Abstract
Most nonenhanced MRA techniques for evaluating peripheral artery disease (PAD) require cardiac synchronization through physiological gating. Electrocardiographic (ECG) gating is the most popular method for cardiac synchronization; however, it is subject to interference from switching magnetic field gradients and radiofrequency pulses. A method is described for self-gated nonenhanced MRA that does not require the use of ECG gating. Imaging was prospectively triggered by detecting the acceleration of blood flow during systole with a reference-less phase contrast navigator. The technique was implemented for non-subtractive nonenhanced MRA using quiescent-interval single-shot (QISS) MRA. The lower extremity peripheral arteries of eight healthy subjects were imaged using ECG-, pulse-, and self-gated QISS. Self-gated QISS triggered with 99% accuracy. There were no significant differences in relative contrast, contrast-to-noise ratio, or image quality between self-gated and ECG-gated QISS MRA (p > 0.05). Image quality with pulse gating was inferior.
Keywords: magnetic resonance angiography, navigator, phase contrast, quiescent-interval single-shot
INTRODUCTION
Peripheral artery disease (PAD) is a significant cause of patient morbidity and can be an indicator for systemic cardiovascular disease (1,2). Contrast-enhanced magnetic resonance angiography (CE-MRA) is routinely used for the evaluation of PAD, especially when therapeutic intervention is contemplated. However, use of gadolinium-based contrast agents is problematic in patients with renal failure due to the risk of nephrogenic systemic fibrosis (NSF)(3). Consequently, nonenhanced MRA techniques may be particularly valuable for such cases.
Current nonenhanced MRA techniques include 2D time-of-flight (TOF)(4), subtractive fast-spin-echo (FBI)(5), and non-subtractive quiescent-interval single-shot (QISS)(6). Unlike CE-MRA, nonenhanced techniques require that data acquisition be consistently synchronized to the cardiac cycle. Electrocardiographic (ECG) gating is typically used for synchronization but requires additional setup time for lead placement. Inconsistent ECG gating may occur due to rapid magnetic field gradient switching and radio-frequency pulses (7,8). ECG gating is especially problematic in patients with low QRS voltage or with poor lead contact. Although techniques such as vector gating can ensure adequate gating in most cases (9), it would be desirable to have an alternative gating technique that did not suffer these limitations. Pulse gating is easy to use but provides less precise triggering than the ECG. It is also susceptible to degradation from finger motion. Inconsistent gating may occur with either ECG or pulse gating in the setting of cardiac arrhythmias.
In this work, we sought to develop a self-gating technique that allows nonenhanced MRA to be performed without the use of ECG gating and with automated selection of trigger delays. Nonenhanced MRA was prospectively gated by detection of the onset of systolic blood flow using a reference-less phase contrast navigator module. The feasibility of the self-gated approach was tested in QISS MRA.
METHODS
Overview of Technique
The self-gated method employs a modified phase contrast navigator to synchronize the image readout with the cardiac cycle. Unlike traditional phase contrast (PC) MRA, which calculates the phase difference (i.e. velocity) from the complex subtraction of a flow-compensated “zero-phase” readout from a flow-sensitized velocity-encoded readout (10), the navigator sequence evaluated here performs pair-wise subtractions of successive velocity-encoded readouts. The pairwise difference data reflects phase changes from acceleration instead of velocity as in traditional PC.
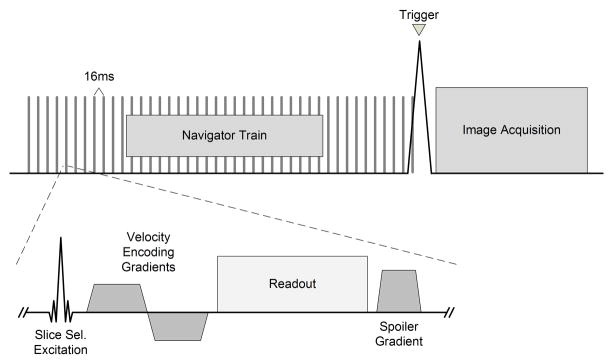
Figure 1 displays a diagram of the self-gated pulse sequence. Prior to each imaging readout, a flow-sensitized axial navigation slice was repeatedly acquired in a fixed position 10mm cephalad to the superior end of the QISS station. The navigation slice was acquired as a line scan with slice and frequency encoding only. Acceleration values were computed in real-time after each navigator acquisition as the phase difference between the prior and current projection. Noise suppression was accomplished by multiplying the acceleration values by the magnitude values and then by a magnitude threshold mask. The mask removed air noise while magnitude multiplication reduced noise in the surrounding tissues. The mask removed all values with a magnitude less than an empirically determined threshold of 15% of the maximum magnitude value in the slice. Real-time computation of acceleration values and post-processing took 6ms for each navigator acquisition. Note that due to magnitude multiplication and partial volume averaging in the non-encoded direction, the acceleration values were not accurate measurements of arterial flow acceleration, but instead were acceleration-weighted values proportional to the arterial flow acceleration.
Fig 1.
Diagram of the self-gated MRA acquisition with the navigator module pulse sequence as inset. The navigator module is repeatedly acquired until flow acceleration is detected, which triggers image acquisition.
The MRA image acquisition was triggered by the detection of acceleration peaks using two statistical thresholds. After each navigator acquisition, the maximum acceleration within the slice was compared to an upper threshold and the minimum acceleration was compared to a lower threshold. The minimum acceleration values were used to improve detection probability with the inherent aliasing in the navigator data. Image acquisition was triggered if the maximum value exceeded the pre-calculated upper threshold or the minimum value fell below the lower threshold. Both thresholds were calculated using an initial set of flow data acquired during a 5 second training period prior to the image acquisition period. This duration encompassed three to four heart beats. The upper threshold was set at two standard deviations above the mean of the maximum values while the lower threshold was set at two standard deviations below the mean of the minimum values. The entire post-processing scheme is diagrammed in Fig. 2.
Fig 2.
Flow chart of the navigator reconstruction and triggering algorithm. Mmax / Mmin and σmax/σmin are the respective means and standard deviations of the maximum/minimum acceleration values acquired during the training period. Each cycle through the flow chart corresponds to a single navigator repetition time of 16ms. The phase difference between navigator (n) and navigator (n-1) is proportional to arterial flow acceleration.
Study Details
ECG-, self-, and pulse-gated QISS MRA was acquired in five contiguous stations of 48 slices each that covered the lower peripheral arteries (foot to lower thigh) of eight healthy subjects. The stations were acquired at isocenter and the scanner was reshimmed for each. Imaging was performed on a 32-channel 1.5T scanner (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany). A phased-array peripheral coil that extends from the feet to upper thigh was used to receive the MR signal. Navigator parameters included a velocity-encoding sensitivity of 20 cm/s, flip angle of 30 degrees, TR 16 ms. QISS MRA parameters included a field of view of 400×260 mm, matrix of 400×260, flip angle of 90 degrees, 48 3 mm axial slices per station, TR/TE 1 R-R/1.43 ms, bandwidth 658 Hz/Px, GRAPPA 2 acceleration. The ECG-gated QISS delay time after R-wave detection was 100ms, while the post-trigger delay times of pulse- and self-gated QISS were both 0ms.
Navigator Parameter Optimization
Prior to the study, navigator velocity encoding and flip angle parameters were optimized in a single volunteer. The optimal parameters were chosen based on combinations that produced maximum navigator signal-to-noise ratio (SNR). The navigator was continuously acquired over fifteen heart beats, where the maximum acceleration value from each acquired slice was collected. Using these values SNR was calculated as Save/σ, where Save is the average of the values at the acceleration peaks minus the average all values, and σ is the standard deviation of all values between the acceleration peaks. In addition, the navigator flip angle was minimized to reduce saturation effects during imaging.
Evaluation
The effectiveness of noise removal in the navigator post-processing scheme was evaluated based on improvements in triggering accuracy and navigator SNR using flow data from the eight subjects. The triggering accuracy was calculated as Ta/Ttot * 100%, where Ta is the number of accurate detections of the systolic flow upslope and Ttot is total number of triggers.
Quantitative and qualitative evaluations were made comparing QISS MRA images utilizing the three different gating schemes. Relative contrast and apparent contrast-to-noise ratios (CNR) were calculated from measurements made on axial source images at the left/right popliteal artery, left/right anterior tibial artery and left/right posterior tibial artery for all three methods using region-of-interest tools from public domain software (ImageJ, National Institutes of Health, Bethesda, MD). Relative contrast and apparent CNR values were averaged between left/right locations and were calculated as Sa/Sb - 1 and (Sa – Sb)/σb respectively, where Sa was the mean of the arterial signal, Sb was the mean of neighboring background signal, and σb was the standard deviation of the background signal. Measurement locations for the popliteal and anterior/posterior tibial arteries were 6 cm proximal and 1.5 cm distal to the arterial trifurcation respectively. Image quality was evaluated for the diagnostic display of arteries using a five point Likert scale (0 = Nondiagnostic, 1 = Poor quality and observer not confident, 2 = Fair quality and observer marginally confident, 3 = Good quality and observer confident, 4 = Excellent quality and observer highly confident) by a licensed radiologist with more than 10 years of experience reading MR angiograms. Coronal maximum intensity projections were performed on the axial source images and were randomized prior to qualitative evaluation. Statistical significance was determined with ANOVA and subsequent Tukey tests (p < 0.05).
RESULTS
Table 1 displays the navigator parameter optimization results. A velocity encoding of 20 cm/s and a flip angle of 90 degrees produced the maximum navigator SNR, however the flip angle was lowered to 30 degrees for all image acquisitions to minimize saturation effects on the QISS images by the navigator excitation. Although aliasing was observed at a velocity encoding of 20 cm/s, this value was necessary to sensitize to the small velocity changes between navigator TR’s associated with flow acceleration during systole. Furthermore aliasing did not detract from triggering accuracy because detection occurred early on the acceleration curve, prior to the aliased section at high velocities.
Table I.
Navigator Parameter Optimization
| VENCa | SNRc | FAb | SNRc |
|---|---|---|---|
| 130 | 5 | 90 | 23 |
| 100 | 5 | 80 | 19 |
| 90 | 3 | 70 | 16 |
| 80 | 7 | 60 | 15 |
| 70 | 7 | 50 | 12 |
| 60 | 9 | 40 | 11 |
| 50 | 6 | 30 | 8 |
| 40 | 13 | 10 | 7 |
| 30 | 28 | 5 | 3 |
| 25 | 24 | ||
| 20 | 39 | ||
| 15 | 36 | ||
| 10 | 23 | ||
| 5 | 28 |
VENC = velocity encoding (cm/s), FA = flip angle (degrees), SNR = signal-to-noise ratio
Performed with FA 30
Performed with VENC 50
Columns were derived from distinct measurements; small differences between them reflect measurement variability
Prior to noise removal, air noise dominated the flow data of the eight subjects resulting in a triggering accuracy of 0% with an SNR of 1. After applying the magnitude threshold mask, SNR increased to 6 and the navigator triggered with 55% accuracy. Subsequent multiplication by the magnitude values further increased SNR and triggering accuracy to 19 and 99% respectively.
ECG-, self-, and pulse-gated QISS images in a single subject are displayed in Fig. 3. In this figure, the ECG- and self-gated images appeared almost identical however select areas of vasculature in the self-gated image showed signal loss, with 13% less CNR and 17% less contrast than the ECG-gated image. The pulse-gated QISS image showed complete signal loss at multiple locations due to frequent misgating or delayed gating. The qualitative results are presented in Table 2, and revealed that there was no significant difference between the diagnostic display ratings of self- and ECG-gated QISS images. Self-gated QISS produced images with 11% less contrast and 9% less CNR than ECG-gated QISS at all measurement locations, yet there was no significant difference between the two (Fig. 4). Pulse-gated QISS contrast and CNR values were about 50% lower than ECG- and self-gated QISS values, showing significant difference from both. The navigator, ECG, and pulse signal traces in a healthy subject are displayed in Fig. 5, where it can be seen that navigator triggering and pulse triggering occurred approximately 250ms and 700ms after the R-wave respectively.
Fig 3.
ECG-, self-, and pulse-gated QISS MR angiograms in a healthy subject. ECG- and self-gated angiograms appear similar and without artifact. The pulse-gated QISS angiogram displays numerous artifacts caused by synchronization of image acquisition to a suboptimal phase of the cardiac cycle.
Table II.
Qualitative Results
Methods were evaluated on a 0–4 Likert scale by a licensed radiologist with more than 10 years experience reading MR angiograms
Scores for each method were averaged over eight subjects, with error shown as standard deviation
No significant difference was found between these methods (p > 0.05)
Fig 4.
Relative contrast and apparent CNR measurements of ECG-, self-, and pulse-gated QISS MRA at three locations. Each column represents average measurements over eight healthy subjects and the left and right sides of the arterial anatomy. Error is shown as standard deviation. Asterisks indicate no significant difference (p > 0.05).
Fig 5.
Navigator, ECG, and pulse signals displayed as a function of time. The y-axis is arbitrary. The ECG trace is shown in blue. The pulse trace (black) was captured using a pulse oximeter on the index finger. The navigator trace (red) represents maximum projected acceleration in a slice acquired at the popliteal artery. Colored squares represent trigger points for each trace.
DISCUSSION AND CONCLUSION
We have demonstrated the feasibility of using self-gating for non-subtractive nonenhanced MRA. Self-gated sequences utilizing prospective (11) and retrospective (12,13) gating have been previously proposed for cardiac cine MRI. In the prospective approach, Vasanawala et al. used polarity flipping of the flow-sensitizing gradients to acquire phase data samples that yielded time-dependent blood flow velocity upon subtraction. Acceleration-based PC methods may be advantageous over velocity-based methods because they detect the initial upslope of the flow profile (and hence onset of systole) rather than the peak velocity. At least for the QISS sequence, we can speculate that triggering on the systolic upslope, rather than the systolic peak, provides superior image contrast because it allows for earlier triggering and potentiates greater arterial inflow into the imaging slice during systole.
The use of a reference-less PC scheme may be advantageous because temporal resolution is doubled compared with traditional flow-rephased/flow-encoded approaches for PC. Moreover, every navigator repetition employs identical flow-sensitizing gradients. Since the gradient waveforms are unchanged over the entire navigator acquisition cycle, phase errors caused by gradient-induced eddy currents (14) are minimized compared with the traditional flow-rephased/flow-encoded approach where the gradient “flipping” required to extract velocity information produces different patterns of phase errors across the FOV that do not cancel during subtraction. An additional phase correction step is normally required to reduce these phase errors (15).
The cause of the reduced image quality in self-gated QISS when compared with ECG-gating may be related to downstream flow of partly saturated spins from the navigator slice. Although this effect was ameliorated by lowering the navigator flip angle to 30 degrees, it was still present, however it did not appreciably affect overall diagnostic image quality.
Low navigator SNR due to noise can produce mistriggering; therefore noise removal methods were implemented to improve SNR. A significant improvement in SNR and triggering accuracy was seen after magnitude masking and subsequent multiplication. The magnitude mask removed noise within air-bearing voxels and increased SNR; however, noise remained in regions of tissue exhibiting substantial saturation by the navigator excitation. Direct multiplication by the magnitude values greatly reduced noise in these regions and ultimately raised the triggering accuracy to 99%.
As currently implemented this technique has several potential limitations. First, being flow dependent, the navigator sequence may mistrigger if flow pulsatility is diminished or absent. Second, as mentioned above, saturation of blood by the navigator excitation leads to intravascular signal loss in the images. This effect was reduced by lowering the navigator flip angle and may be reduced further placing the navigator slice below the imaging slice. Also, being acceleration based, the navigator might trigger off the systolic downslope if the upslope signal is below the triggering threshold. Finally, it should be noted that the navigator parameters were optimized only in healthy subjects. Additional optimization will be required for robust performance in patients with abnormal flow patterns due to peripheral artery disease.
In conclusion, we have demonstrated the feasibility of a prospective, acceleration-based PC navigator for nonenhanced MRA. In the future, we plan to improve triggering accuracy by employing more sophisticated post-processing algorithms, and will perform clinical studies to determine the robustness of the approach in patients with PAD.
Acknowledgments
This work was supported in part by NIH R01HL096916 and a grant from the Grainger Foundation.
References
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 2.Heald CL, Fowkes FG, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis. 2006;189(1):61–69. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 4.Keller PJ, Drayer BP, Fram EK, Williams KD, Dumoulin CL, Souza SP. MR angiography with two-dimensional acquisition and three-dimensional display. Work in progress Radiology. 1989;173(2):527–532. doi: 10.1148/radiology.173.2.2798885. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki M, Sugiura S, Tateishi F, Wada H, Kassai Y, Abe H. Non-contrast-enhanced MR angiography using 3D ECG-synchronized half-Fourier fast spin echo. J Magn Reson Imaging. 2000;12(5):776–783. doi: 10.1002/1522-2586(200011)12:5<776::aid-jmri17>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Edelman RR, Sheehan JJ, Dunkle E, Schindler N, Carr J, Koktzoglou I. Quiescent-interval single-shot unenhanced magnetic resonance angiography of peripheral vascular disease: Technical considerations and clinical feasibility. Magn Reson Med. 63(4):951–958. doi: 10.1002/mrm.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felblinger J, Lehmann C, Boesch C. Electrocardiogram acquisition during MR examinations for patient monitoring and sequence triggering. Magn Reson Med. 1994;32(4):523–529. doi: 10.1002/mrm.1910320416. [DOI] [PubMed] [Google Scholar]
- 8.Polson MJ, Barker AT, Gardiner S. The effect of rapid rise-time magnetic fields on the ECG of the rat. Clin Phys Physiol Meas. 1982;3(3):231–234. doi: 10.1088/0143-0815/3/3/008. [DOI] [PubMed] [Google Scholar]
- 9.Felblinger J, Slotboom J, Kreis R, Jung B, Boesch C. Restoration of electrophysiological signals distorted by inductive effects of magnetic field gradients during MR sequences. Magn Reson Med. 1999;41(4):715–721. doi: 10.1002/(sici)1522-2594(199904)41:4<715::aid-mrm9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Walker MF, Souza SP, Dumoulin CL. Quantitative flow measurement in phase contrast MR angiography. J Comput Assist Tomogr. 1988;12(2):304–313. doi: 10.1097/00004728-198803000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Vasanawala SS, Sachs TS, Brittain JH, Meyer CH, Nishimura DG. Prospective MR signal-based cardiac triggering. Magn Reson Med. 1999;42(1):82–86. doi: 10.1002/(sici)1522-2594(199907)42:1<82::aid-mrm12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med. 2004;51(1):93–102. doi: 10.1002/mrm.10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowe ME, Larson AC, Zhang Q, Carr J, White RD, Li D, Simonetti OP. Automated rectilinear self-gated cardiac cine imaging. Magn Reson Med. 2004;52(4):782–788. doi: 10.1002/mrm.20212. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein MA, Zhou XJ, Polzin JA, King KF, Ganin A, Pelc NJ, Glover GH. Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med. 1998;39(2):300–308. doi: 10.1002/mrm.1910390218. [DOI] [PubMed] [Google Scholar]
- 15.Lankhaar JW, Hofman MB, Marcus JT, Zwanenburg JJ, Faes TJ, Vonk-Noordegraaf A. Correction of phase offset errors in main pulmonary artery flow quantification. J Magn Reson Imaging. 2005;22(1):73–79. doi: 10.1002/jmri.20361. [DOI] [PubMed] [Google Scholar]