Abstract
Objective
Major Depressive Disorder (MDD) is a likely risk factor for dementia, but some cases of MDD in older adults may actually represent a prodrome of this condition. The purpose of this study was to use neuropsychological test scores to predict conversion to dementia in a sample of depressed older adults diagnosed as nondemented at time of neuropsychological testing.
Design
Longitudinal, with mean follow-up of 5.45 years.
Setting
Outpatient depression treatment study at Duke University
Participants
30 nondemented individuals depressed at time of neuropsychological testing and later diagnosed with incident dementia; 149 nondemented individuals depressed at time of neuropsychological testing and a diagnosis of cognitively normal.
Methodology
All participants received clinical assessment of depression, were assessed to rule out prevalent dementia at time of study enrollment, completed neuropsychological testing at time of study enrollment, and were diagnosed for cognitive disorders on an annual basis.
Results
Non-demented, acutely depressed older adults who converted to dementia during the study period exhibited broadly lower cognitive performances at baseline than acutely depressed individuals who remained cognitively normal. Discriminant function analysis indicated that 2 neuropsychological tests, CERAD Recognition Memory and Trail Making B, best predicted dementia conversion.
Conclusions
Depressed older adults with cognitive deficits in the domains of memory and executive functions during acute depression are at higher risk for developing dementia. Some cases of late-life depression may reflect a prodrome of dementia in which clinical manifestation of mood changes may co-occur with emerging cognitive deficits.
Keywords: geriatric depression, dementia, neuropsychology, memory, executive function
Early identification of dementia risk is an increasingly important target for research and clinical practice. Research suggests that one risk factor for dementia may be Major Depressive Disorder (MDD) (1, 2), and that some cases of MDD in older adults may actually represent a prodrome of this condition (3, 4). Depression symptoms in a very early stage of dementia may present as more noticeable than the early cognitive changes that characterize prodromal dementia conditions like Mild Cognitive Impairment (MCI); however, it is also reasonable to expect that neuropsychologial deficits consistent with prodromal dementia may be detectable in MDD. Many older adults with MDD have neuropsychological deficits in domains like episodic memory and executive functions (5, 6), which can persist even in remission (7). These deficits can be difficult to differentiate from or early dementia (8), and may reflect a common pathophysiology (9). Because incident dementia in nondepressed samples is consistently associated with lower performances on tests of memory and executive functions (10), a common pathway perspective would suggest that lower performances on tests of memory and executive functions in older adults with MDD should also predict individuals with this diagnosis who convert to dementia.
The goal of the current study was to use neuropsychological test scores to predict conversion to dementia in a sample of older adults enrolled in a treatment study for MDD and diagnosed as nondemented at time of neuropsychological testing. These individuals completed neuropsychological testing during an episode of MDD, were followed longitudinally, and were assessed for dementia on an annual basis by a consensus panel of clinicians and researchers. We hypothesized that neuropsychological performances would be lower in the group that converted to dementia relative to the group that remained cognitively normal, particularly on tests of memory and executive functions. We used discriminant function analysis to identify the combination of individual neuropsychological measures that best discriminated individuals who converted to dementia from those who did not. This approach was repeated on a subset that included Alzhheimer’s Disease (AD) diagnoses only in order to examine if the results deviated from the broader dementia group.
Methods
Participants
Participants were enrolled in the Neurocognitive Outcomes of Depression in the Elderly study (NCODE). Although the study includes a nondepressed comparison group, incident dementia in that group (n = 2) precludes statistical comparison in the current study. Participants in this study were enrolled if they met criteria for a current episode of unipolar major depression, were age 60 or older, did not have another primary psychiatric illness, and were free from neurological illnesses affecting cognition. Per NCODE criteria, participants with contraindications to brain MRI were also excluded. Comprehensive methods of the NCODE study have been reported previously (11).
Initial Clinical Assessment
At the time of study enrollment, a geriatric psychiatrist interviewed each depressed participant and assessed depression symptoms with a number of standardized clinical assessments (11). Briefer clinical assessments were repeated when clinically indicated, but at least every three months. For the current study, the Montgomery-Asberg Depression Rating Scale (MADRS) was used as an index of depression severity.
Participants were excluded if they had dementia or suspected dementia at the time of neuropsychological testing based on information available to the assigned geriatric psychiatrist, who examined the participant, reviewed medical records, and conferred with referring physicians for all patients. Per MHCRC protocol, any depressed participants entering the study with a Mini-Mental State Exam (MMSE) (12) score less than 25 were followed through their treatment to determine if cognition improved. In the current study, this included 20 individuals who initially scored below 25, and 13 of these individuals later improved to a score of 25 or better. The remaining 7 individuals had MMSE scores ranging from 20–24 during follow-up, but were included in the study based on a psychiatric diagnosis of nondemented at the time of their neuropsychological testing. Thus, in the clinical judgment of the study geriatric psychiatrist and by established study protocol, clinically evident dementia was excluded at or close to baseline in all participants.
Clinical Follow-up of Depressed Participants
The NCODE study operated in a naturalistic treatment milieu using treatment guidelines established by the Duke Affective Disorders Program (13). Treatment was monitored to ensure that clinical guidelines were followed appropriately. The protocol recommended that patients receive continuation treatment for at least one to two years (some indefinitely) once they achieve remission.
Neuropsychological Assessment
Neuropsychological assessment occurred at study enrollment for most participants. The earliest participants in the longitudinal study did not receive neuropsychological testing at enrollment, but did receive testing if there was a relapse of major depression. We included individuals whose clinical status was still considered to be non-demented at the time of testing (n = 38). The neuropsychological assessment was based on a battery of tests that has been successfully employed in a number of clinical and epidemiological studies of dementia (14, 15), and which is fully described in Steffens et al. (2004) (11). We chose 15 individual measures from the neuropsychological battery to use as independent variables: 1) CERAD Word List Learning (sum of 3 trials), 2) CERAD Word List Delayed Recall (total score), 3) CERAD Recognition Memory, 4) CERAD Boston Naming (total score), 5) CERAD Constructional Praxis Drawing (total score), 6) Delayed Praxis Recall (total score), 7) Logical Memory I (immediate recall, total score), 8) Logical Memory II (delayed recall, total score), 9) Benton Visual Retention Test (total correct), 10) Digit Span (total score), 11) Symbol-Digit Modalities Test (total score), 12) Trail Making A (time in seconds up to 91), 13) Trail Making B (time in seconds up to 301), 14) Animal Naming (total score), 15) Controlled Oral Word Association (total score). For CERAD Recognition Memory, we employed the Recognition Discriminability score used by Chandler et al. (16), which was calculated by subtracting the number of false positive responses from the number of true positive responses and setting these scores to a lower boundary of 0. A licensed clinical neuropsychologist trained the psychometric technicians on standardized administration and scoring of each test and supervised data collection.
Consensus Diagnostic Conference
Participants were assigned to be reviewed by the yearly consensus panel if they met one of the following criteria: 1) the study geriatric psychiatrist suspected dementia or clinically significant cognitive decline, 2) neuropsychological test performances were consistent with cognitive impairment, or 3) neurological consultation resulted in a diagnosis of dementia or cognitive impairment. Individuals with no indications of cognitive difficulty were reviewed periodically to keep their diagnostic status up-to-date. Using a model developed in our epidemiological studies of dementia (17), we convened a panel of experts to review each case, including the treating geriatric psychiatrists, a cognitive neuroscientist, 1–2 neuropsychologists specializing in memory disorders, and a neurologist specializing in memory disorders. Panel members reviewed the following information for each participant: 1) initial evaluation and most recent clinical depression study notes, 2) neuropsychological testing profiles for all participants who underwent cognitive testing, 3) informant report of cognitive decline based on the Dementia Severity Rating Scale (18), and 4) additional neurological and clinical neuropsychological consultations when available. The treating study psychiatrist briefly presented the case, a neuropsychologist summarized the neuropsychological findings, and the panel would discuss the case until a consensus cognitive diagnosis was reached. Panel members chose among several clinical diagnoses; however, only diagnoses reflecting AD or dementia were used in the current study (Table 1). We used published criteria for diagnoses of probable and possible Alzheimer disease (19), probable and possible vascular dementia (20), and Lewy body dementia (21). The category of “dementia of undetermined etiology” was used when a participant met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (22) criteria for dementia, but the clinical presentation was too complex or atypical to permit a diagnosis of Possible AD and no other apparent cause for dementia could be identified. The final diagnostic category in the current study was cognitively normal/no dementia.
Table 1.
List of Possible Clinical Diagnoses of Dementia Available at Consensus Diagnostic Conference, with Diagnoses Used in Current Study Highlighted in Bold with Sample Size
| Probable Alzheimer’s disease (n = 8) |
| Possible Alzheimer’s disease (n = 13) |
| Probable vascular dementia (n = 1) |
| Possible vascular dementia (n = 2) |
| Dementia of Parkinson’s disease |
| Alcoholic dementia |
| Lewy body dementia |
| Huntington’s dementia |
| Progressive supranuclear palsy |
| Frontal lobe dementia |
| Severe head trauma with residual dementia |
| Hypoperfusion dementia |
| Postencephalic dementia |
| Normal pressure hydrocephalus |
| Dementia of undeterimined etiology (n = 6) |
| Normal/noncase (n = 149) |
Note: Diagnosis of cognitive impairment, no dementia (CIND) not used in the current study; see Steffens et al. (2004) (10) for comprehensive description of diagnoses and subtypes.
Statistical Methods
The analysis cohort was composed of 179 participants. Incident dementia was diagnosed for 30 of these participants, with 21 also meeting criteria for AD. The remaining participants were classified as cognitively normal/no dementia.
A dichotomous outcome measure denoting ‘caseness’ was coded as follows: case (dementia) = 1; non case (cognitively normal) = 0. This outcome was subsequently evaluated to determine its level of association with the neuropsychological variables.
Prior to the analyses, missing values were imputed using chained MCMC imputation procedures (23) implemented in SAS 9.2 (PROC MI) (24). Imputed values in the final complete data set were based on mean values computed from 10 imputations. Eight of the candidate scales contained no missing data, six had less than 5% missing; the final scale (Digit Span) had less than 10% missing.
For the discriminant analysis, data reduction techniques, based on the branch and bound algorithm of Furnival and Wilson (25) were used to derive a specified number of reduced models based on the highest likelihood score (chi-square) statistic for all possible sizes from one-to-five-effect models. Using logistic regression procedures (SAS 9.2; PROC LOGISTIC), the dichotomous outcome measure was regressed on the 15 candidate neuropsychological measures to derive optimal one-, two-, three-, four-, and five-variable solutions as determined by score criteria. Among the five estimated models, a given model was selected over the next-most-simple model only if the difference in likelihood scores (as tested by a 1 df chi-square statistic) indicated that the increase in model fit was significantly improved by the additional measure (p < 0.05). All models included age as a covariate.
Results
Mean time of follow up was 6.33 (SD = 3.07) years for the dementia group and 5.27 (SD =3.46) years for the no dementia group, which was not a statistically significant difference based on a Wilcoxon-Mann-Whitney test. A chi-square test indicated no significant difference in the proportion of dementia diagnoses when neuropsychological testing occurred at enrollment versus relapse. Means and standard deviations of participant demographics and neuropsychological performances are presented in Table 2 by diagnostic group. Age was significantly higher among subjects meeting criteria for dementia (t = −6.77; df = 177; p < 0.0001) and the MMSE score was significantly lower (Wilcoxon-Mann-Whitney Z = −3.70; p < 0.0002). The median MMSE score was 26.5 for the dementia group and 29.0 for the no dementia/cognitively normal group. Only age remained significantly associated with dementia when both covariates were included in succeeding models. Among individual neuropsychological measures, baseline scores of dementia cases were significantly worse than those of cognitively normal individuals on all 15 measures, based on both conventional t-tests and Wilcoxon-Mann-Whitney tests (all p < 0.01). Similar results occurred for the AD only group. We calculated Cohen’s d as a measure of effect size between groups, which ranged from .67 – 1.89 (Table 2). We also adopted Cohen’s (26) conventions in interpreting effect sizes estimated from d as small (≥.20), medium (≥.50), and large (≥.80). As seen in Table 1, we found large effect sizes for 14 out of 15 neuropsychological measures.
Table 2.
Group Differences on Demographic Characteristics and Neuopsychological Performance, with Effect Sizes
| Normal/Noncase n = 149 |
Dementia n = 30 |
Prob | cohen’s d | |||
|---|---|---|---|---|---|---|
| mean | SD | mean | SD | |||
| Age | 67.25 | 5.49 | 75.10 | 7.13 | ** | |
| % female | 59% | 63% | ||||
| MADRS | 19.87 | 8.01 | 21.26 | 7.37 | ||
| Years education | 14.88 | 2.90 | 13.63 | 4.19 | ||
| MMSE | 28.24 | 1.69 | 25.23 | 4.36 | * | 1.18 |
| Animal Naming | 17.68 | 4.29 | 11.73 | 4.98 | ** | 1.35 |
| CERAD Naming | 14.58 | 0.83 | 12.97 | 2.66 | ** | 1.23 |
| COWA | 37.19 | 12.42 | 26.01 | 10.67 | ** | 0.93 |
| CERAD Praxis | 9.94 | 1.02 | 9.20 | 1.47 | * | 0.67 |
| CERAD Praxis Delay | 8.79 | 1.84 | 5.67 | 3.04 | ** | 1.50 |
| CERAD Word List | 20.21 | 4.11 | 14.30 | 3.61 | ** | 1.47 |
| CERAD Word List Delay | 7.02 | 1.74 | 3.93 | 2.41 | ** | 1.66 |
| CERAD Recognition | 9.59 | 0.81 | 7.67 | 2.15 | ** | 1.68 |
| Logical Memory | 25.63 | 7.41 | 18.13 | 9.16 | ** | 0.97 |
| Logical Memory II | 21.96 | 8.30 | 12.70 | 9.53 | ** | 1.09 |
| BVRT # correct | 6.04 | 1.81 | 3.82 | 2.64 | ** | 1.13 |
| Trail Making A (sec) | 37.99 | 14.03 | 83.67 | 53.82 | ** | 1.81 |
| Trail Making B (sec) | 104.45 | 46.27 | 208.08 | 86.52 | ** | 1.89 |
| SDMT | 40.61 | 9.69 | 23.58 | 13.41 | ** | 1.64 |
| Digit Span | 16.39 | 3.97 | 11.89 | 3.14 | ** | 1.18 |
Wilcoxon-Mann-Whitney two-sample test: Control vs Dementia
p < 0.0001
p < 0.001
Note. SD = standard deviation. Chi-square used to test differences in % female. Age ranges: Normal/Noncase (60–83 years), Dementia (61–88 years). MADRS = Montgomery-Asberg Depression Rating Scale. MMSE = Mini-Mental State Examination. COWA = Controlled Oral Word Association. CERAD = Consortium to Establish a Registry for Alzheimer’s Disease. BVRT = Benton Visual Retention Test. SDMT = Symbol-Digit Modalities Test.
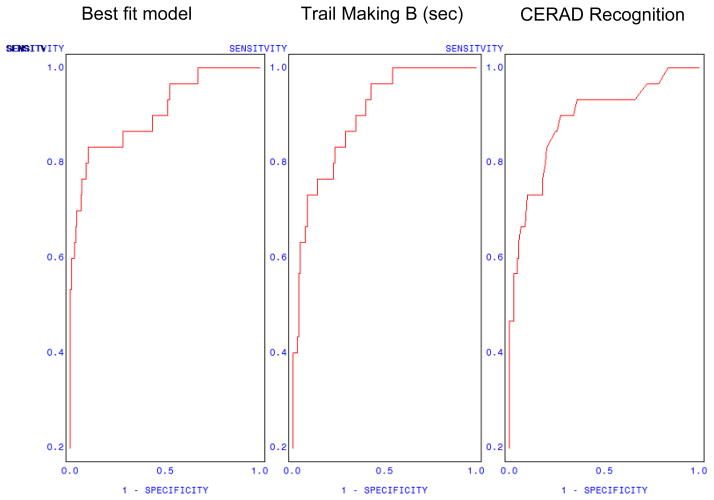
Derived multivariate models are presented in Tables 3 and 4. Optimal discrimination of the 30 participants meeting criteria for dementia was obtained based on only two indices: CERAD Recognition and Trail Making B (Table 3). Subsequent addition of one-, two-, or three-candidate variables produced no significant increment in model fit (Table 4). The optimal two-variable model (Table 3) yielded a max-rescaled R2 = 0.61, and a concordance index c = 0.91 (area under the ROC curve). Better recognition memory performance, as indexed by CERAD Recognition, was associated with reduced odds of dementia (OR = 0.46). Slower time per second to complete Trail Making B was associated with increased odds of dementia (OR = 1.02). Using a classification criterion of p = 0.75, the model correctly classified 91.6% of the responses; rates of false positives and false negatives at this criterion level were 5.9% and 8.6%, respectively (Table 5). The ROC curve for the selected two-variable model is presented in Figure 1. In addition, we examined these models with AD diagnosis only, and found similar results to the full dementia group.
Table 3.
Results of discriminative best-subset model predicting dementia vs. noncase from demographic and neuropsychological predictors.
| Parameter | DF | Odds Ratio | −95%CI | +95%CI | χ2 = | Prob < χ2 |
|---|---|---|---|---|---|---|
| Age | 1 | 1.180 | 1.075 | 1.295 | 12.12 | 0.0005 |
| TMT B (seconds) | 1 | 1.015 | 1.006 | 1.023 | 11.03 | 0.0009 |
| Recognition | 1 | 0.461 | 0.296 | 0.719 | 11.67 | 0.0006 |
| Model Fit: | ||||||
| Max-rescaled R-Square = 0.6085 | ||||||
| Concordance Index c = 0.907 |
Note. TMT B = Trail Making Test Part B.
Table 4.
Regression models leading to optimal solution (Table 3), based on 15 candidate neuropsychological tests
| Number of Variables | Score χ2 | Change χ2 * | Variables Included in the Model |
|---|---|---|---|
| 1 | 36.85 | n/a | Age |
| 2 | 73.54 | 36.69 | Age, Recognition |
| 3 | 84.33 | 10.80 | Age, Recognition, TMT-B |
| 4 | 86.17 | 1.84 | Age, Recognition, TMT B, Digit Span |
| 5 | 89.19 | 3.02 | Age, Recognition, LM I, TMT B, Digit Span |
| 6 | 90.87 | 1.68 | Age, Praxis Delay, Recognition, LM I, TMT B, Digit Span |
χ2 Critical Value (p < 0.05) = 3.84; df = 1
Note. TMT B = Trail Making Test Part B. Recognition = CERAD Recognition Discriminability. LM I = Logical Memory I (immediate recall).
Table 5.
Classification Table for combined Trail Making B (seconds) and Recognition Discriminability performances at ascending levels of dementia probability.
| Prob event | Correct | Incorrect | Percentages | Predicted TMT B | Predicted CERAD Recognition | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | Non-event | Event | Non-event | Correct | Sensitivity | Specificity | False Pos. | False Neg. | |||
| 0.30 | 22 | 139 | 10 | 8 | 89.9% | 73.3% | 93.3% | 31.3% | 5.4% | 148 | 8.8 |
| 0.35 | 22 | 140 | 9 | 8 | 90.5% | 73.3% | 94.0% | 29.0% | 5.4% | 157 | 8.6 |
| 0.40 | 20 | 141 | 8 | 10 | 89.9% | 66.7% | 94.6% | 28.6% | 6.6% | 167 | 8.4 |
| 0.45 | 19 | 142 | 7 | 11 | 89.9% | 63.3% | 95.3% | 26.9% | 7.2% | 177 | 8.2 |
| 0.50 | 18 | 145 | 4 | 12 | 91.1% | 60.0% | 97.3% | 18.2% | 7.6% | 186 | 8.1 |
| 0.55 | 18 | 146 | 3 | 12 | 91.6% | 60.0% | 98.0% | 14.3% | 7.6% | 196 | 7.9 |
| 0.60 | 17 | 146 | 3 | 13 | 91.1% | 56.7% | 98.0% | 15.0% | 8.2% | 206 | 7.7 |
| 0.65 | 17 | 147 | 2 | 13 | 91.6% | 56.7% | 98.7% | 10.5% | 8.1% | 216 | 7.5 |
| 0.70 | 17 | 148 | 1 | 13 | 92.2% | 56.7% | 99.3% | 5.6% | 8.1% | 225 | 7.3 |
| 0.75 | 16 | 148 | 1 | 14 | 91.6% | 53.3% | 99.3% | 5.9% | 8.6% | 235 | 7.2 |
| 0.80 | 13 | 148 | 1 | 17 | 89.9% | 43.3% | 99.3% | 7.1% | 10.3% | 245 | 7.0 |
| 0.85 | 10 | 149 | 0 | 20 | 88.8% | 33.3% | 100.0% | 0.0% | 11.8% | 255 | 6.8 |
| 0.90 | 8 | 149 | 0 | 22 | 87.7% | 26.7% | 100.0% | 0.0% | 12.9% | 264 | 6.6 |
Note. Prob level = probability of dementia case. TMT B = Trail Making B time in seconds. CERAD Recognition (Discriminability) = true positives − false positives: # correct Yes responses − (10 − # correct No responses).
Figure 1.
Receiver-Operator Curves (ROC) Predicting Dementia: Best-Fit Model, Trail Making only, Recognition Discriminability only.
Note. CERAD Recognition = Discriminability score.
To derive appropriate scale scores at a given cut-point, CERAD Recognition and Trail Making B were each individually regressed on predicted scores estimated from the same model. The ensuing regression equations were then used to generate predicted scores for each scale over a range of cut-points (Table 5, final two columns). The ROC curves for CERAD Recognition and Trail Making B are presented as part of Figure 1.
Conclusions
The current study found that non-demented, acutely depressed older adults who convert to dementia exhibit broadly lower cognitive performances than acutely depressed individuals who were diagnosed as cognitively normal. Although there were significant group differences across all neuropsychological measures we examined, bivariate comparisons between dementia-converted and nonconverted individuals indicated the largest effect sizes on tests of memory and executive functions. Discriminant function analysis indicated that 2 neuropsychological tests, CERAD Recognition memory and Trail Making B, best predicted dementia conversion. Results were similar when the group included AD diagnoses only.
The current results share similarities with three lines of research related to neuropsychological function in MDD and/or dementia: 1) neuropsychological predictors of dementia in MDD, 2) neuropsychological differences between MDD and AD, and 3) neuropsychological prediction of dementia among cognitively normal individuals. Only a few studies have examined neuropsychological predictors of incident dementia or cognitive decline in the context of MDD. One retrospective study of 44 MDD patients criteria found that individuals who met formal diagnostic criteria for dementia (n = 14; 7 AD) over a 7.5 year follow-up had worse performance on attention and memory items from a cognitive screening measure (27). Another study did not formally diagnose dementia, but did find that verbal recall at baseline was deficient in individuals experiencing cognitive decline over a 15-month period, and who are presumably at greater risk of dementia (28). Our study found comparable neuropsychological measures to be associated with dementia risk, with the largest effect sizes on measures of episodic memory and executive functions.
Research on neuropsychological differentiation of MDD and dementia has found that measures of delayed recall best differentiate between dementia and depression (29–31). One study using approximately the same CERAD neuropsychological measures as the current study found that Delayed Word Recall and Constructional Praxis Recall were the two tests that best differentiated between late-onset depression and AD (31), while our study found Delayed Word Recall and Praxis Recall showing the largest effect sizes between those with MDD who converted to dementia and those who remained cognitively normal. Our multivariate model, however, found that Recognition Memory and Trail Making B were most highly predictive of depressed individuals with incident dementia relative to the cognitively normal group.
Research indicates that nondepressed individuals with prodromal AD can show subtle deficits across a broad range of neuropsychological domains, invariably including episodic memory and executive functions (32, 33), which is similar to the current study. One representative study that used similar measures to the current study found that CERAD measures of Word List Learning, Delayed Recall, and Praxis Recall predicted incident probable AD, as did both Trail Making A and Trail Making B (34). In addition, our study’s finding of the sensitivity of recognition memory to incident dementia in depression is interesting because of research and clinical opinion that recognition memory is relatively less affected by the symptoms of depression than is delayed recall (35–37) and typically does not show the level of impairment in depression that is seen in AD (38, 39) or amnestic MCI (40). Although delayed free recall is generally regarded as the most sensitive measure to early dementia in nondepressed samples (41), it is possible that added vulnerability of delayed recall to MDD (42) confounds the sensitivity of delayed recall to a certain extent when MDD is comorbid with underlying dementia pathology. Delayed recognition discrimination may be especially sensitive to the integrity of the entorhinal cortex (43), which is also the region that first evidences the neurofibrillary tangles that characterize AD (44). Thus, while recognition memory tests may be useful in detecting AD-like impairments in the context of late-life depression, further research is needed.
The major clinical implication of the current study is that individuals presenting with acute MDD who also present with deficits in memory and executive functions should be considered at risk for conversion to AD. Means and effects sizes in Table 2 can provide clinicians with raw scores by which to gauge the relative performance of their own patients. We have also provided information in Table 5 by which a clinician can compare CERAD Recognition and Trail Making B scores at different levels of dementia probability. Although we present two optimal tests based on our sample and our specific statistical approaches, other selection procedures may produce a different combination of optimal predictors. We also note Bondi et al.’s (33) caution that the heterogeneity of dementia makes it unlikely that a single cognitive test or single cognitive domain will be sufficient to accurately identify prodromal dementia.
The current study has potential limitations and methodologic challenges that merit discussion. One limitation is that while the sample size of the dementia group is large relative to extant prospective studies of clinically diagnosed MDD and dementia, the number of cases is still relatively small. Despite this potential constraint to statistical power, we detected statistically significant effects between groups on all of the neuropsychological measures. The current study did not examine how age of first depression onset is related to dementia, which was due to sample size constraints. We also did not include individuals in the category of cognitive impairment, no dementia (CIND). Although many individuals with CIND or MCI-like conditions are certainly at higher risk of dementia (45), there is also much heterogeneity (46), and many of the diagnostic endpoints of this group do not fit within the scope of the current study. Although outcomes of depression and CIND have been studied (45), future research on these classifications could help better identify which of these individuals are at the highest risk of AD and other adverse outcomes.
Our study presents the methodologic challenge of predicting a prospective diagnosis of dementia based on a number of individuals that were retrospectively characterized to be in a prodromal stage of this condition at baseline. As described in our methods, we employed a number of procedures to exclude individuals with dementia at entry to the study and at time of baseline neuropsychological testing, but it is possible that other approaches would lead to a different decision in some cases. On this point, we did include 20 individuals with a baseline MMSE below 25 at time of testing, and note that all 7 individuals who did not subsequently improve their MMSE above the cut-off level were ultimately diagnosed with dementia. Nonetheless, these scores occurred in the context of a thorough clinical evaluation at their study baseline that did not yield a diagnosis of dementia for these individuals. This finding suggests that a persistently low MMSE score-especially in the context of symptom improvement-should raise suspicion for dementia and warrant a more extensive neuropsychological evaluation.
Our study question has the potential confound that our independent variables (neuropsychological performance) can influence the definition of our dependent variable (clinical diagnosis). On this point, we emphasize that neuropsychological testing was one aspect of a comprehensive assessment that included a clinical and medical history, other informant reports of cognition and functional performance, applicable medical records, and in some cases additional neurological evaluation and/or a clinical neuropsychological assessment. In addition, 93% (28/30) of dementia cases had 2 or more neuropsychological testing visits prior to diagnosis, such that baseline neuropsychological performance was not necessarily the most influential information contributing to clinical diagnosis. Thus, while baseline neuropsychological testing performance was available at the time of diagnosis, it is unlikely that these scores were overly influential in the diagnostic process.
Finally, this study does not answer the question of whether depression is a risk factor or a prodrome of AD. The mechanisms by which depression would lead to dementia are well reviewed by others, and include possibilities such as cortisol-induced atrophy of the hippocampus, increased amyloid plaque deposition, and increased production of pro-inflammatory cytokines (47). Although the risk vs. prodrome debate has been well reviewed by others (1, 2), our view is that the ideas of depression as a risk factor or prodrome of dementia are not mutually exclusive, but rather reflect the heterogeneous clinical presentations and etiologies of both conditions. In this context, our question concerns which neuropsychological performances are potential indicators of conversion to dementia among depressed individuals, and we highlight that individuals with early dementia may manifest clinically significant symptoms of depression before the disease is otherwise clinically apparent. The task ahead is to continue investigating the mechanisms by which depression at different points in the lifespan may contribute to dementia, to characterize the range of tests that will identify dementia as early as possible, and to use this knowledge to optimize the benefits of intervention approaches.
Acknowledgments
This research was supported by the following grants: R01MH054846, P50MH060451, K23MH087741, K24MH070027, P30AG028377. The authors wish to acknowledge the contributions of the study psychiatrists (John Beyer, Eric Christopher, Kenneth Gersing, Mugdha Thakur, Warren Taylor) and the NCODE study team.
Footnotes
The authors have no conflict of interest disclosures to report.
References
- 1.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust NZ J Med. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 2.Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 4.Schweitzer I, Tuckwell V, O’Brien J, et al. Is late onset depression a prodrome to dementia? Int J Geriatr Psychiatry. 2002;17:997–1005. doi: 10.1002/gps.525. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann LL, Goodwin GM, Ebmeier KP. The cognitive neuropsychology of depression in the elderly. Psychol Med. 2007;37:1693–1702. doi: 10.1017/S0033291707001134. [DOI] [PubMed] [Google Scholar]
- 6.Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: Possible implications for functional neuropathology. Br J Psychiatry. 2001;178:206–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- 7.Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 8.Swainson R, Hodges JR, Galton CJ, et al. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- 9.Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: Differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647–654. doi: 10.1016/j.jpsychires.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Albert M, Blacker D, Moss MB, et al. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- 11.Steffens DC, Welsh-Bohmer KA, Burke JR, et al. Methodology and preliminary results from the Neurocognitive Outcomes of Depression in the Elderly Study. J Geriatr Psychiatry Neurol. 2004;17:202–211. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- 12.Folstein M, Folstein S, Fanjiang G. Mini-Mental State Examination: Clinical Guide and User’s Guide. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 13.Steffens DC, McQuoid DR, Krishnan KRR. The Duke Somatic Treatment Algorithm for Geriatric Depression (STAGED) approach. Psychopharmacol Bull. 2002;36:58–68. [PubMed] [Google Scholar]
- 14.Tschanz JT, Welsh-Bohmer KA, Skoog I, et al. Dementia diagnoses from clinical and neuropsychological data compared. Neurology. 2000;54:1290–1296. doi: 10.1212/wnl.54.6.1290. [DOI] [PubMed] [Google Scholar]
- 15.Plassman BL, Langa KM, Fisher GG, et al. The prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandler MJ, Lacritz LH, Hynan LS, et al. A total score for the CERAD neuropsychological battery. Neurology. 2005;65:102–106. doi: 10.1212/01.wnl.0000167607.63000.38. [DOI] [PubMed] [Google Scholar]
- 17.Plassman BL, Khachaturian AS, Townsend JJ, et al. Comparison of clinical and neuropathologic diagnoses of Alzheimer’s disease in 3 epidemiologic samples. Alzheimers Dement. 2006;2:2–11. doi: 10.1016/j.jalz.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Clark CM, Ewbank DC. Performance of the Dementia Severity Rating Scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10:31–39. [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 21.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 23.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 24.SAS Institute I. SAS Release 9.2. Cary, NC: 2008. [Google Scholar]
- 25.Furnival G, Wilson R. Regressions by leaps and bounds. Technometrics. 1974;16:499–511. [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, N.J.: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 27.Jean L, Simard M, van Reekum R, et al. Differential cognitive impairment in subjects with geriatric depression who will develop Alzheimer’s disease and other dementias: a retrospective study. Int Psychogeriatr. 2005;17:289–301. doi: 10.1017/s1041610205001511. [DOI] [PubMed] [Google Scholar]
- 28.von Gunten A, Giannakopoulos P, Duc R. Cognitive and demographic determinants of dementia in depressed patients with subjective memory complaints. Eur Neurol. 2005;54:154–158. doi: 10.1159/000090104. [DOI] [PubMed] [Google Scholar]
- 29.Lachner G, Engel RR. Differentiation of dementia and depression by memory tests. A meta-analysis. J Nerv Ment Dis. 1994;182:34–39. doi: 10.1097/00005053-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Coen RF, Kirby M, Swanwick GRJ, et al. Distinguishing between patients with depression or very mild Alzheimer’s disease using the delayed-word-recall test. Dement Geriatr Cogn Disord. 1997;8:244–247. doi: 10.1159/000106638. [DOI] [PubMed] [Google Scholar]
- 31.Kunig G, Jager M, Stief V, et al. The impact of the CERAD-NP on diagnosis of cognitive deficiencies in late onset depression and Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:911–916. doi: 10.1002/gps.1579. [DOI] [PubMed] [Google Scholar]
- 32.Bäckman L, Jones S, Berger AK, et al. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 33.Bondi MW, Jak AJ, Delano-Wood L, et al. Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychol Rev. 2008;18:73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jungwirth S, Zehetmayer S, Bauer P, et al. Prediction of Alzheimer dementia with short neuropsychological instruments. J Neural Transm. 2009;116:1513–1521. doi: 10.1007/s00702-009-0318-6. [DOI] [PubMed] [Google Scholar]
- 35.Pfennig A, Littmann E, Bauer M. Neurocognitive impairment and dementia in mood disorders. J Neuropsychiatry Clin Neurosci. 2007;19:373–382. doi: 10.1176/jnp.2007.19.4.373. [DOI] [PubMed] [Google Scholar]
- 36.Wright S, Persad C. Distinguishing between depression and dementia in older persons: Neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189–198. doi: 10.1177/0891988707308801. [DOI] [PubMed] [Google Scholar]
- 37.Caine ED. Pseudodementia. Current concepts and future directions. Arch Gen Psychiatry. 1981;38:1359–1364. doi: 10.1001/archpsyc.1981.01780370061008. [DOI] [PubMed] [Google Scholar]
- 38.Miller E, Lewis P. Recognition memory in elderly patients with depression and dementia: a signal detection analysis. J Abnorm Psychol. 1977;86:84–86. doi: 10.1037//0021-843x.86.1.84. [DOI] [PubMed] [Google Scholar]
- 39.Taylor R, Gilleard CJ. Signal-detection analysis of nonverbal recognition memory in dementia. Percept Mot Skills. 1990;71:1255–1258. doi: 10.2466/pms.1990.71.3f.1255. [DOI] [PubMed] [Google Scholar]
- 40.Ritter E, Despres O, Monsch AU, et al. Topographical recognition memory sensitive to amnestic mild cognitive impairment but not to depression. Int J Geriatr Psychiatry. 2006;21:924–929. doi: 10.1002/gps.1581. [DOI] [PubMed] [Google Scholar]
- 41.Welsh K, Butters N, Hughes J, et al. Detection of abnormal memory decline in mild cases of Alzheimer’s disease using CERAD neuropsychological measures. Arch Neurol. 1991;48:278–281. doi: 10.1001/archneur.1991.00530150046016. [DOI] [PubMed] [Google Scholar]
- 42.Portella MJ, Marcos T, Rami L, et al. Residual cognitive impairment in late-life depression after a 12-month period follow-up. Int J Geriatr Psychiatry. 2003;18:571–576. doi: 10.1002/gps.895. [DOI] [PubMed] [Google Scholar]
- 43.Wolk DA, Dickerson BC. Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage. 2011;54:1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18:S85–S88. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 45.Steffens DC, McQuoid DR, Potter GG. Outcomes of older cognitively impaired individuals with current and past depression in the NCODE study. J Geriatr Psychiatry Neurol. 2009;22:52–61. doi: 10.1177/0891988708328213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monastero R, Palmer K, Qiu C, et al. Heterogeneity in risk factors for cognitive impairment, no dementia: population-based longitudinal study from the Kungsholmen Project. Am J Geriatr Psychiatry. 2007;15:60–69. doi: 10.1097/01.JGP.0000229667.98607.34. [DOI] [PubMed] [Google Scholar]
- 47.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]