Abstract
Background
The associations between nicotine dependence and specific variants in the nicotinic receptor CHRNA5-A3-B4 subunit genes are irrefutable with replications in many studies. The relationship between the newly identified genetic risk variants for nicotine dependence and comorbid psychiatric disorders is unclear. We examined whether these genetic variants were associated with comorbid disorders and whether comorbid psychiatric disorders modified the genetic risk of nicotine dependence.
Methods
In a case control study of nicotine dependence with 2032 subjects of European descent, we used logistic regression models to examine the pleiotropy and risk moderation. Comorbid disorders examined were alcohol dependence, cannabis dependence, major depressive disorder, panic attack, social phobia, posttraumatic stress disorder (PTSD), attention deficit hyperactivity disorder (ADHD), conduct disorder, and antisocial personality disorder (ASPD).
Results
Nicotine dependence was associated with every examined comorbid psychiatric disorders, with odds ratio varying from 1.75 to 3.33. No evidence supported the associations between the genetic variants and the comorbid disorders (pleiotropy). No evidence suggested that the risks for nicotine dependence associated with the genetic variants vary with comorbid psychiatric disorders in general, but the power was limited in detecting interactions.
Conclusions
The genetic risks of nicotine dependence associated with the CHRNA5-A3-B4 subunit genes are specific, and not shared among commonly comorbid psychiatric disorders. The risks for nicotine dependence associated with these genetic variants are not modified by comorbid psychiatric disorders such as major depressive disorder or alcohol dependence. However, the power is an important limitation in studying the interplay of comorbidity and genetic variants.
Keywords: nicotine dependence, nicotinic receptor genes, case control study, comorbidity, pleiotropy, genetic epidemiology
1. Introduction
Tobacco smoking continues to be a serious public health problem. The proportion of United States (US) adults who are current cigarette smokers remains high; approximately 20% of the population continues to smoke despite the public health efforts to reduce tobacco use (CDC, 2009). Smoking is known to be associated with significant morbidity and mortality by causing 443,000 premature deaths and costing $193 billion in direct health-care and productivity loss every year in the US (CDC, 2008). In addition, smoking co-occurs with many mental disorders.
Smoking commonly co-occurs with substance dependence, mood disorders, anxiety disorders, and other behavioral disorders (Agrawal et al., 2008; Breslau, 1995; Breslau et al., 2004a, b; Falk et al., 2006; Grant et al., 2004; Griesler et al., 2008; John et al., 2003; Klungsoyr et al., 2006; Pasco et al., 2008; Pedersen and von Soest, 2009; Sihvola et al., 2008). For example, daily smoking is associated with the onset of alcohol dependence and major depressive disorder (Breslau et al., 2004a). In general, individuals with nicotine dependence have a two to three fold increased odds for having another psychiatric disorder.
There are multiple potential reasons for comorbidity. One possible mechanism is that one disorder causes another. It is also possible that some underlying environmental risk factor increases the likelihood of developing both disorders. For example, traumatic events such as severe abuse can increase the risk of developing multiple disorders such as alcohol dependence, post-traumatic stress disorder (PTSD), and major depressive disorder. Another possible explanation for the increased comorbidity is that a common underlying genetic risk leads to both illnesses.
The common vulnerability theory posits an underlying vulnerability factor predisposes the clustering of disorders. Twin studies support the common vulnerability theory and demonstrate common genetic influence shared between nicotine dependence and many comorbid disorders. Twin studies show that nicotine and alcohol dependence share genetic influences (Golub and Johnson, 2001; Hettema et al., 1999; True et al., 1999; Young et al., 2006). Twin studies also show common genetic influence for both nicotine dependence and major depressive disorder (Kendler et al., 1993; Lyons et al., 2008), and an overlapping genetic risk for nicotine dependence, conduct disorder, and antisocial personality disorder (ASPD; Fu et al., 2007). This work provides evidence that some genetic variants may increase the risk of developing nicotine dependence, other substance dependence as well as other psychiatric disorders.
Recent findings from large scale genetic studies demonstrate that specific genetic variants contribute to the development of nicotine dependence and this research brings unprecedented opportunities for further characterization of the genetic risk for nicotine dependence and comorbid disorders. Recent genome-wide association meta-analyses of large samples with European descent identify associations between smoking quantity (cigarettes per day) and multiple genetic variants. The most consistent genetic finding points to a locus in the CHRNA5-CHRNA3-CHRNB4 gene cluster on chromosome 15 (Liu et al., 2010; TAG, 2010; Thorgeirsson et al., 2010). Evidence shows that the genetic risk for nicotine dependence is influenced by at least two distinct biological mechanisms: receptor functional variation in CHRNA5 marked by rs16969968 and variability in CHRNA5 mRNA expression tagged by rs588765 (Bierut et al., 2008; Wang et al., 2009). Joint analysis of the uncorrelated Single Nucleotide Polymorphisms (SNPs) rs16969968 and rs588765 indicates that these two variants each exert independent influence on nicotine dependence vulnerability (Saccone et al., 2010).
These strong genetic findings for nicotine dependence now allow us to increase our knowledge on how genetic risk factors influence nicotine dependence and comorbid psychiatric disorders. For instance, we can test whether these genetic variants in the chromosome 15 region which increase the risk for developing nicotine dependence are also associated with commonly comorbid disorders such as alcohol dependence and major depressive disorder. In addition, we can test whether or not comorbid psychiatric disorders moderate the genetic risk for nicotine dependence. Potential genetic risk moderation is important from both etiologic and preventive standpoints (Hunter, 2005). It can provide clues about how genetic variants may affect the disease risk, through potential interactions with other genetic or environmental factors associated with a comorbid disorder. In addition, this work can help identify subpopulations that are more susceptible to the genetic risks. For example, depressed individuals may be at increased genetic risk for developing nicotine dependence.
Using data from the Collaborative Genetic Study of Nicotine Dependence (COGEND), we employed a comprehensive approach to examine the relationship between nicotine dependence, comorbid psychiatric disorders, and specific high priority genetic variants for nicotine dependence. Because of the evidence of multiple genetic factors that independently contribute to nicotine dependence in the chromosome 15 region, we used diplotypes of rs16969968 and rs588765 to represent the genetic risk in the CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster. In this paper we examined the following: (1) whether dependent smokers (as compared to non-dependent smokers) had increased risk for comorbid psychiatric disorders, (2) whether the diplotypes were associated with each comorbid disorder (alcohol dependence, cannabis dependence, major depressive disorder, panic attack, social phobia, posttraumatic stress disorder (PTSD), attention deficit hyperactivity disorder (ADHD), conduct disorder, and antisocial personality disorder (ASPD)) and whether the associations remained significant after controlling for nicotine dependence, (3) whether the genetic risk for nicotine dependence associated with the diplotypes of SNPs (rs16969968, rs588765) was moderated by comorbid psychiatric disorders, and (4) the statistical power to test the above hypotheses. The answers to questions of pleiotropy (the genetic effect of a single gene on multiple phenotypic traits) between nicotine dependence and other psychiatric disorders and of possible genetic risk moderation by comorbid disorders provide a critical step to further our understanding of common versus specific vulnerability for nicotine dependence and comorbid disorders.
2. METHODS
2.1. Subjects
The COGEND sample consists of individuals aged 25-44 who were recruited through telephone screening in St. Louis, Missouri and Detroit, Michigan. Subjects included 1054 nicotine dependence cases and 978 controls selected from the COGEND study who self-identified as being of European descent (Bierut et al., 2007). Nicotine dependent cases were defined as current smokers with a Fagerström Test for Nicotine Dependence (FTND) score of 4 or more (maximum score of 10; Heatherton et al., 1991). The FTND is an ordinal scale commonly used to measure nicotine dependence. Controls were defined as smokers (individuals who smoked at least 100 cigarettes lifetime), who never experienced any symptoms of dependence (lifetime FTND=0 or 1). The threshold of 100 cigarettes smoked over the lifetime is a commonly used threshold for significant smoking exposure (Bondy et al., 2009). By selecting controls who smoked, we focused our study on those genetic effects specific to the development of nicotine dependence. Only subjects of European American descent were included to decrease the genetic heterogeneity. The study was approved by the Institutional Review Board at each data collection site, and subjects provided informed consent prior to participating.
2.2. Measurements
2.2.1. Phenotypic Data
All subjects were personally interviewed using the Semi-Structured Assessment for Nicotine Dependence (SSAND) which was developed specifically for COGEND and was modeled after the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994; Hesselbrock et al., 1999) and the Composite International Diagnostic Interview (CIDI; Robins et al., 1988). Nicotine dependence was assessed with Fagerström Test for Nicotine Dependence (FTND). In addition, the SSAND included assessments of DSM IV diagnoses of alcohol dependence, cannabis dependence, major depressive disorder, panic attacks, social phobia, PTSD, ADHD, conduct disorder, and ASPD.
2.2.2. Genetic Data
Blood samples were collected for genetic analyses, and genotypic data were cleaned extensively (Bierut et al., 2007; Saccone et al., 2009; Saccone et al., 2007). We analyzed diplotypes using two distinct genetic variants in nicotinic receptor genes: rs16969968 and rs588765, which are located in the CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster on chromosome 15 and have previously been shown to be associated with nicotine dependence and function of CHRNA5 nicotinic receptor subunit (Saccone et al., 2009).
2.3. Analyses
The purpose of these analyses is to examine 1) the relationship between nicotine dependence and other psychiatric disorders, 2) the association between these genetic variants and comorbid psychiatric disorders, 3) the potential risk moderation by comorbid psychiatric disorder for nicotine dependence, and 4) the power. First, we examined the distribution of comorbid disorders among cases and control, and then modeled the lifetime associations between nicotine dependence and comorbid disorders in logistic regression models adjusting for age, gender, and education. Because lifetime prevalences of many psychiatric disorders were known to differ between men and women, we compared the associations of nicotine dependence with comorbid disorders across gender. The differences in comorbidity patterns across gender were then tested in logistic regression models.
Second, a series of analyses examined the potential genetic contribution of these variants to the comorbid disorder. We first modeled the risk for each of the comorbid disorders associated with age, gender, education, and the diplotype, before adding nicotine dependence as an additional covariate to test for potential mediation.
Third, in order to examine possible moderation of genetic risks for nicotine dependence, we examined the main effects of the diplotypes (rs16969968, rs588765) and comorbid psychiatric disorder risk factors jointly by examining the additive effect of genetic and comorbid risks for nicotine dependence using logistic regression models. Next, a standard series of regression equations was used to test main effects and diplotype × comorbidity interactions.
Last, we conducted a simulation to compute power for detecting various effect sizes with the constraints of our sample size and study design using SAS. These estimation results were critical in the interpretation of our results. We performed the power calculation for our sample size of 2,032 in three steps. First, a simulated dataset was produced under a logistic regression model with assumed proportions and effect sizes (odds radios) for the variables in the model (including the interaction term if it was included in the model). Next, the regression model was executed using the simulated data and subsequently tested to determine whether the assumed effect size was significantly different from zero. The Wald Chi-square statistic (including the degrees of freedom) resulting from the test was taken as a non-centrality parameter of the non-central Chi-square distribution. Finally, power was calculated by subtracting the probability of the non-central Chi-square distribution from 1. Type I error was set to 0.05.
We calculated power for three sets of models: (1) testing pleiotropy between nicotine dependence and each of the comorbid disorders, (2) testing pleiotropy while adjusting for nicotine dependence, and (3) testing genetic risk moderation for nicotine dependence by each of the comorbid disorders. To compute the power for detecting the various interaction effect sizes, we focus on the power for the interaction of AA_CC diplotype for rs16969968 and rs588765 with comorbid disorders.
3. RESULTS
3.1. Associations between Nicotine Dependence and Comorbid Psychiatric Disorders
Table 1 shows the distribution of age, gender, education, and comorbid psychiatric disorders among nicotine dependence cases and non-dependent smoking controls. Nicotine dependent smokers, when compared to non-dependent smokers, were significantly more likely to have a history of alcohol dependence, cannabis dependence, major depressive disorder, panic attacks, social phobia, PTSD, ADHD, conduct disorder, and ASPD. For example, 17.2% of nicotine dependent smoking cases, had a lifetime diagnosis of alcohol dependence compared to 7.2% of non-nicotine dependence controls (Odds Ratio (OR)=2.31, 95% Confidence Interval (CI)=1.67-3.20, p=4.8E-07). Similarly, 26.7% of nicotine dependent cases and 16.4% of non-nicotine dependent controls had a history of major depressive disorder (OR=2.17, 95% CI=1.70-2.78, p=7.5E-10). Though all of the subjects in the study have a history of smoking, the nicotine dependent smokers had a higher rate of being afflicted with all the comorbid disorders, compared to non-dependent smokers.
Table 1.
Nicotine Dependence and Comorbid Psychiatric Disorders
| Cases (n=1054) | Controls (n=978) | 95% C.I. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | LB | UB | P value | ||
| Age (mean, SD) | 36.9 | 5.39 | 35.9 | 5.52 | 1.02 | 1.01 | 1.04 | 0.014 | |
| Sex | Male | 487 | 46.2% | 300 | 30.7% | 2.15 | 1.75 | 2.63 | 1.5E-13 |
| Female | 567 | 53.8% | 678 | 69.3% | ref | ref | ref | ref | |
| Education (above high school)* | No | 646 | 61.3% | 193 | 19.7% | ref | ref | ref | ref |
| Yes | 408 | 38.7% | 785 | 80.3% | 0.15 | 0.12 | 0.19 | 4.8E-73 | |
| Alcohol dependence | 178 | 17.2% | 70 | 7.2% | 2.31 | 1.67 | 3.20 | 4.8E-07 | |
| Cannabis dependence | 152 | 15.1% | 43 | 4.5% | 3.09 | 2.11 | 4.54 | 8.4E-09 | |
| Major depressive disorder | 279 | 26.7% | 160 | 16.4% | 2.17 | 1.70 | 2.78 | 7.5E-10 | |
| Panic attack | 344 | 33.0% | 219 | 22.4% | 2.10 | 1.67 | 2.63 | 1.6E-10 | |
| Social Phobia | 236 | 23.5% | 130 | 13.9% | 1.75 | 1.34 | 2.28 | 3.3E-05 | |
| Posttraumatic stress disorder | 97 | 9.7% | 56 | 6.0% | 2.08 | 1.42 | 3.04 | 1.8E-04 | |
| Attention deficit hyperactive disorder | 158 | 15.7% | 71 | 7.6% | 2.00 | 1.44 | 2.78 | 3.4E-05 | |
| Conduct disorder | 137 | 13.2% | 41 | 4.2% | 2.82 | 1.90 | 4.19 | 2.9E-07 | |
| Antisocial personality disorder | 112 | 11.1% | 27 | 2.9% | 3.33 | 2.09 | 5.31 | 4.2E-07 | |
OR: odds ratio. 95%C.I.: confidence interval. LB: lower bound. UB: upper bound. ref: reference. SD: standard deviation.
ORs are adjusted for socio-demographic factors (age, gender, and education).
Education is split by median to two levels (above high school; high school graduation or less).
Table 2 shows rates and odds ratios for comorbid disorders among cases and controls stratified by gender, adjusted for age and education. Nicotine dependence was associated with examined psychiatric disorders similarly across gender with few exceptions. In women, nicotine dependence was associated with an increased risk of social phobia (OR=2.16, 95% CI=1.58-2.97, p=1.7E-06), but not in men (OR=1.14, 95%CI=0.72-1.81, p=0.58), and the difference was significant (wald=4.90, p=0.027). The associations between nicotine dependence and other examined disorders were not statistically different across gender, so in the subsequent analyses the genders were analyzed together.
Table 2.
Comorbid Disorders among Nicotine Dependent Cases and Controls, Stratified by Gender
| Men | Women | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cases | controls | 95%CI | cases | controls | 95%CI | ||||||||
| N=487 | N=300 | OR | LB | UB | p | N=567 | N=678 | OR | LB | UB | p | ||
| Alcohol dependence | n | 103 | 27 | 2.14 | 1.30 | 3.52 | 3.5E-03 | 75 | 43 | 2.37 | 1.54 | 3.63 | 8.1E-05 |
| % | 21.4% | 9.1% | 13.5% | 6.4% | |||||||||
| Cannabis dependence | n | 92 | 23 | 2.44 | 1.44 | 4.13 | 1.0E-03 | 60 | 20 | 3.95 | 2.26 | 6.89 | 1.4E-06 |
| % | 20.0% | 7.8% | 11.0% | 3.0% | |||||||||
| Major depressive disorder | n | 93 | 40 | 1.67 | 1.07 | 2.61 | 2.5E-02 | 186 | 120 | 2.42 | 1.81 | 3.23 | 2.0E-09 |
| % | 19.2% | 13.4% | 33.2% | 17.7% | |||||||||
| Panic attack | n | 123 | 47 | 1.97 | 1.31 | 2.98 | 1.2E-03 | 221 | 172 | 2.05 | 1.57 | 2.67 | 1.4E-07 |
| % | 25.2% | 15.7% | 39.5% | 25.4% | |||||||||
| Social phobiaa | n | 82 | 40 | 1.14 | 0.72 | 1.81 | 5.8E-01 | 154 | 90 | 2.16 | 1.58 | 2.97 | 1.7E-06 |
| % | 17.7% | 14.2% | 28.4% | 13.8% | |||||||||
| Posttraumatic stress disordera | n | 16 | 10 | 1.25 | 0.52 | 2.99 | 6.2E-01 | 81 | 46 | 2.37 | 1.57 | 3.59 | 4.5E-05 |
| % | 3.4% | 3.5% | 15.2% | 7.1% | |||||||||
| Attention deficit hyperactive disorder | n | 98 | 39 | 1.75 | 1.12 | 2.75 | 1.5E-02 | 60 | 32 | 2.38 | 1.47 | 3.86 | 4.1E-04 |
| % | 20.9% | 13.6% | 11.2% | 4.9% | |||||||||
| Conduct disorder | n | 92 | 27 | 2.37 | 1.44 | 3.91 | 1.6E-03 | 45 | 14 | 4.14 | 2.16 | 7.94 | 1.8E-05 |
| % | 19.1% | 9.2% | 8.1% | 2.1% | |||||||||
| Antisocial personality disorder | n | 77 | 19 | 2.81 | 1.59 | 4.98 | 3.7E-04 | 35 | 8 | 5.36 | 2.37 | 12.1 | 5.6E-05 |
| % | 16.3% | 6.7% | 6.5% | 1.2% | |||||||||
OR: odds ratio. 95%C.I.: confidence interval. LB: lower bound. UB: upper bound.
ORs were computed from respective logistic regression models using nicotine dependence as a response variable.
ORs are adjusted for socio-demographic factors (age and education) as these models were stratified by gender.
The comorbidity between nicotine dependence and comorbid disorder is stronger in women than in men, indicated by interactions significant for social phobia (wald=4.90, p=.027).
Table 3 shows the association between nicotine dependence and the diplotype (rs16969968 and rs588765). The relationship between these two SNPs is shown in Table 3(a), with three sparse cells representing the evolutionary history between rs16969968 and rs588765. Table 3(b) shows the association between the diplotypes and nicotine dependence using the GG_CC diplotype (lowest risk) as the reference group, and that the AA_CC diplotype is associated with the highest risk for nicotine dependence.
Table 3.
Association of nicotine dependence with the diplotypes (rs16969968, rs588765)
| (a) Joint Distribution of the Diplotypes (rs16969968, rs588765) | ||||
|---|---|---|---|---|
| Rs588765 genotype |
||||
| CC | CT | TT | ||
| Rs16969968 genotype | GG | 103 | 384 | 365 |
| GA | 314 | 585 | 3a | |
| AA | 258 | 1a | 0 | |
individuals with these rare diplotypes were excluded from the model in Table 3(b).
| (b) Genetic Risk of Nicotine Dependence Associated with the Diplotypes (rs16969968-rs588765) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | 95%CI | ||||||||
| n | % | n | % | OR | LB | UB | Wald | P value | ||
| Diplotype (rs16969968, rs588765) | 21.6 | 6.3E-04b | ||||||||
| GG_CC | 41 | 3.9% | 62 | 6.4% | ref | ref | ref | |||
| GG_CT | 187 | 17.8% | 207 | 21.3% | 1.29 | 0.79 | 2.10 | 1. 6 | 0.32 | |
| GG_TT | 182 | 17.3% | 183 | 18.9% | 1.54 | 0.94 | 2.52 | 2.9 | 0.09 | |
| GA_CC | 157 | 15.0% | 157 | 16.2% | 1.43 | 0.86 | 2.43 | 3.2 | 0.17 | |
| GA_CT | 311 | 29.6% | 274 | 28.2% | 1.66 | 1.03 | 2.67 | 5.7 | 0.037 | |
| AA_CC | 171 | 16.3% | 87 | 9.0% | 2.70 | 1.60 | 4.57 | 18.9 | 2.1E-04 | |
OR: odds ratio. 95%C.I.: confidence interval. LB: lower bound. UB: upper bound.
omnibus p for overall diplotypic effect. ref: reference.
ORs were computed from a logistic regression model using nicotine dependence as a response variable, the diplotypes, age, gender, and education as covariates.
3.2. Genetic Pleiotropy: Testing the Association of the Genetic Variants and Psychiatric Disorders
Table 4 shows the associations between the risk diplotype (rs16969968, rs588765) and each of the comorbid disorders to test the potential pleiotropic effect of these variants for nicotine dependence and the comorbid psychiatric disorders. We examined the risk for each comorbid disorder with age, gender, education, and diplotype in Model 1, and tested the potential mediating effect of nicotine dependence in Model 2. We found no evidence of associations between the diplotypes and any comorbid disorders. Although there were trends of association, none of the genetic associations were significant when adjusted for multiple comparisons. We found similar results when defining PTSD by excluding subjects without prior exposure to traumatic events.
Table 4.
Logistic Regression Models of Comorbid Disorders: Associations with the Diplotypes (rs16969968, rs588765) While adjusting for Nicotine Dependence
| Alcohol Dependence (Response Variable) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| model 1: without adjusting for nicotine dependence | model 2: adjusting for nicotine dependence | ||||||||
| Covariates | OR | LB | UB | p | Covariates | OR | LB | UB | p |
| Age | 1.01 | 0.99 | 1.04 | 0.33 | Age | 1.01 | 0.98 | 1.04 | 0.46 |
| Sex (male) | 1.85 | 1.41 | 2.43 | 7.7E-06 | Sex (male) | 1.65 | 1.25 | 2.17 | 3.6E-04 |
| Education (above high school) | 0.63 | 0.48 | 0.83 | 9.0E-04 | Education (above high school) | 0.88 | 0.65 | 1.18 | 0.38 |
| Diplotype (rs16969968- | 0.68a | Diplotype (rs16969968-rs588765) | 0.73a | ||||||
| GG_CC (reference) | GG_CC (reference) | ||||||||
| GG_CT | 0.88 | 0.45 | 1.70 | 0.70 | GG_CT | 0.85 | 0.43 | 1.65 | 0.63 |
| GG_TT | 0.76 | 0.39 | 1.50 | 0.44 | GG_TT | 0.71 | 0.36 | 1.40 | 0.32 |
| GA_CC | 1.00 | 0.51 | 1.96 | 0.99 | GA_CC | 0.95 | 0.48 | 1.88 | 0.88 |
| GA_CT | 0.83 | 0.44 | 1.58 | 0.57 | GA_CT | 0.77 | 0.40 | 1.47 | 0.43 |
| AA_CC | 1.11 | 0.56 | 2.18 | 0.77 | AA_CC | 0.96 | 0.48 | 1.91 | 0.90 |
| Nicotine dependence | 2.35 | 1.70 | 3.26 | 2.9E-07 | |||||
| Marijuana Dependence (Response Variable) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| model 1: without adjusting for nicotine dependence | model 2: adjusting for nicotine dependence | ||||||||
| Covariates | OR | LB | UB | p | Covariates | OR | LB | UB | p |
| Age | 1.00 | 0.97 | 1.02 | 0.73 | Age | 0.99 | 0.96 | 1.02 | 0.50 |
| Sex (male) | 2.48 | 1.83 | 3.37 | 5.1E-09 | Sex (male) | 2.16 | 1.59 | 2.95 | 1.0E-06 |
| Education (above high school) | 0.52 | 0.38 | 0.70 | 2.4E-05 | Education (above high school) | 0.81 | 0.58 | 1.12 | 0.20 |
| Diplotype (rs16969968- | 0.16a | Diplotype (rs16969968-rs588765) | 0.14a | ||||||
| GG_CC (reference) | GG_CC (reference) | ||||||||
| GG_CT | 1.88 | 0.82 | 4.33 | 0.14 | GG_CT | 1.81 | 0.78 | 4.20 | 0.17 |
| GG_TT | 1.12 | 0.47 | 2.67 | 0.80 | GG_TT | 1.01 | 0.42 | 2.42 | 0.99 |
| GA_CC | 1.36 | 0.57 | 3.25 | 0.49 | GA_CC | 1.27 | 0.53 | 3.06 | 0.59 |
| GA_CT | 1.17 | 0.51 | 2.69 | 0.71 | GA_CT | 1.06 | 0.46 | 2.46 | 0.89 |
| AA_CC | 1.70 | 0.72 | 4.03 | 0.23 | AA_CC | 1.41 | 0.59 | 3.38 | 0.44 |
| Nicotine dependence | 3.24 | 2.19 | 4.78 | 3.7E-09 | |||||
| Major Depression (Response Variable) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| model 1: without adjusting for nicotine dependence | model 2: adjusting for nicotine dependence | ||||||||
| Covariates | OR | LB | UB | p | Covariates | OR | LB | UB | p |
| Age | 0.99 | 0.97 | 1.01 | 0.36 | Age | 0.99 | 0.97 | 1.01 | 0.21 |
| Sex (male) | 0.62 | 0.49 | 0.77 | 3.1E-05 | Sex (male) | 0.54 | 0.43 | 0.69 | 3.5E-07 |
| Education (Above high school) | 0.84 | 0.67 | 1.04 | 0.11 | Education (above high school) | 1.14 | 0.90 | 1.45 | 0.28 |
| Diplotype (rs16969968- | 0.16a | Diplotype (rs16969968-rs588765) | 0.29a | ||||||
| GG_CC (reference) | ref | ref | ref | GG_CC (reference) | |||||
| GG_CT | 1.42 | 0.81 | 2.49 | 0.22 | GG_CT | 1.37 | 0.78 | 2.42 | 0.28 |
| GG_TT | 1.23 | 0.69 | 2.18 | 0.48 | GG_TT | 1.16 | 0.65 | 2.07 | 0.61 |
| GA_CC | 1.41 | 0.79 | 2.51 | 0.24 | GA_CC | 1.34 | 0.75 | 2.40 | 0.32 |
| GA_CT | 1.16 | 0.67 | 2.01 | 0.60 | GA_CT | 1.07 | 0.62 | 1.87 | 0.80 |
| AA_CC | 1.77 | 0.99 | 3.18 | 0.054 | AA_CC | 1.55 | 0.86 | 2.79 | 0.15 |
| Nicotine dependence | 2.17 | 1.69 | 2.77 | 8.7E-10 | |||||
| Panic Attack (Response Variable) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| model 1: without adjusting for nicotine dependence | model 2: adjusting for nicotine dependence | ||||||||
| Covariates | OR | LB | UB | p | Covariates | OR | LB | UB | p |
| Age | 0.99 | 0.97 | 1.01 | 0.33 | Age | 0.99 | 0.97 | 1.01 | 0.19 |
| Sex (male) | 0.59 | 0.48 | 0.73 | 8.2E-07 | Sex (male) | 0.52 | 0.42 | 0.65 | 3.8E-09 |
| Education (above high school) | 0.88 | 0.72 | 1.08 | 0.22 | Education (above high school) | 1.18 | 0.95 | 1.48 | 0.14 |
| Diplotype (rs16969968- | 0.71a | Diplotype (rs16969968-rs588765) | 0.67a | ||||||
| GG_CC (reference) | ref | ref | ref | GG_CC (reference) | |||||
| GG_CT | 0.97 | 0.60 | 1.59 | 0.92 | GG_CT | 0.93 | 0.57 | 1.53 | 0.79 |
| GG_TT | 0.88 | 0.54 | 1.45 | 0.63 | GG_TT | 0.83 | 0.50 | 1.37 | 0.47 |
| GA_CC | 1.13 | 0.69 | 1.86 | 0.62 | GA_CC | 1.08 | 0.65 | 1.78 | 0.76 |
| GA_CT | 0.92 | 0.57 | 1.47 | 0.72 | GA_CT | 0.85 | 0.53 | 1.37 | 0.50 |
| AA_CC | 1.06 | 0.64 | 1.78 | 0.81 | AA_CC | 0.93 | 0.55 | 1.56 | 0.77 |
| Nicotine dependence | 2.07 | 1.65 | 2.60 | 3.2E-10 | |||||
| Social Phobia (Response Variable) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| model 1: without adjusting for nicotine dependence | model 2: adjusting for nicotine dependence | ||||||||
| Covariates | OR | LB | UB | p | Covariates | OR | LB | UB | p |
| Age | 1.01 | 0.98 | 1.03 | 0.62 | Age | 1.00 | 0.98 | 1.02 | 0.80 |
| Sex (male) | 0.75 | 0.59 | 0.95 | 0.02 | Sex (male) | 0.68 | 0.53 | 0.87 | 2.5E-03 |
| Education (above high school) | 0.60 | 0.48 | 0.76 | 2.0E-05 | Education (above high school) | 0.76 | 0.59 | 0.98 | 0.03 |
| Diplotype (rs16969968- | 0.78a | Diplotype (rs16969968-rs588765) | 0.83a | ||||||
| GG_CC (reference) | ref | ref | ref | GG_CC (reference) | |||||
| GG_CT | 0.94 | 0.52 | 1.69 | 0.83 | GG_CT | 0.90 | 0.50 | 1.64 | 0.74 |
| GG_TT | 1.22 | 0.68 | 2.20 | 0.50 | GG_TT | 1.17 | 0.65 | 2.11 | 0.60 |
| GA_CC | 1.09 | 0.60 | 1.98 | 0.79 | GA_CC | 1.05 | 0.57 | 1.92 | 0.88 |
| GA_CT | 1.18 | 0.67 | 2.08 | 0.57 | GA_CT | 1.12 | 0.63 | 1.98 | 0.70 |
| AA_CC | 1.13 | 0.61 | 2.10 | 0.69 | AA_CC | 1.02 | 0.55 | 1.90 | 0.95 |
| Nicotine dependence | 1.79 | 1.37 | 2.33 | 1.8E-05 | |||||
| Posttraumatic Stress Disorder (Response Variable) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| model 1: without adjusting for nicotine dependence | model 2: adjusting for nicotine dependence | ||||||||
| Covariates | OR | LB | UB | p | Covariates | OR | LB | UB | p |
| Age | 0.99 | 0.96 | 1.03 | 0.69 | Age | 0.99 | 0.96 | 1.02 | 0.54 |
| Sex (male) | 0.28 | 0.18 | 0.44 | 1.2E-08 | Sex (male) | 0.25 | 0.16 | 0.39 | 7.2E-10 |
| Education (above high school) | 0.83 | 0.59 | 1.16 | 0.27 | Education (above high school) | 1.10 | 0.76 | 1.59 | 0.61 |
| Diplotype (rs16969968- | 0.056a | Diplotype (rs16969968-rs588765) | 0.082a | ||||||
| GG_CC (reference) | ref | ref | ref | GG_CC (reference) | |||||
| GG_CT | 2.08 | 0.71 | 6.11 | 0.18 | GG_CT | 2.03 | 0.69 | 5.97 | 0.20 |
| GG_TT | 2.73 | 0.94 | 7.94 | 0.065 | GG_TT | 2.66 | 0.91 | 7.76 | 0.07 |
| GA_CC | 1.65 | 0.55 | 4.99 | 0.37 | GA_CC | 1.58 | 0.52 | 4.80 | 0.42 |
| GA_CT | 1.67 | 0.58 | 4.82 | 0.34 | GA_CT | 1.57 | 0.54 | 4.56 | 0.40 |
| AA_CC | 3.14 | 1.06 | 9.31 | 0.039 | AA_CC | 2.80 | 0.94 | 8.33 | 0.06 |
| Nicotine dependence | 2.09 | 1.43 | 3.07 | 1.5E-04 | |||||
| Attention Deficit Hyperactive Disorder (Response Variable) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| model 1: without adjusting for nicotine dependence | model 2: adjusting for nicotine dependence | ||||||||
| Covariates | OR | LB | UB | p | Covariates | OR | LB | UB | p |
| Age | 0.97 | 0.95 | 1.00 | 0.04 | Age | 0.97 | 0.95 | 1.00 | 0.02 |
| Sex (male) | 2.54 | 1.91 | 3.38 | 1.3E-10 | Sex (male) | 2.31 | 1.73 | 3.08 | 1.4E-08 |
| Education (above high school) | 0.71 | 0.54 | 0.95 | 0.02 | Education (above high school) | 0.94 | 0.69 | 1.28 | 0.68 |
| Diplotype (rs16969968- | 0.09a | Diplotype (rs16969968-rs588765) | 0.15a | ||||||
| GG_CC (reference) | ref | ref | ref | GG_CC (reference) | |||||
| GG_CT | 1.17 | 0.54 | 2.53 | 0.68 | GG_CT | 1.15 | 0.53 | 2.48 | 0.73 |
| GG_TT | 1.56 | 0.73 | 3.33 | 0.25 | GG_TT | 1.48 | 0.69 | 3.17 | 0.31 |
| GA_CC | 1.43 | 0.66 | 3.11 | 0.37 | GA_CC | 1.36 | 0.62 | 2.98 | 0.44 |
| GA_CT | 1.04 | 0.49 | 2.19 | 0.92 | GA_CT | 0.98 | 0.46 | 2.07 | 0.95 |
| AA_CC | 1.91 | 0.88 | 4.13 | 0.10 | AA_CC | 1.71 | 0.79 | 3.73 | 0.17 |
| Nicotine dependence | 1.99 | 1.42 | 2.77 | 5.7E-05 | |||||
| Conduct Disorder (Response Variable) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| model 1: without adjusting for nicotine dependence | model 2: adjusting for nicotine dependence | ||||||||
| Covariates | OR | LB | UB | p | Covariates | OR | LB | UB | p |
| Age | 0.97 | 0.94 | 0.99 | 0.02 | Age | 0.96 | 0.93 | 0.99 | 0.01 |
| Sex (male) | 3.61 | 2.60 | 5.02 | 1.9E-14 | Sex (male) | 3.15 | 2.26 | 4.40 | 1.4E-11 |
| Education (above high school) | 0.62 | 0.45 | 0.85 | 3.0E-03 | Education (above high school) | 0.95 | 0.67 | 1.34 | 0.76 |
| Diplotype (rs16969968- | 0.40a | Diplotype (rs16969968-rs588765) | 0.25a | ||||||
| GG_CC (reference) | ref | ref | ref | GG_CC (reference) | |||||
| GG_CT | 0.98 | 0.45 | 2.14 | 0.96 | GG_CT | 0.94 | 0.43 | 2.08 | 0.88 |
| GG_TT | 0.83 | 0.37 | 1.84 | 0.65 | GG_TT | 0.74 | 0.33 | 1.67 | 0.47 |
| GA_CC | 1.34 | 0.61 | 2.94 | 0.46 | GA_CC | 1.25 | 0.56 | 2.77 | 0.58 |
| GA_CT | 0.82 | 0.38 | 1.77 | 0.62 | GA_CT | 0.74 | 0.34 | 1.61 | 0.45 |
| AA_CC | 0.85 | 0.37 | 1.95 | 0.70 | AA_CC | 0.71 | 0.31 | 1.64 | 0.42 |
| Nicotine dependence | 3.06 | 2.05 | 4.58 | 4.5E-08 | |||||
| Antisocial Personality Disorder (Response Variable) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| model 1: without adjusting for nicotine dependence | model 2: adjusting for nicotine dependence | ||||||||
| Covariates | OR | LB | UB | p | Covariates | OR | LB | UB | p |
| Age | 0.96 | 0.93 | 1.00 | 0.03 | Age | 0.96 | 0.93 | 0.99 | 0.01 |
| Sex (male) | 3.88 | 2.67 | 5.65 | 1.4E-12 | Sex (male) | 3.33 | 2.27 | 4.87 | 6.0E-10 |
| Education (above high school) | 0.58 | 0.41 | 0.83 | 2.8E-03 | Education (above high school) | 0.93 | 0.63 | 1.36 | 0.70 |
| Diplotype (rs16969968- | 0.24a | Diplotype (rs16969968-rs588765) | 0.17a | ||||||
| GG_CC (reference) | ref | ref | ref | GG_CC (reference) | |||||
| GG_CT | 1.20 | 0.48 | 3.04 | 0.69 | GG_CT | 1.16 | 0.45 | 2.96 | 0.76 |
| GG_TT | 1.02 | 0.40 | 2.62 | 0.96 | GG_TT | 0.91 | 0.35 | 2.37 | 0.85 |
| GA_CC | 1.79 | 0.71 | 4.50 | 0.22 | GA_CC | 1.65 | 0.65 | 4.22 | 0.29 |
| GA_CT | 0.93 | 0.37 | 2.32 | 0.88 | GA_CT | 0.83 | 0.33 | 2.10 | 0.70 |
| AA_CC | 1.09 | 0.41 | 2.86 | 0.87 | AA_CC | 0.90 | 0.34 | 2.40 | 0.84 |
| Nicotine dependence | 3.65 | 2.28 | 5.85 | 7.2E-08 | |||||
omnibus p for overall diplotypic effect. ADHD: Attention deficit hyperactivity disorder. ASPD: Antisocial personality disorder.
OR: odds ratio. LB: lower bound of 95% confidence interval. UB: upper bound of 95% confidence interval. ref: reference.
ORs were computed from a logistic regression model using each psychiatric disorder as response variable, the diplotypes, age, gender, and education (above high school=1; high school graduation or less=0) as covariates.
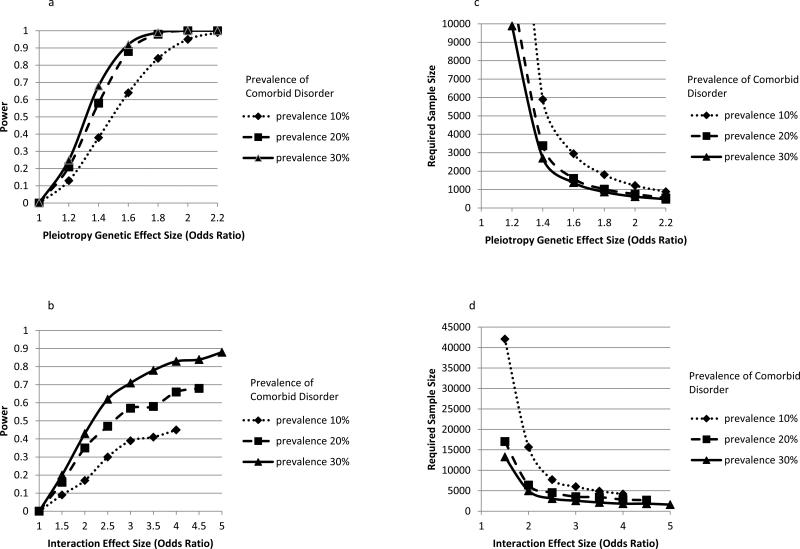
We conducted the power analysis to demonstrate the power regarding the findings of null associations. We had sufficient power (80%) to detect an association with an OR of 1.50 for comorbid disorder with 30% prevalence such as panic attacks (Figure 1). For comorbid disorders with 20% prevalence such as major depressive disorder and social phobia, our sample had sufficient power (80%) to detect an association with an OR of 1.55. As the prevalence of comorbid disorders decreased to 10% as with alcohol dependence, cannabis dependence, PTSD, ADHD, conduct disorder, and ASPD, our sample had power (80%) to detect the association with an OR of 1.75. Therefore, we had power to detect only strong associations between the diplotypes and the comorbid disorders. In light of this limited power, we found no evidence of associations between the diplotypes and comorbid disorders and thus no evidence for pleiotropy.
Figure 1. Power and Required Sample Size in Testing of Pleiotropy and Interaction.
Figure 1a. Power of Testing Pleiotropy When Adjusting for Nicotine Dependence: Effect Size by Prevalence of Comorbid Disorder (Response Variable)
Figure 1b. Power of Testing Interaction: Effect Size by Prevalence of Comorbid Disorder (Covariate)
Figure 1c. Required Sample Size for Testing Pleiotropy with 80% Power: Effect Size by Prevalence of Comorbid Disorder (Response Variable)
Figure 1d. Required Sample Size for Testing Interaction with 80% Power: Effect Size by Prevalence of Comorbid Disorder (Covariate)
3.3. Risk Moderation: Testing the Interaction of Genetic Variants and Psychiatric Disorders for Risk of Nicotine Dependence
The hypothesis of a multiplicative interaction between the diplotypes and psychiatric disorder was tested1. For example, both comorbid alcohol dependence and diplotype risks remained significantly associated with the risk for nicotine dependence in an additive model. However, there is no evidence for a multiplicative interaction between the diplotypes and alcohol dependence (Interaction wald=2.52, d.f.=5, p=0.77). Similarly, we found that psychiatric disorders and diplotypes independently contribute to the risk of nicotine dependence and saw no evidence of genetic risk moderation by any comorbid disorders.
We showed the limited power of our study to detect a diplotype and comorbid disorder interaction, with the power varying as a function of the prevalence rates of comorbid disorders used as predicting variables and the effect size (Figure 1b). For a comorbid disorder with 10% prevalence such as cannabis dependence, power is very limited. Even when the comorbid disorder covariate is more common (e.g., 30%, the prevalence of panic attacks in our sample), we only had 80% power to detect a large interaction effect size, such as an OR of 3.6 or higher. In other words, a much larger sample size is needed to detect an interaction of smaller effect size. In addition, we confirmed our results by computing the power to detect the reported estimates from our actual analyses with all covariates in our sample.
3.4. Required Sample Size to Detect Pleiotropy and Interaction
Required sample size for the detection of pleiotropy varies with the detectable effect size and prevalence rates of comorbid disorders. Figure 1c shows the required sample size by effect size of the diplotype and the comorbid disorder when power is fixed at 80%. For example, a sample size of 1,378 is needed to detect an OR of 1.60 with 92% power, while a sample size of 1,806 is needed to detect an OR of 1.80 with 84% power.
Figure 1d shows the required sample size by interaction effect size for comorbid disorders with a prevalence of 10%, 20%, and 30%, while the power is fixed at 80%. In order to detect an interaction effect size of 2.0 with a comorbid disorder of 30% prevalence, we need a sample of 4,987 subjects, we need 6,376 subjects to detect the same interaction with a comorbid disorder of 20% prevalence such as major depressive disorder, and we need 15,663 subjects to detect the same interaction with a comorbid disorder of 10% prevalence. The required sample size increases as detectable effects size and prevalence decrease.
4. DISCUSSION
With the recent discovery of specific genetic variants for nicotine dependence, this study examined the joint contribution of comorbid psychiatric disorders and genetic risks to nicotine dependence. Importantly, we tested if the genetic variants for nicotine dependence were also associated with other commonly comorbid psychiatric disorders (pleiotropy), and if other psychiatric disorders moderated these known genetic risk for nicotine dependence. The strength of our study lies in the specific case control design contrasting the difference between being a smoker to becoming nicotine dependent, the assessments of DSM-IV diagnoses of other psychiatric disorders, and the opportunity to characterize identified genetic findings.
First, the phenotypic pattern of psychiatric comorbidity with nicotine dependence was what we expected. We found that nicotine dependent smokers, when compared with non-dependent smokers, had a two-to-three fold increase in risk for other psychiatric disorders, which is consistent with findings from the National Epidemiologic Survey of Alcohol Related Conditions (NESARC) and the National Comorbidity Survey (NCS). The development of nicotine dependence is clearly associated with other mental disorders (Dierker and Donny, 2008; Goodwin et al., 2008; Hasin et al., 2011; Swendsen et al., 2010).
Next, we found no evidence to support a significant association between the risk diplotype in the CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster and any of the comorbid psychiatric disorders, and we demonstrated the sufficient power to detect associations of moderate effect sizes with an odds ratio range of 1.50-1.75. These null association results should be interpreted with caution as we had limited power to detect an effect size smaller than an odds ratio of 1.50. Modest genetic effects identified for common complex disorders can increase the risk by 1.3 or less. Overall, our findings indicate the genetic risk associated with the CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster is a robust specific risk factor for nicotine dependence, and not a shared risk with other comorbid psychiatric disorders. In other words, there is no support for pleiotropy.
In addition, we found the influence of both psychiatric comorbidity and genetic risks (CHRNA5-CHRNA3-CHRNB4), were distinct, additive risks for nicotine dependence. There was no evidence to support any significant moderation of the genetic risks for nicotine dependence by the examined comorbid psychiatric disorders. Having alcohol or cannabis dependence, depressive disorder or anxiety disorder did not alter the genetic risk associated with the CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster for nicotine dependence.
We performed the power analysis to demonstrate 1) the sufficient statistical power in testing pleiotropy, 2) the limited power in testing interaction, and 3) the power of contemporary sample sizes in answering these important questions. Understanding the statistical power is especially critical when interpreting the null association results in our study. These negative results need to be interpreted in the context of sample size, frequency and hypothesized effect size of genotype, prevalence and relative risk of environmental factors, and type I error level (Cohen, 1988; Gauderman and Morrison, 2006). We found no evidence for the genetic risk moderation by examined psychiatric disorders, but the null moderation results should be interpreted with caution as we had compromised power to detect an interaction effect size smaller than an odds ratio of 3.6. We can only state that there are not likely to be large (Interaction OR>3.6) moderating influences of psychiatric comorbidity on the genetic risk of developing nicotine dependence. Extremely large samples sizes, in the order of tens of thousands of individuals, will be needed to detect a modest moderation effect if it is present.
The questions of pleiotropy and effect moderation with comorbid psychiatric disorders regarding the CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster have not been previously studied, as this type of work involves comprehensive assessments of multiple phenotypes. Our results suggest no pleiotropy or moderation for nicotine dependence and examined psychiatric disorders. In contrast, there is strong evidence that these variants on chromosome 15 contribute to comorbid lung cancer and chronic obstructive pulmonary disease. There have been efforts to tease apart the potential pleiotropy between nicotine dependence, lung cancer, and COPD associated with this particular gene cluster and the results are controversial (Chanock and Hunter, 2008). Some studies have reported that the genetic association with lung cancer is completely or partially mediated by smoking or nicotine dependence, while others have reported a direct, non-mediated association between lung cancer and the gene cluster (Amos et al., 2008; Hung et al., 2008; Thorgeirsson et al., 2008; Whitfield et al., 2002).
There are several limitations of our study. Because this is a cross-sectional study and these analyses rely on self report and are subject to recall bias, we assumed no temporal sequence in our studies of comorbidity. Our sample size may be sufficient to test main effect associations, but power is quite limited to detect any gene-comorbidity interactions of moderate effect size, so the results must be interpreted with caution. An estimated sample of 10,000-20,000 is required for such interaction analyses. Lastly, our sampling selection for cases and controls was based on FTND scores, and we need to be cautious about the interpretation of the relationship of comorbid psychiatric disorders and nicotine dependence over the full range of smoking behaviors. In addition, comorbid psychiatric illness defined in this study does not represent all psychiatric disorder found in the community, but represents a subset of individuals who smoke cigarettes and have lifetime psychiatric illness. Thus, further examination of the relationship between psychiatric disorders and nicotine dependence needs to be done in different community based samples to increase generalizability.
Despite the above limitations, these results represent an important first effort to characterize the identified genetic risk associated with the CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster in the context of comorbid disorders given that nicotine dependence is highly comorbid with many other psychiatric disorders. The concept of common liability to addiction supported by twin studies suggests a substantial proportion of genetic factors account for a general vulnerability for all substance dependence. However, we found that variants in the CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster are specific risks for nicotine dependence, and not shared among comorbid psychiatric disorders. We also found that the risk associated with these genetic variants and comorbid disorders are additive and this genetic risk was not modified by comorbid psychiatric disorders such as major depressive disorder or alcohol dependence. Future association studies of a larger sample size using a novel phenotype definition capturing the common liability to addiction may help identify genetic variants responsible for multiple substance dependence.
Supplementary Material
Acknowledgements
In memory of Theodore Reich, founding Principal Investigator of COGEND, we are indebted to his leadership in the establishment and nurturing of COGEND and acknowledge with great admiration his seminal scientific contributions to the field. Lead investigators directing data collection are Laura Bierut, Naomi Breslau, Dorothy Hatsukami, and Eric Johnson. The authors thank Heidi Kromrei and Tracey Richmond for their assistance in data collection and Sherri Fisher for her assistance in editing the manuscript. This research was supported by NIH grants P01 CA089392 from the National Cancer Institute, U01HG04422-02 from the National Human Genome Institute, KL2RR023249 (PI, Fraser) by NIH/NCRR, and GA305231 from the Global Research Awards for Nicotine Dependence (GRAND) by Pfizer.
Role of Funding Source
This research was supported by NIH grants P01 CA089392 from the National Cancer Institute, U01HG04422-02 from the National Human Genome Institute, R01DA026911 and K08DA030398 from the National Institute of Drug Abuse, K08DA030398, KL2RR023249 by NIH/NCRR, and GA305231 from the Global Research Awards for Nicotine Dependence (GRAND) by Pfizer. Genotyping work at Perlegen Sciences was performed under NIDA Contract HHSN271200477471C. Phenotypic and genotypic data are stored in the NIDA Center for Genetic Studies (NCGS) at http://zork.wustl.edu/ under NIDA Contract HHSN271200477451C (PIs J Tischfield and J Rice). Genotyping services were also provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268200782096. The funding organizations are not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
Authors Bierut and Chen designed the study. Bierut, Breslau, Hatsukami, and Johnson collected the data and analyzed the results. Chen and Xian undertook the statistical analysis. Grucza, Saccone, and Wang helped collection and analysis of the data. Chen wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
LB Bierut and JC Wang are listed as an inventor on a patent (US 20070258898) covering the use of certain SNPs in diagnosing, prognosing, and treating addiction. Laura Bierut served as a consultant to Pfizer in 2008. All other authors declare that they have no conflict of interest.
Supplementary Table 1 can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:... .
REFERENCES
- Agrawal A, Lynskey MT, Pergadia ML, Bucholz KK, Heath AC, Martin NG, Madden PA. Early cannabis use and DSM-IV nicotine dependence: a twin study. Addiction. 2008;103:1896–1904. doi: 10.1111/j.1360-0443.2008.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, Sullivan K, Matakidou A, Wang Y, Mills G, Doheny K, Tsai YY, Chen WV, Shete S, Spitz MR, Houlston RS. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat. Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum. Mol. Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Morgan SD, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PAF, Mayo K, Nurnberger JJ, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan GT, J. A, Edenberg HJ, Rice JP, Goate AM. Nicotine dependence and the a5-a3-b4 nicotinic receptor gene cluster: variants in the nicotinic receptors alter the risk for nicotine dependence. Am. J. Psychiatry. 2008:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tob. Control. 2009;18:317–323. doi: 10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav. Genet. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Daily smoking and the subsequent onset of psychiatric disorders. Psychol. Med. 2004a;34:323–333. doi: 10.1017/s0033291703008869. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol. Psychiatry. 2004b;55:69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr., Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J. Stud. Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- CDC Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000-2004. MMWR. 2008;57:1226–1228. [PubMed] [Google Scholar]
- CDC Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR. 2009;58:1227–1232. [PubMed] [Google Scholar]
- Chanock SJ, Hunter DJ. Genomics: when the smoke clears. Nature. 2008;452:537–538. doi: 10.1038/452537a. [DOI] [PubMed] [Google Scholar]
- Cohen L. Statistical Power Analysis for the Behavioral Sciences. Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Dierker L, Donny E. The role of psychiatric disorders in the relationship between cigarette smoking and DSM-IV nicotine dependence among young adults. Nicotine Tob. Res. 2008;10:439–446. doi: 10.1080/14622200801901898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of cooccurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res. Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Lyons MJ, Tsuang MT, True WR, Eisen SA. Common genetic risk of major depression and nicotine dependence: the contribution of antisocial traits in a United States veteran male twin cohort. Twin Res. Hum. Genet. 2007;10:470–478. doi: 10.1375/twin.10.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman W, Morrison J. QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies. Univ. of Southern California. 2006 [Google Scholar]
- Golub A, Johnson BD. Variation in youthful risks of progression from alcohol and tobacco to marijuana and to hard drugs across generations. Am. J. Public Health. 2001;91:225–232. doi: 10.2105/ajph.91.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Zvolensky MJ, Keyes KM. Nicotine dependence and mental disorders among adults in the USA: evaluating the role of the mode of administration. Psychol. Med. 2008;38:1277–1286. doi: 10.1017/S0033291708003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Griesler PC, Hu MC, Schaffran C, Kandel DB. Comorbidity of psychiatric disorders and nicotine dependence among adolescents: findings from a prospective, longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1340–1350. doi: 10.1097/CHI.0b013e318185d2ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Fenton MC, Skodol A, Krueger R, Keyes K, Geier T, Greenstein E, Blanco C, Grant B. Personality disorders and the 3-year course of alcohol, drug, and nicotine use disorders. Arch. Gen. Psychiatry. 2011;68:1158–1167. doi: 10.1001/archgenpsychiatry.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Corey LA, Kendler KS. A multivariate genetic analysis of the use of tobacco, alcohol, and caffeine in a population based sample of male and female twins. Drug Alcohol Depend. 1999;57:69–78. doi: 10.1016/s0376-8716(99)00053-8. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G, Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F, Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F, Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S, Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P, Trichopoulos D, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A, Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [see comment] [DOI] [PubMed] [Google Scholar]
- Hunter DJ. Gene-environment interactions in human diseases. Nat .Rev. Genet. 2005;6:287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Probabilities of alcohol high-risk drinking, abuse or dependence estimated on grounds of tobacco smoking and nicotine dependence. Addiction. 2003;98:805–814. doi: 10.1046/j.1360-0443.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Arch. Gen. Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Klungsoyr O, Nygard JF, Sorensen T, Sandanger I. Cigarette smoking and incidence of first depressive episode: an 11-year, population-based follow-up study. Am. J. Epidemiol. 2006;163:421–432. doi: 10.1093/aje/kwj058. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, Vollenweider P, Preisig M, Wareham NJ, Zhao JH, Loos RJ, Barroso I, Khaw KT, Grundy S, Barter P, Mahley R, Kesaniemi A, McPherson R, Vincent JB, Strauss J, Kennedy JL, Farmer A, McGuffin P, Day R, Matthews K, Bakke P, Gulsvik A, Lucae S, Ising M, Brueckl T, Horstmann S, Wichmann HE, Rawal R, Dahmen N, Lamina C, Polasek O, Zgaga L, Huffman J, Campbell S, Kooner J, Chambers JC, Burnett MS, Devaney JM, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Epstein S, Wilson JF, Wild SH, Campbell H, Vitart V, Reilly MP, Li M, Qu L, Wilensky R, Matthai W, Hakonarson HH, Rader DJ, Franke A, Wittig M, Schafer A, Uda M, Terracciano A, Xiao X, Busonero F, Scheet P, Schlessinger D, St Clair D, Rujescu D, Abecasis GR, Grabe HJ, Teumer A, Volzke H, Petersmann A, John U, Rudan I, Hayward C, Wright AF, Kolcic I, Wright BJ, Thompson JR, Balmforth AJ, Hall AS, Samani NJ, Anderson CA, Ahmad T, Mathew CG, Parkes M, Satsangi J, Caulfield M, Munroe PB, Farrall M, Dominiczak A, Worthington J, Thomson W, Eyre S, Barton A, Mooser V, Francks C, Marchini J. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat. Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons M, Hitsman B, Xian H, Panizzon MS, Jerskey BA, Santangelo S, Grant MD, Rende R, Eisen S, Eaves L, Tsuang MT. A twin study of smoking, nicotine dependence, and major depression in men. Nicotine Tob. Res. 2008;10:97–108. doi: 10.1080/14622200701705332. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Williams LJ, Jacka FN, Ng F, Henry MJ, Nicholson GC, Kotowicz MA, Berk M. Tobacco smoking as a risk factor for major depressive disorder: population-based study. Br. J. Psychiatry. 2008;193:322–326. doi: 10.1192/bjp.bp.107.046706. [DOI] [PubMed] [Google Scholar]
- Pedersen W, von Soest T. Smoking, nicotine dependence and mental health among young adults: a 13-year population-based longitudinal study. Addiction. 2009;104:129–137. doi: 10.1111/j.1360-0443.2008.02395.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch. Gen. Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, Ma JZ, Short SE, Stephens SH, Stevens VL, Sun L, Wang Y, Wenzlaff AS, Aggen SH, Breslau N, Broderick P, Chatterjee N, Chen J, Heath AC, Heliovaara M, Hoft NR, Hunter DJ, Jensen MK, Martin NG, Montgomery GW, Niu T, Payne TJ, Peltonen L, Pergadia ML, Rice JP, Sherva R, Spitz MR, Sun J, Wang JC, Weiss RB, Wheeler W, Witt SH, Yang BZ, Caporaso NE, Ehringer MA, Eisen T, Gapstur SM, Gelernter J, Houlston R, Kaprio J, Kendler KS, Kraft P, Leppert MF, Li MD, Madden PA, Nothen MM, Pillai S, Rietschel M, Rujescu D, Schwartz A, Amos CI, Bierut LJ. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2009;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum. Mol. Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvola E, Rose RJ, Dick DM, Pulkkinen L, Marttunen M, Kaprio J. Early-onset depressive disorders predict the use of addictive substances in adolescence: a prospective study of adolescent Finnish twins. Addiction. 2008;103:2045–2053. doi: 10.1111/j.1360-0443.2008.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Glantz M, Jin R, Merikangas KR, Sampson N, Kessler RC. Mental disorders as risk factors for substance use, abuse and dependence: results from the 10-year follow-up of the National Comorbidity Survey. Addiction. 2010;105:1117–1128. doi: 10.1111/j.1360-0443.2010.02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAG Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmundsson J, Jonsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, Kristjansson K, Skuladottir H, Isaksson HJ, Gudbjartsson T, Jones GT, Mueller T, Gottsater A, Flex A, Aben KKH, de Vegt F, Mulders PFA, Isla D, Vidal MJ, Asin L, Saez B, Murillo L, Blondal T, Kolbeinsson H, Stefansson JG, Hansdottir I, Runarsdottir V, Pola R, Lindblad B, van Rij AM, Dieplinger B, Haltmayer M, Mayordomo JI, Kiemeney LA, Matthiasson SE, Oskarsson H, Tyrfingsson T, Gudbjartsson DF, Gulcher JR, Jonsson S, Thorsteinsdottir U, Kong A, Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, Gieger C, Rawal R, Mangino M, Prokopenko I, Magi R, Keskitalo K, Gudjonsdottir IH, Gretarsdottir S, Stefansson H, Thompson JR, Aulchenko YS, Nelis M, Aben KK, den Heijer M, Dirksen A, Ashraf H, Soranzo N, Valdes AM, Steves C, Uitterlinden AG, Hofman A, Tonjes A, Kovacs P, Hottenga JJ, Willemsen G, Vogelzangs N, Doring A, Dahmen N, Nitz B, Pergadia ML, Saez B, De Diego V, Lezcano V, Garcia-Prats MD, Ripatti S, Perola M, Kettunen J, Hartikainen AL, Pouta A, Laitinen J, Isohanni M, Huei-Yi S, Allen M, Krestyaninova M, Hall AS, Jones GT, van Rij AM, Mueller T, Dieplinger B, Haltmayer M, Jonsson S, Matthiasson SE, Oskarsson H, Tyrfingsson T, Kiemeney LA, Mayordomo JI, Lindholt JS, Pedersen JH, Franklin WA, Wolf H, Montgomery GW, Heath AC, Martin NG, Madden PA, Giegling I, Rujescu D, Jarvelin MR, Salomaa V, Stumvoll M, Spector TD, Wichmann HE, Metspalu A, Samani NJ, Penninx BW, Oostra BA, Boomsma DI, Tiemeier H, van Duijn CM, Kaprio J, Gulcher JR, McCarthy MI, Peltonen L, Thorsteinsdottir U, Stefansson K. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 2010;42:448–453. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch. Gen. Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP, Duan W, Fox L, Grucza RA, Kern J, Mayo K, Reyes O, Rice J, Saccone SF, Spiegel N, Steinbach JH, Stitzel JA, Anderson MW, You M, Stevens VL, Bierut LJ, Goate AM. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum. Mol. Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB, Zhu G, Nestler JE, Heath AC, Martin NG. Genetic covariation between serum gamma-glutamyltransferase activity and cardiovascular risk factors. Clin. Chem. 2002;48:1426–1431. [PubMed] [Google Scholar]
- Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific? Behav. Genet. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.