Abstract
Background
Paclitaxel causes an acute pain syndrome (P-APS), occurring within days after each dose and usually abating within days. Paclitaxel also causes a more classic peripheral neuropathy, which steadily increases in severity with increasing paclitaxel total doses. Little detail is available regarding the natural history of these two syndromes, or any relationship between them, although a recent publication does provide natural history data about weekly paclitaxel, supporting an association between the severity of P-APS and eventual peripheral neuropathy symptoms.
Methods
Patients entering this study were about to receive paclitaxel and carboplatin every 3 weeks. Daily questionnaires were completed for the first week after every chemotherapy dose and EORTC QLQ-CIPN 20 instruments were completed weekly.
Results
The P-APS severity peaked on day 4 after the initial chemotherapy dose, with 12%, 29%, 23%, and 36% of patients having maximal pain scores of 0, 1–4, 5–6, or 7–10 during the first week after the first dose of therapy, respectively. Patients with P-APS scores of 0–4 with the first dose of chemotherapy had less eventual sensory neuropathy than did patients with P-APS scores of 5–10 (p=0.001).
With regard to the more peripheral neuropathy, sensory neuropathy was more problematic than was either motor or autonomic neuropathy. Numbness and tingling were more common components of the sensory neuropathy, than was pain.
Conclusions
Patients with worse P-APS severities appear to have more eventual chemotherapy induced peripheral neuropathy. This provides support for the concept that the P-APS is a form of nerve pathology.
Keywords: Paclitaxel, arthralgia, myalgia, paclitaxel acute pain syndrome, neuropathy
INTRODUCTION
Paclitaxel, a staple chemotherapeutic agent used in breast, ovarian, lung, head and neck cancers, is associated with a syndrome of subacute aches and pains, which has commonly been referred to as `paclitaxel-induced arthralgias and myalgias'1, 2 and more recently as the “paclitaxel-associated acute pain syndrome” (P-APS)3, 4 This pain syndrome causes significant morbidity, with an incidence of up to 70% of patients receiving paclitaxel.5 Despite the widespread use of paclitaxel and the high frequency of P-APS, little is known about the natural history of this pain syndrome.
In addition to this acute paclitaxel toxicity, peripheral neuropathy can be a devastating long-term consequence of this drug. As with P-APS, peripheral neuropathy has been described in up to 70% of patients.6
The current prospective study serves to further the understanding about P-APS and its possible association with peripheral neuropathy. This report evaluates paclitaxel given in an every 3 week fashion, concurrently with carboplatin.
METHODS
The patients presented here are the second reported cohort of a clinical trial which was designed to study four cohorts.4 Eligible patients for the present cohort must have been scheduled to receive paclitaxel at a dose of least 175 mg/m2 at 2–4 week intervals and slated to receive, concurrently, another neurotoxic chemotherapy agent. Carboplatin was on the list of other neurotoxic agents for this trial, understanding that it does not cause as much neurotoxicity as do other platinum drugs, like cisplatin or oxaliplatin. It turned out that all patients on this trial received paclitaxel combined with carboplatin.
A previously-reported cohort from this study, the first to accrue its projected sample size, involved patients receiving weekly paclitaxel at doses of 70–90 mg/m2. Two additional cohorts of this trial are still accruing patients; one involves patients receiving this lower dose weekly paclitaxel with another concurrent neurotoxic agent and the other involves patients receiving higher dose paclitaxel every 3 weeks without another neurotoxic agent. Data from these cohorts will be reported when they complete their accrual; they will be used to try to confirm data presented now, as an independent data set, and to contrast findings between the slightly different data sets.
Patients on this study must have been at least 18 years old, must have been able to provide written consent and needed to be able to complete study questionnaires. In addition, they had to have a life expectancy of greater than 6 months and an ECOG performance status of 0 or 1. Patients were excluded if they had ever been diagnosed with peripheral neuropathy from any cause, ever been diagnosed with fibromyalgia, planned to receive concurrent neutrophil colony stimulating factor therapy, or had a previous exposure to paclitaxel or other neurotoxic chemotherapy drugs (e.g. other taxanes, platinum agents, or vinca alkaloids). Participants were to have been told to use a non-opioid analgesic (for example acetaminophen or a nonsteroidal antiinflammatory agent as needed) for “breakthrough” pain with the option of opioid pain medications (for example oxycodone 5mg every 1–2 hours as needed). There was no stipulation in this trial as to what doses of corticosteroids were concomitantly administered in this trial, acknowledging that 10–20 mg of dexamethasone is usually administered with paclitaxel doses, to prevent allergic reactions. No information was collected regarding comorbidities such as diabetes or lifestyle choices such as alcohol use. Institutional review board approval and individual signed patient consent were obtained for this study at each participating institution, per United States federal guidelines.
P-APS symptoms were measured by asking patients to keep a daily symptom log, comprising 10 items regarding pain symptoms and the use of pain medications on days 2–7 following each paclitaxel dose. These questions were derived and adapted from Cleeland's Brief Pain Inventory, which has been widely used in cancer populations.7 The modified items asked about aches as well as pain and anchored these directly to the paclitaxel treatment. As well, a 22 item summary questionnaire regarding symptom quality, location, alleviating/aggravating factors, and medication use was given on the 8th day following each paclitaxel dose.
To measure CIPN symptoms, the EORTC (European Organization for Research and Treatment of Cancer) QLQ (Quality of Life Questionnaire) -CIPN 20 instrument was administered at baseline and on day 1 with each subsequent paclitaxel dose. The EORTC QLQ-CIPN20 is a 20-item CIPN-specific questionnaire which includes three scales assessing sensory (9 items), motor (8 items), and autonomic (3 items) symptoms and functioning, with each item measured on a 1–4 scale (1 – not at all; 4 – very much). The EORTC QLQ-CIPN20 tool has been tested in cancer patients receiving a variety of chemotherapy agents and has been shown to have internal consistency reliability based on Cronbach's alpha coefficients of 0.82, 0.73, and 0.76 for the three scales, respectively.8
Patient reported outcome (PRO) for acute pain was also collected and evaluated on a 0 to 10 scale, with 0 being the best possible score. All other PRO scores were converted into a 0 – 100 point scale with 100 being the best possible score9–11.
The primary goal for this trial was to describe the incidence and characteristics of, and change in, pain (P-APS) related to paclitaxel infusions over several cycles. This analysis is descriptive, intended to detail the unique nature of the P-APS syndrome qualitatively. The frequency of the incidence and severity variables were plotted over time with related confidence intervals.
Depending on the variable of interest, mean (SD), median (range) and frequency (percentage) were used to summarize data in a descriptive manner. Nonparametric Wilcoxon rank-sum tests, two-sample t-tests and chi-square tests (or Fisher's exact tests) were used to compare differences in scores by pain groups defined in the first cycle.
To investigate the association between the P-APS symptoms and eventual chemotherapy-induced neuropathy, correlation coefficients were produced, relating the worst pain scores for the first dose of therapy and the subsequent neuropathy scores, as judged from the daily and every 3 week questions.
EORTC differences in sensory, autonomic, and motor sub-scales were evaluated by the actual changes from baseline to week 18 and percent changes from baseline to week 18. Changes from baseline were tested between each pair of variables and the differences in AUC (Area Under the Curve) for the reported protocol outcome variables were tested using Kruskal-Wallis non-parametric procedures. Mixed models were evaluated as well.
No adjustments were made for multiple comparisons.
RESULTS
A total of 85 patients were accrued on the above defined patient cohort, from 20 NCCTG institutions between February 2009 and December 2010. A consort diagram (Figure 1) illustrates patient flow on this study. Patient characteristics are reported in Table 1. All patients received chemotherapy every 3 weeks and all received concurrent carboplatin.
Figure 1.
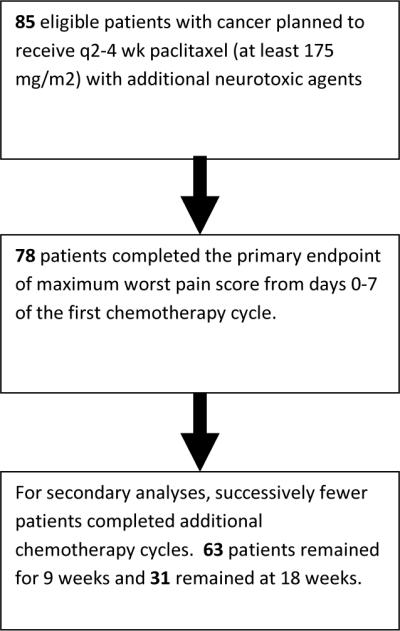
Consort diagram
TABLE 1.
PATIENT CHARACTERISTICS
| Total (N=81) | |
|---|---|
| Age | |
| N | 81 |
| Mean (SD) | 60.5 (10.50) |
| Median | 61.0 |
| Q1, Q3 | 54.0, 67.0 |
| Range | (33.0–89.0) |
| Gender | |
| F | 60 (74.1%) |
| M | 21 (25.9%) |
| Race | |
| White | 64 (79%) |
| Black or African American | 13 (16%) |
| Asian | 1 (1.2%) |
| American Indian or Alaska Native | 2 (2.5%) |
| Not Available | 1 (1.2%) |
| Concurrent Bevacizumab/Avastin | |
| Unsure (on clinical trial) | 1(1%) |
| Yes | 18 (22%) |
| No | 62 (77%) |
| Number of Cycles Completed | |
| Mean (SD) | 4.4 (1.8) |
| Median | 4.5 |
| Range | 1 to 9 |
Paclitaxel-associated acute pain syndrome
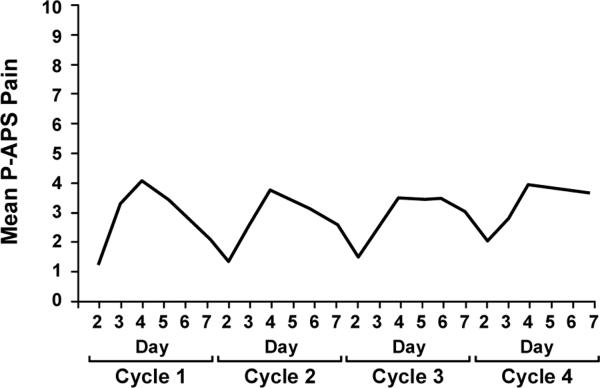
To assess pain, patients were asked “Please rate any aches/pains that are NEW since your last dose of paclitaxel, and that you think might be related to your chemotherapy treatment by circling ONE number that best describes your aches/pains at its WORST in the last 24 hours”. Subjects were asked to record this number on days 0–7 after each administration of paclitaxel. Eighty eight percent (69/78) of the patients noted pain during the first cycle, ranging from 1–10 on a 0–10 scale. The pain peaked on day 4 after beginning each paclitaxel treatment (Figure 2). Worst individual pain scores for the 7 days after the first chemotherapy dose were grouped according to commonly accepted pain severity classes12. During the first cycle of therapy, 12%, 29%, 23%, and 36% of participants had maximal pain scores, on a 0–10 point scale, of 0, 1–4, 5–6, and 7–10, respectively. Mean worst pain score severities associated with the first cycle of paclitaxel, appeared to predict pain difficulties with subsequent paclitaxel doses (Figure 3), with correlations of 0.7 with the second cycle scores and 0.6 with the third cycle scores (p<0.0001).
Figure 2.
Mean maximal daily pain scores during the first paclitaxel week (from the PRO questions related to the P-APS).
Figure 3.
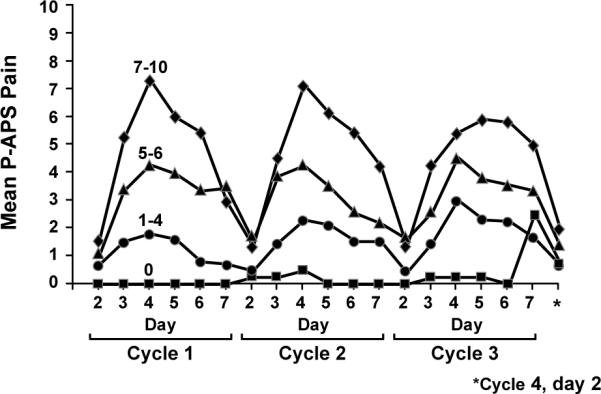
Worst mean maximum P-APS scores over the 12 weeks of paclitaxel, segregated by the worst mean maximum P-APS scores for the first week (from the PRO questions related to the P-APS).
The character and location of pain as experienced by study subjects, following their first paclitaxel infusion, was assessed by questionnaire, a week after therapy. The pain was most prominent in the lower extremities and was most often described as being aching (Table 2). Severity was such that non-prescription medications were used by 46–64% of patients per week, while opioids were used by 23–41% in the week after each chemotherapy dose (Figure 4).
TABLE 2.
Characteristics and locations of pain from the PRO questions related to the P-APS (N=67)
| Pain Characteristics | Pain Locations | ||
|---|---|---|---|
| Aching | 55% | Legs, lower | 42% |
| Sharp | 30% | Feet | 31% |
| Cramping | 31% | Hips | 28% |
| Throbbing | 31% | Abdomen | 25% |
| Dull | 27% | Legs, upper | 22% |
| Shooting | 18% | Back, lower | 22% |
| Gnawing | 15% | Arms, upper | 22% |
| Heavy | 15% | Shoulders | 16% |
| Pulsating | 15% | Head | 16% |
| Stinging | 15% | Arms, lower | 16% |
| Stabbing | 15% | Hands | 16% |
| Hot | 10% | Other | 15% |
| Splitting | 6% | Neck | 13% |
| Chest | 12% | ||
| Back, upper | 10% | ||
Figure 4.
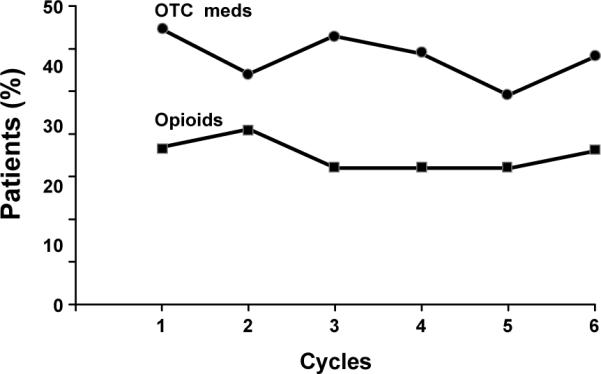
Rates of analgesic agents used for the treatment of the P-APS, over time. (OTC-over the counter)
Chemotherapy induced peripheral neuropathy
The EORTC QLQ CIPN-20 instrument was utilized to record symptoms associated with the evolution of peripheral neuropathy over the series of paclitaxel infusions for each patient. Figure 5 illustrates the percent changes in sensory, motor, and autonomic scores over time. Sensory nerve function was most impaired by paclitaxel, while symptoms associated with changes in autonomic or motor nerve function were more limited. Sensory nerve scale changes, per mixed model statistics, were more marked than were motor changes (p=0.0001) or autonomic changes (p= 0.01).
Figure 5.
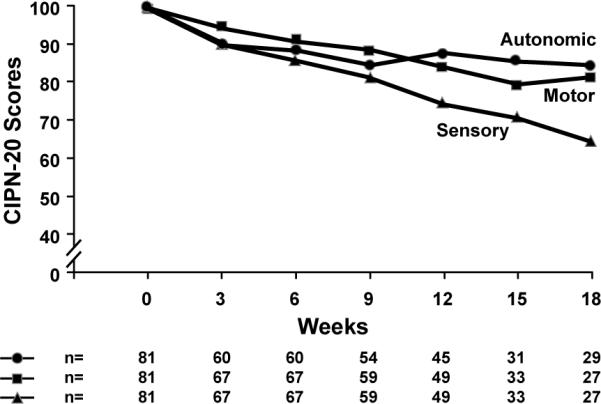
Sensory, motor, and autonomic sub-scores from the EORTC CIPN-20 instrument, in terms of percent of baseline over time.
Figure 6 illustrates the 3 main components of the sensory neurotoxicity, those being numbness, tingling, and shooting/burning pain, in the fingers and hands over time, based on individual EORTC CIPN-20 questions addressing each of these items. These data illustrate that numbness and tingling are very closely related to each other, while burning/shooting pain is a less prominent finding. Similar relationships were observed for the same questions regarding the toes and feet. The differences in AUC, testing upper versus lower extremities for tingling (p=0.95) and numbness (p=0.18), demonstrates that symptom severity was similar. However, shooting or burning pain (p=0.003) was shown to be worse in the lower extremities, as compared to the upper extremities.
Figure 6.
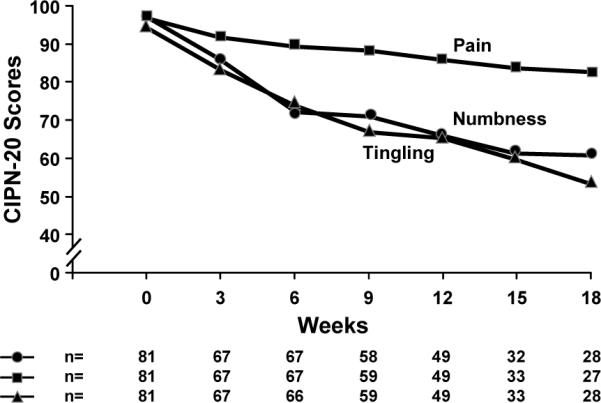
Mean numbness, tingling, and pain scores for hands and fingers (from the EORTC CIPN-20 instrument).
To determine whether there was any relationship between the P-APS and the development of peripheral neuropathy, EORTC CIPN-20 scores were compared between first cycle P-APS pain score groups (Figure 7). Mean sensory nerve function scores declined over 6 cycles (18 weeks) by 40 points in the group with the most severe (7–10) pain with the first dose of paclitaxel. Those with mild (1–4) or moderate (5–6) pain during the first dose of paclitaxel also experienced a decline in mean sensory scores over time, both by 33 points (p= 0.007 and p=0.125, respectively). Subjects who did not experience pain with the first paclitaxel cycle, had no significant sensory EORTC CIPN-20 score change from baseline. Subjects who experienced less acute pain (scores of 0–4) with the first paclitaxel cycle had less neuropathy over 18 weeks of treatment than did patients with 5–10 pain score severities (Table 3). Given that there was a greater dropout of patients after 4 cycles (12 weeks) of therapy, it is reasonable to ask whether the degree of the P-APS in the first cycle of therapy positively correlated with eventual neuropathy, if all of the data were censored at 12 weeks. Indeed, similar results were seen, as are illustrated in Figure 7. Over 12 weeks, mean sensory nerve function scores declined by 30 points in the group with severe pain (7–10) (p=0.0001), 34 points in patients with moderate pain (5–6) (p=0.0005), 22 points in patients with mild pain (1–4) (p=0.0001), and no significant change for patients with no pain in the first cycle (p=0.75).
Figure 7.
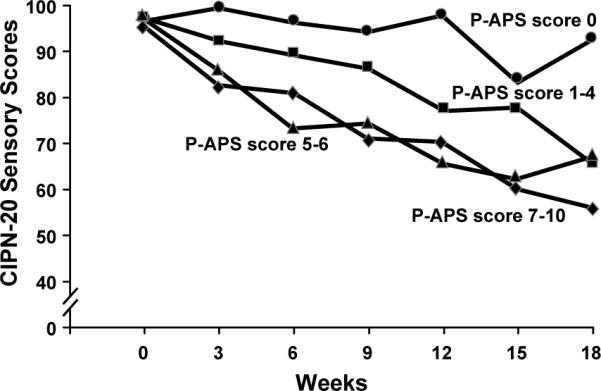
Total sensory neuropathy, numbness, tingling, and burning/shooting pain scores (from the EORTC CIPN-20 instrument), segregated by lower paclitaxel acute pain scores during the week after receiving the first dose of paclitaxel versus higher scores (from the PRO questions related to the P-APS).
Table 3.
Mixed Model P Values for CIPN Severity Over Time, Comparing P-APS Severity in the First Week
| EORTC CIPN-20 Endpoint | Mean Decrease in CIPN Severity for P-APS Score 0–4 (versus 5–10)* | P-Value** | Mean Decrease in CIPN Severity for P-APS Score 0–4 (versus 5–10)* Using Cycles 1–4 | P-Value** |
|---|---|---|---|---|
| Autonomic Subscale | 7.8 | 0.007 | 10.2 | 0.003 |
| Motor Subscale | 7.9 | 0.004 | 10.4 | 0.0008 |
| Sensory Subscale | 11.3 | 0.001 | 14.3 | 0.0005 |
| Numbness-Feet | 17.0 | 0.001 | 20.4 | 0.002 |
| Burning/Shooting Pain-Feet | 11.5 | 0.02 | 13.7 | 0.02 |
| Tingling-Feet | 13.7 | 0.02 | 18.6 | 0.007 |
| Numbness-Hands | 16.0 | 0.002 | 20.2 | 0.001 |
| Burning/Shooting Pain-Hands | 8.7 | 0.02 | 10.9 | 0.02 |
| Tingling-Hands | 11.0 | 0.04 | 14.9 | 0.02 |
On a 0–100 point scale
Mixed modeling p values adjusted for age and race
Pain scores were compared between subjects receiving bevacizumab and subjects not receiving bevacizumab. Subjects on placebo-controlled clinical clinicals, where their bevacizumab use was not known, were excluded from these analyses. Based on univariate comparisons and multivariate mixed models, there was no indication that these two groups of patients experienced different levels of P-APS or peripheral neuropathy.
DISCUSSION
The present study provides further understanding of the paclitaxel-associated acute pain syndrome (P-APS). Eighty eight percent of subjects experienced pain that peaked at day 4 after their first paclitaxel cycle, with 59% noting pain scores of at least 5 out of 10. This is in contrast to seventy percent in a weekly paclitaxel (70–90 mg/m2) group having pain and 20% noting pain scores of at least 5 out of 10,4 consistent with this problem being related to paclitaxel dosing13. Like the previous weekly paclitaxel study4, the pain was most prominent in the hips and lower extremities. Also, as was observed in the previous cohort, aching was the most common pain descriptor.
One of the two major differences in this current cohort and the previous weekly cohort is that the individual paclitaxel doses were different, with the every 3 week dose group receiving 2–3 times the amount of paclitaxel as individual doses. The other difference is that the present group also received concurrent carboplatin. It is likely that the increased intensity of the P-APS in the current trial is related to the paclitaxel dose, as opposed to the concurrent administration of carboplatin. This is because carboplatin is not generally known to be associated with acute aches and pains. Further data to confirm and/or deny this contention should be available when data from another cohort of this trial have been collected, composed of patients receiving a similar dose of paclitaxel every 3 weeks without the concurrent administration of carboplatin.
Since carboplatin does not cause much neuropathy, it is also likely that this drug had little to do with the eventual CIPN that was seen in this trial, which was probably mainly caused by the paclitaxel, although, theoretically, the combination might cause worse CIPN than would paclitaxel alone.
Both reported cohorts, those being from the current manuscript and the previously published manuscript4, demonstrate evidence of an association between the presence and severity of P-APS and the eventual development of sensory neuropathy. It is reasonable to ask the question as to whether the available data are now strong enough to confirm an association between the amount of P-APS pain and the eventually more classic CIPN observed with paclitaxel. While the initially reported weekly paclitaxel cohort supported that there was a relationship, this was not claimed to be definitive based on the data from this group, even though the hypothesized relationship was observed. With this second cohort showing similar findings, it would be reasonable to claim that it actually is true that patients with more P-APS pain in the first week after their first dose of paclitaxel have more eventual peripheral neuropathy months later. However, a more conservative stance would be to wait to see if this association would again be observed in the other two cohorts of this trial, which are currently still accruing patients.
This potential association further supports that, while the P-APS and the longer term peripheral neuropathy are separate clinical entities, P-APS and this longer term neuropathy may both be manifestations of nerve pathology. However, proving an association between the severity of the P-APS and eventual CIPN does not prove that the P-APS is actually nerve pathology. Another explanation for an association between the degree of P-APS pain and eventual CIPN is that this might be because some individuals might have increased pain perception relative to any pathologic process or from any noxious stimuli. Additionally, it could be from a pharmacogenomic process, whereby some individuals have higher active drug concentrations that might cause more drug related toxicities regardless of them being from different pathologic mechanisms.
While it would be nice to have supporting evidence from objective neuro-diagnostic physiologic testing and imaging, it should be noted that there is no physiologic testing or imaging to support that the P-APS is from muscle of joint pathology. The notation that there is demonstrated dorsal root ganglion injury 24 hours following a clinically appropriate dose of paclitaxel in a rat model14, 15 and that the clinical descriptions of the pain syndrome sound like they come from neurologic injury3, supports that the P-APS could be a form of neurologic toxicity. It does appear to be as reasonable to hypothesize that the acute largely-transient symptoms of the P-APS is a manifestation of neurologic toxicity as it is to accept the commonly believed claim that the acute largely-transient symptoms of oxaliplatin (e.g. cold induced hyperalgesia and cramps) is a form of acute neurologic toxicity16.
The mechanistic link between P-APS and the development of peripheral neuropathy has not yet been fully elucidated. Similar axial pains have been described in cases of an immune sensory polyradiculopathy in pork workers, wherein there was prominent inflammation of the nerve roots and dorsal root ganglia.17 In vivo studies in rodents support DRG inflammation following paclitaxel administration as a possible pain mechanism: rapid infiltration of macrophages within DRG are seen following activation of markers of nerve injury.14, 15 Further, immune suppression with prednisone in humans has been reported to decrease P-APS18,19, although these observations are not universal20 and there have been no associated randomized-controlled trials. Minocycline, which is a selective microglia/macrophage inhibitor, and the anti-inflammatory cytokine IL-10 have also been reported to reduce paclitaxel-associated mechanical allodynia in a rat model21–23. Taken together, it is possible that early inflammation in the DRG is at least, in part, responsible for the pain associated with P-APS and continued inflammation may lead to downstream effects responsible for the development of peripheral neuropathy.
The primary mechanism of action of paclitaxel is by stabilizing microtubules, preventing microtubule polymerization of mitotically active cells, thereby leading to inhibition of cell division. How paclitaxel then leads to toxicity of non-mitotically active sensory neurons remains unclear. It has been proposed that microtubule-dependent axonal transport may be disrupted by paclitaxel. Mitochondrial transport may be affected, which could explain the predilection of neuropathy to the hands and feet, which are the longest axons and therefore require the most mitochondrial support. Indeed, disruption of mitochondrial energetics has been implicated in the development of paclitaxel-associated peripheral neuropathy.24, 25 Evidence suggests that paclitaxel may directly target mitochondria by binding to β-tubulin that is associated with the mitochondrial permeability transition pore, thereby leading to mitochondrial swelling.24 Acetyl-L-carnitine, which plays an essential role in transporting long-chain fatty acids within mitochondria, has been reported to have a preventive effect on development of peripheral neuropathy in a small pilot report.26 Olesoxime, a small cholesterol-like molecule exerting effects on mitochondria, has also shown promise in preventing paclitaxel-associated peripheral neuropathy in a human cell culture line and in rats.27, 28
To date, no therapy has been proven to be beneficial for preventing the paclitaxel associated acute pain syndrome or paclitaxel associated peripheral neuropathy. The only randomized, double-blinded, placebo-controlled trial for alleviating the P-APS evaluated glutamine, with negative study results29. While a variety of other agents have been proposed as being potentially helpful, data from prospective placebo-controlled trials are not currently available. Ideally, the treatment would prevent both the P-APS and resultant peripheral neuropathy, and be well-tolerated. Until effective agents are available, patients should be advised of P-APS and instructed on appropriate use of over-the-counter analgesics and/or opioid pain medications to ameliorate short-term toxicities.
In summary, the current study adds to the understanding of the P-APS, which has a high incidence in those treated with paclitaxel and is a quite bothersome side-effect that may herald the onset of peripheral neuropathy. Further research is needed to better elucidate underlying mechanisms and effective treatments.
Acknowledgments
Funding: This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-124477. CA-63848, CA-52352, CA-35090, CA-35101, CA-35269, CA-37417, CA-35448, CA-63844, CA-35267, CA-35272, CA-35113, CA-35103, CA-35415 and CA-35431.
Footnotes
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-124477. CA-63848, CA-52352, CA-35090, CA-35101, CA-35269, CA-37417, CA-35448, CA-63844, CA-35267, CA-35272, CA-35113, CA-35103, CA-35415 and CA-35431. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Additional participating institutions: Columbus CCOP, Columbus, OH 53215 (J. Philip Kuebler, M.D., Ph.D.); Grand Rapids Clinical Oncology Program, Grand Rapids, MI 49503 (Martin Bury, M.D.); Montana Cancer Consortium, Billings, MT 59101 (Benjamin T. Marchello, M.D.); Colorado Cancer Research Program, Denver, CO 80224 (Eduardo R. Pajon, Jr., M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Miroslaw Mazurczak, M.D.); Siouxland Hematology-Oncology Associates, Sioux City, IA 51105 (Donald B. Wender, M.D.); Virginia Commonwealth University, Richmond, VA 23298 (Mary Helen Hackney, M.D.); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416 (Patrick J. Flynn, M.D.); Duluth CCOP, Duluth, MN 55805 (Daniel A. Nikcevich, M.D.); Upstate Carolina CCOP, Spartanburg, SC (James D. Bearden, III, M.D.); Mayo Clinic Florida, Jacksonville, FL 32224 (Kurt A. Jaeckle, M.D.); Hawaii Minority-Based CCOP, Honolulu, HI 96813 (William S. Loui, M.D.); Heartland Cancer Research CCOP, St. Louis, MO 63131 (Alan P. Lyss, M.D.); Toledo Community Hospital Oncology Program CCOP, Toledo, OH 43623 (Rex B. Mowat, M.D.); Medical College of Georgia, Augusta, GA 30912 (Anand P. Jillella, M.D.); CentraCare Clinic, St. Cloud, MN 56301 (Donald J. Jurgens, M.D.); Columbia River Oncology Program, Portland, OR 97225 (Janet C. Ruzich, M.D.); Virgina Mason CCOP, Seattle, WA 98101 (Jacqueline Vuky, M.D.); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN 46601 (Robin T. Zon, M.D.); Meritcare Hospital CCOP, Fargo, ND 58122 (Preston D. Steen, M.D.); Iowa Oncology Research Association CCOP, Des Moines, IA 50309-1014 (Robert J. Behrens, M.D.); Michigan Cancer Research Consortium, Ann Arbor, MI 48106 (Philip J. Stella, M.D.); Hematology & Oncology of Dayton, Inc, Dayton OH (Howard M. Gross, M.D.); Marshfield Clinical Research Foundation, Minocqua, WI 54548 (Matthias Weiss, M.D.); Cancer Care Associates, Tulsa, OK 74136 (Alan M. Keller, M.D.); Montana Cancer Consortium, Billings, MT 59101 (Benjamin T. Marchello, M.D.); Geisinger Clinic & Medical Center CCOP, Danville, PA 17822 (Albert M. Bernath, M.D.)
REFERENCES
- 1.Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20(4 Suppl 3):1–15. [PubMed] [Google Scholar]
- 2.Garrison JA, McCune JS, Livingston RB, et al. Myalgias and arthralgias associated with paclitaxel. Oncology (Williston Park) 2003;17(2):271–7. discussion 81–2, 86–8. [PubMed] [Google Scholar]
- 3.Loprinzi CL, Maddocks-Christianson K, Wolf SL, et al. The Paclitaxel acute pain syndrome: sensitization of nociceptors as the putative mechanism. Cancer J. 2007;13(6):399–403. doi: 10.1097/PPO.0b013e31815a999b. [DOI] [PubMed] [Google Scholar]
- 4.Loprinzi CL, Reeves BN, Dakhil SR, et al. Natural History of Paclitaxel-Associated Acute Pain Syndrome: Prospective Cohort Study NCCTG N08C1. J Clin Oncol. 2011;29(11):1472–8. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42–7. doi: 10.3747/co.v17i4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argyriou AA. Tracing the incidence of paclitaxel-induced peripheral neuropathy. Eur J Cancer Care (Engl) 2009;18(5):522–3. doi: 10.1111/j.1365-2354.2009.01140.x. [DOI] [PubMed] [Google Scholar]
- 7.Cleeland C. How to assess cancer pain. Handbook of Pain Assessment: The Guilford Press; 1992. pp. 360–87. [Google Scholar]
- 8.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–9. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Sloan J, O'Fallon J, Suman V. Incorporating Quality of Life Measurement Into Oncology Clinical Trials. Proc Am Stat Assoc. 1998 [Google Scholar]
- 10.Sloan J, Symonds T, Vargas-Chanes D, Fridley B. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Information Journal. 2003;37:23–31. [Google Scholar]
- 11.Sloan J, Dueck A. Issues for Statisticians in Conducting Analyses and Translating Results for Quality of Life End Points in Clinical Trials. Journal of Biopharmaceutical Statistics. 2004;14(1):73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 12.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–84. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 13.Moulder SL, Holmes FA, Tolcher AW, et al. A randomized phase 2 trial comparing 3-hour versus 96-hour infusion schedules of paclitaxel for the treatment of metastatic breast cancer. Cancer. 2010;116(4):814–21. doi: 10.1002/cncr.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimenez-Andrade JM, Peters CM, Mejia NA, Ghilardi JR, Kuskowski MA, Mantyh PW. Sensory neurons and their supporting cells located in the trigeminal, thoracic and lumbar ganglia differentially express markers of injury following intravenous administration of paclitaxel in the rat. Neurosci Lett. 2006;405(1–2):62–7. doi: 10.1016/j.neulet.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Peters CM, Jimenez-Andrade JM, Jonas BM, et al. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol. 2007;203(1):42–54. doi: 10.1016/j.expneurol.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Grothey A, Nikcevich DA, Sloan JA, et al. Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol. 2011;29(4):421–7. doi: 10.1200/JCO.2010.31.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachance DH, Lennon VA, Pittock SJ, et al. An outbreak of neurological autoimmunity with polyradiculoneuropathy in workers exposed to aerosolised porcine neural tissue: a descriptive study. Lancet Neurol. 2010;9(1):55–66. doi: 10.1016/S1474-4422(09)70296-0. [DOI] [PubMed] [Google Scholar]
- 18.Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J. Use of low-dose oral prednisone to prevent paclitaxel-induced arthralgias and myalgias. Gynecol Oncol. 1999;72(1):100–1. doi: 10.1006/gyno.1998.5226. [DOI] [PubMed] [Google Scholar]
- 19.Schiller JH, Storer B, Tutsch K, et al. Phase I trial of 3-hour infusion of paclitaxel with or without granulocyte colony-stimulating factor in patients with advanced cancer. J Clin Oncol. 1994;12(2):241–8. doi: 10.1200/JCO.1994.12.2.241. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen VH, Lawrence HJ. Use of gabapentin in the prevention of taxane-induced arthralgias and myalgias. J Clin Oncol. 2004;22(9):1767–9. doi: 10.1200/JCO.2004.99.298. [DOI] [PubMed] [Google Scholar]
- 21.Liu CC, Lu N, Cui Y, et al. Prevention of paclitaxel-induced allodynia by minocycline: Effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol Pain. 2010;6:76. doi: 10.1186/1744-8069-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cata JP, Weng HR, Dougherty PM. The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain Res. 2008;1229:100–10. doi: 10.1016/j.brainres.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledeboer A, Jekich BM, Sloane EM, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21(5):686–98. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122(3):245–57. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasr P, Sullivan PG, Smith GM. Mitochondrial imaging in dorsal root ganglion neurons following the application of inducible adenoviral vector expressing two fluorescent proteins. J Neurosci Methods. 2008;172(2):185–94. doi: 10.1016/j.jneumeth.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchi G, Vitali G, Caraceni A, et al. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur J Cancer. 2005;41(12):1746–50. doi: 10.1016/j.ejca.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Rovini A, Carre M, Bordet T, Pruss RM, Braguer D. Olesoxime prevents microtubule-targeting drug neurotoxicity: selective preservation of EB comets in differentiated neuronal cells. Biochem Pharmacol. 2010;80(6):884–94. doi: 10.1016/j.bcp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Xiao WH, Zheng FY, Bennett GJ, Bordet T, Pruss RM. Olesoxime (cholest-4-en-3-one, oxime): analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel. Pain. 2009;147(1–3):202–9. doi: 10.1016/j.pain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson SD, Loprinzi CL, Sloan JA, et al. Glutamine does not prevent paclitaxel-associated myalgias and arthralgias. J Support Oncol. 2003;1(4):274–8. [PubMed] [Google Scholar]