Abstract
Background
Traditional testing for gastroparesis with gastric emptying scintigraphy (GES) likely misses a subset of patients because of the heterogeneous nature of the disease. The primary aim of this study is to determine the prevalence of simultaneously measured transit and pressure abnormalities in patients with gastroparesis. The secondary aim is to assess diagnostic gain realized by measuring antroduodenal pressure and gastric transit with wireless motility capsule (WMC) compared to gastric transit measured by GES. Identification of abnormalities beyond gastric transit delay in gastroparesis may yield novel targets for pharmacological therapies.
Methods
43 subjects with symptoms of gastroparesis and previous abnormal GES within 2 years were enrolled in the study. Subjects underwent simultaneous GES and WMC to assess gastric transit. Gastric and small bowel pressure profiles were measured by WMC to determine the contribution of pressure to diagnostic gain realized with WMC
Key Results
51% of subjects had abnormal GES while 70% of subjects had either abnormal GET or antroduodenal pressure. GET was abnormal in 60% of subjects while gastric or small bowel pressure was abnormal in 47% of subjects. The overall diagnostic gain of WMC compared to GES was 19% (p=0.04). 7% of subjects had abnormal small bowel pressure profiles when both GES and GET were normal. Conclusions:(i) Gastroparesis is a heterogeneous disorder and testing only solid food emptying by scintigraphy may miss a significant amount of pathology. (ii)_Measuring complementary aspects of gastric and small bowel function simultaneously results in greater detection of physiologic abnormalities that may underlie patient symptoms.
Keywords: Gastric emptying scintigraphy, Gastroparesis, Wireless motility capsule
INTRODUCTION
Gastroparesis is a chronic disorder characterized by impaired gastric emptying and altered motility in the upper GI tract in the absence of mechanical obstruction (1). Numerous physiologic factors may contribute to symptoms such as abnormalities in liquid and solid meal emptying (fed state), emptying of indigestible objects (fasted state), and gastric and proximal small bowel contractility (2).
The pathophysiology behind gastroparesis is varied and may include vagal and/or autonomic neuropathy (3, 4), interruption of the interstitial cells of Cajal (5), and possibly genetic factors (6). This can lead to impaired antral contractions, tonic motor defects, altered intragastric distribution, increased outflow resistance in the pylorus or small intestine, and impaired distal regulatory mechanisms.
The most commonly used test for diagnosis of gastroparesis is currently solid-phase gastric emptying scintigraphy (GES), which measures only gastric emptying of solid food. However, gastroparesis is a complex disease with potential abnormalities in both the fed and fasted state and so GES may miss potential physiologic abnormalities (7, 8).
Antroduodenal manometry is used to assess pressure profiles in the stomach and proximal small bowel but is not widely available and few clinicians have the expertise to interpret these motility studies. The procedure is also inconvenient, invasive, and uncomfortable.
A Wireless Motility Capsule (WMC) is available to measure GI transit and pressures. The device is FDA approved, non-invasive, and non-digestible (9, 10). After ingestion with a standardized meal, the capsule travels through the GI tract and measures luminal pH, pressure, and temperature. This can provide regional (gastric, small bowel, and colonic) transit profiles as well as whole gut transit times. Gastric emptying time (GET) of less than 5 hours is considered normal and can differentiate healthy and gastroparetic subjects (10, 11). In addition, the capsule can characterize pressure patterns and motility indices in the entire GI tract (12-15).
GES and antroduodenal manometry were compared to WMC to determine mechanisms of emptying of a non-digestible solid. Cassilly et al. found that WMC primarily emptied via high amplitude antral contractions, mainly phase III migrating motor complexes (MMC) but also occasionally through isolated antral contractions. The authors reported that emptying of a meal measured by GES was followed by initiation of the fasted state and phase III portion of the MMC with subsequent gastric emptying of WMC. They also found that WMC and antroduodenal manometry had similar pressure patterns around the time period of WMC emptying (10).
Gastroparesis remains a challenging condition with multifactorial physiologic abnormalities contributing to symptoms. Multiple tests may uncover other pathophysiologic mechanisms contributing to symptoms of gastroparesis. The primary aim of this analysis was to evaluate the prevalence of abnormalities of gastric and proximal small bowel function (gastric transit as well as gastric and small bowel pressure measurements) in symptomatic gastroparetic subjects. The second aim was to assess the additional diagnostic gain realized with measurement of gastric transit and antroduodenal pressure by WMC as a replacement for the measurement of gastric emptying of a meal by GES alone.
MATERIALS AND METHODS
Subject enrolment
This data for analysis was taken from a study that was conducted at seven medical centers from March 2005 to October 2007 and the Institutional Review Board of each participating center approved the study protocol. Subjects with a history of gastroparesis and continued symptoms were enrolled. Subjects entered the study after the nature and purpose of the study had been explained and after they granted written informed consent. The subjects in our analysis were selected from the clinical study previously described by Kuo (11) who met the following inclusion/exclusion criteria.
Subjects with history of gastroparesis
Males and females between ages 18 and 65 years with history of nausea and vomiting, early satiety, epigastric pain or discomfort for at least 6 months and documented abnormal scintigraphy by local standards within two years were enrolled.
Subjects with previous gastrointestinal surgery were excluded. Medications that can affect gastrointestinal parameters or may cause delayed gastric emptying were stopped at least 3 days prior to WMC ingestion and during the study.
Experimental Protocol
Subjects swallowed the WMC capsule with 50 ml of water followed by ingestion of a standard eggbeaters meal mixed with 1 mCi 99mTc sulphur-colloid marker to ensure standardized conditions for measurement of gastric emptying scintigraphy. The eggbeater meal has a total caloric value of 255 kcal (72% carbohydrate, 24% protein, 2% fat, and 2% fiber), which is nutritionally identical to a Smartbar meal that is routinely taken with WMC (16, 17). Gastric emptying scintigraphy was performed simultaneously with the WMC examination. Scintigraphic images were taken in the 140 keV 99Tc peak with a 20% window (140 keV ± 10%); 1 min of anterior and 1 min of posterior measurements were taken for each scan. Data were corrected for time decay of technetium. The region of interest was drawn around the image of the stomach for each time frame and the geometric mean was calculated as the square root of the product of the counts measured on the anterior and posterior images. Data were expressed as per cent of the meal retained at 2 h (GES-2 h) and per cent of the meal retained at 4 h (GES-4h) as previously described (11).
Six hours after capsule ingestion, subjects consumed 250 ml Ensure®. Approximately 8 hours after capsule ingestion, subjects left the study center with the data receiver to enable continued data acquisition from the capsule. At 48 to 120 hours post ingestion, subjects returned with the data receiver and diary. Capsule exit was confirmed for each subject by plain abdominal radiograph (KUB) unless the subject retrieved and returned the capsule (18-20).
Pressure and pH Monitoring System
pH and pressure data were obtained by using the WMC wireless capsule system (The SmartPill Corporation, Buffalo, NY). The capsule houses sensors for pH, temperature, and pressure and transmits the data to a receiver worn by the subject during ambulatory monitoring. The wireless motility capsule is 13mm across and 26mm long. The capsule and receiver have battery lives rated for 5 days. pH is accurate to within 0.5 units and pressure is accurate to +/- 5mmHg below 100 mmHg. After completion of the test, data was downloaded to a computer from the data receiver through a docking station and was analyzed using the WMC pressure analysis software (GIMS 1.8).
Assessment of gastroduodenal motility
Pressure data recorded by the WMC capsule was analyzed for 1 hour before and after the WMC empties the stomach (GET). Subjects were included for analysis if pressure profiles in these regions included ≥ 85% of the pressure data available. The lower limits for the pressure parameters were based on the lowest 5th percentile of the normal population. The transition from stomach to small bowel was marked by an abrupt pH rise (> 3 pH units) from gastric baseline to a pH greater than 4 (21-23). This pH change marked the end of the gastric pressure analysis window and the beginning of the small bowel pressure analysis window. Pressure analysis of the small bowel did not commence until pH was consistently 3 pH units above baseline indicating that the capsule had passed into the small intestine (10). Any transitory drops in pH could not exceed 10 minutes in length for the initial abrupt pH rise to be considered gastric emptying. The analyzed parameters were: number of contractions (Ct), and motility index defined as: MI = Ln (sum of pressure amplitudes * number of contractions +1) (24, 25). Pressure peaks exceeding 10 mmHg but less than 300 mmHg were included in the analyses. Motility parameters in the stomach and proximal small bowel of these patients were compared to healthy volunteers as previously described in the literature (11, 26). Gastric emptying time (GET) is defined as the duration of time from capsule ingestion to entry of the capsule into the duodenum. The cut-off value for GET reported by Kuo is 5h while normal value for GES reported by Tougas is < 10% gastric retention at 4h (11, 16).
Statistical Analysis
Study variables were summarized using frequencies and relative frequencies. To statistically assess differences between proportions McNemar's test was used. Reported p-vales were based on the exact distribution of the test statistic. A nominal significance level of 0.05 was used in all testing and all analyses were done using SAS (version 9.2). Device agreement compared the agreement of GET to GES.
Calculation of Diagnostic Gain in Upper GI Tract
The total number of subjects with an abnormal motility parameter detected by WMC (GET, gastric Ct, SB Ct, gastric MI, and/or SB MI) with normal GES was adjusted for the total number of subjects with abnormal GES but normal WMC to arrive at diagnostic gain. Diagnostic gain was then expressed as a percentage of the total number of subjects.
Abnormal pressure measurements (PM) are defined as number of Ct and/or MI that did not meet threshold values for the lowest 5th percentile of the normal population (26). Data was then analyzed to determine whether individual pressure parameters (Ct, MI) as well as total PM provided additional diagnostic gain compared to GES.
RESULTS
Study Subjects and Demographics
48 subjects were initially enrolled in the study. 5 subjects were excluded because pressure profiles in the analyses windows were incomplete. Data from 43 subjects (8 male, 35 female, mean age 42 years) met the inclusion criteria of this analysis. 27 subjects had idiopathic gastroparesis while 16 subjects had gastroparesis secondary to underlying diabetes mellitus.
Prevalence of Physiologic Abnormalities
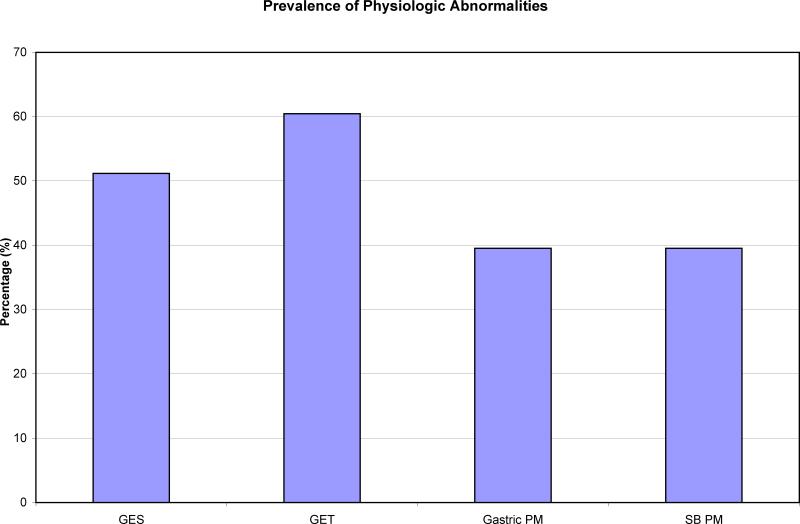
26/43 (60%) subjects had an abnormal GET and 22/43 (51%) had an abnormal GES (Figure 1). Overall device agreement was calculated as 77% (positive agreement = 86%, negative agreement = 66%) between GET and GES. Seven subjects had delayed GET when GES was normal while 3 subjects had delayed GES when GET was normal.
FIGURE 1.
Prevalence of physiologic abnormalities calculated by GES, GET, as well as gastric and proximal small bowel pressure measurements (PM) by WMC.
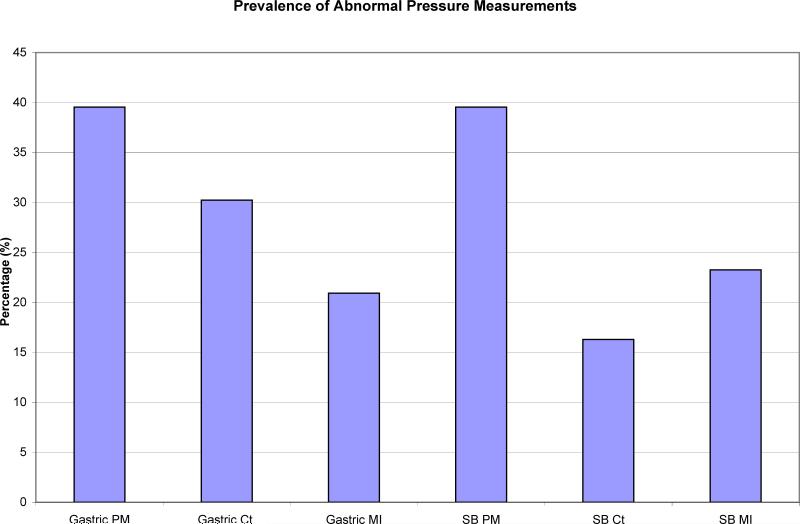
Overall, a total of 47% had abnormal gastric and SB PM. 40% of these subjects had an abnormal gastric PM (Ct and/or MI) while 40% of subjects had abnormal small bowel PM (Figure 1). The remaining 20% of subjects with abnormal PM had both abnormal gastric and small bowel PM. 30% of subjects overall had abnormal gastric Ct and 16% of subjects had abnormal SB Ct. Gastric MI was abnormal in 21% of subjects while 23% had abnormal SB MI (Figure 2).
FIGURE 2.
Percentage of abnormal pressure measurements (PM) by contractility (Ct) and motility index (MI) in the stomach as well as proximal small bowel.
Additional Diagnostic Gain of WMC over GES
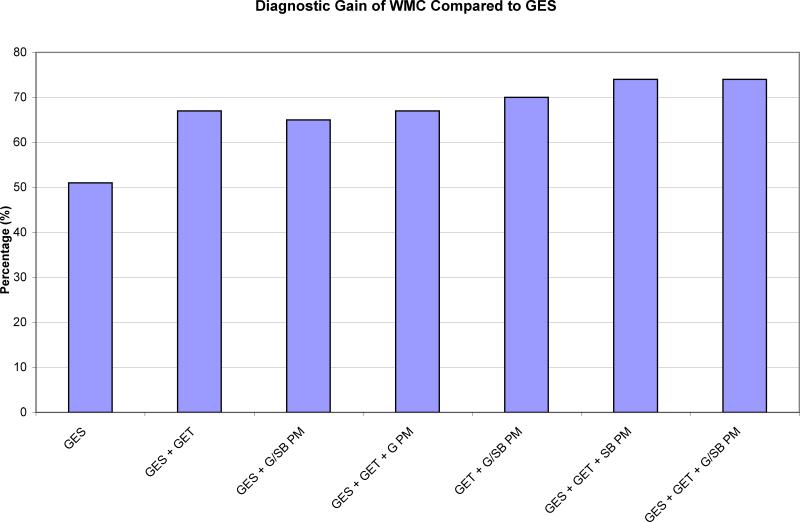
Ten of the twenty-one subjects with normal GES had abnormalities identified by WMC (GET, gastric Ct, gastric MI, SB Ct, or SB MI). There were two subjects who had normal parameters by WMC but abnormal GES. The overall diagnostic gain with WMC compared to GES was 19% (p = 0.04).
The diagnostic gain associated with individual pressure parameters was calculated as well. In the 21 subjects with normal GES, seven had an abnormal GET, three had abnormal gastric pressure, and six had abnormal SB pressure. Of the 14 subjects with normal GES and GET, there were no subjects with abnormal gastric PM but there were 3 subjects with abnormal SB PM (Figure 3).
FIGURE 3.
Comparison of diagnostic gain of WMC compared to GES. WMC and GES were performed simultaneously and each subject acted as their own control. All parameters showed statistical significance compared to GES (p < 0.05).
Compared to GES, there was a statistically significant improvement in diagnostic gain discovered by GES + GET (p = 0.02), GES + gastric PM + SB PM (p = 0.03), GES + GET + gastric PM (p = 0.02), GET + gastric PM + SB PM (p = 0.04), as well as GES + GET + SB PM and GES + GET + gastric PM + SB PM (p = 0.0020).
DISCUSSION
Gastroparesis is a heterogeneous disorder with multiple physiologic etiologies underlying symptoms, which can potentially impact emptying of solids and indigestible objects. The conventional measure of gastric transit, GES, measures only one aspect of gastric function, the emptying of a meal corresponding to the gastric fed state. This may fail to uncover other physiologic reasons for symptoms and may result in under detection of abnormalities in symptomatic patients. Our study is the first to quantify three simultaneously obtained variables (GES, GET, and pressure measurements) in gastroparetics. We found that measuring multiple simultaneously acquired motility variables reveals physiologic abnormalities including small bowel dysmotility that may have been overlooked by testing focused mainly on gastric meal emptying. We also found that WMC produces an additional statistically significant diagnostic gain compared to GES in gastroparesis.
There are few published studies looking at multiple variables measured simultaneously in gastroparesis. Sogabe et al. used ultrasound to measure gastric emptying, gastric contractions, and gastric motility index in gastroparetics (27). Darwiche et al. found a strong correlation between simultaneously acquired ultrasound and GES to evaluate gastric emptying in gastroparetics (28). However, ultrasound has many limitations, such as being operator dependent and does not have the capability to measure small bowel dysfunction. Pfaffenbach et al. simultaneously compared elctrogastrography (EGG) with GES in gastroparetics but could not find a correlation between electrical abnormalities measured by EGG with delayed gastric emptying shown on GES (29). Braden et al. have compared stable isotope breath tests simultaneously with GES and showed reliable correlation with liquid emptying. However, solid food emptying by breath tests in gastroparetics has not been validated to date.(30, 31).
Camilleri et al. performed same day testing with antroduodenal manometry and GES on subjects with gastroparesis (32). This study is closest in design to our study as both measure gastric meal emptying as well as gastric and small bowel motility patterns in gastroparetics. Camilleri shows that antral contractility dysfunction and/or small bowel dysmotility is important in the pathogenesis of gastroparesis, which may be missed by only measuring solid food emptying. We have also found gastric and small bowel dysmotility present in gastroparesis. However, in contrast to Camilleri's study, we performed GES and WMC simultaneously allowing direct comparison of the two diagnostic tests.
WMC is also less invasive, more widely available, and easier to tolerate than antroduodenal manometry. Even though there was not a control population used in this study, each subject acted as his or her own control to calculate diagnostic gain and a previous study by Cassilly et al. has already characterized normal pressure patterns of the stomach and proximal small bowel with simultaneously performed ADM and WMC in healthy controls (10).
Gastroparesis is a clinical diagnosis based on typical symptoms and physiologic tests showing delayed gastric emptying. GES is the most commonly used modality in the diagnosis of gastroparesis. However, there are significant disadvantages in relying on this technique as a modality to evaluate for gastroparesis. It is not standardized in terms of meals, diagnostic cutoffs, and test duration times across institutions. We found that in our population of subjects with gastroparetic symptoms, only 51% had an abnormal GES despite having a previously documented abnormal scintigraphy study. Degen et al. has shown there is variation with gastric emptying scintigraphy (CV = 15%) (33). Furthermore, there can be variability with GES given the lack of standardization across institutions with most institutions measuring gastric emptying for only 90 or 120 minutes. Our study measured gastric emptying for 4h, which is the current recommended time period (34). We also utilized a standardized test meal currently recommended by the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine (34). Previous scintigraphic examinations in some subjects utilized variable time frames with non-standardized test meals for measuring gastric emptying, which may also explain intraindividual variation seen in our study.
The overall device agreement between GES and GET was 77%, which again confirms that these two modalities measure similar but not identical physiology. The two measures have also been shown to be highly correlated (11). GES measures solid food emptying in the stomach while GET measures emptying of a non-digestible object. Gastroparesis may involve multiple physiologic abnormalities including meal emptying, trituration, coordinated pressure profiles, pyloric coordination and small bowel regulation. GES alone may fail to detect mechanisms not dependent on meal emptying that contribute to symptoms. We were able to identify 7 additional subjects with delayed GET that appeared normal by GES. Overall, we showed a 19% increase in diagnostic gain of WMC compared to GES. WMC and GES both measure parameters of gastric transit. However, because WMC has increased diagnostic gain compared to GES, it suggests that both tests do not have to be performed simultaneously in most clinical situations.
We also found that small bowel dysfunction is present in some subjects with symptoms of gastroparesis. Small bowel regulatory mechanisms are believed to be important for normal gastric emptying. This has been measured typically in the past by antroduodenal manometry with multiple sensors in fixed locations in the antrum and proximal small bowel (35). Dysregulation of these regulatory mechanisms may play an important role in the pathogenesis of gastroparesis (7, 8). Uncoordinated and reduced small intestinal pressure patterns have been shown to be important in the development of gastroparesis. Disturbed sympathetic innervation could also play a role in this dysfunction (36-39). This suggests interplay between stomach and small bowel regulates normal gastric emptying. However, disruption of this relationship may play an important role in the pathogenesis of gastroparesis. Clinically, this may have an impact on therapy as the pharmacological dosing in the treatment of small bowel dysmotility is typically less aggressive compared to gastric stimulation with a motilide agent (40, 41).
The primary concern of WMC is it may provide only an indirect measure of solid meal emptying as it measures gastric emptying of a non-digestible object. Although WMC and GES are highly correlated, emptying of indigestible objects relies on return of the phase III MMC in addition to the meal emptying, which can introduce some variability (10). Additionally, WMC is a free floating object and so the exact location where pressure profiles are generated from is indeterminate which may limit interpretation. Finally, the classical peristaltic pattern obtained as a result of multiple pressure sensors on the catheter based ADM is not available with the single, moving WMC pressure sensor. As a result, WMC cannot directly measure peristalsis and has led to concerns that it cannot differentiate between artifact and true peristaltic waves. However, several authors have found that WMC accurately measures upper GI motility patterns in a noninvasive manner with good fidelity to simultaneously performed ADM during the last hour prior to and the first hour after gastric emptying in both healthy and gastroparetic subjects (10, 26). Kloetzer and Thumshirn have reported similar percent declines in contraction frequency utilizing WMC and ADM, respectively, in gastroparetics compared to healthy controls. This further suggests the two modalities sense similar overall pressure patterns (10, 42).
In conclusion, gastroparesis is a heterogeneous condition with likely multiple pathophysiologic mechanisms. Traditional scintigraphic testing methods typically measure only a single variable, which may fail to uncover other potential physiologic abnormalities and thus miss the correct diagnosis in a significant subset of patients. This missed subset might benefit from appropriate use of pro-motility agents. The multiple gastric and small bowel motility parameters may not always agree in terms of normal/abnormal profile and explains why correlation of symptoms with a single parameter may be poor. Testing multiple aspects of gastric function can uncover different but complementary physiologic abnormalities that all may contribute to symptoms of gastroparesis. Measuring multiple variables, such as gastric transit as well as gastric and small bowel PM provides additional insight into the pathophysiology of gastroparesis and can improve diagnostic gain compared to GES.
ACKNOWLEDGMENTS AND DISCLOSURES
Allen Lee wrote the paper and analyzed the data; Gregory Wilding analyzed the data; Braden Kuo wrote the paper, analyzed the data, and designed the research study.
This work was supported by International Foundation of Functional Gastrointestinal Disorders and NIH NIDDK K23K069614
Footnotes
Disclosures:
Braden Kuo and Gregory Wilding serve as consultants for SmartPill and have performed clinical trials in the past funded by SmartPill.
Allen Lee has no conflicts of interest.
REFERENCES
- 1.Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli-Labate AM, Monetti N, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996 Apr;110(4):1036–42. doi: 10.1053/gast.1996.v110.pm8612991. [DOI] [PubMed] [Google Scholar]
- 2.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998 Nov;43(11):2398–404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 3.Kristensson K, Nordborg C, Olsson Y, Sourander P. Changes in the vagus nerve in diabetes mellitus. Acta Pathol Microbiol Scand. 1971;79(6):684–5. doi: 10.1111/j.1699-0463.1971.tb01872.x. [DOI] [PubMed] [Google Scholar]
- 4.Merio R, Festa A, Bergmann H, Eder T, Eibl N, Stacher-Janotta G, et al. Slow gastric emptying in type I diabetes: relation to autonomic and peripheral neuropathy, blood glucose, and glycemic control. Diabetes Care. 1997;20(3):419–23. doi: 10.2337/diacare.20.3.419. [DOI] [PubMed] [Google Scholar]
- 5.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum R. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9(1):102–8. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Nohara S, Iwase M, Imoto H, Sasaki N, Nakamura U, Uchizono Y, et al. Gastric emptying in patients with Type 2 diabetes mellitus and diabetes associated with mitochondrial DNA 3243 mutation using 13C-octanoic acid breath test. J Diabetes Complications. 2006;20(5):295–301. doi: 10.1016/j.jdiacomp.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Khoo J, Rayner CK, Feinle-Bisset C, Jones KL, Horowitz M. Gastrointestinal hormonal dysfunction in gastroparesis and functional dyspepsia. Neurogastroenterol Motil. Dec;22(12):1270–8. doi: 10.1111/j.1365-2982.2010.01609.x. [DOI] [PubMed] [Google Scholar]
- 8.Fraser R, Horowitz M, Maddox A, Dent J. Postprandial antropyloroduodenal motility and gastric emptying in gastroparesis--effects of cisapride. Gut. 1994;35(2):172–8. doi: 10.1136/gut.35.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo B, McCallum RW, Koch K, Chey WD, Wo JM, Sitrin M, et al. SmartPill, A Novel Ambulatory Diagnostic Test for Measuring Gastric Emptying in Health and Disease. Gastroenterology. 2006;(130):M2205. [Google Scholar]
- 10.Cassilly D, Kantor S, Knight L, Maurer A, Fisher R, Semler J, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20(4):311–9. doi: 10.1111/j.1365-2982.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuo B, McCallum R, Koch K, Sitrin M, Wo J, Chey W, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27(2):186–96. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 12.Sarosiek I, Reddymasu S, Koch K, Hasler W, Lackner J, Katz L, et al. Relationship Between the Frequency of Gastric Contractions and Gastric Emptying of a Non-Digestible Solid in Controls and Gastroparetics Using Smartpill Pressure Recording Capsule: Its Future Clinical Potential. Gastroenterology. 2007;(M1175):A378. [Google Scholar]
- 13.Kloetzer L, Gaman A, Napadow V, McCallum R, Koch K, Sitrin M, et al. Spectral Analysis of Contractile Frequency in the Ileum and Proximal Colon: Characterization of Motility Patterns Using An Ambulatory Capsule, Smartpill. Gastroenterology. 2008;(W1303):302. [Google Scholar]
- 14.Kloetzer L, Gaman A, McCallum R, Koch K, Sitrin M, Chey W, et al. Characterization of Pressure Patterns in Distal Colonic and Rectal Motility Activity Between Healthy Volunteers and Patients with Dyssynergic Defecation Using An Ambulatory Capsule, Smartpill. Gastroenterology. 2008;(S1838):176. [Google Scholar]
- 15.Kloetzer L, Chey WD, McCallum RW, Koch KL, Wo JM, Sitrin M, et al. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil. May;22(5):527–33. e117. doi: 10.1111/j.1365-2982.2010.01468.x. [DOI] [PubMed] [Google Scholar]
- 16.Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000 Jun;95(6):1456–62. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 17.Bucur MC, Michalek W, McCallum RW, Koch KL, Sitrin MD, Chey WD, et al. The Impact of Using Different Standardized Meals in the Assessment of Motility of Upper Gastrointestinal Tract. Gastroenterology. 2009;136(5S1):A781. [Google Scholar]
- 18.Faegenburg D, Kryle LS, Kashiwabara H, Doctor NM, Pai P. Intestinal obstruction caused by ingestion of a Heidelberg capsule: report of a case. Am J Gastroenterol. 1985 Oct;80(10):787–9. [PubMed] [Google Scholar]
- 19.Jones BH, Fleischer DE, Sharma VK, Heigh RI, Shiff AD, Hernandez JL, et al. Yield of repeat wireless video capsule endoscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005 May;100(5):1058–64. doi: 10.1111/j.1572-0241.2005.40722.x. [DOI] [PubMed] [Google Scholar]
- 20.Fleischer DE, et al. Video capsule endoscopy (VCE) is useful in the evaluation of unexplained abdominal pain (AP). Gastroenterology. 2003;200(Pt 2):245. [Google Scholar]
- 21.Mojaverian P, Chan K, Desai A, John V. Gastrointestinal transit of a solid indigestible capsule as measured by radiotelemetry and dual gamma scintigraphy. Pharm Res. 1989 Aug;6(8):719–24. doi: 10.1023/a:1015998708560. [DOI] [PubMed] [Google Scholar]
- 22.Mojaverian P, Ferguson RK, Vlasses PH, Rocci ML, Jr., Oren A, Fix JA, et al. Estimation of gastric residence time of the Heidelberg capsule in humans: effect of varying food composition. Gastroenterology. 1985 Aug;89(2):392–7. doi: 10.1016/0016-5085(85)90342-7. [DOI] [PubMed] [Google Scholar]
- 23.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988 Aug;29(8):1035–41. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollen RM, Salvioli B, Camilleri M, Burton D, Kost LJ, Phillips SF, et al. The effects of biofeedback on rectal sensation and distal colonic motility in patients with disorders of rectal evacuation: evidence of an inhibitory rectocolonic reflex in humans? Am J Gastroenterol. 1999 Mar;94(3):751–6. doi: 10.1111/j.1572-0241.1999.00947.x. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri M, Malagelada JR, Brown ML, Becker G, Zinsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1985 Nov;249(5 Pt 1):G580–5. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- 26.Kloetzer L, Gaman A, McCallum R, Koch K, Wo J, Sitrin M, et al. Motility of the Antroduodenum in Healthy and Gastroparetic Populations Characterized by Wireless Motility Capsule. Neurogastroenterology and Motility. 2009 doi: 10.1111/j.1365-2982.2010.01468.x. [DOI] [PubMed] [Google Scholar]
- 27.Sogabe M, Kimura Y, Iwaki H, Okita Y, Hibino S, Sawda S, et al. Ultrasonographic comparison of gastric motility between diabetic gastroparesis patients with and without metabolic syndrome. J Gastroenterol Hepatol. 2008 Jul;23(7 Pt 2):e17–22. doi: 10.1111/j.1440-1746.2007.05055.x. [DOI] [PubMed] [Google Scholar]
- 28.Darwiche G, Bjorgell O, Thorsson O, Almer LO. Correlation between simultaneous scintigraphic and ultrasonographic measurement of gastric emptying in patients with type 1 diabetes mellitus. J Ultrasound Med. 2003 May;22(5):459–66. doi: 10.7863/jum.2003.22.5.459. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffenbach B, Wegener M, Adamek RJ, Schaffstein J, Lee YH, Ricken D. Antral myoelectric activity, gastric emptying, and dyspeptic symptoms in diabetics. Scand J Gastroenterol. 1995 Dec;30(12):1166–71. doi: 10.3109/00365529509101626. [DOI] [PubMed] [Google Scholar]
- 30.Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, et al. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995 Apr;108(4):1048–55. doi: 10.1016/0016-5085(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 31.Choi MG, Camilleri M, Burton DD, Zinsmeister AR, Forstrom LA, Nair KS. [13C]octanoic acid breath test for gastric emptying of solids: accuracy, reproducibility, and comparison with scintigraphy. Gastroenterology. 1997 Apr;112(4):1155–62. doi: 10.1016/s0016-5085(97)70126-4. [DOI] [PubMed] [Google Scholar]
- 32.Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986 Jul;91(1):94–9. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- 33.Degen LP, Phillips SF. Variability of gastrointestinal transit in healthy women and men. Gut. 1996 Aug;39(2):299–305. doi: 10.1136/gut.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008 Mar;103(3):753–63. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 35.Camilleri M, Hasler W, Parkman H, Quigley E, Soffer E. Measurement of Gastrointestinal Motility in the GI Laboratory. Gastroenterology. 1998;115:747–62. doi: 10.1016/s0016-5085(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 36.Camilleri M, Malagelada J. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur J Clin Invest. 1984;14(6):420–7. doi: 10.1111/j.1365-2362.1984.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 37.Allescher H, Daniel E. Role of NO in pyloric, antral, and duodenal motility and its interaction with other inhibitory mediators. Dig Dis Sci. 1994;39(12 Suppl):73S–5S. doi: 10.1007/BF02300376. [DOI] [PubMed] [Google Scholar]
- 38.Edelbroek M, Horowitz M, Dent J, Sun W, Malbert C, Smout A, et al. Effects of duodenal distention on fasting and postprandial antropyloroduodenal motility in humans. Gastroenterology. 1994;106(3):583–92. doi: 10.1016/0016-5085(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 39.Glatzle J, Darcel N, Rechs A, Kalogeris T, Tso P, Raybould H. Apolipoprotein A-IV stimulates duodenal vagal afferent activity to inhibit gastric motility via a CCK1 pathway. Am J Physiol Regul Integr Comp Physiol. 2004;287(2):R354–9. doi: 10.1152/ajpregu.00705.2003. [DOI] [PubMed] [Google Scholar]
- 40.Bortolotti M, Annese V, Mari C, Lopilato C, Porrazzo G, Miglioli M. Dose-related stimulatory effect of clarithromycin on interdigestive gastroduodenal motility. Digestion. 2000;62(1):31–7. doi: 10.1159/000007775. [DOI] [PubMed] [Google Scholar]
- 41.Tack J, Janssens J, Vantrappen G, Peeters T, Annese V, Depoortere I, et al. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology. 1992 Jul;103(1):72–9. doi: 10.1016/0016-5085(92)91097-n. [DOI] [PubMed] [Google Scholar]
- 42.Thumshirn M, Bruninga K, Camilleri M. Simplifying the evaluation of postprandian antral motor function in patients with suspected gastroparesis. Am J Gastroenterol. 1997;92(9):1496–500. [PubMed] [Google Scholar]