ||||
| Baseline | 87.9 (9.1) | 80.7 (10.9) | 80.8 (11.5) | |
| 6 months | 87.5 (7.4) | 82.8 (9.3)b | 91.8 (8.7) | |
| adjusted mean change* (95% CI) | 1.2 (-3.3, 5.9) | 0.5 (-4.8, 18.4) | 8.8 (5.2, 12.4) | 0.01 |
| Effects of Kidney Dz | ||||
| Baseline | 69.5 (23.3) | 64.8 (28.3) | 72.4 (19.8) | |
| 6 months | 67.7 (18.7)b | 78.7 (16.3)bc | 95.1 (5.4) | |
| adjusted mean change* (95% CI) | -1.9 (-8.1, 4.3) | 10.9 (3.5, 18.4) | 18.6 (5.2, 12.4) | <0.001 |
| Burden of Kidney Dz | ||||
| Baseline | 64.9 (27.5) | 42.3 (29.3)a | 46.1 (24.2)a | |
| 6 months | 60.5 (28.2)b | 49.3 (9.1)bc | 76.5 (20.3) | |
| adjusted mean change* (95% CI) | 0.9 (-8.8, 4.3) | 4.2 (-7.2, 15.5) | 24.4 (16.8, 32.1) | 0.001 |
| WORK | ||||
| Baseline | 50.0 (45.6) | 50.0 (43.3) | 77.5 (37.9) | |
| 6 months | 46.1 (47.7)b | 45.0 (36.6)b | 85.0 (28.5) | |
| adjusted mean change* (95% CI) | -8.8 (-24.3,6.7) | -1.9 (-20.0, 16.2) | 13.9 (1.2, 26.6) | 0.07 |
| Cognitive Function | ||||
| Baseline | 89.7 (13.5) | 85.1 (10.9) | 79.6 (21.7) | |
| 6 months | 85.6 (15.1) | 91.3 (7.0) | 88.3 (14.9) | |
| adjusted mean change* (95% CI) | -1.1 (-8.7, 6.5) | 6.2 (-2.6, 15.1) | 2.4 (-3.6, 8.5) | 0.45 |
| SLEEP | ||||
| Baseline | 39.8 (13.4) | 45.0 (8.6) | 41.6 (11.4) | |
| 6 months | 42.3 (10.0) | 44.0 (15.5) | 36.2 (10.8) | |
| adjusted mean change* (95% CI) | 2.02 (-2.7,6.8) | -2.5 (-8.2, 3.1) | -5.3 (-9.0, -1,4) | 0.21 |
| Sexual Functioning | ||||
| Baseline | 85.5 (11.1) | 90.3 (17.4) | 83.1 (23.0) | |
| 6 months | 82.7 (14.1) | 87.5 (9.8) | 90.0 (21.6) | |
| adjusted mean change* (95% CI) | 6.5 (-7.1,20.2) | 4.3 (-10.7, 19.4) | 5.8 (-4.1, 15.8) | 0.97 |
Unless otherwise indicated, values are given as mean ± standard deviation.
Abbreviations:
CHD-CHD, remaining on conventional hemodialysis therapy; CHD-DHD, conventional hemodialysis to daily hemodialysis therapy; TRANSPLANT, received kidney transplant
adjusted mean change for baseline values (95% confidence intervals)
p<0.01 compared to CHD at visit 1
p<0.01 compared to TRANSPLANT at visit 2
p<0.01 compared to CHD at visit 2
The CHD-CHD and CHD-DHD groups differed in the effects of- and burden of kidney disease scale score at visit 2, with those changing to DHD showing a higher score that reflected less effects of kidney disease but their scores reflected greater burden of disease.
Discussion
Overall, we found that modality change to daily hemodialysis and to transplantation resulted in significant positive change in the PF scale of the SF-36 and in the PCS score, which incorporates the PF and other individual scales, whereas those remaining on CHD did not change. The magnitude of change was similar in the two groups (DHD 4.7 and transplant 6.7 points on PF; DHD 5.3 and transplant 7.1 on PCS). The magnitude of change on the PCS in the DHD was greater than that reported in the FHN study (14), which reported increase in PHC of 3.4 over 1 year. The changes observed in our study would be considered to be clinically important as described by Samsa, et al (15) who suggest that a change in 3-5 points in an individual scale score to be meaningful. Minimally important differences can also be evaluated using estimates of effect size (change divided by baseline standard deviation) (16). Effect sizes range from small to large effect according to Cohen's (17) rules of thumb, where 0.20 is considered a small effect, 0.50 is a medium effect and 0.80 or above is a large effect. Expressed as estimates of effect size, changing to DHD had near moderate effect on PF (effect size = 0.47) and PCS (effect size = 0.46) whereas transplant had a moderate effect on PF (effect size = 0.68) and a large effect on PCS (effect size = 0.84).
We also found significant positive change in 5 of the remaining 7 generic scale scores in patients who received kidney transplants (effect size ranging from 0.48 to 0.64); achieving scores similar to healthy controls in all domains except general health and vitality. With the exception of significant positive changes in the vitality and social function scores in the transplant group, changes observed in this study were in the physical scales. The mental health scores (i.e. role emotional, social functioning, mental health) were, overall, not affected by the changes in treatment modality.
Transplantation resulted in large effects on disease-targeted scales, specifically symptoms (effect size =0.76), effects of kidney disease (effect size =0.93) and burden of kidney disease (effect size=1.0). The effects of kidney disease were also reduced in the group that changed to DHD (small effect = 0.38), but the burden of kidney disease did not change, and actually remained lower than those who remained on conventional hemodialysis.
As expected, due to the strict inclusion criteria, our patient groups had higher scores at baseline than other published studies of the general population of dialysis patients (4, 18). Despite the highly selected group of patients, they still scored significantly lower on 6 of the 8 generic scales on the SF-36 at baseline compared to the control group. Nevertheless, we were able to document significant changes in most of the HRQoL domains in those receiving transplant and in several scales in those changing modality to short daily hemodialysis. Following transplant, the SF-36 scale scores of our subjects were much higher than reported in other studies of transplant recipients (4, 5) although the disease-specific scale scores were similar.
The options available for renal replacement therapy are increasing, with significant interest in more intensive hemodialysis such as short daily dialysis. Our data are in agreement with many reports of improvements in perceived quality of life in patients who change to more frequent hemodialysis treatments (19-23) (8) and in those who receive kidney transplants (1-4). Because of our selection criteria, our data are better able to show that the changes observed are due to the modality change, since the patients were of similar age and those with significant comorbidity were excluded from the study and all subjects were either on the wait list for transplant or qualified for transplant. Other studies have suggested that differences previously reported in HRQoL between dialysis and transplant patients are due to selection bias, with one of the most important unadjusted factors being comorbidities (5, 24). Rosenberger 13 and Kovac 5 adjusted for comorbidities in comparison groups of transplant recipients and wait listed hemodialysis patients and found that differences only remained in the disease-specific scales.
Limitations in this study include small sample sizes and non-randomized groups. The study was also powered to detect changes in physiologic measures rather than HRQoL (25). However, given the expected large standard deviations on most HRQoL scores, the magnitude and significance of change observed in the DHD and the transplant groups, suggest that the data represents statistical and clinically meaningful change. The lack of randomization does not allow us to determine if the improvements observed in the DHD group are because they were discontent with in-center dialysis and the change was due to a change to home treatment vs. incenter treatment. Although the study was initially designed to recruit a separate group of patients for transplant who were treated with hemodialysis in addition to a group that was scheduled to receive pre-emptive transplant, we were unable to schedule the hemodialysis group for a full day of testing prior to transplant, since most of those patients were employed and were unable to take additional time off for the testing prior to transplant. Thus we opted to combine the 5 patients in that group with those who were not on dialysis prior to one transplant group, thus we are unable to determine if the changes with transplant in our group would be similar in patients treated with CHD prior to transplant. Our sample may also be biased in that we were unable to obtain repeat HRQoL measures in those who were dropped from the study due to reasons related to the primary study design. The exclusion criteria for this study clearly limits the generalizability of this study to the overall dialysis population because our subjects were relatively higher functioning, however it is probable that the effects of modality change would be greater in patients starting at lower levels of HRQoL than the subjects in this study.
Despite the limitations of a small and non-randomized sample, we were able to detect differences in change in several domains of HRQoL. Although the inclusion criteria were quite strict, they allowed us to evaluate changes in HRQoL without the confounding effects of comorbidities that are known to affect HRQoL. Since there may be a ceiling effect of change in HRQoL, it is possible that the changes observed in these relatively healthy patients with modality change may be less than in patients starting at lower levels of HRQoL. No studies have compared changes in HRQoL in patients changing modality to daily hemodialysis and to transplant. This study is also unique in having a healthy control group for comparison purposes. The longitudinal design and the exclusion of patients with significant comorbidities enhances the internal validity of the results.
Renal transplant is universally preferred over dialysis as a treatment modality because of the enhanced survival and reduced morbidity. However, with the growing interest in and access to more intensive hemodialysis treatments, reports have emerged of patients who opt against transplantation to remain on intensive dialysis (26). We have previously reported that changing to daily hemodialysis does not change exercise capacity (as measured by peak oxygen uptake) or the physiologic determinants of exercise capacity (25), whereas there is a significant improvement in these physiologic measurements with receipt of a kidney transplant (25). Likewise, although the changes in HRQoL observed with DHD were primarily in the self-reported physical function, those receiving a transplant had positive change scores in more HRQoL domains, had greater magnitude of change and most importantly, greater positive change in the disease-specific scales. Given that HRQoL is strongly predictive of outcomes in dialysis patients (27-30), transplant should still be considered the treatment modality most likely to optimize HRQoL.
Acknowledgements
The authors would like to acknowledge the following dialysis centers: Satellite Healthcare (San Francisco Bay area), Mt Zion / UCSF Outpatient Hemodialysis, DaVita Dialysis in San Francisco and Minneapolis, Clarian Home Dialysis program (Indianapolis) and Barnes Jewish Dialysis Center at Washington University School of Medicine (St. Louis, MO).
This study was funded by a grant from the National Institute of Nursing Research (RO1-NR008286)
Funding: The sponsor had no involvement in study design, collection, analysis or interpretation of data, or writing the report or any decision related to submission of the report for publication.
Footnotes
Financial Disclosures:
Patricia Painter, Ph.D.: no currently; has received funding from Amgen
Joanne B. Krasnoff Ph.D. none
Michael Kuskowski,Ph.D. none
Lynda Frassetto, M.D.: none
Kirsten L. Johansen, M.D.: has received funding from Abbott and Amgen
Conflict of Interest Statement: The authors of this study have no conflicts of interest to declare. The results presented in this paper have not bee published previously in whole or part, except in abstract format.
References
- 1.Valderrabano F, Jofre R, Lopez-Gomez JM. Quality of life in end-stage renal disease patients. Am J Kidney Dis. 2001;38:443–464. doi: 10.1053/ajkd.2001.26824. [DOI] [PubMed] [Google Scholar]
- 2.Dew MA, Switzer GE, Goycoolea JM, et al. Does Transplantation Produce Quality of Life Benefits? Transplantation. 1997;64:1261–1273. doi: 10.1097/00007890-199711150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Evans RW, Manninen DL, Garrison LP, et al. The quality of life of patients with end-stage renal disease. New England Journal of Medicine. 1985;312:553–559. doi: 10.1056/NEJM198502283120905. [DOI] [PubMed] [Google Scholar]
- 4.Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink MGM. Quality of Life Assessed with teh Medical Outcomes Study Short Form 36-Item Health Survey of Patients on Renal Replacement Therapy: A systematic Review and Meta-Analysis. Value in Health. 2007;10:390–397. doi: 10.1111/j.1524-4733.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs AZ, Molnar MZ, Szeifert L, et al. Sleep disorders, depressive symptoms and health-related quality of life- a cross-sectinal comparison between kidney transplant recipients and waitlisted patients on maintenance dialysis. Nephrol Dial Transplant. 2011;26:1058–1065. doi: 10.1093/ndt/gfq476. [DOI] [PubMed] [Google Scholar]
- 6.Punal J, Lema LV, Sanhez-Guisande D, Ruano-Ravina A. Clinical effectiveness and quality of life of conventional hemodialysis versus short daily haemodialysis: a systematic review. Nephrol Dial Transplant. 2008;23:2634–2646. doi: 10.1093/ndt/gfn010. [DOI] [PubMed] [Google Scholar]
- 7.Kurella M, Suri RS, Chertow GM. Frequent Hemodialysis and Psychosocial Function. Seminars in Dialysis. 2005;18:132–136. doi: 10.1111/j.1525-139X.2005.18216.x. [DOI] [PubMed] [Google Scholar]
- 8.Group. TFT In center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Sports Medicine . Guidelines for Exercise Testing and Prescription. 5th edition Williams & Wilkins; Philadelphia: 1995. [Google Scholar]
- 10.Ibrahim HN, Foley R, Tan L, et al. Long-Term Consequences of Kidney Donation. N Engl J Med. 2009;360:459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the Kidney Disease Quality of Life (KDQOL) Instrument. Quality of Life Research. 1994;3:329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 12.Glover C, Banks P, Carson A, Martin CR, Duffy T. Understanding and Assessing the Impact of End-Stage Renal Disease on Quality of LIfe. Patient. 2011;4:19–30. doi: 10.2165/11584650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health summary scales: a User's Manual. ed2 Health Institute; Boston, MA: 1994. [Google Scholar]
- 14.Hall YN, Larive B, Painter P, et al. Effects of Six versus Three Times per Week Hemodialysis on Physical Performance, Health and Functioning: Frequent Hemodialysis Network (FHN) Randomized Trials. Journal of the American Society of Nephrology. 2011 doi: 10.2215/CJN.10601011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samsa G, Edelman D, Rothman ML, et al. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15:141–155. doi: 10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- 16.Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2005;2:63–67. doi: 10.1081/copd-200050663. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J. Statistical Power Analysis for the Behavioral Sciences. revised edition Academic Press, Inc.; New York: 1989. [Google Scholar]
- 18.Lopes AA, Bragg-Gresham JL, Goodkin DA, et al. Factors associated with health-related quality of life among hemodialysis patients in the DOPPS. Qual Life Res. 2007;16:545–557. doi: 10.1007/s11136-006-9143-7. [DOI] [PubMed] [Google Scholar]
- 19.Andre MB, Rembold SM, Pereira CM, Lugon JR. Prospective evaluation of an incenter daily hemodialysis program: results of two years of treatment. Am J Nephrol. 2002;22:473–479. doi: 10.1159/000065280. [DOI] [PubMed] [Google Scholar]
- 20.Heidenheim AP, Muirhead N, Moist L, et al. Patient quality of life on quotidian hemodialysis. Am J Kidney Dis. 2003;42:36–41. doi: 10.1016/s0272-6386(03)00536-5. [DOI] [PubMed] [Google Scholar]
- 21.Kooistra MP, Vos J, Koomans HA, Vos PF. Daily home haemodialysis in The Netherlands: effects on metabolic control, haemodynamics, and quality of life. Nephrol Dial Transplant. 1998;13:2853–2869. doi: 10.1093/ndt/13.11.2853. [DOI] [PubMed] [Google Scholar]
- 22.Ting GO, Kjellstrand C, Freitas T, Carrie BJ, Zarghamee S. Long-term studyof high-comorbidity ESRD patients converted from conventional to short daily hemodialysis. Am J Kidney Dis. 2003;42:1020–1035. doi: 10.1016/j.ajkd.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Williams AW, Chebrolu SB, Ing TS, et al. Early clinical, quality of life, and biochemical changes of “daily hemodialysis” (6 dialyses per week). Am J Kidney Dis. 2004;43:90–102. doi: 10.1053/j.ajkd.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberger J, Vandijk JP, Prihodova I, et al. Differences in perceived health status between kidney transplant recipients and dialyzed patietns are based mainly on the selection process. Clin Transplant. 2006;24:358–365. doi: 10.1111/j.1399-0012.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 25.Painter P, Krasnoff JB, Kuskowski M, Frassetto L, Johansen K. Effects of Modality Change and Transplant on Peak Oxygen Uptake in Patients with Kidney Failure. Am J Kidney Dis. 2011;57:113–122. doi: 10.1053/j.ajkd.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macfarlane PA. Should Patients Remain on Intensive Hemodialysis Rather than Choosing to Receive a Kidney Transplant? Seminars in Dialysisq. 2010;23:516–519. doi: 10.1111/j.1525-139X.2010.00740.x. [DOI] [PubMed] [Google Scholar]
- 27.Deoreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization and dialysis-attendance compliance. American Journal of Kidney Diseases. 1997;30:204–212. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 28.Knight E, Ofsthun N, Teng M, Lazarus JM, Curhan GC. The Association between mental health, physical function and hemodialysis mortality. Kidney International. 2003;63:1843–1851. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 29.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 30.Parkerson GR, Gutman RA. Health-related quality of life predictors of survival and hospital utilization. Health Care Financing Review. 2001;21:171–184. [PMC free article] [PubMed] [Google Scholar]


