Abstract
Background
Although metabolic risk factors are known to cluster in individuals who are prone to developing diabetes and cardiovascular disease, the underlying biological mechanisms remain poorly understood.
Methods and Results
To identify pathways associated with cardiometabolic risk, we used liquid chromatography/mass spectrometry to determine the plasma concentrations of 45 distinct metabolites and examine their relation to cardiometabolic risk in the Framingham Heart Study (FHS; N=1015) and the Malmö Diet and Cancer Study (MDC; N=746). We then interrogated significant findings in experimental models of cardiovascular and metabolic disease. We observed that metabolic risk factors (obesity, insulin resistance, high blood pressure, dyslipidemia) were associated with multiple metabolites including branched-chain amino acids, other hydrophobic amino acids, tryptophan breakdown products, and nucleotide metabolites. We observed strong associations of insulin resistance traits with glutamine (standardized regression coefficients −0.04 to −0.22, per 1-SD change in log-glutamine, P<0.001), glutamate (0.05 to 0.14, P<0.001), and glutamine-glutamate ratio (−0.05 to −0.20, P<0.001) in the discovery sample (FHS); similar associations were observed in the replication sample (MDC). High glutamine-glutamate ratio was associated with lower risk of incident diabetes in FHS (OR 0.79; adjusted P=0.03), but not in MDC. In experimental models, administration of glutamine in mice led to both increased glucose tolerance (P=0.01) and to lower blood pressure (P<0.05).
Conclusions
Biochemical profiling identified circulating metabolites not previously associated with metabolic traits. Experimentally interrogating one of these pathways demonstrated that excess glutamine relative to glutamate, resulting from exogenous administration, is associated with reduced metabolic risk in mice.
Keywords: epidemiology, metabolic syndrome, risk factors, metabolomics
Introduction
Certain clinical risk factors are known to cluster in individuals who are prone to developing diabetes and future cardiovascular events.1 These risk factors are generally associated with insulin resistance and include abdominal obesity, elevated fasting glucose levels, hypertension, and dyslipidemia. The term ‘metabolic syndrome’ has been used to describe a clustering of these traits among individuals.2–4 The prevalence of the metabolic syndrome is high, affecting up to 25% of adults in the United States, and is increasing.5
Pathways underlying metabolic risk factors in humans remain unknown, but are likely related to derangements in primary metabolism.6 Recent advances in liquid chromatography tandem mass spectrometry (LC/MS) allow the acquisition of high-throughput profiles of the metabolic status of whole organisms (e.g. metabolomics), providing a comprehensive assessment of molecules that are substrates or products of metabolic pathways.7–11 Performing metabolite profiling in individuals with metabolic disorders could elucidate potential roles for specific metabolites in the development of metabolic disease and its sequelae.12–15 Therefore, we performed metabolite profiling in two community-based cohorts comprising individuals spanning a spectrum of metabolic risk. We then performed focused experimental studies in an animal model to test whether interventions aimed at modifying an adverse metabolite profile could attenuate manifestations of metabolic disease.
Methods
Human Study Samples
All human study protocols were approved by the Institutional Review Boards of Boston University Medical Center, Massachusetts General Hospital, and Lund University, Sweden. All study participants provided written informed consent. The Framingham Heart Study (FHS) Offspring cohort was formed in 1971, with the enrollment of 5,124 individuals into a community-based longitudinal cohort study.16 Of the 2,413 attendees who were free of diabetes and cardiovascular disease (CVD) at the 5th examination cycle (1991 through 1995), 650 had metabolite profiling performed as part of 2 nested case-control studies designed to investigate predictors of diabetes and cardiovascular disease; an additional 365 randomly-selected individuals also had metabolite profiling performed.17 Thus, 1,015 individuals were eligible for the current analyses.
Findings in FHS were examined for replication in the Malmö Diet and Cancer Study (MDC), an investigation of 6,103 individuals who were originally enrolled between 1991 and 1996 as part of a longitudinal population-based epidemiologic cohort. Of the 4,577 participants who had complete covariate data and were free of diabetes and CVD at the original examination, 746 had metabolite profiling performed as part of a nested case-control study modeled after that in FHS; these individuals make up the replication sample for the present analyses.
Clinical and Dietary Assessment
All FHS and MDC participants underwent a baseline examination in addition to longitudinal surveillance for incident diabetes, as described in the Supplemental Methods.
Metabolite Profiling
For blood specimens collected from the FHS and MDC study participants, profiles of plasma metabolites were obtained, as previously described17 (see Supplemental Methods for details).
Murine Studies
Weight-matched C57Bl6 mice were obtained at 7 weeks of age from Jackson Laboratories (Bar Harbor, ME) and were housed in pairs with free access to water. Details regarding the experimental models of glucose tolerance and blood pressure response are provided in the Supplemental Methods.
Statistical Analyses
All metabolite values were natural logarithmically transformed, due to their non-normal distribution, and then standardized (to mean=0, standard deviation=1) within each cohort (FHS and MDC). Age- and sex-adjusted Pearson correlation coefficients were estimated to determine correlations between metabolites known to cluster within well-defined groups (e.g. amino acids, urea cycle metabolites, etc.) in each study sample. Regression analyses were performed in each study sample to examine the relation of each metabolite (predictor variable) with each clinical metabolic traits (response variables): BMI, waist circumference (WC), fasting glucose, log fasting insulin, log HOMA, systolic blood pressure (SBP), diastolic blood pressure (DBP), log triglycerides, and high-density lipoprotein (HDL) cholesterol. We analyzed each trait against each metabolite with individual regressions, adjusting for sex and age. In secondary analyses, we adjusted for BMI in addition to age and sex. Regression analyses were performed using mixed linear models18 that accounted for the sampling algorithm. Given the 45 metabolites analyzed, we employed a Bonferonni-corrected P value threshold of 0.001 (=0.05/45) to account for the number of metabolites analyzed. Because the majority of metabolites were correlated within well-defined biological groups (e.g. amino acids, urea cycle metabolites, etc.), this correction was conservative.
We estimated statistical power to detect associations in the FHS and MDC samples. At the specified significance threshold, 0.001, we had power >80% to detect association of a trait with a metabolite if the true partial correlation is at least 0.129 (FHS) or 0.151 (MDC).
In tertiary analyses, we analyzed the relation of select metabolites (based on cross-sectional analyses results) with risk of future diabetes in the FHS sample of individuals who were identified as diabetes cases or randomly selected controls (N=601), all of whom were free of diabetes at baseline. These analyses were performed using logistic regression models adjusting for age, sex, BMI, and baseline fasting glucose.
Analyses of data from the experimental models of glucose tolerance and blood pressure response are described in the Supplemental Methods.
All analyses were performed using SAS software version 9.1.3 (SAS Institute, Cary, NC).
Results
Characteristics of the human study sample, including participants of the Framingham Heart Study (FHS) and Malmö Diet and Cancer study (MDC), are shown in Table 1 and Supplemental Table 1, respectively. In FHS, the prevalence of obesity (body mass index [BMI] ≥30 kg/m2) was 31%, and 45% of individuals met criteria for metabolic syndrome. In the FHS sample, mean Pearson correlations within groups of related analytes were moderate to high for branched-chain amino acids (age- and sex-adjusted r=0.82), the larger group of hydrophobic amino acids (r=0.45), and urea cycle metabolites (r=0.46).
Table 1.
Characteristics of the FHS Study Participants
| FHS (N=1,015) |
|
|---|---|
| Age, years | 57±9 |
| Women, % | 47 |
| Body mass index, kg/m2 | 28.3±5.0 |
| Waist circumference, cm | 95.6±13.8 |
| Systolic blood pressure, mmHg | 129±18 |
| Diastolic blood pressure, mmHg | 76±10 |
| Hypertension, % | 41 |
| Serum triglycerides, mg/dL | 156±105 |
| Serum HDL, mg/dL | 48±14 |
| Fasting glucose, mg/dL | 98±10 |
| Fasting insulin, uU/mL | 10±8 |
| Metabolic syndrome, % | 45 |
| HOMA | 2.5±2.2 |
| Insulin resistance, %* | 33 |
FHS, Framingham Heart Study; HOMA, homeostasis model assessment of insulin resistance.
Values are shown as means ± standard deviation or percentages.
Defined as having HOMA value >75th percentile of the derivation sample.
Analytes Associated With Metabolic Traits
In regression analyses adjusted for age and sex (Figure 1 and Table 2), multiple analytes demonstrated significant associations (P<0.001) with three or more categories of clinical metabolic traits, including body size (BMI or waist circumference), glucose and insulin metabolism (fasting glucose, insulin, or HOMA), systolic and diastolic blood pressure (SBP or DBP), and lipid abnormalities (high-density lipoprotein [HDL] cholesterol or triglycerides). Individuals with metabolic traits had highly significant elevations in the branched-chain amino acids (leucine, isoleucine, and valine) and other hydrophobic amino acids, including alanine and the aromatic amino acids (phenylalanine and tyrosine). We identified additional metabolites not previously associated with an adverse metabolic profile, including tryptophan breakdown products (kynurenic acid, anthranillic acid) and nucleotide metabolites (xanthosine, n-carbamoyl-β-alanine). Notably, the majority of these analytes were not correlated with fasting glucose.
Figure 1.
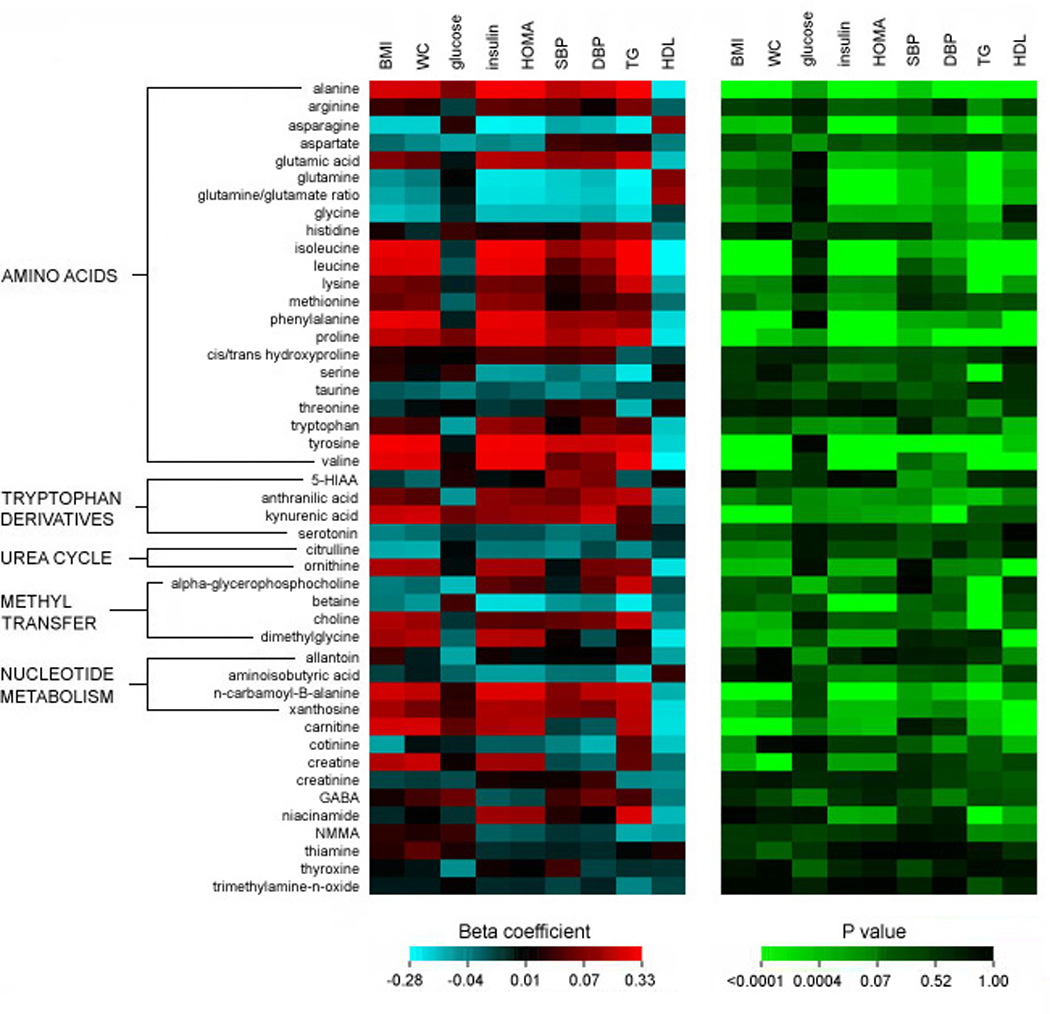
Beta coefficient and P values generated from age- and sex-adjusted regression analyses of the relation of each metabolite (standardized and log transformed) with insulin resistance phenotypes and metabolic traits (standardized) in the FHS sample.
Table 2.
Associations of Metabolites with Insulin Resistance Phenotypes and Metabolic Traits in FHS
| Regression Coefficient (Standard Error)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI | WC | Glucose | Insulin | HOMA | SBP | DBP | Log TG | HDL | |
| 5-HIAA | −0.01 (0.04) | −0.03 (0.03) | 0.02 (0.03) | 0.00 (0.04) | 0.01 (0.04) | 0.09 (0.03)† | 0.07 (0.04)† | −0.02 (0.04) | 0.02 (0.03) |
| alanine | 0.16 (0.03)‖ | 0.15 (0.03)‖ | 0.07 (0.03)‡ | 0.25 (0.03)‖ | 0.25 (0.03)‖ | 0.11 (0.03)§ | 0.14 (0.03)‖ | 0.33 (0.03)‖ | −0.17 (0.03)‖ |
| allantoin | 0.03 (0.03) | 0.00 (0.03) | −0.07 (0.03)† | 0.02 (0.03) | 0.01 (0.03) | 0.01 (0.03) | 0.01 (0.03) | 0.02 (0.03) | −0.06 (0.03)† |
| a-glycerophosphocholine | −0.04 (0.03) | −0.03 (0.03) | −0.08 (0.02)§ | 0.05 (0.03) | 0.03 (0.03) | 0.00 (0.03) | 0.05 (0.03) | 0.14 (0.03)‖ | −0.02 (0.03) |
| aminoisobutyric acid | −0.01 (0.03) | 0.00 (0.03) | −0.03 (0.03) | −0.06 (0.03) | −0.06 (0.03) | −0.04 (0.03) | −0.03 (0.03) | −0.10 (0.03)‡ | 0.02 (0.03) |
| anthranilic acid | 0.06 (0.03) | 0.04 (0.03) | −0.05 (0.02)† | 0.08 (0.03)‡ | 0.07 (0.03)† | 0.06 (0.03)† | 0.10 (0.03)‡ | 0.11 (0.03)§ | −0.06 (0.03) |
| arginine | 0.03 (0.03) | 0.02 (0.03) | −0.01 (0.03) | 0.05 (0.03) | 0.05 (0.03) | 0.04 (0.03) | 0.01 (0.03) | 0.07 (0.03)† | −0.03 (0.03) |
| asparagine | −0.10 (0.03)§ | −0.10 (0.03)§ | 0.02 (0.02) | −0.22 (0.03)‖ | −0.20 (0.03)‖ | −0.07 (0.03)† | −0.08 (0.03)† | −0.19 (0.03)‖ | 0.08 (0.03)‡ |
| aspartate | −0.03 (0.03) | −0.04 (0.03) | −0.07 (0.03)† | −0.04 (0.03) | −0.05 (0.03) | 0.03 (0.03) | 0.02 (0.03) | 0.02 (0.03) | −0.04 (0.03) |
| betaine | −0.04 (0.03) | −0.05 (0.03) | 0.03 (0.03) | −0.14 (0.03)‖ | −0.14 (0.03)‖ | −0.05 (0.03) | −0.04 (0.03) | −0.19 (0.03)‖ | −0.03 (0.03) |
| carnitine | 0.15 (0.03)‖ | 0.16 (0.03)‖ | 0.05 (0.03) | 0.10 (0.03)‡ | 0.11 (0.03)§ | −0.01 (0.03) | −0.02 (0.03) | 0.11 (0.03)§ | −0.15 (0.03)‖ |
| choline | 0.10 (0.03)§ | 0.09 (0.03)‡ | −0.01 (0.03) | 0.05 (0.03) | 0.04 (0.03) | 0.05 (0.03) | 0.06 (0.03)† | 0.13 (0.03)‖ | −0.05 (0.03) |
| cis/trans hydroxyproline | 0.02 (0.03) | 0.01 (0.03) | 0.01 (0.02) | 0.04 (0.03) | 0.04 (0.03) | 0.03 (0.03) | 0.04 (0.03) | −0.02 (0.03) | −0.01 (0.03) |
| citrulline | −0.07 (0.03)† | −0.07 (0.03)† | 0.01 (0.03) | −0.04 (0.03) | −0.04 (0.03) | −0.04 (0.03) | −0.01 (0.03) | −0.04 (0.03) | −0.01 (0.03) |
| cotinine | −0.07 (0.03)† | 0.00 (0.03) | 0.00 (0.02) | −0.02 (0.03) | −0.02 (0.03) | −0.04 (0.03) | −0.08 (0.03)‡ | 0.05 (0.03) | −0.09 (0.03)‡ |
| creatine | 0.11 (0.03)‡ | 0.14 (0.03)‖ | 0.01 (0.03) | 0.10 (0.03)‡ | 0.09 (0.03)‡ | −0.02 (0.03) | −0.03 (0.03) | 0.05 (0.04) | −0.03 (0.03) |
| creatinine | −0.01 (0.03) | −0.01 (0.03) | −0.02 (0.03) | 0.02 (0.03) | 0.01 (0.03) | 0.01 (0.03) | 0.03 (0.03) | −0.04 (0.04) | −0.05 (0.03) |
| dimethylglycine | 0.10 (0.03)‡ | 0.11 (0.03)§ | −0.03 (0.03) | 0.11 (0.03)§ | 0.10 (0.03)‡ | 0.01 (0.03) | −0.02 (0.03) | 0.02 (0.03) | −0.17 (0.03)‖ |
| GABA | 0.02 (0.03) | 0.03 (0.03) | 0.06 (0.03)† | −0.02 (0.03) | −0.01 (0.03) | 0.03 (0.03) | 0.06 (0.03) | 0.04 (0.03) | −0.04 (0.03) |
| glycine | −0.09 (0.03)‡ | −0.07 (0.03)† | 0.00 (0.03) | −0.09 (0.03)‡ | −0.09 (0.03)‡ | −0.09 (0.03)‡ | −0.07 (0.03)† | −0.12 (0.03)§ | −0.01 (0.03) |
| histidine | 0.02 (0.03) | 0.00 (0.03) | 0.03 (0.03) | 0.02 (0.03) | 0.02 (0.03) | 0.02 (0.03) | 0.07 (0.03)† | 0.08 (0.03)‡ | −0.04 (0.03) |
| isoleucine | 0.22 (0.03)‖ | 0.21 (0.03)‖ | −0.01 (0.03) | 0.26 (0.03)‖ | 0.25 (0.03)‖ | 0.09 (0.03)‡ | 0.11 (0.03)‡ | 0.29 (0.03)‖ | −0.28 (0.03)‖ |
| kynurenic acid | 0.13 (0.03)‖ | 0.14 (0.03)‖ | 0.05 (0.03)† | 0.08 (0.03)‡ | 0.09 (0.03)‡ | 0.09 (0.03)‡ | 0.14 (0.03)‖ | 0.04 (0.03) | −0.04 (0.03) |
| leucine | 0.19 (0.03)‖ | 0.19 (0.03)‖ | −0.02 (0.03) | 0.23 (0.03)‖ | 0.22 (0.03)‖ | 0.04 (0.03) | 0.08 (0.03)† | 0.27 (0.03)‖ | −0.25 (0.03)‖ |
| lysine | 0.07 (0.03)† | 0.06 (0.03) | 0.00 (0.03) | 0.06 (0.03) | 0.06 (0.03) | 0.02 (0.03) | 0.04 (0.03) | 0.14 (0.03)‖ | −0.07 (0.03)† |
| methionine | 0.05 (0.03) | 0.07 (0.03)† | −0.03 (0.03) | 0.08 (0.03)‡ | 0.08 (0.03)† | 0.01 (0.03) | 0.03 (0.03) | 0.04 (0.03) | −0.03 (0.03) |
| N-carbomoyl-beta-alanine | 0.15 (0.03)‖ | 0.12 (0.03)‖ | 0.02 (0.03) | 0.19 (0.03)‖ | 0.19 (0.03)‖ | 0.08 (0.03)‡ | 0.11 (0.03)§ | 0.13 (0.03)‖ | −0.08 (0.03)‡ |
| niacinamide | 0.00 (0.03) | 0.01 (0.03) | −0.01 (0.03) | 0.09 (0.03)‡ | 0.09 (0.03)‡ | 0.02 (0.03) | 0.01 (0.03) | 0.18 (0.03)‖ | −0.08 (0.03)‡ |
| NMMA | 0.02 (0.03) | 0.02 (0.03) | 0.03 (0.03) | −0.02 (0.03) | −0.02 (0.03) | −0.01 (0.03) | −0.01 (0.03) | −0.07 (0.03)† | −0.05 (0.03) |
| ornithine | 0.10 (0.03)§ | 0.10 (0.03)§ | 0.00 (0.03) | 0.10 (0.03)‡ | 0.10 (0.03)‡ | 0.00 (0.03) | 0.06 (0.03) | 0.07 (0.03)† | −0.16 (0.03)‖ |
| phenylalanine | 0.23 (0.03)‖ | 0.21 (0.03)‖ | 0.00 (0.03) | 0.21 (0.03)‖ | 0.21 (0.03)‖ | 0.09 (0.03)‡ | 0.09 (0.03)‡ | 0.08 (0.03)† | −0.13 (0.03)‖ |
| proline | 0.12 (0.03)‖ | 0.11 (0.03)§ | 0.06 (0.03)† | 0.19 (0.03)‖ | 0.19 (0.03)‖ | 0.10 (0.03)§ | 0.12 (0.03)‖ | 0.17 (0.03)‖ | −0.17 (0.03)‖ |
| serine | 0.02 (0.03) | 0.01 (0.03) | 0.02 (0.03) | −0.06 (0.03)† | −0.06 (0.03) | −0.03 (0.03) | −0.04 (0.03) | −0.17 (0.03)‖ | 0.02 (0.03) |
| serotonin | −0.04 (0.03) | −0.03 (0.03) | −0.01 (0.02) | −0.02 (0.03) | −0.02 (0.03) | −0.04 (0.03) | −0.03 (0.03) | 0.03 (0.03) | 0.00 (0.03) |
| taurine | −0.02 (0.03) | −0.03 (0.03) | −0.04 (0.03) | −0.02 (0.03) | −0.02 (0.03) | −0.04 (0.03) | −0.04 (0.03) | −0.02 (0.03) | −0.02 (0.03) |
| thiamine | 0.02 (0.03) | 0.05 (0.03) | 0.02 (0.02) | −0.01 (0.03) | 0.00 (0.03) | 0.00 (0.03) | 0.00 (0.03) | 0.01 (0.03) | 0.02 (0.03) |
| threonine | −0.01 (0.03) | 0.01 (0.03) | 0.01 (0.02) | −0.01 (0.03) | 0.00 (0.03) | 0.02 (0.03) | 0.03 (0.03) | −0.08 (0.03)‡ | 0.02 (0.03) |
| thyroxine | 0.01 (0.03) | 0.00 (0.03) | −0.05 (0.03) | 0.02 (0.03) | 0.01 (0.03) | 0.03 (0.03) | −0.01 (0.03) | 0.00 (0.03) | −0.01 (0.03) |
| trimethylamine-N-oxide | 0.00 (0.03) | 0.00 (0.03) | 0.01 (0.03) | 0.00 (0.03) | 0.00 (0.03) | −0.01 (0.03) | 0.00 (0.03) | −0.04 (0.03) | −0.01 (0.03) |
| tryptophan | 0.04 (0.03) | 0.03 (0.03) | −0.06 (0.03)† | 0.09 (0.03)‡ | 0.08 (0.03)† | 0.01 (0.03) | 0.05 (0.03) | 0.04 (0.03) | −0.10 (0.03)‡ |
| tyrosine | 0.26 (0.03)‖ | 0.24 (0.03)‖ | 0.00 (0.03) | 0.25 (0.03)‖ | 0.24 (0.03)‖ | 0.14 (0.03)† | 0.14 (0.03)‖ | 0.18 (0.03)‖ | −0.11 (0.03)§ |
| valine | 0.21 (0.03)‖ | 0.20 (0.03)‖ | 0.02 (0.03) | 0.24 (0.03)‖ | 0.23 (0.03)‖ | 0.05 (0.03) | 0.07 (0.03)† | 0.23 (0.03)‖ | −0.24 (0.03)‖ |
| xanthosine | 0.10 (0.03)‡ | 0.07 (0.03)† | 0.02 (0.03) | 0.10 (0.03)§ | 0.10 (0.03)§ | 0.07 (0.03)† | 0.06 (0.03) | 0.12 (0.03)§ | −0.15 (0.03)‖ |
| glutamine/isoleucine ratio | −0.10 (0.03)‡ | −0.09 (0.03)‡ | 0.01 (0.02) | −0.22 (0.03)‖ | −0.21 (0.03)‖ | −0.12 (0.03)† | −0.11 (0.03)§ | −0.27 (0.03)‖ | 0.14 (0.03)‖ |
| glutamine/leucine ratio | −0.09 (0.03)‡ | −0.08 (0.03)‡ | 0.01 (0.02) | −0.21 (0.03)‖ | −0.20 (0.03)‖ | −0.11 (0.03)§ | −0.10 (0.03)‡ | −0.27 (0.03)‖ | 0.13 (0.03)‖ |
| glutamine/valine ratio | −0.09 (0.03)‡ | −0.08 (0.03)‡ | 0.00 (0.02) | −0.21 (0.03)‖ | −0.21 (0.03)‖ | −0.11 (0.03)† | −0.10 (0.03)‡ | −0.26 (0.03)‖ | 0.12 (0.03)‖ |
BMI, body mass index; WC, waist circumference; HOMA, homeostasis model assessment of insulin resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; HDL, high-density lipoprotein cholesterol.
Regression coefficients represent the standardized change in dependent variable per 1-SD change in the log-transformed metabolite. For example, a 1-SD increment in log alanine was associated with a 0.25 SD change in insulin, or 2 uU/mL. Separate regressions were fitted for each combination of trait (response) and metabolite (predictor), adjusted for age and sex.
P<0.05;
P<0.01;
P<0.001;
P<0.0001
After adjustment for BMI in addition to age and sex, several metabolites remained significantly associated with multiple metabolic traits including blood pressure. These metabolites included glutamine, glutamate, proline (a derivative of glutamate), and several hydrophobic amino acids (alanine, tyrosine). The hydrophobic amino acids have been linked to insulin resistance in prior studies, as has the metabolite glutamate.19 By contrast, the association of glutamine with multiple metabolic risk factors in humans has not been reported previously. After further adjustment for BMI, in addition to age and sex, glutamine was inversely associated with insulin, HOMA, SBP, DBP, and log triglycerides, and positively associated with HDL (Table 3; P<0.0001 to P=0.008). Conversely, glutamate was positively associated with insulin, HOMA, SBP, DBP, and log triglycerides, and inversely associated with HDL (P<0.0001 to P=0.024). Since glutamine and glutamate are in a precursor-product relationship, we also examined the glutamine-to-glutamate ratio (Supplemental Figures 1–3). In age- and sex-adjusted analyses, the glutamine-to-glutamate ratio was inversely associated with BMI, insulin, HOMA, SBP, DBP, and log triglycerides, and positively associated with HDL (Table 3; P<0.0001 to P=0.024).
Table 3.
Associations of Select Metabolites with Insulin Resistance Phenotypes and Metabolic Traits in FHS
| Regression Coefficient (P value)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI | WC | Glucose | Insulin | HOMA | SBP | DBP | Log TG | HDL | |
| Models adjusting for age and sex | |||||||||
| glutamate | 0.08 (0.011) | 0.05 (0.07) | 0.00 (0.90) | 0.11 (0.0004) | 0.10 (0.0006) | 0.08 (0.003) | 0.09 (0.005) | 0.14 (<0.0001) | −0.09 (0.001) |
| glutamine | −0.05 (0.09) | −0.04 (0.16) | 0.01 (0.78) | −0.17 (<0.0001) | −0.16 (<0.0001) | −0.10 (0.0003) | −0.08 (0.005) | −0.22 (<0.0001) | 0.08 (0.008) |
| glutamine/glutamate ratio | −0.07 (0.024) | −0.05 (0.08) | 0.00 (0.91) | −0.15 (<0.0001) | −0.15 (<0.0001) | −0.10 (0.0003) | −0.09 (0.002) | −0.20 (<0.0001) | 0.09 (0.002) |
| Models adjusting for age, sex, and BMI | |||||||||
| glutamate | — | −0.01 (0.54) | −0.01 (0.84) | 0.07 (0.009) | 0.07 (0.011) | 0.07 (0.016) | 0.07 (0.024) | 0.13 (<0.0001) | −0.08 (0.008) |
| glutamine | — | 0.00 (0.86) | 0.01 (0.62) | −0.14 (<0.0001) | −0.13 (<0.0001) | −0.09 (0.0012) | −0.07 (0.017) | −0.20 (<0.0001) | 0.06 (0.023) |
| glutamine/glutamate ratio | — | 0.01 (0.69) | 0.01 (0.69) | −0.12 (<0.0001) | −0.11 (<0.0001) | −0.09 (0.0017) | −0.08 (0.011) | −0.19 (<0.0001) | 0.08 (0.007) |
BMI, body mass index; WC, waist circumference; HOMA, homeostasis model assessment of insulin resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; HDL, high-density lipoprotein cholesterol.
Regression coefficients represent the standardized change in dependent variable per 1-SD change in the log-transformed metabolite. For example, a 1-SD increment in log glutamine was associated with a −0.17 SD change in insulin, or −1.4 uU/mL. Separate regressions were fitted for each combination of trait (response) and metabolite (predictor).
In analyses of effect modification by sex on associations with lipid traits, there was no significant sex interaction on the relation of log triglycerides with glutamine, glutamate, or the ratio (P>0.37) in the FHS or MDC cohorts. There was also no significant sex interaction on the relation of HDL with glutamine (P>0.15), although borderline significant sex interactions were noted for the association of HDL with glutamate (P=0.04) and the ratio (P=0.048). In sex-specific analyses, the directionality of relations between these metabolites and HDL was similar but attenuated in men compared with women (Supplemental Tables 2 and 3). In analyses that included additional adjustment for dietary glutamine intake and physical activity, results remained unchanged (data not shown).
We repeated the analyses of glutamate and glutamine in the MDC cohort (Table 4, Supplemental Results, Supplemental Table 3, and Supplemental Figure 4). In age- and sex-adjusted regression analyses (Table 4), glutamate was significantly associated with BMI, WC, fasting glucose, insulin, HOMA, and log triglycerides, and inversely associated with HDL (P<0.0001 to P=0.001). Glutamine was inversely associated with BMI, WC, fasting glucose, insulin, and HOMA (P=0.001 to P=0.02). The glutamine-to-glutamate ratio was inversely associated with BMI, WC, fasting glucose, insulin, HOMA, and log triglycerides, and positively associated with HDL (P<0.0001 to P=0.0006). These associations remained significant in analyses that also adjusted for BMI (Table 4 and Supplemental Table 3).
Table 4.
Associations of Select Metabolites with Insulin Resistance Phenotypes and Metabolic Traits in MDC
| Regression Coefficient (P value)* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI | WC | Glucose | Insulin | HOMA | SBP | DBP | Log TG | HDL | |
| Models adjusting for age and sex | |||||||||
| glutamate | 0.16 (<0.0001) | 0.17 (<0.0001) | 0.09 (0.002) | 0.14 (0.0001) | 0.17 (<0.0001) | 0.08 (0.031) | 0.05 (0.15) | 0.23 (<0.0001) | −0.15 (<0.0001) |
| glutamine | −0.12 (0.001) | −0.08 (0.006) | −0.07 (0.022) | −0.10 (0.008) | −0.11 (0.006) | −0.05 (0.15) | −0.03 (0.44) | −0.02 (0.52) | 0.06 (0.081) |
| glutamine/glutamate ratio | −0.19 (<0.0001) | −0.19 (<0.0001) | −0.11 (0.0002) | −0.17 (<0.0001) | −0.20 (<0.0001) | −0.09 (0.010) | −0.06 (0.10) | −0.22 (<0.0001) | 0.17 (<0.0001) |
| Models adjusting for age, sex, and BMI | |||||||||
| glutamate | — | 0.06 (<0.0001) | 0.09 (0.004) | 0.10 (0.006) | 0.12 (0.0006) | 0.05 (0.19) | 0.02 (0.61) | 0.19 (<0.0001) | −0.11 (0.0006) |
| glutamine | — | 0.00 (0.74) | −0.07 (0.038) | −0.07 (0.069) | −0.07 (0.013) | −0.03 (0.45) | 0.00 (0.97) | 0.01 (0.76) | 0.03 (0.41) |
| glutamine/glutamate ratio | — | −0.05 (0.0002) | −0.11 (0.0005) | −0.12 (0.0009) | −0.14 (<0.0001) | −0.05 (0.13) | −0.02 (0.64) | −0.18 (<0.0001) | 0.12 (0.0004) |
BMI, body mass index; WC, waist circumference; HOMA, homeostasis model assessment of insulin resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; HDL, high-density lipoprotein cholesterol.
Regression coefficients represent the standardized change in dependent variable per 1-SD change in the log-transformed metabolite. For example, a 1-SD increment in log glutamine was associated with a −0.10 SD change in insulin, or −1.0 uU/mL. Separate regressions were fitted for each combination of trait (response) and metabolite (predictor).
Relation of Glutamine and Precursor Amino Acids to Incident Diabetes in Humans
In a sample of 601 participants from the FHS cohort who were free of diabetes at baseline, we examined the relation of glutamine, glutamate, and the ratio of glutamine to glutamate with incident diabetes over 12 years. Multivariable logistic regression analyses revealed that glutamine (adjusted OR 0.83, 95% CI 0.68–1.02; P=0.08), glutamate (OR 1.29, 95% CI 1.04–1.60; P=0.02), and the glutamine-glutamate ratio (OR 0.79, 95% CI 0.64–0.98; P=0.03) were associated with the risk of diabetes, after adjusting for age, sex, BMI, and baseline fasting glucose. The glutamine-glutamate ratio was only weakly correlated with the branched-chain amino acids, which we have previously identified as predictors of incident diabetes (r=−0.25 with isoleucine, r=−0.15 with leucine, and r=−0.07 with valine).17 Accordingly, the glutamine-glutamate ratio was associated with decreased risk for future diabetes even after adjusting for the branched-chain amino acids (data not shown). In a sample of 409 participants from the MDC cohort free of diabetes at baseline, glutamine was inversely associated with incident diabetes as captured in a registry database (OR 0.81, 95% CI 0.67–0.00; P=0.039); however relations of glutamate (P=0.31) and the glutamine-glutamate ratio (P=0.91) were non-significant.
Amino Acid Administration and Metabolic Phenotypes in Mice
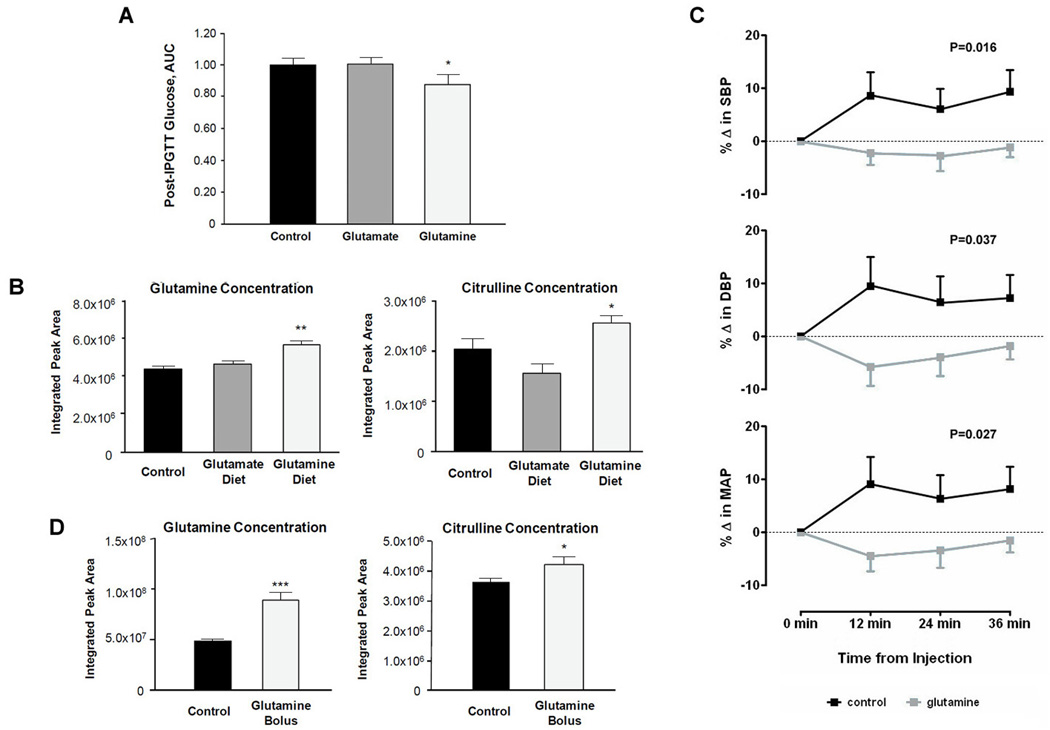
To test the hypothesis that glutamine or glutamate administration modulates glucose tolerance, we performed a dietary intervention study in mice. Three groups of C57Bl6 mice received 1 of 3 diets for 8 weeks: glutamine plus standard chow (n=10), glutamate plus standard chow (n=10), and standard chow alone (n=10). Baseline weight and fasting glucose did not differ significantly among the 3 groups. There was no significant difference in the change in weight (P=0.19) or total caloric intake (P=0.25) across the three groups. At the end of the dietary intervention, and following 6-hour fast, all animals received a glucose load injected into the peritoneal cavity (intra-peritoneal glucose tolerance test [IPGTT]). Compared with mice in the control and glutamate groups, mice in the glutamine group had the lowest plasma glucose levels measured at each time point following glucose administration. Total glucose excursions, represented by the area under the curve (AUC) of serial glucose measures, are shown in Figure 2A, and were significantly lower in the glutamine group compared with the other groups (P=0.01).
Figure 2.
Experimental results. (A) Mean (± standard error) area under the curve of serial glucose levels measured after intraperitoneal glucose tolerance test in mice treated for 8 weeks with dietary glutamine, glutamate, or standard chow alone (control). (B) Mean (± standard error) measures of glutamine and citrulline are shown in mice treated with dietary glutamine, glutamate, or standard chow alone (control). (C) Mean (± standard error) measures of systolic, diastolic, and mean blood pressure measurements are shown for mice following either intraperitoneal injection of glutamine plus saline (glutamine) or saline alone (control). (D) Mean (± standard error) measures of glutamine and citrulline are shown for mice following either intraperitoneal injection of glutamine plus saline (glutamine) or saline alone (control).
At the end of the dietary intervention, metabolite profiling confirmed that circulating levels of glutamine and glutamate were significantly higher in the glutamine- and glutamate-fed mice, respectively, compared with the control group. Metabolite profiling also demonstrated that several analytes had a strong inverse correlation with directly measured levels of glutamine, including the branched-chain amino acids and other hydrophobic amino acids: isoleucine (r=−0.65; P=0.002), leucine (r=−0.73, P<0.001), valine (r=−0.62; P=0.003), tryptophan (r=−0.62; P=0.003), and tyrosine (r=−0.68; P<0.001). On the other hand, there was a strong positive correlation between glutamine and citrulline: r=0.77 (P<0.0001). There were significantly higher plasma concentrations of both glutamine (P<0.01) and citrulline (P=0.01) in the glutamine-fed mice compared with the control group (Figure 2B).
To test the hypothesis that glutamine administration modulates blood pressure, we performed intraperitoneal injection of either glutamine plus saline or saline alone (vehicle control) in two groups of 10 mice. Serial blood pressure measurements were recorded using the CODA non-invasive tail blood pressure system (Kent Scientific, Torrington, CT). SBP (P=0.016), DBP (P=0.037), and MAP (P=0.027) were significantly reduced glutamine-treated mice versus controls (Figure 2C). As in the feeding experiment, levels of both glutamine (P<0.001) and citrulline (P=0.04), a known precursor to nitric oxide, were significantly higher in the glutamine treated animals (Figure 2D). In a parallel experiment of glutamate administration, there was no significant difference in blood pressure observed between glutamate plus saline versus saline alone (data not shown).
Discussion
High throughput metabolite profiling provides the opportunity to perform a systematic, unbiased investigation of the possible pathways underlying complex phenotypes such as the ‘metabolic syndrome’. Therefore, we applied metabolite profiling in two community-based cohorts and observed that individuals with metabolic risk factors have higher circulating concentrations of glutamate, branched-chain amino acids, and other amino acid derivatives, and lower concentrations of glutamine. We observed this metabolomic signature in association with multiple components of the “metabolic syndrome,” including central adiposity, glucose intolerance, dyslipidemia, and hypertension. Furthermore, we found that an excess of glutamine relative to glutamate in the circulation was associated with a reduced risk of future diabetes. Concordant with the findings in humans, we observed that glutamine supplementation improved glucose tolerance and lowered blood pressure in mice. Together, these data suggest that diminished glutamine, particularly in relation to its precursor amino acids, is not only a marker of metabolic risk but may also contribute to the development of metabolic disease. Our study also identified unanticipated associations of tryptophan breakdown products (kynurenic acid, anthranillic acid) and nucleotide metabolites (xanthosine, n-carbamoyl-β-alanine) with cardiometabolic disease.
Several patterns noted in our metabolomic data are consistent with results of prior experimental and physiologic studies. Felig and colleagues originally reported that selected amino acids were associated with fasting insulin levels in 10 obese and 10 non-obese individuals.19 More recent investigations in small samples have highlighted associations of the branched-chain amino acids (isoleucine, leucine, and valine) and other hydrophobic amino acids (e.g. alanine, phenylalanine, and tyrosine) with obesity, impaired glucose tolerance, and insulin resistance.12–15,19 A population study also observed an association of 24-hour urinary excretion of alanine with higher blood pressure.11 The current investigation extends the prior work by demonstrating robust and reproducible associations of circulating levels of branched-chain amino acids with not only obesity and impaired glucose tolerance but also with dyslipidemia and blood pressure, in two large human cohorts.
Our metabolomic data also highlight several patterns that have not been previously well-described in association with metabolic disease. Glutamine is best known for its role in providing intermediates to the tricarboxyclic acid cycle (anapleurosis) and for its ammonia carrying capacity, which is critical for maintaining overall nitrogen balance.20–22 Total body glutamine is depleted in catabolic states such as trauma, critical illness, infection, and sepsis, and parenteral glutamine supplementation in the intensive care setting is associated with reduced morbidity and mortality.23 These findings have been attributed to the effects of glutamine on the regulation of white cell function, cell volume, response to cytotoxins, and cellular redox potential via glutathione-mediated pathways.21 We now demonstrate in two large human cohorts that glutamine is inversely associated with a wide array of metabolic traits, including hypertension and hypertriglyceridemia, in addition to measures of obesity and HOMA-IR. In our data, standard deviation increments in metabolite concentrations were generally associated with meaningful differences in metabolic trait measures (for instance, a 1.2 to 2.0 uU/mL difference in plasma insulin). The consistent associations observed between multiple glutamine-related metabolites and metabolic traits further support the hypothesis that glutamine-cycling pathways are prominently involved in the development of metabolic risk.
Interestingly, a prior study of 24 adults with and without diabetes showed that glutamine supplementation was associated with improved glucose intolerance.24 In support of the hypothesis that glutamine may be more than a marker of metabolic risk, animal studies have shown that glutamine administration improves glucose and insulin metabolism in the setting of exercise25 or a high-fat diet.26 Our experimental data extend these findings by distinguishing the protective effect of glutamine versus glutamate supplementation, showing the corresponding changes in metabolite profiles, and demonstrating that the improvements in glucose tolerance are independent of changes in weight. Furthermore, we found that glutamine administration decreases the blood pressure following acute administration.
Taken together, these data underscore the potentially beneficial effects of glutamine on cardiometabolic risk, which may be due to a number of mechanisms such as enhancing release of glucagon-like peptide 1 (GLP-1), externalization of glucose transporter type 4 (GLUT-4), transcription of insulin-dependent enzymes, pancreatic beta-cell insulin secretion, and insulin sensitivity of adipose tissue.24,27,28 The association of glutamine with blood pressure may be further related to direct or indirect effects on the regulation of nitric oxide production,29–31 as suggested by our finding of strong correlations between glutamine and citrulline, a known precursor to nitric oxide.
The distinct roles of glutamine versus glutamate have not been highlighted previously. Endogenous glutamine is continually synthesized in the skeletal muscle, predominantly from glutamate but also from branched-chain amino acids. Cycling between glutamine and glutamate is regulated by the activity of glutamine synthetase and glutaminase, enzymes that have wide tissue distribution.21,32 Prior studies have observed an association of dietary glutamate with lower blood pressure,33 but without accounting for its effect on endogenous glutamine levels.34 A recent investigation of metabolomic profiles in humans observed that obesity and insulin resistance are associated with an analyte identified as both glutamine and glutamate.12 Our platform distinguishes the two analytes, highlighting the unanticipated finding that glutamate may confer adverse metabolic risk whereas glutamine may be protective. In contrast to the likely favorable effects of glutamine, glutamate has been shown to stimulate glucagon release from pancreatic α cells35 and increase transmination of pyruvate to alanine, a strong promoter of gluconeogenesis that is abundant in obesity.12 Glutamate is also well known as a direct precursor to α-ketoglutarate, an intermediate in the Krebs cycle that serves as an energy source for multiple cell types and that exerts anabolic as well as anti-catabolic activity.36
Several limitations of the present study merit consideration. We used an LC/MS platform that does not provide full coverage of the plasma metabolome. However, our focus on abundant metabolites permitted the measure of 45 analytes; by providing key clinical correlates for a large panel of plasma metabolites, these data complement ongoing efforts to annotate the human metabolome.37 The present analysis was focused on interrogating the relationship of glutamine (and its precursor analytes) with metabolic traits and potential functional roles. The biology underlying the correlates of other analytes remains the subject of future investigation. A small number of metabolites had coefficients of variation exceeding 20%, but were retained in analyses for completeness; the results of analyzing these specific metabolites may have been biased to the null. As a surrogate measure of insulin resistance, HOMA-IR is less precise than measures obtained from the hyperinsulinemic euglycemic glucose clamp technique or the insulin suppression test. Nonetheless, it is regarded as a reasonably reliable surrogate in individuals without severely impaired pancreatic beta-cell function and it is considered practical for epidemiological studies.38,39 Because the MDC cohort was relatively small in size and consisted exclusively of individuals previously identified as having a high baseline risk for developing incident diabetes or CVD, analyses of incident diabetes in this cohort were limited by sampling in addition to possible ascertainment bias. Thus, additional investigations of the relation of glutamine and glutamate with incident diabetes are needed in unselected populations. Because many of the metabolites included in the present study were amino acids or amino acid precursors or derivatives, the possible associations of non-amino acid molecules with cardiometabolic risk remain a subject for further investigation. Our clinical cohorts were predominantly comprised of middle-aged to elderly individuals of European ancestry; thus, the generalizability of our findings to younger individuals and other racial/ethnic groups is not yet known.
The present study demonstrates the potential value of integrating metabolomic and clinical data in human cohorts with detailed phenotyping. While highlighting select amino acids of interest, we recognize that our data relating all of the measured metabolites to metabolic traits in a large sample of ambulatory individuals may be of broader scientific interest. Indeed, we submit that several features favor the potential of our data to serve as a resource for future investigations. First, these data demonstrate the feasibility and reproducibility of analyzing the relations of metabolomic with clinical data across two separate community-based cohorts. Second, our results provide an important clinical dimension to numerous plasma metabolites, complementing ongoing efforts to annotate the human metabolome.37 Third, the range of observed associations between metabolites and metabolic traits highlights opportunities for further research aimed at elucidating pathways underlying cardiometabolic disease and identifying potential therapeutic targets. Because metabolites represent intermediate traits that may play functional roles (either adaptive or maladaptive) in disease pathogenesis, future applications of metabolomics technology can provide additional insights regarding the mechanisms by which established risk factors are associated with clinically important cardiovascular and metabolic outcomes.
Supplementary Material
Clinical Perspective.
Although metabolic risk factors are known to cluster in individuals who are prone to developing diabetes and cardiovascular disease, the underlying biological mechanisms remain poorly understood. To acquire a more detailed understanding of the biochemical pathways, we applied high-throughput metabolite profiling to samples from 1,761 individuals from two large, well-characterized clinical cohorts. We observed that the presence of metabolic risk factors (including obesity, insulin resistance, high blood pressure, dyslipidemia) was significantly associated with variation in select metabolites, including branched-chain amino acids, other hydrophobic amino acids, tryptophan breakdown products, and nucleotide metabolites. We observed particularly strong associations of insulin resistance traits with decreased glutamine and increased glutamate. We followed up these findings in experimental models and demonstrated that glutamine administration in mice resulted in both increased glucose tolerance and lower blood pressure. Taken together, our clinical and experimental data highlight the glutamine-glutamate metabolic pathway as a potential target for interventions aimed at attenuating metabolic risk in humans. Furthermore, by demonstrating the feasibility and utility of biochemical profiling in large clinical samples, we anticipate that our data could serve as a resource for future studies of the human metabolome and its relevance to cardiovascular and metabolic diseases. Because metabolites represent intermediate traits that may play functional roles (either adaptive or maladaptive) in disease pathogenesis, future applications of metabolomics technology can provide additional insights regarding the mechanisms by which established risk factors are associated with clinically important outcomes.
Acknowledgments
Funding Sources: This work was supported by NIH contract N01-HC-25195, R01-DK-HL081572, the Donald W. Reynolds Foundation, and the Leducq Foundation. Dr. Cheng is supported by the Ellison Foundation. Dr. Dejam is supported by American Heart Association grant 10CRP2660009.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: Drs. Larson, Vasan, Gerszten, and Wang are named as co-inventors on a patent application to the US Patent Office pertaining to metabolite predictors of diabetes.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Golden SH, Folsom AR, Coresh J, Sharrett AR, Szklo M, Brancati F. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: the atherosclerosis risk in communities study. Diabetes. 2002;51:3069–3076. doi: 10.2337/diabetes.51.10.3069. [DOI] [PubMed] [Google Scholar]
- 3.Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 4.Sundstrom J, Vallhagen E, Riserus U, Byberg L, Zethelius B, Berne C, Lind L, Ingelsson E. Risk associated with the metabolic syndrome versus the sum of its individual components. Diabetes Care. 2006;29:1673–1674. doi: 10.2337/dc06-0664. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 6.Salmenniemi U, Ruotsalainen E, Pihlajamaki J, Vauhkonen I, Kainulainen S, Punnonen K, Vanninen E, Laakso M. Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation. 2004;110:3842–3848. doi: 10.1161/01.CIR.0000150391.38660.9B. [DOI] [PubMed] [Google Scholar]
- 7.Allen J, Davey HM, Broadhurst D, Heald JK, Rowland JJ, Oliver SG, Kell DB. High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat Biotechnol. 2003;21:692–696. doi: 10.1038/nbt823. [DOI] [PubMed] [Google Scholar]
- 8.An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson JK, Wilson ID. Opinion: understanding 'global' systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov. 2003;2:668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- 10.Raamsdonk LM, Teusink B, Broadhurst D, Zhang N, Hayes A, Walsh MC, Berden JA, Brindle KM, Kell DB, Rowland JJ, Westerhoff HV, van Dam K, Oliver SG. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat Biotechnol. 2001;19:45–50. doi: 10.1038/83496. [DOI] [PubMed] [Google Scholar]
- 11.Holmes E, Loo RL, Stamler J, Bictash M, Yap IK, Chan Q, Ebbels T, De Iorio M, Brown IJ, Veselkov KA, Daviglus ML, Kesteloot H, Ueshima H, Zhao L, Nicholson JK, Elliott P. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, Carr SA, Thadhani R, Gerszten RE, Mootha VK. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wopereis S, Rubingh CM, van Erk MJ, Verheij ER, van Vliet T, Cnubben NH, Smilde AK, van der Greef J, van Ommen B, Hendriks HF. Metabolic profiling of the response to an oral glucose tolerance test detects subtle metabolic changes. PLoS One. 2009;4:e4525. doi: 10.1371/journal.pone.0004525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao X, Peter A, Fritsche J, Elcnerova M, Fritsche A, Haring HU, Schleicher ED, Xu G, Lehmann R. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab. 2009;296:E384–E393. doi: 10.1152/ajpendo.90748.2008. [DOI] [PubMed] [Google Scholar]
- 16.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SAS/STAT User's Guide, Version 9.22. Cary, NC: SAS Institute Inc.; 2010. [Google Scholar]
- 19.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 20.Bruce M, Constantin-Teodosiu D, Greenhaff PL, Boobis LH, Williams C, Bowtell JL. Glutamine supplementation promotes anaplerosis but not oxidative energy delivery in human skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E669–E675. doi: 10.1152/ajpendo.2001.280.4.E669. [DOI] [PubMed] [Google Scholar]
- 21.Roth E. Nonnutritive effects of glutamine. J Nutr. 2008;138:2025S–2031S. doi: 10.1093/jn/138.10.2025S. [DOI] [PubMed] [Google Scholar]
- 22.Young VR, Ajami AM. Glutamate: an amino acid of particular distinction. J Nutr. 2000;130:892S–900S. doi: 10.1093/jn/130.4.892S. [DOI] [PubMed] [Google Scholar]
- 23.Bongers T, Griffiths RD, McArdle A. Exogenous glutamine: the clinical evidence. Crit Care Med. 2007;35:S545–S552. doi: 10.1097/01.CCM.0000279193.23737.06. [DOI] [PubMed] [Google Scholar]
- 24.Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, Reimann F, Holst JJ, Gribble FM. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89:106–113. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwashita S, Williams P, Jabbour K, Ueda T, Kobayashi H, Baier S, Flakoll PJ. Impact of glutamine supplementation on glucose homeostasis during and after exercise. J Appl Physiol. 2005;99:1858–1865. doi: 10.1152/japplphysiol.00305.2005. [DOI] [PubMed] [Google Scholar]
- 26.Opara EC, Petro A, Tevrizian A, Feinglos MN, Surwit RS. L-glutamine supplementation of a high fat diet reduces body weight and attenuates hyperglycemia and hyperinsulinemia in C57BL/6J mice. J Nutr. 1996;126:273–279. doi: 10.1093/jn/126.1.273. [DOI] [PubMed] [Google Scholar]
- 27.Bakalar B, Duska F, Pachl J, Fric M, Otahal M, Pazout J, Andel M. Parenterally administered dipeptide alanyl-glutamine prevents worsening of insulin sensitivity in multiple-trauma patients. Crit Care Med. 2006;34:381–386. doi: 10.1097/01.ccm.0000196829.30741.d4. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Buettger C, Kwagh J, Matter A, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Stanley CA, Matschinsky FM. A signaling role of glutamine in insulin secretion. J Biol Chem. 2004;279:13393–13401. doi: 10.1074/jbc.M311502200. [DOI] [PubMed] [Google Scholar]
- 29.Bryk J, Ochoa JB, Correia MI, Munera-Seeley V, Popovic PJ. Effect of citrulline and glutamine on nitric oxide production in RAW 264.7 cells in an arginine-depleted environment. JPEN J Parenter Enteral Nutr. 2008;32:377–383. doi: 10.1177/0148607108319807. [DOI] [PubMed] [Google Scholar]
- 30.Nishiyama A, Yokote Y, Sakagami H. Changes in Amino Acid Metabolism During Activation of Mouse Macrophage-like Cell Lines. In Vivo. 2010;24:857–860. [PubMed] [Google Scholar]
- 31.Ligthart-Melis GC, van de Poll MC, Boelens PG, Dejong CH, Deutz NE, van Leeuwen PA. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am J Clin Nutr. 2008;87:1282–1289. doi: 10.1093/ajcn/87.5.1282. [DOI] [PubMed] [Google Scholar]
- 32.Labow BI, Souba WW, Abcouwer SF. Mechanisms governing the expression of the enzymes of glutamine metabolism--glutaminase and glutamine synthetase. J Nutr. 2001;131:2467S–2474S. doi: 10.1093/jn/131.9.2467S. discussion 2486S–2467S. [DOI] [PubMed] [Google Scholar]
- 33.Stamler J, Brown IJ, Daviglus ML, Chan Q, Kesteloot H, Ueshima H, Zhao L, Elliott P. Glutamic acid, the main dietary amino acid, and blood pressure: the INTERMAP Study (International Collaborative Study of Macronutrients, Micronutrients and Blood Pressure) Circulation. 2009;120:221–228. doi: 10.1161/CIRCULATIONAHA.108.839241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mourtzakis M, Graham TE. Glutamate ingestion and its effects at rest and during exercise in humans. J Appl Physiol. 2002;93:1251–1259. doi: 10.1152/japplphysiol.00111.2002. [DOI] [PubMed] [Google Scholar]
- 35.Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Kohler M, Fachado A, Vieira E, Zierath JR, Kibbey R, Berman DM, Kenyon NS, Ricordi C, Caicedo A, Berggren PO. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. 2008;7:545–554. doi: 10.1016/j.cmet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cynober LA. The use of alpha-ketoglutarate salts in clinical nutrition and metabolic care. Curr Opin Clin Nutr Metab Care. 1999;2:33–37. doi: 10.1097/00075197-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016957. e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 39.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.