Abstract
Decorin (DCN), a component of the extracellular matrix of the peritubular wall and the interstitial areas of the human testis, can interact with growth factor (GF) signaling, thereby blocking downstream actions of GFs. In the present study the expression and regulation of DCN using both human testes and two experimental animal models, namely the rhesus monkey and mouse, were examined. DCN protein was present in peritubular and interstitial areas of adult human and monkey testes, while it was almost undetectable in adult wild-type mice. Interestingly, the levels and sites of testicular DCN expression in the monkeys were inversely correlated with testicular maturation markers. A strong DCN expression associated with the abundant connective tissue of the interstitial areas in the postnatal through prepubertal phases was observed. In adult and old monkeys the DCN pattern was similar to the one in normal human testes, presenting strong expression at the peritubular region. In the testes of both infertile men and in a mouse model of inflammation associated infertility (aromatase-overexpressing transgenic mice), the fibrotic changes and increased numbers of Tumor necrosis factor (TNF)-α-producing immune cells were shown to be associated with increased production of DCN. Furthermore, studies with human testicular peritubular cells isolated from fibrotic testis indicated that TNF-α significantly increased DCN production. The data, thus, show that an increased DCN level is associated with impaired testicular function, supporting our hypothesis that DCN interferes with paracrine signaling of the testis in health and disease.
INTRODUCTION
The components of the extracellular matrix (ECM) of the human testis and their specific functions are not well known, but different collagen types, fibronectin, laminin (Davidoff et al. 1990, Dobashi et al. 2003, Gulkesen et al. 2002, Haider et al. 1999, Pollanen et al. 1985, Santamaria et al. 1990, Siu and Cheng 2008, Takaba 1990) and the proteoglycans, decorin (DCN) and biglycan, have been identified (Ungefroren et al. 1995). The latter were found both in the peritubular wall and in interstitial areas. Testicular DCN, to our knowledge, has not been described in any other species or has been reported to be absent in the developing mouse gonad (Miqueloto and Zorn 2007).
Recently, we confirmed the presence of DCN in adult normal human testes and found that DCN expression is strikingly increased in testes of men with Mixed Atrophy (MA)-syndrome, in which focal tubular damage and fibrosis coexist along with tubules bearing normal spermatogenesis (Adam et al. 2011). Accumulation of DCN was strong and patchy in the thickened peritubular wall both in ECM deposits and in testicular peritubular myoid cells. The latter, human testicular peritubular cells (HTPCs) produce DCN also when cultured and were used to study possible actions of DCN in a human cellular model of the peritubular wall (Adam et al. 2011).
DCN is a small chondroitin/dermatan sulfate proteoglycan, belonging to the small leucine-rich proteoglycan (SLRP) family, which consits of a 40 kDa core protein and one glycosaminoglycan chain (Iozzo 1997, Iozzo 1999, Reed and Iozzo 2002). It plays a fundamental role in the organization of the ECM as it interacts with collagen type I (Jarvelainen et al. 2006, Orgel et al. 2009, Reed and Iozzo 2002). In addition, DCN can bind growth factors (GF), e.g. platelet-derived GF (PDGF) (Nili et al. 2003), and importantly, it can serve as a ligand of several growth factor receptors (GFRs), including epidermal GFR (EGFR) and insulin-like GF1R (IGF1R) (Schaefer and Iozzo 2008), hepatocyte GFR (HGFR) (Goldoni et al. 2009) and vascular endothelial GFR (VEGFR) (Iacob et al. 2008). As clearly documented for the EGFR, DCN is able to block the EGF/EGFR signaling (Zhu et al. 2005) in several cells, including isolated HTPCs. In these cells DCN interferes with EGF/EGFR and PDGF/PDGFR signaling (Adam et al. 2011). We found that DCN first stimulated the initial signaling cascade and then inhibited the proliferative actions of EGF and PDGF in HTPCs. Consequently, we have suggested that DCN most likely is involved in the paracrine signaling events in the human testis (Adam et al. 2011). This point is of special importance in conditions where the levels of DCN are physiologically increased or pathologically imbalanced, as e.g. in MA patients. We hypothesize that altered focal changes of the levels of DCN negatively interfere with testicular functions in MA patients.
Increased levels of ECM, associated with interstitial and peritubular fibrosis, are typical for male infertility patients exhibiting a range of changes, including not only MA-, but also Sertoli cell only syndrome (SCOS) and Germ cell arrest (GA) syndrome. The ECM of the fibrotically remodeled peritubular walls contains collagens, including collagen type I, and currently unidentified components (Gülkesen et al. 2002, Takaba, 1990, Davidoff et al. 1990; Haider et al. 1999), which may include DCN. If so, interferences of DCN with GF signaling in the peritubular wall and between tubular, peritubular and interstitial compartments of the testis are assumed.
Interestingly, a transgenic mouse, overexpressing aromatase (AROM+) and therefore having increased intratesticular estrogen/testosterone ratio shares many features found in human male infertility. In both fibrosis, impaired spermatogenesis and increased numbers of immune cells producing Tumor necrosis factor (TNF)-α, are found (Frungieri et al. 2002, Li et al. 2006, Meineke et al. 2000, Li et al. 2006, Strauss et al. 2009). AROM+ mice, which develop these changes after the age of 2–3 months, can be viewed as a unique model for male infertility. Whether DCN may be expressed in the testes of AROM+ animals and could be furthermore causally involved in the development of infertility is not known.
Likewise, it is unclear whether DCN plays a role in the development of the testis. A changing ECM environment is thought to be important for testicular formation and development (Pelliniemi et al. 1984). Yet, DCN was reported to be absent in the developing mouse testis (Miqueloto and Zorn 2007, Soto-Suazo et al. 2002), and there are no studies from other animal species or humans to confirm or refute this observation. EGF and PDGF, in particular, i.e. possible interacting partners of DCN, promote cell proliferation, regulate tissue differentiation, and modulate organogenesis and have a fundamental role in testicular development (Basciani et al. 2010, Nurmio et al. 2007, Yan et al. 1998).
In the present study we sought to explore the role of DCN in the testis by studying its expression and regulation. We examined expression of DCN in fibrotically altered human testes of GA and SCOS patients and in testes of AROM+ mice. We also employed a recently described unique cellular model, HTPCs isolated from normal human testis and corresponding cells stemming from men suffering from testicular fibrosis and impaired spermatogenesis (HTPC-Fs; Albrecht et al. 2006, Schell et al. 2008, Schell et al. 2010, Spinnler et al. 2010) to study the question whether DCN may be regulated by TNF-α. Finally, as human and mouse were found to be inappropriate models to study DCN expression during the process of testicular development, DCN expression during ontogeny of the gonad was examined in rhesus monkeys.
MATERIALS AND METHODS
Human testicular samples, isolation and cultivation of HTPC/HTPC-Fs
Human testicular biopsies, embedded in paraffin were isolated from men with normal (n=3) or pathologically altered spermatogenesis, diagnosed either with Sertoli cell only (n= 4) or Germ cell arrest (n=4). They were sectioned and used for immunohistochemistry, as described previously (Albrecht et al. 2006, Meineke et al. 2000). The local ethics committee approved the use of the samples.
Isolation of HTPC/-Fs was performed as described in previous studies (Albrecht et al. 2006, Spinnler et al. 2010, Adam et al. 2011). While HTPCs stem from patients with normal spermatogenesis, HTPC-Fs were isolated from men suffering from impaired spermatogenesis and testicular fibrosis. The latter, HTPC-Fs, were in part characterized previously (Spinnler et al. 2010, Adam et al. 2011). All participants granted written informed consent. The local ethics committee approved the study. Cells, passages 3–12, were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS; both from PAA GmbH, Cölbe, Germany), unless indicated otherwise. After initial screening with several concentrations (1, 5 and 50 ng/ml) in order to determine optimal response, treatments with 5 ng/ml human recombinant TNF-α (Sigma Aldrich, Hamburg, Germany) were performed for three days, as detailed below. H2O, as solvent of for TNF-α, was included as control in all experiments.
Transgenic mice expressing human P450 aromatase
Transgenic mice expressing the human P450 aromatase (AROM+ mice) were generated as described previously (Li et al. 2003, Li et al. 2001, Li et al. 2006). The mice were fed with Soya free diet, and they were maintained in a specific pathogen-free stage at Central Animal Laboratory at the University of Turku, complying with international guidelines on the care and use of laboratory animals. All animal experiments were approved by the Finnish Animal Ethics Committee.
Genotyping of the AROM+ mice was carried out as reported before (Li et al. 2001). Testicular samples from at least three-five adult AROM+ and age-matched wild type mice (age: 2–3 months, 5 months and 9–10 months) were used for different experiments, including RT-PCR and morphology. For the latter, they were fixed in Bouin’s fluid, embedded in paraffin wax, and sectioned for immunohistochemistry, as described (Li et al. 2006). In addition, testes from at least three young (3 months) and three old (9–10 months) wild type and AROM+ mice were fixed in 5% glutaraldehyde/phosphate buffered saline (PBS) and processed for electron microscopy, as described (Strauss et al. 2009), or were frozen and kept until isolation of RNA and RT-PCR.
Rhesus monkey testicular samples
Samples used for immunohistochemistry were identical to ones studied previously (Frungieri et al. 2000). In brief, the testes were obtained through the Oregon National Primate Research Center (ONPRC) Tissue Distribution Program, from rhesus monkeys (Macaca mulatta) that were primarily involved in other unrelated studies. They were classified as: infantile (0–1 year; n=4), juvenile (1–2 years; n=1), peripubertal (3–4 years; n=2), young adult (5–6 years; n=3) and old adult (24–30 years; n=5). The animals were cared for by the ONPRC in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. They were housed in a 12L:12D photoperiod (i.e., 12 h of light per day) and fed twice daily with Purina monkey chow; fresh fruit was also provided daily and drinking water was made available ad libitum. They were painlessly killed using an overdose of ketamine and pentobarbital, according to procedure established by the Panel on Euthanasia of the American Veterinary Society. For the purpose of this study, the testes were fixed for at least 48 h in Bouin’s fluid, followed by 70% ethanol, and then embedded in paraffin wax. Sections (5 μm) were prepared for immunohistochemistry.
Microarray analysis
Testicular samples from 9 monkeys (3 juvenile (1–2 years), 3 young adult (5–6 years) and 3 old adult (24–30 years)) were homogenized with a PowerGen rotor-stator homogenizer (Fisher Scientific, Pittsburgh, PA, USA) and total RNA was isolated using a Qiagen RNeasy Mini Kit, according to the manufacturer’s instructions (QIAGEN, Valencia, CA, USA). Briefly, tissues were homogenized in RLT buffer with 1% β-mercaptoethanol, and RNA was collected onto RNeasy mini columns and eluted in water. The concentration of RNA was measured by spectroscopy, with an expected A260/A280 ratio close to 2, denoting an acceptably pure nucleic acid sample. RNA integrity and quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara CA), and met requirements for hybridization to Affymetrix GeneChip® microarrays. As previously described (Lemos et al. 2006, Urbanski et al. 2009), microarray analysis was performed by the Affymetrix Microarray Core (AMC) of the Oregon Health and Science University (OHSU), using the human HG_U133A GeneChip® microarray platform. The samples were prepared using the AMC One-cycle cDNA IVT (in vitro transcription) amplification/labeling protocol (Standard Labeling), and hybridized to a microarray chip. The image processing and expression analysis were performed using Affymetrix GeneChip® Operating Software (GCOS) version 1.2. After GCOS analysis, absolute analyses were re-run using global scaling to an average target intensity of 200. In this way, scaling allowed for direct comparison of the hybridization values from the two targets. The parameters α1 and α2, which set the point at which the probe set is called present, marginally present, or undetectable, were set to 0.05 and 0.065 (Affymetrix defaults) respectively. These parameters were used to determine expression levels for each probe set based on the detection P-value of the probe set. An assay performance assessment was conducted to confirm that the quality of the RNA used was acceptable and that genome hybridizations performed well. The performance assessment (conducted by AMC) was based on the GCOS analysis, and pair-wise scatter plots of Affymetrix Microarray Suite (MAS) absolute analyses.
Immunohistochemistry
Immunohistochemistry was performed as described elsewhere (Albrecht et al. 2006, Schell et al. 2008). A specific antibody against DCN (goat anti-human cross-reacting with monkey and mouse DCN; R&D Systems, Wiesbaden-Nordenstadt, Germany; 1:50 or 1:100) was used. Controls consisted of incubation with normal non-immune serum instead of the specific antibody or omission of the primary antibody.
RT-PCR and quantitative (q)RT-PCR
HTPCs and HTPC-Fs, respectively from at least 3 different patients were grown in 60-mm dishes (Nunc GmbH & Co. KG, Wiesbaden, Germany) in DMEM containing 10% FCS to sub-confluence and incubated with/without 5 ng/ml TNF-α in DMEM medium containing 10% FCS for 3 days. Subsequently, the cells were washed twice with DMEM without FCS and RNA was isolated using the QIAGEN RNeasy minikit (QIAGEN GmbH, Hilden, Germany). Reverse transcription was performed using random hexamer primers (Frungieri et al. 2002, Schell et al. 2010).
Deparaffinzed sections of rhesus monkey testes served as an additional source of RNA. Tissues were scraped from the glass slide, RNA was extracted using the RNeasy FFPE kit (QIAGEN), and reverse transcription was performed using Oligo-dT primers.
Mouse whole testes samples were homogenized in 1 ml of TRIsure (Bioline GmbH, Luckenwalde, Germany), and total RNA was isolated according to the manufacturer’s instructions. One μg of total RNA was thereafter treated with DNase (DNase I Amplification Grade Kit; Invitrogen, Life Technologies, Paisley, UK). Reverse transcription using random hexamer primer (300 ng/μl) and M-Mul VRT RNase H+ (Finnzymes Oy, Espoo, Finland) was performed.
The following primer pairs were used for PCR: human DCN: forward 5′-GGA ATT GAA AAT GGG GCT TT -3′ and reverse 5′-GCC ATT GTC AAC AGC AGA GA -3′ (35 cycles; annealing temperature: 59 °C), yielding a 221-bp fragment; human nested DCN: forward 5′-CAC CAG CAT TCC TCA AGG TC-3′and reverse 5′-TCA ATC CCA ACT TAG CCA AA-3′ (35 cycles, annealing temperature: 59 °C), yielding a 122-bp fragment; mouse DCN: forward 5′-TGA GCT TCA ACA GCA TCA CC-3′ and reverse 5′-AAG TCA TTT TGC CCA ACT GC-3′ (35 cycles; annealing temperature: 60 °C), yielding a 181-bp fragment. The DCN mRNA expression levels were determined in proportion to the ribosomal protein L19 (RLP19) housekeeping gene: human forward 5′-AGG CAC ATG GGC ATA GGT AA -3′ and reverse 5′-CCA TGA GAA TCC GCT TGT TT -3′ (annealing temperature: 58 °C); mouse forward 5′-GGA CAG AGT CTT GAT GAT CTC-3′ and reverse 5′-CTG AAG GTC AAA GGG AAT GTG-3′ (annealing temperature: 55 °C).
PCR products were visualized by ethidium bromide staining in 2% agarose gels. Commercially available cDNA from human testes (Clontech, Palo Alto, CA, USA) was used as a positive control. Identities of all PCR products were verified by sequencing (Schell et al. 2010).
Amplification of cDNAs for qRT-PCR was performed employing the DyNAmo two-step SYBR Green qRT-PCR kit (Finnzymes). The Engine Opticon system (MJ Research, Inc., Waltham, MA, USA) for continuous fluorescent detection was used. Samples and standards were amplified in triplicates. The expression levels were determined in proportion to the ribosomal protein L19 (RPL19) housekeeping gene (Adam et al. 2011).
Transmission electron microscopy (TEM)
Testicular biopsies from 3 patients with GA and 3 AROM+ and wild type mice were examined by electron microscopy (Zeiss EM 10 microscope; Zeiss, Oberkochen, Germany). Samples were identical to the ones previously reported (Meineke et al. 2000, Strauss et al. 2009). In addition a human testicular biopsy was used for visualizing DCN. To this end a small piece was fixed in 2.5% Glutaraldehye/PBS over night and was then processed using an established method with Cupromeronic blue (CMB) as cationic phthalocyanin dye (Haigh and Scott 1986, Scott and Thomlison 1998). When tissues are stained with CMB reagent under specific electrolyte concentrations, the anionic glycosaminoglycans appear as regularly and orthogonally bridging filaments between and across collagen fibrils in electron micrographs.
Western blotting
In order to examine functionality of TNFR-1 and -2 in HTPC-Fs, the cells were incubated either in the presence or absence of TNF-α (5 ng/ml) for various time periods (10, 20, and 60 min), as described in a previous study for HTPCs (Schell et al. 2008). Immunoblots were performed, as described (Frungieri et al. 2002), using a polyclonal rabbit anti-human ERK 1/2 (Extracellular regulated kinase) antibody (Cell Signaling Technology, New England Biolabs GmbH, Frankfurt am Main, Germany; 1:1,000) and a monoclonal mouse anti-human phospho-ERK 1/2 antibody (Cell Signaling Technology; 1:1,000). HTPC/-Fs were also incubated in the presence or absence of different concentrations of TNF-α (1, 5 or 50 ng/ml) in DMEM medium containing 10% FCS for 3 days. Immunoblotting was performed by using the same DCN antibody (1:1,000, R&D Systems) as for immunohistochemistry. The use of a β-actin antibody (1:5,000, Sigma) allowed corrections for small differences in loading. Signals were detected with chemiluminescent reagents (SuperSignal® West Femto Maximum Sensitivity Substrate, Pierce, Thermo Scientific, Rockford IL, USA), analyzed densitometrically with ImageJ Software (National Institutes of Health, Bethesda, MD, USA; version 1.40g) and the results were normalized to β-actin results. The experiments were repeated with cells from at least three different patients of each group, HTPCs and HTPC-Fs.
Enzyme-linked immunosorbent assay (ELISA)
DCN was determined in conditioned media (3 days) using a commercial immunoassay (R&D Systems), according to the manufacturer’s protocol (100 μl of the conditioned media diluted 1:1,000). The medium supernatants (total 2 ml per 60-mm dish) of the same TNF-α-treated (5 ng/ml) and untreated control cells, which were used in the Western blot experiments, were examined. The results were expressed as secreted DCN per total cellular protein.
Data analysis and statistics
Results obtained were analyzed using PRISM 4.0 (GraphPad Software, Inc., San Diego, CA). Student t-tests or one-way analysis of variances (ANOVAs) were performed, as indicated. Differences between the groups were evaluated with the appropriate post hoc tests (Newman-Keuls Multiple Comparison). All of the data are expressed as mean ± SEM.
RESULTS
DCN is strongly expressed in adult human and rhesus monkey testes, but is barely detectable in mouse testes
RT-PCR revealed DCN mRNA in the testes of all the species that we examined, i.e. adult human, rhesus monkey and mouse (Fig. 1A). However, DCN protein was detected in the interstitial ECM and in the peritubular compartment, as well as in peritubular cells, of human and rhesus monkey testes (Fig. 1C,D). In contrast, very little, if any, DCN protein expression was found in testes samples of adult mice (Fig. 1E). In human testis, DCN precipitates decorated collagen fibrils, as expected, and were found in interstitial and peritubular areas. Strikinly compact bundles of collagen fibrils surround the testicular tubules. Fine and distinct CMB precipitates, representing DCN (Scott and Bosworth 1990, Scott and Thomlison 1998, Orgel et al. 2009), interconnect neighbouring collagen fibrils at very regular distances of 70 nm (Fig. 1B). This observation is in agreement with findings on CMB distribution in other mammalian connective tissues (Scott and Bosworth 1990, Scott and Thomlison 1998) and can be best seen, if the collagen fibrils are cut longitudinally. In cross sections through collagen fibrils the CMB precipitates can be seen to form a complex system of straight or oblique interconnecting precipitates. CMB precipitates that may represent DCN were also found at the inner and outer aspect of the markedly thickened basal lamina of the testicular tubules.
Figure 1. Testicular DCN expression in adult man, monkey and mouse.
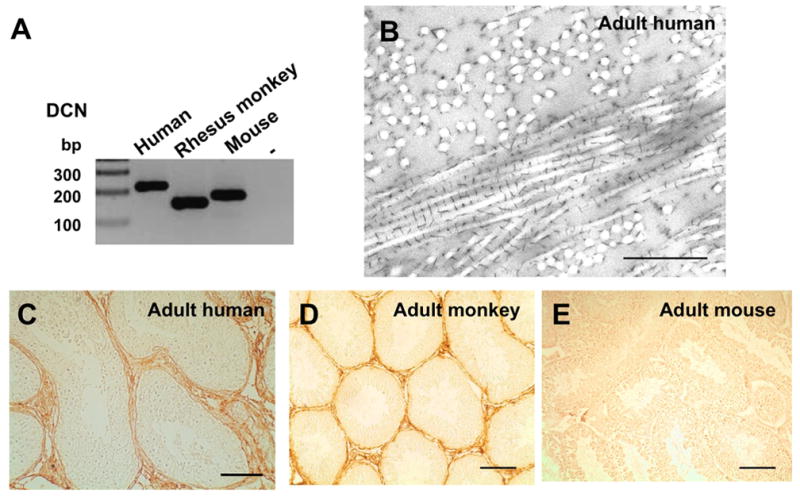
A: Result of RT-PCR: DCN is found in commercial testicular human cDNA, in whole testis tissue samples from monkeys and mice (-: no input cDNA).
B: DCN precipitates are decorating collagen fibrils in the peritubular wall of human testis. Bar: Approximately: 0.1 μm.
C: In normal adult human testis DCN is located to the peritubular wall and interstitial areas. Bar: Approximately 100 μm.
D: In the testis of an adult monkey (age: 27 years) DCN staining is seen in the peritubular area. Bar: Approximately 50 μm.
E: DCN staining in the testis of a wild type mouse at the age of 5 months is almost undetectable. Bar: Approximately 100 μm.
DCN accumulates in the ECM of the interstitial and peritubular compartment of testes of infertile men
As expected, sections of GA biopies examined by TEM showed abundant interstitial and peritubular collagen fibrilles (Fig. 2A). The distribution pattern of immunodetectable DCN in the testes of men with GA and SCOS, and interstitial and tubular fibrosis, was similar to that observed in the normal human testis. However, the DCN staining in the fibrotically remodeled tubular wall was much more abundant, mainly due to DCN deposits in the thickened layers of the ECM. DCN accumulation in interstitial areas of the testes was also found (Fig. 2B, C). All of the controls, performed with non-immune serum and omission of primary antibody, showed negative immunohistochemical staining (Fig. 2D).
Figure 2. DCN in the testis of infertile men with GA and SCOS.
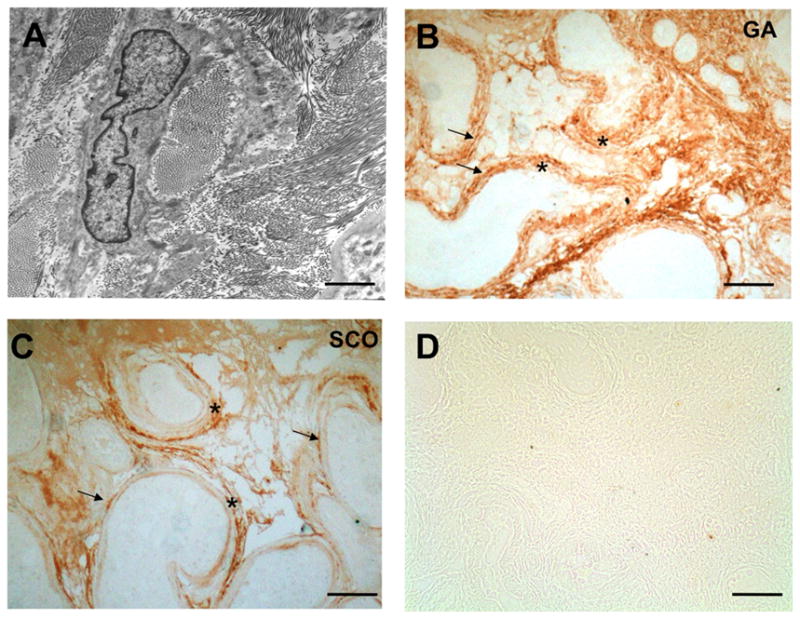
A: Electron micrographic view of a peritubular region of a patient with GA revealing abundant interstitial and peritubular collagen fibrils, in line with fibrotic remodeling of this compartment. Bar: Approximately 1 μm.
B: Immunoreactive DCN is detected in cells and extracellular sites in a biopsy sample of a GA patient: Strongest staining is seen in the peritubular cells lining the peritubular walls (arrows) and interstitial cells, which presumably are fibroblasts. Clear staining of DCN is also observed in the thickened ECM of the peritubular walls (asterisks) and interstitial areas. Bar: Approximately 100 μm.
C: DCN deposits in massively remodeled tubular walls (asterisks) stain strongly in a testis sample of SCOS patient. Arrows point to peritubular cells. Bar: Approximately 100 μm.
D: Control of an SCOS patient sample in which the antiserum was replaced by buffer. Bar: Approximately 200 μm.
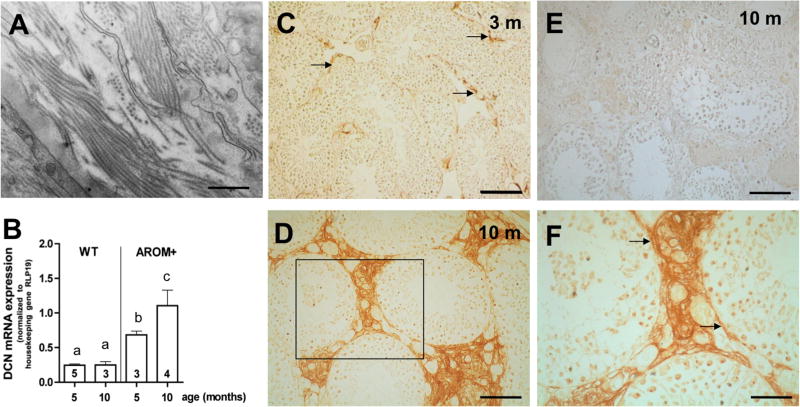
Increased DCN mRNA levels in testis of AROM+ mice
In line with previous observations of testicular fibrosis (Li et al. 2006) electron microscopy revealed massive collagen fibrils in AROM+ testes (Fig. 3A). Results of qRT-PCR measurements showed low DCN mRNA levels in the testes of wild type (WT) mice at the age of 5 respectively 10 months. Compared to WT, significantly increased DCN mRNA expression levels in the testes of 5-month-old transgenic AROM+ mice (Fig. 3B) were observed. While DCN mRNA expression level is not significantly altered in testes of aged WT mice, it further increases in AROM+ mouse testes. AROM+ mice below the age of 2–3 months have normal testicular morphology and function, but then develop progressive age-dependent testicular fibrosis, inflammatory reactions and impaired spermatogenesis (Li et al. 2001). These changes, as we found, were highly correlated with high levels of testicular DCN (Fig. 3B, D, F). In 3-month-old AROM+ mice DCN staining is likewise low as in testicular samples of WT, but DCN is already evident in the interstitial areas (Fig. 3C). However, at the age of 10 months a massive DCN expression in the peritubular region and the enlarged interstitial areas was apparent in testes of AROM+ animals (Fig. 3D, F). Controls with non-immune serum and omission of primary antibody were negative (Fig. 3E).
Figure 3. Testicular fibrosis and DCN in the testes of AROM+ mice.
A: Electron micrographic view of an AROM+ mouse testicular sample showing abundant collagen fibrils. Bar: Approximately 0.1 μm.
B: Summary of qRT-PCR screening of whole testis samples of Wild type (WT) mice (5- and 10-month-old) and of AROM+ mice (5- and 10-month-old). Results (means + SEM) were normalized to the housekeeping gene RPL19. DCN does not change in WT, but fibrotically altered testes of infertile AROM+ show a significantly higher DCN mRNA level than WT testes, which increases with age and the deterioration of spermatogenesis (p<0.05). Different letters above the columns indicate statistically significant differences (p<0.05). Numbers within the columns indicate the number of animals per group.
C: Immunhistochemical staining for DCN in the testis of an AROM+ mouse at 3 months of age shows low expression of DCN. However DCN can be detected already in the peritubular region and in the interstitial regions (arrows). Bar: Approximately 100 μm.
D: DCN accumulates massively in testicular interstitial cells of a 10-month-old AROM+ mouse. Bar: Approximately 50 μm.
E: Control in which the antiserum was replaced by buffer. Bar: Approximately 50 μm.
F: Higher magnification (of boxed area in D) of DCN staining in the testis of a 10-month-old AROM+ mouse shows that DCN is expressed, in addition to the interstitial region, in peritubular cells (arrows). Bar: Approximately 20 μm.
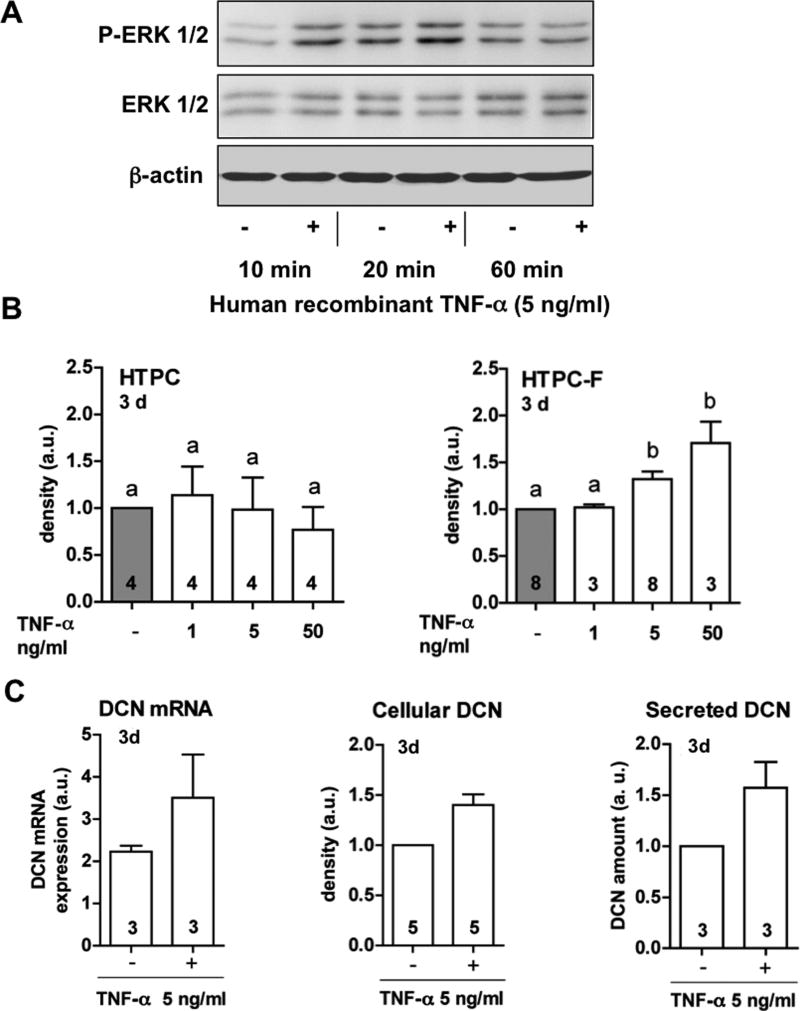
TNF-α stimulates DCN production and secretion in isolated peritubular cells of fibrotic human testes
HTPC-Fs, like HTPCs (Schell et al. 2008, Spinnler et al. 2010) express functional TNFRs as recombinant TNF-α (5 ng/ml) increased phosphorylation of ERK 1/2 in HTPC-Fs from three patients after 10, 20, but not 60 min of stimulation (Fig. 4A). Western blot results with total, non-phosphorylated ERK and β-actin revealed equal protein loading (Fig. 4A). However HTPC-Fs, but not HTPCs, responded to TNF-α by increasing DCN production and secretion after 3 days. TNF-α increased not only the amounts of cellular DCN, but also DCN mRNA and secreted protein after 3 days in HTPC-Fs (Fig. 4B, C).
Figure 4. TNF-α, stimulates DCN production and secretion by HTPC-Fs.
A: Western blot experiments revealed phosphorylation of ERK1/2 (P-ERK1/2) after 10 and 20 min, but not after 60 min, of TNF-α treatment (5 ng/ml) of HTPC-Fs. Levels of total ERK1/2 and β-actin are shown below.
B: Summary view of Western blot experiments: DCN protein levels in HTPCs were not affected by the treatment with different concentrations of TNF-α (1, 5 and 50 ng/ml) for 3 days (left graph), while in HTPC-Fs, DCN is regulated by TNF-α in dose-dependent manner. Results were normalized to β-actin protein levels (a.u.). Data shown are means + SEM of blots performed with cells of at least three different patients per group.
C: DCN mRNA expression, cellular and secreted protein levels after 72 h in HTPC-Fs exposed to 5 ng/ml TNF-α. DCN mRNA and protein levels in stimulated HTPC-Fs, are significantly higher than in untreated control cells (p<0.05). QRT-PCR results were normalized to the housekeeping gene RPL19. Western blot results were normalized to β-actin protein levels. ELISA values were normalized to total proteins amount and controls. Results are shown in arbitrary units (a.u.) and means +SEM. Numbers within columns show the number of patients used for each experiment.
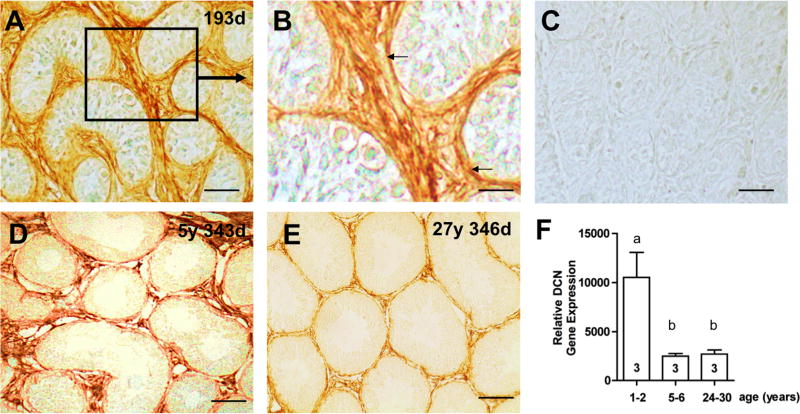
DCN in development of rhesus monkey testes
Testicular DCN during ontogeny was studied in the non-human primate. In young, prepubertal rhesus monkeys, immunohistochemical staining showed strong DCN immunoreactive signals associated with connective tissue cells (Fig. 5A, B). In contrast, in adult monkeys DCN distinctly marked the peritubular wall and interstitial areas, comparable to normal human testes (Fig. 1B; 5 D, E). Significant differences in testicular DCN expression levels were disclosed by gene microarray analysis of prepubertal (1–2 years, n=3), young adult (5–6 years, n=3), and old adult (26–30 years, n=3) animals (Fig. 5F). Testicular DCN levels were significantly higher in prepubertal animals than in young or old adults, and were inversely correlated with the expression of VASA (a spermatogenesis gene) and SMA (smooth muscle actin; a gene expressed in mature tubular wall cells), as well as testicular weight (Table 1). In contrast, the expression levels of ECM genes (collagen type I and IV and laminin) showed a positive correlation with DCN levels. Collagen type I and type IV expression in the testis were significantly reduced in the young and old adults, compared to prepubertal animals. Laminin expression did not change significantly but showed the same tendency as collagens. As expected, the expression levels of house keeping genes HPRT1 and SDHA were similar in each of the three age groups.
Figure 5. DCN during testicular development in rhesus monkey.
A: Immunohistochemical staining for DCN in the testis of a prepubertal rhesus monkey (193 days of age). DCN is strongly expressed in the peritubular region and in the interstitial regions. Bar: Approximately 50 μm.
B: Magnified view of the boxed area in A. DCN staining of cells of the peritubular wall (arrows) and the interstitial area of a prepubertal monkey testis. Bar: Approximately 25 μm.
C: Control (prepubertal animal) in which the antiserum was replaced by buffer. Bar: Approximately 50 μm.
D: Testicular DCN can be detected in a rhesus monkey at the age of 5 years and 343 days. DCN staining is seen in peritubular and interstitial regions. Bar: Approximately 50 μm.
E: In the testis of monkey of 27 years and 205 days of age a low DCN expression in the peritubular area can be detected. Bar: Approximately 50 μm.
F: Gene microarray data: Gene expression profiling of rhesus monkey testes show that DCN is highly expressed in prepubertal animals (1–2 years of age) and is significantly lower in young adults (5–6 years of age) and old adults (24–30 years of age). Numbers within the columns indicate the number of animals per group.
Table 1.
Summary of testes weights and gene array data of rhesus monkeys. Values are means of relative gene expression ± SEM. Different letters indicate statistically significant differences (p < 0.05).
| 1–2 years | 5–6 years | 24–30 years | ||
|---|---|---|---|---|
| Body weight (kg) | 2.2 ± 0.89 a | 8.4 ± 0.92 b | 7.3 ± 2.4 b | |
| Testis weight (g) | 0.3 ± 0.04 a | 16.2 ± 2.08 b | 21.1 ± 3.36 b | |
|
| ||||
| DCN | 10531 ± 2545 a | 2489 ± 271 b | 2703 ± 425 b | ECM components |
| Collagen type 1 | 6030 ± 1072 a | 730 ± 441 b | 420 ± 33 b | |
| Collagen type 4 | 1045 ± 138 a | 436 ± 60 b | 406 ± 148 b | |
| Laminin | 311 ± 150 a | 118 ± 26 a | 142 ± 31 a | |
|
| ||||
| SMA | 239 ± 47 a | 588 ± 191 b | 690 ± 47 b | SMC marker |
|
| ||||
| CD90 | 303 ± 26 a | 305 ± 11 a | 276 ± 32 a | Fibroblast marker |
|
| ||||
| VASA | 432 ± 148 a | 3760 ± 1349 b | 3446 ± 671 b | Germ cell marker |
|
| ||||
| HPRT1 | 5523 ± 1178 a | 4237 ± 1690 a | 3681 ± 989 a | Testicular house-keeping genes |
| SDHA | 536 ± 125 a | 665 ± 123 a | 744 ± 130 a | |
DISCUSSION
The results show that a high level of testicular DCN is inversely related to testicular functionality, namely spermatogenesis, in health and disease. In rhesus monkeys, the high DCN levels observed in the prepubertal, quiescent testes showed a marked decrease during sexual maturation and subsequently remained low even in the testes of old healthy animals. Furthermore, in a typical case of human male infertility and in a rodent model of infertility, testicular DCN levels increased significantly. In the latter case, this is partly due to stimulatory actions of immunological factors, which at least in HTPC-Fs stimulate DCN. Although the correlative nature of the present study does not prove a causative link between DCN and infertility, the known inhibitory actions of DCN at GF/GFR signaling, including recent studies using HTPCs/HTPC-Fs (Adam et al. 2011) strongly suggest that the regulation of DCN is an unexplored mechanism controlling paracrine signaling in the testis.
In our study DCN levels were associated with the amounts of testicular connective tissue, levels of fibrosis and expression of collagen type I. This is not surprising, because DCN is “decorating” collagen fibrills, a fact also clearly shown for human testicular samples in the present study. Interestingly, the DCN core protein is able to bind different collagen types, including types I, II, III, V and VI (Reed and Iozzo 2002), while its dermatan sulfate side chain can bind to the collagens type XII and XIV (Iozzo 1997). Interaction of DCN with the trypical basement membrane collagen type IV has not been reported although motives for DCN binding sites are present in collagen type IV gene (Parkin et al. 2011).
DCN is overexpressed in a number of human diseases, which involve fibrotic remodeling processes (e.g. pulmonary and nephrogenic systemic fibrosis (Fadic et al. 2006, Gambichler et al. 2009, van Straaten et al. 1999). In a recent study we reported increased focal DCN production in the fibrotic microenvironment of MA patients (Adam et al. 2011). In the present study we examined DCN expression in human SCOS and GA patients, which are distinct from MA patients. Indeed the patchy DCN pattern found in MA (Adam et al. 2011) was replaced by a rather homogeneous staining of the peritubular and interstitial compartment, which showed signs of fibrosis and abundant ECM material.
In normal mice, DCN is not detectable throughout postnatal development (Miqueloto and Zorn 2007, Soto-Suazo et al. 2002, and our unpublished data) and in the present study we found that it is barely detectable in adult gonads. However, adult transgenic AROM+ mice above the age of 2–3 months develop progressive age-dependent testicular fibrosis, inflammatory reactions and impaired spermatogenesis (Li et al. 2001). As we detected by qRT-PCR, these changes were correlated with high levels of DCN. Immunohistochemistry confirmed massive DCN levels in the interstitial areas and to some degree also in the peritubular areas of AROM+ mice, a result in line with the general changes in infertile men. It is possible that AROM+ mice present with a stronger interstitial fibrosis and DCN accumulation, than humans because rodents possess only one layer of peritubular cells (Nurmio et al. 2007), while humans have several.
TNF-α is a product of macrophages and mast cells in testes of mice and men and is significantly increased in AROM+ and in infertile men (Frungieri et al. 2002, Li et al. 2006.). Hence we tested whether it may be involved in the stimulation of DCN using primary human testicular cells isolated from peritubular walls. As reported, HTPCs isolated from normal human testis secrete less DCN than HTPC-Fs from fibrotically altered testes with deranged spermatogenesis (Adam et al. 2011) and TNF-α significantly stimulated DCN mRNA and secreted protein in HTPC-Fs. This action of TNF-α is in line with its effect on isolated human chondrocytes (Dodge et al. 1998), while in human dermal fibroblast cultures DCN expression decreased after TNF-α addition (Mauviel et al. 1995). As the prototype product of mast cells, the protease tryptase, also significantly increases DCN production in HTPC-Fs (Adam et al. 2011), immune cells appear to be involved in the stimulation of DCN in the human testis.
Reports on DCN in the testis are sparse, but one of the few studies did not find DCN in the pre- and postnatal mouse testis (Soto-Suazo et al. 2002, Miqueloto and Zorn 2007). Hence DCN may not play a role in murine gonadal development. Similarily, DCN may also not be of importance in the adult mouse either, where DCN is barely detectable. In contrast, human and monkey testes show robust DCN expression. Furthermore the degree of staining of testicular connective tissue and the levels of DCN gene expression are even more pronounced in the prepubertal monkey gonad than in the adult. What causes this physiological reduction of DCN during maturation remains to be shown. Yet, DCN is clearly inversely related to the degree of the activity of the testis, namely spermatogenesis.
In conclusion, the ECM proteoglycan DCN is present in the normal human and non-human primate testis (Ungefroren et al. 1995, Adam et al. 2011). In mice DCN appears of low abundance, however in infertile AROM+ mice DCN becomes strongly expressed. Thus in infertile men and mice, the observed increases in DCN levels correlate well with physiological or pathological states of infertility. DCN acts as a ligand to GFRs in many cells including human testicular cells (Adam et al. 2011). Based on this fact, we propose that this ECM component is an unexplored regulator of GF signaling in the testis. Consequently factors regulating testicular DCN levels are regulators of testicular function.
Acknowledgments
We thank Astrid Tiefenbacher, Gabriele Terfloth, Daniel Einwang and Sabine Tost for technical assistance. This work was financially supported by DFG MA 1080/16-3 and MA1080/21-1; DAAD, the Academy of Finland; NIH grants AG036670 and RR000163.
M.A. performed the majority of the work with peritubular cells and analyzed the results. H.F.U. and V.T.G. performed research on monkey tissues and analyzed the results. U.W. contributed to the electron microscopical studies, F.M.K. and J.U.S. contributed essential biopsies and helped in the interpretation of the data. L.S. and M.P. contributed essential animal models, significantly helped in the acquisition of expression data and the drafting of the manuscript. A.M. conceived of the study and designed the research. Together with M.A. he wrote the manuscript.
LIST OF ABBREVIATIONS
- AMC
Affymetrix GeneChip®
- CMB
Cupromeronic blue
- DCN
Decorin
- DMEM
Dulbecco’s modified Eagle’s medium
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- ELISA
Enzyme-linked immunosorbent assay
- ErbB2
v-erb-b2 erythroblastic leukemia viral oncogene homolog 2
- ErbB3
v-erb-b2 erythroblastic leukemia viral oncogene homolog 3
- ErbB4
v-erb-a erythroblastic leukemia viral oncogene homolog 4
- ERK 1/2
Extracellular regulated kinase 1/2
- FCS
Fetal calf serum
- GA
Germ cell arrest
- GCOS
GeneChip® Operating Software
- GDNF
glial cell line derived neurotrophic factor
- GF
Growth factor
- GFR
Growth factor receptor
- HGFR
Hepatocyte growth factor receptor
- HTPC
Human testicular peritubular cell
- HTPC-F
Human testicular peritubular cell from fibrotic testes
- HPRT1
Homo sapiens hypoxanthine phosphoribosyltransferase
- IGF1R
Insulin-like growth factor I receptor
- MA
Mixed atrophy
- MC
Mast cell
- PBS
Phosphate buffered saline
- PDGF
Platelet-derived growth factor
- PDGFR
Platelet-derived growth factor receptor
- RPL19
Ribosomal protein L19
- RTK
Receptor tyrosine kinase
- SCOS
Sertoli cell-only syndrome
- SDHA
Succinate dehydrogenase complex, subunit A
- SLRP
Small leucine-rich proteoglycans
- SMA
Smooth muscle actin
- TEM
Transmission electron microscopy
- TNF-α
Tumor necrosis factor α
- TNFR-1
Tumor necrosis factor receptor 1
- TNFR-2
Tumor necrosis factor receptor 2
- VASA
DDX4 DEAD (Asp-Glu-Ala-Asp) box polypetide 4
- VEGFR
Vascular endothelial growth factor receptor
- WT
Wild type
Footnotes
Disclosure summary
The authors have nothing to declare. This work was done in partial fulfillment of the requirements of a Dr. rer nat. thesis of M.A.
All authors contributed to the final version of the paper and approved it.
References
- Adam M, Schwarzer JU, Kohn FM, Strauss L, Poutanen M, Mayerhofer M. Mast cell tryptase stimulates production of decorin by human testicular peritubular cells: possible role in male infertility with growth factor signaling? Hum Reprod. 2011;26:2613–25. doi: 10.1093/humrep/der245. [DOI] [PubMed] [Google Scholar]
- Albrecht M, Ramsch R, Kohn FM, Schwarzer JU, Mayerhofer A. Isolation and cultivation of human testicular peritubular cells: a new model for the investigation of fibrotic processes in the human testis and male infertility. J Clin Endocrinol Metab. 2006;91:1956–60. doi: 10.1210/jc.2005-2169. [DOI] [PubMed] [Google Scholar]
- Basciani S, Mariani S, Spera G, Gnessi L. Role of platelet-derived growth factors in the testis. Endocr. 2010;31:916–39. doi: 10.1210/er.2010-0004. Rev. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Breucker H, Holstein AF, Seidl K. Cellular architecture of the lamina propria of human seminiferous tubules. Cell Tissue Res. 1990;262:253–61. doi: 10.1007/BF00309880. [DOI] [PubMed] [Google Scholar]
- Dobashi M, Fujisawa M, Naito I, Yamazaki T, Okada H, Kamidono S. Distribution of type IV collagen subtypes in human testes and their association with spermatogenesis. Fertil Steril. 2003;80(Suppl 2):755–60. doi: 10.1016/s0015-0282(03)00775-1. [DOI] [PubMed] [Google Scholar]
- Dodge GR, Diaz A, Sanz-Rodriguez C, Reginato AM, Jimenez SA. Effects of interferon-gamma and tumor necrosis factor alpha on the expression of the genes encoding aggrecan, biglycan, and decorin core proteins in cultured human chondrocytes. Arthritis Rheum. 1998;41:274–83. doi: 10.1002/1529-0131(199802)41:2<274::AID-ART11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fadic R, Mezzano V, Alvarez K, Cabrera D, Holmgren J, Brandan E. Increase in decorin and biglycan in Duchenne Muscular Dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. J Cell Mol Med. 2006;10:758–69. doi: 10.1111/j.1582-4934.2006.tb00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frungieri MB, Calandra RS, Lustig L, Meineke V, Kohn FM, Vogt HJ, Mayerhofer A. Number, distribution pattern, and identification of macrophages in the testes of infertile men. Fertil Steril. 2002;78:298–306. doi: 10.1016/s0015-0282(02)03206-5. [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Urbanski HF, Hohne-Zell B, Mayerhofer A. Neuronal elements in the testis of the rhesus monkey: ontogeny, characterization and relationship to testicular cells. Neuroendocrinology. 2000;71:43–50. doi: 10.1159/000054519. [DOI] [PubMed] [Google Scholar]
- Gambichler T, Kreuter A, Skrygan M, Burkert B, Altmeyer P, Schieren G. Decorin is significantly overexpressed in nephrogenic systemic fibrosis. Am J Clin Pathol. 2009;132:139–43. doi: 10.1309/AJCPGB55YDURJXZC. [DOI] [PubMed] [Google Scholar]
- Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–54. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulkesen KH, Erdogru T, Sargin CF, Karpuzoglu G. Expression of extracellular matrix proteins and vimentin in testes of azoospermic man: an immunohistochemical and morphometric study. Asian J Androl. 2002;4:55–60. [PubMed] [Google Scholar]
- Haider SG, Talati J, Servos G. Ultrastructure of peritubular tissue in association with tubular hyalinization in human testis. Tissue Cell. 1999;31:90–8. doi: 10.1054/tice.1999.0005. [DOI] [PubMed] [Google Scholar]
- Haigh M, Scott JE. A method of processing tissue sections for staining with cu-promeronic blue and other dyes, using CEC techniques, for light and electron microscopy. Basic Appl Histochem. 1986;30:479–86. [PubMed] [Google Scholar]
- Iacob D, Cai J, Tsonis M, Babwah A, Chakraborty C, Bhattacharjee RN, Lala PK. Decorin-mediated inhibition of proliferation and migration of the human trophoblast via different tyrosine kinase receptors. Endocrinology. 2008;149:6187–97. doi: 10.1210/en.2008-0780. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–74. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–6. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- Jarvelainen H, Puolakkainen P, Pakkanen S, Brown EL, Hook M, Iozzo RV, Sage EH, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–52. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Downs JL, Urbanski HF. Twenty-four-hour rhythmic gene expression in the rhesus macaque adrenal gland. Mol Endocrinol. 2006;20:1164–76. doi: 10.1210/me.2005-0361. [DOI] [PubMed] [Google Scholar]
- Li X, Makela S, Streng T, Santti R, Poutanen M. Phenotype characteristics of transgenic male mice expressing human aromatase under ubiquitin C promoter. J Steroid Biochem Mol Biol. 2003;86:469–76. doi: 10.1016/s0960-0760(03)00376-5. [DOI] [PubMed] [Google Scholar]
- Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142:2435–42. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- Li X, Strauss L, Kaatrasalo A, Mayerhofer A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Transgenic mice expressing p450 aromatase as a model for male infertility associated with chronic inflammation in the testis. Endocrinology. 2006;147:1271–7. doi: 10.1210/en.2005-0654. [DOI] [PubMed] [Google Scholar]
- Mauviel A, Santra M, Chen YQ, Uitto J, Iozzo RV. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-alpha. J Biol Chem. 1995;270:11692–700. doi: 10.1074/jbc.270.19.11692. [DOI] [PubMed] [Google Scholar]
- Meineke V, Frungieri MB, Jessberger B, Vogt H, Mayerhofer A. Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril. 2000;74:239–44. doi: 10.1016/s0015-0282(00)00626-9. [DOI] [PubMed] [Google Scholar]
- Miqueloto CA, Zorn TM. Characterization and distribution of hyaluronan and the proteoglycans decorin, biglycan and perlecan in the developing embryonic mouse gonad. J Anat. 2007;211:16–25. doi: 10.1111/j.1469-7580.2007.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nili N, Cheema AN, Giordano FJ, Barolet AW, Babaei S, Hickey R, Eskandarian MR, Smeets M, Butany J, Pasterkamp G, Strauss BH. Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am J Pathol. 2003;163:869–78. doi: 10.1016/S0002-9440(10)63447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmio M, Toppari J, Zaman F, Andersson AM, Paranko J, Soder O, Jahnukainen K. Inhibition of tyrosine kinases PDGFR and C-Kit by imatinib mesylate interferes with postnatal testicular development in the rat. Int J Androl. 2007;30:366–76. doi: 10.1111/j.1365-2605.2007.00755.x. discussion 376. [DOI] [PubMed] [Google Scholar]
- Orgel JP, Eid A, Antipova O, Bella J, Scott JE. Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PLoS One. 2009;4:e7028. doi: 10.1371/journal.pone.0007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin JD, San Antonio JD, Pedchenko V, Hudson B, Jensen ST, Savige J. Mapping structural landmarks, ligand binding sites and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions and variation in phenotypes in inherited diseases affecting basement membranes. Hum Mutat. 2011;32:127–43. doi: 10.1002/humu.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelliniemi LJ, Paranko J, Grund SK, Frojdman K, Foidart JM, Lakkala-Paranko T. Extracellular matrix in testicular differentiation. Ann N Y Acad Sci. 1984;438:405–16. doi: 10.1111/j.1749-6632.1984.tb38302.x. [DOI] [PubMed] [Google Scholar]
- Pollanen PP, Kallajoki M, Risteli L, Risteli J, Suominen JJ. Laminin and type IV collagen in the human testis. Int J Androl. 1985;8:337–47. doi: 10.1111/j.1365-2605.1985.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–55. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- Santamaria L, Martinez-Onsurbe P, Paniagua R, Nistal M. Laminin, type IV collagen, and fibronectin in normal and cryptorchid human testes. An immunohistochemical study. Int J Androl. 1990;13:135–46. doi: 10.1111/j.1365-2605.1990.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–9. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell C, Albrecht M, Mayer C, Schwarzer JU, Frungieri MB, Mayerhofer A. Exploring human testicular peritubular cells: identification of secretory products and regulation by tumor necrosis factor-alpha. Endocrinology. 2008;149:1678–86. doi: 10.1210/en.2007-1064. [DOI] [PubMed] [Google Scholar]
- Schell C, Albrecht M, Spillner S, Mayer C, Kunz L, Kohn FM, Schwarzer U, Mayerhofer A. 15-Deoxy-delta 12–14-prostaglandin-J2 induces hypertrophy and loss of contractility in human testicular peritubular cells: implications for human male fertility. Endocrinology. 2010;151:1257–68. doi: 10.1210/en.2009-1325. [DOI] [PubMed] [Google Scholar]
- Scott JE, Bosworth TR. A comparative biochemical and ultrastructural study of proteoglycan-collagen interactions in corneal stroma. Functional and metabolic implications. Biochem J. 1990;270:491–7. doi: 10.1042/bj2700491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JE, Thomlinson AM. The structure of interfibrillar proteoglycan bridges (shape modules’) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J Anat. 1998;192(Pt 3):391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MK, Cheng CY. Extracellular matrix and its role in spermatogenesis. Adv Exp Med Biol. 2008;636:74–91. doi: 10.1007/978-0-387-09597-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Suazo M, San Martin S, Ferro ES, Zorn TM. Differential expression of glycosaminoglycans and proteoglycans in the migratory pathway of the primordial germ cells of the mouse. Histochem Cell Biol. 2002;118:69–78. doi: 10.1007/s00418-002-0414-2. [DOI] [PubMed] [Google Scholar]
- Spinnler K, Kohn FM, Schwarzer U, Mayerhofer A. Glial cell line-derived neurotrophic factor is constitutively produced by human testicular peritubular cells and may contribute to the spermatogonial stem cell niche in man. Hum Reprod. 2010;25:2181–7. doi: 10.1093/humrep/deq170. [DOI] [PubMed] [Google Scholar]
- Strauss L, Kallio J, Desai N, Pakarinen P, Miettinen T, Gylling H, Albrecht M, Makela S, Mayerhofer A, Poutanen M. Increased exposure to estrogens disturbs maturation, steroidogenesis, and cholesterol homeostasis via estrogen receptor alpha in adult mouse Leydig cells. Endocrinology. 2009;150:2865–72. doi: 10.1210/en.2008-1311. [DOI] [PubMed] [Google Scholar]
- Takaba H. A morphological study of the testes in patients with idiopathic male infertility--immunohistochemical analysis of collagens and laminin in human testes. Hinyokika Kiyo. 1990;36:1173–80. [PubMed] [Google Scholar]
- Ungefroren H, Ergun S, Krull NB, Holstein AF. Expression of the small proteoglycans biglycan and decorin in the adult human testis. Biol Reprod. 1995;52:1095–105. doi: 10.1095/biolreprod52.5.1095. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Noriega NC, Lemos DR, Kohama SG. Gene expression profiling in the rhesus macaque: experimental design considerations. Methods. 2009;49:26–31. doi: 10.1016/j.ymeth.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straaten JF, Coers W, Noordhoek JA, Huitema S, Flipsen JT, Kauffman HF, Timens W, Postma DS. Proteoglycan changes in the extracellular matrix of lung tissue from patients with pulmonary emphysema. Mod Pathol. 1999;12:697–705. [PubMed] [Google Scholar]
- Yan YC, Sun YP, Zhang ML. Testis epidermal growth factor and spermatogenesis. Arch Androl. 1998;40:133–46. doi: 10.3109/01485019808987936. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–79. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]