Abstract
To investigate the potential role of vitamin or mineral supplementation on the risk of head and neck cancer (HNC), we analyzed individual-level pooled data from 12 case-control studies (7,002 HNC cases and 8,383 controls) participating in the International Head and Neck Cancer Epidemiology consortium. There were a total of 2,028 oral cavity cancer, 2,465 pharyngeal cancer, and 874 unspecified oral/pharynx cancer, 1,329 laryngeal cancer and 306 overlapping HNC cases. Odds ratios (OR) and 95% confidence intervals (CIs) for self reported ever use of any vitamins, multivitamins, vitamin A, vitamin C, vitamin E, and calcium, beta-carotene, iron, selenium, and zinc supplements were assessed. We further examined frequency, duration and cumulative exposure of each vitamin or mineral when possible and stratified by smoking and drinking status. All ORs were adjusted for age, sex, race/ethnicity, study center, education level, and pack-years of smoking, frequency of alcohol drinking and fruit/vegetable intake. A decreased risk of HNC was observed with ever use of vitamin C (OR=0.76, 95% CI=0.59-0.96) and with ever use of calcium supplement (OR=0.64, 95% CI=0.42-0.97). The inverse association with HNC risk was also observed for 10 or more years of vitamin C use (OR=0.72, 95% CI=0.54-0.97) and more than 365 tablets of cumulative calcium intake (OR=0.36, 95% CI=0.16-0.83), but linear trends were not observed for the frequency or duration of any supplement intake. We did not observe any strong associations between vitamin or mineral supplement intake and the risk of head and neck cancer.
Keywords: vitamin supplement, mineral supplement, head and neck cancer
Introduction
Head and neck cancer (HNC) includes cancers originating in the oral cavity, the oropharynx, the hypopharynx, and the larynx. Worldwide, more than half a million HNC cases and over 300,000 deaths due to HNC are estimated to occur each year1. Tobacco smoking and alcohol consumption are the predominant risk factors for HNC 2,3, but the role of diet has been recognized. Increased fruit and vegetable consumption has been repeatedly shown to be associated with a reduced risk of HNC 4-6. However, the mechanisms underlying these associations are complex. There are a large number of compounds in plant foods that may influence the risk of cancer, including both micronutrients for normal metabolism and other bioactive compounds with unknown metabolic significance. Therefore, whether dietary supplements containing micronutrients found in plant foods would be effective chemopreventive agents is of considerable public health interest.
Numerous in vitro studies and animal studies have suggested favorable effects of several vitamins and minerals on angiogenesis, immunity, cell differentiation, proliferation, and apoptosis 7-9. In epidemiologic studies, the precise nature and magnitude of the inverse relationships between multivitamin and mineral supplements and the risk of HNC, however, have not been clearly established because of inconsistent results 10-20. In the early 1990s, case-control studies in the US suggested that vitamin E and vitamin C supplement intake was inversely associated with oral cavity and pharyngeal cancers 13,14. Recently published meta-analysis of randomized trials suggest no association between cancer and vitamin or mineral supplement intake 19-23. In a large randomized controlled trial, supplementation with alpha-tocopheryl acetate and beta-carotene were not associated with upper aerodigestive tract cancer incidence; however, a protective effect was suggested for early stage laryngeal cancers 11.The results from the three secondary prevention trials for HNC, beta-carotene supplement had no significant effect for second primary HNC risk 15-17. Therefore, this large pooled analysis was conducted to investigate the potential role of vitamin or mineral supplementation in the development of HNC.
We analyze pooled data from the International Head and Neck Cancer Epidemiology (INHANCE) Consortium (http://inhance.iarc.fr) that was established in 2004 based on the collaboration of research groups leading large, molecular epidemiology studies of HNC. The primary goal of the consortium is to explore potential risk factors of HNC that are difficult to evaluate in individual studies.
Material and Methods
Study subjects
Within version 1.3 of the INHANCE consortium pooled data set, twelve case-control studies from Europe, Latin America, and the United States with information on vitamin supplementation included data on 7,085 HNC cases and 8,491 controls 24-33. In this current analysis, we excluded the India center (576 cases and 582 controls), but not the other centers from the IARC multicenter study because information on vitamin supplementation was not available in the India center questionnaire. We also excluded 169 cases and 43 controls from Boston study because they had not been interviewed with the vitamin supplementation questionnaire. Finally, subjects with missing data on age, sex or race/ethnicity and cases with missing information on the site of origin of their cancer were excluded (87 cases and 108 controls). Thus the data for this analysis included 7,002 HNC cases and 8,383 controls.
Characteristics of the individual studies in the consortium are provided in Supplemental table 1. Most of the studies were hospital-based case-control studies, and in most studies, the control subjects were frequency matched to the case subjects on age, sex and additional factors (such as study center, hospital, and race/ethnicity). Interviews in all studies were conducted face-to-face. Written informed consent was obtained from study subjects, and the investigations were approved by institutional review boards at each of the institutes involved. Questionnaires were collected from the individual studies to assess the comparability of the data and wording of interview questions. Each data item was checked for illogical or missing values. Queries were sent to investigators and inconsistencies were resolved.
Cases included patients with invasive tumors of the oral cavity, oropharynx, hypopharynx, oral cavity or pharynx not otherwise specified (NOS), larynx, or HNC unspecified as defined previously 34. Cancers of salivary gland (International Classification of Diseases for Oncology version 2 C07-C08) were excluded from our analysis due to the different etiology from other HNC 35. Studies provided tumor site data using either the International Classification of Diseases for Oncology version 2 or International Classification of Diseases, Ninth or Tenth Edition. In the overall dataset there were a total of 2,028 oral cases, 2,465 pharyngeal cases (496 hypopharynx and 1,969 oropharynx), 874 unspecified oral/pharynx cases, 1,329 laryngeal cases and 306 overlapping head and neck cases. In the 12 individual studies, each study subject was asked whether he or she had taken any vitamins or minerals. The definitions for ever use of vitamin or mineral supplements were: at least once a week for a year (New York multicenter and Tampa studies), at least once a week (Seattle study), at least once a month (Los Angeles study), on a regular basis for six months or longer (US multicenter study), on a regular basis (Puerto Rico, France, Boston, Memorial Sloan-Kettering Cancer Center (MSKCC) studies), prior to one year ago (North Carolina study), in the last two years (IARC multicenter and Latin America studies). Variables on the type of vitamin supplementation were available in nine studies (New York multicenter, Seattle, North Carolina, Tampa, Los Angeles, Puerto Rico, Boston, MSKCC and US multicenter studies). Variables on the frequency of the type of vitamin supplementation were available in four studies (Seattle, Los Angeles, US multicenter and MSKCC studies). Variables on duration of vitamin supplementation were available in eight studies (France, New York multicenter, North Carolina, Tampa, Los Angeles, Puerto Rico, Boston and US multicenter studies). The lifetime number of tablets of vitamin supplementation was calculated based on the number of tablets per week or day and duration reported to estimate the cumulative and daily consumption in four studies (New York multicenter, Tampa, North Carolina and Puerto Rico studies). In short, for any vitamin and mineral supplement intake, 3 questions were included in the France, Latin America and IARC studies;10 questions were included in Seattle study; 15 questions were included in the MSKCC (14) and Tampa (16) studies; and more than 20 questions were included in New York multicenter (21), Puerto Rico (24), North Carolina (25), Los Angeles (27), US multicenter (28) and Boston (32).
Variables on pack-years of tobacco smoking and the frequency of alcohol drinking were available in all studies. The detailed description on the method used for pooling data on smoking and alcohol across different studies is provided in a previous paper 34. Variables on dietary information included overall vegetables and fruit intake in quartiles based on center-specific controls.
Statistical Methods
The association between head and neck cancer risk and vitamin supplementation was assessed by estimating odds ratios (ORs) and 95% confidence intervals (CIs) using unconditional logistic regression models for each case-control study. The model included age, sex, education, race/ethnicity, pack-years of smoking (continuous), the frequency of alcohol drinking (continuous), vegetable and fruit intake (quartiles of center-specific controls) to adjust to potential confounders. To calculate the summary estimates of association, the study-specific estimates were included in a two-stage random-effects logistic regression model with the maximum likelihood method, which allows for unexplained sources of heterogeneity between studies 36. Pooled ORs were also estimated with a fixed-effects logistic regression models, adjusted for all the factors mentioned before and study center.
For subjects missing information on education level (634 cases and 461 controls), we applied multiple imputation with the PROC MI procedure in SAS. We assumed that the education data were missing at random, i.e. whether or not education was missing did not depend on any other unobserved or missing values 37. We used the logistic regression model 38 to predict education level using age, sex, race/ethnicity, study center, and case/control status as the covariates within each of the geographic region.
We tested for heterogeneity between the study-specific ORs by conducting the likelihood ratio test, for the head and neck combined and for each of the subsites, by testing the difference between the log likelihood of a model with the product terms between study and the variable of interest, and that of a model with no such product terms, based on a χ2 distribution with a df one less than the number of studies. If any heterogeneity was detected, we reported the random-effects estimates and examined whether the results from the two-stage random-effects model and the fixed-effects logistic regression model were comparable in magnitude of effect. Fixed-effects estimates are reported for all other models. We also conducted meta-regression analysis between studies, adjusting for some potential sources of heterogeneity, including case source(hospital vs. cancer registry), year of study (<= 1990s vs. >=2000s), geographic location of the study (Europe, North America vs. South/central America), and sample size(<400 vs. >400 cases). We also conducted influence analysis, where each study was excluded one at a time to assure that the statistical significance and magnitude of the overall summary estimate was not dependent on any one study.
Results
Characteristics of Studies and Subjects
Selected demographic characteristics of cases and controls are shown in Table 1. The largest number of cases and controls were from the Latin America study (2,191 cases/1,706 controls), followed by the US multicenter study (1,114 cases/1,268 controls). There was a predominance of male cases (77%). The distributions of age, race/ethnicity and educational level were different between case and control groups.
Table 1.
Demographic characteristics of the head and neck cases and controls
| Demographic characteristics | Cases | (%) | Controls | (%) |
|---|---|---|---|---|
| Total | 7002 | 8383 | ||
| Study | ||||
| France | 323 | 4.61 | 234 | 2.79 |
| New York Multicenter | 497 | 7.10 | 271 | 3.23 |
| Seattle, WA | 191 | 2.73 | 400 | 4.77 |
| North Carolina | 180 | 2.57 | 202 | 2.41 |
| Tampa, FL | 207 | 2.96 | 897 | 10.70 |
| Los Angeles, CA | 417 | 5.96 | 1005 | 11.99 |
| Puerto Rico | 350 | 5.00 | 521 | 6.21 |
| Latin America | 2191 | 31.29 | 1706 | 20.35 |
| IARC Multicenter | 983 | 14.04 | 1094 | 13.05 |
| Boston | 415 | 5.93 | 616 | 7.35 |
| US multicenter | 1114 | 15.91 | 1268 | 15.13 |
| Memorial Sloan-Kettering Cancer Center | 134 | 1.91 | 169 | 2.02 |
| Age | ||||
| <40 | 303 | 4.33 | 500 | 5.96 |
| 40_<45 | 403 | 5.76 | 597 | 7.12 |
| 45_<50 | 760 | 10.85 | 893 | 10.65 |
| 50_<55 | 996 | 14.22 | 1347 | 16.07 |
| 55_<60 | 1259 | 17.98 | 1455 | 17.36 |
| 60_<65 | 1117 | 15.95 | 1244 | 14.84 |
| 65_<70 | 950 | 13.57 | 1034 | 12.33 |
| 70_<75 | 707 | 10.10 | 724 | 8.64 |
| ≥75 | 507 | 7.24 | 589 | 7.03 |
| P for χ2test | <0.0001 | |||
| Sex | ||||
| Men | 5392 | 77.01 | 5791 | 69.08 |
| Women | 1610 | 22.99 | 2592 | 30.92 |
| P for χ2test | <0.0001 | |||
| Race/Ethnicity | ||||
| Nonhispanic white | 3922 | 56.01 | 5406 | 64.49 |
| Black | 557 | 7.95 | 659 | 7.86 |
| Hispanic/Latino | 156 | 2.23 | 359 | 4.28 |
| Asian/Pacific islanders | 37 | 0.53 | 72 | 0.86 |
| Others | 139 | 1.99 | 181 | 2.16 |
| Latin Americans* | 2191 | 31.29 | 1706 | 20.35 |
| P for χ2test | <0.0001 | |||
| Education | ||||
| None | 139 | 2.18 | 129 | 1.63 |
| <Junior high school | 2444 | 38.38 | 2073 | 26.17 |
| some high school | 1185 | 18.61 | 1117 | 14.10 |
| High school graduate | 728 | 11.43 | 960 | 12.12 |
| Vocational, some college | 1224 | 19.22 | 2042 | 25.78 |
| ≥College | 648 | 10.18 | 1601 | 20.21 |
| Missing | 634 | 461 | ||
| P for χ2test | <0.0001 | |||
| HNC subtype | ||||
| Oral cavity | 2028 | 0.29 | ||
| Pharynx | 2465 | 0.28 | ||
| Oral / pharynx not otherwise specified | 874 | 0.20 | ||
| Larynx | 1329 | 0.19 | ||
| Overlapping head and neck cancer | 306 | 0.04 |
: Information on ethnicity was not collected in the Latin America study. We organized subjects as “Latin American”. We adjusted for study center in all logistic regression models as a proxy variable for race/ethnicity because each center has an expected predominant ethnic group distribution.
Ever Use of Vitamin or Mineral Supplement Intake
Table 2 presents the ORs of HNC according to select vitamin supplements. Ever use of any vitamin supplement was reported by 2,448 cases (37.6%) and 3,921 controls (48.3%), but not associated with the risk of HNC. For individual vitamin supplements, a decreased risk of HNC was observed with ever use of vitamin C (OR=0.76, 95% CI 0.59-0.96) and with ever use of calcium supplements (OR=0.64, 95% CI 0.42-0.97) after adjustment for age, sex, race/ethnicity, study center, education level, pack-years of smoking, frequency of alcohol drinking and fruit/vegetable intake. The inverse association with calcium supplement use persisted after adjustment for ever use of other vitamins (OR=0.67, 95% CI 0.48-0.95). The odds ratios for vitamin C (OR=0.75, 95% CI 0.55-0.95) did not change substantially when the analysis was restricted to the American studies (including Seattle, North Carolina, Tampa, Los Angeles, Puerto Rico, Boston, US multicenter and MSKCC studies; data not shown in the table).
Table 2.
Vitamin or mineral supplement use and risk of head and neck cancer, INHANCE pooled analysis
| Cases / controls | ORa (95% CI) | ORb (95% CI) | |
|---|---|---|---|
| Any vitaminsc | |||
| Never | 4016/4139 | 1.00 | 1.00 |
| Ever | 2448/3921 | 0.87 (0.71-1.06) | 0.94(0.69-1.29) |
| Missing | 41/52 | ||
| P heterogeneity | <0.01 | <0.01 | |
| Multiple vitaminsd | |||
| Never | 1650/2702 | 1.00 | 1.00 |
| Ever | 1341/2351 | 0.99 (0.82-1.19) | 1.04(0.90-1.19) |
| Missing | 17/25 | ||
| P heterogeneity | 0.12 | 0.10 | |
| Vitamin Ad | |||
| Never | 2861/4772 | 1.00 | 1.00 |
| Ever | 130/281 | 1.04(0.54-1.98) | 1.20(0.66-2.16) |
| Missing | 17/25 | ||
| P heterogeneity | <0.01 | <0.01 | |
| Vitamin Cd | |||
| Never | 2449/3787 | 1.00 | 1.00 |
| Ever | 542/1266 | 0.76(0.59-0.96) | 0.82(0.65-1.02) |
| Missing | 17/25 | ||
| P heterogeneity | <0.01 | <0.01 | |
| Vitamin Ed | |||
| Never | 2574/3930 | 1.00 | 1.00 |
| Ever | 417/1123 | 0.71(0.45-1.11) | 0.83(0.54-1.26) |
| Missing | 17/25 | ||
| P heterogeneity | <0.01 | <0.01 | |
| Calciumd | |||
| Never | 1709/3164 | 1.00 | 1.00 |
| Ever | 171/624 | 0.64(0.42-0.97) | 0.67(0.48-0.95) |
| Missing | 14/22 | ||
| P heterogeneity | 0.01 | <0.01 | |
| Beta-carotened | |||
| Never | 1435/2406 | 1.00 | 1.00 |
| Ever | 50/92 | 1.35(0.65-2.81) | 1.45(0.89-2.38) |
| Missing | 11/15 | ||
| P heterogeneity | 0.33 | 0.35 | |
| Irond | |||
| Never | 2445/3488 | 1.00 | 1.00 |
| Ever | 151/275 | 0.79(0.54-1.16) | 0.92(0.69-1.23) |
| Missing | 14/18 | ||
| P heterogeneity | 0.59 | 0.54 | |
| Seleniumd | |||
| Never | 1100/1914 | 1.00 | 1.00 |
| Ever | 35/63 | 1.21(0.35-4.22) | 1.22(0.67-2.24) |
| Missing | 11/15 | ||
| P heterogeneity | 0.73 | 0.74 | |
| Zincd | |||
| Never | 1080/1860 | 1.00 | 1.00 |
| Ever | 55/17 | 0.88(0.34-2.26) | 0.88(0.55-1.43) |
| Missing | 11/15 | ||
| P heterogeneity | 0.74 | 0.72 |
: Odds ratio adjusted for age, sex, race/ethnicity, study center, educational level, pack-years of smoking (continuous), the frequency of alcohol drinking (continuous), vegetable and fruit intake (quartiles of center-specific controls).
: additionally adjusted for other vitamins (Multiple vitamins, vitamin A, vitamin C and vitamin E).
: New York study was not included in the model a; France, New York, Latin America and IARC studies were not included in the model b. So, in model b, there were 1492 cases and 2098 controls with never use of any vitamin supplementation; and 1499 cases and 2955 controls with the ever use of any vitamin supplementation.
: multiple vitamins, vitamin A, vitamin C and vitamin E do not include France, New York, Latin America, MSKCC and IARC studies; calcium do not include France, New York, Latin America, IARC, MSKCC and US multicenter studies; selenium and zinc include North Carolina, Los Angeles, Boston and MSKCC studies; iron include North Carolina, Los Angeles, Puerto Rico, Boston, US multicenter and MSKCC studies; beta-carotene include North Carolina, Los Angeles, Puerto Rico, Boston and MSKCC studies.
Figure 1 and 2 show the study specific estimates for vitamin C and calcium supplements. The point estimates ranged between 0.60 and 1.24 for vitamin C and between 0.14 and 0.94 for calcium. For vitamin C, in 2 out of 7 studies the OR was above unity (Los Angeles and Puerto Rico). For calcium, ORs of all studies were below unity.
Figure 1.
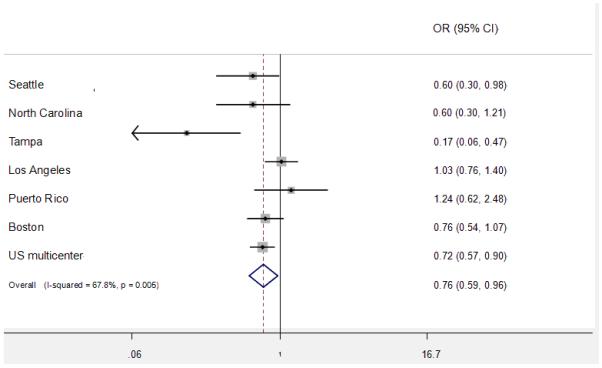
Study specific and pooled estimates for vitamin C
Figure 2.
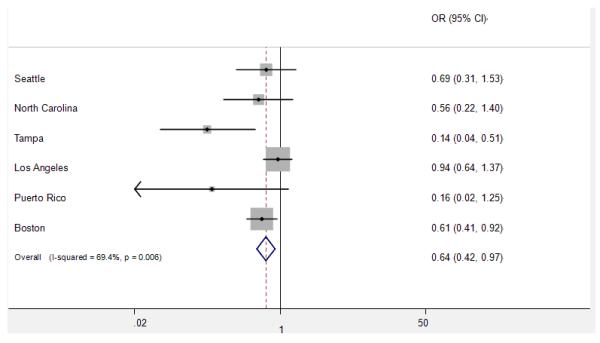
Study specific and pooled estimates for calcium supplement
Heterogeneity was detected for the overall effect of vitamin C and calcium supplements and in a few strata. The random effect estimates, however, did not differ substantially from the fixed effect ones, although the CIs were wider. Meta-regression was conducted for the sources of heterogeneity between studies. The potential sources included the case source (hospital vs. cancer registry), year of study (<= 1990s vs. >=2000s), geographic location of the study (Europe, North America vs. South/central America), and sample size (<400 vs. >400 cases). The results showed that the geographic location of the study was the possible explanation for the heterogeneity of vitamin C supplement (P=0.047). There did not appear to be any associations between HNC risk and ever use of multiple vitamins, vitamin A, beta-carotene, iron, selenium or zinc.
When the result was analyzed by gender, we did not observe any difference in the results stratified by gender. For calcium, the borderline protective effect was also observed for calcium supplement among female subjects (OR=0.49, 95%CI=0.24-1.00), and there was no significant association among male subjects (OR=0.77, 95%CI=0.55-1.09).
The analysis was further stratified by cancer subsite (Supplemental Table 2). There was no difference in the reduction of risk associated with any vitamins, multiple vitamins, vitamin A, vitamin C, vitamin E and calcium supplement use by site of oral cavity, pharynx and larynx cancer. A 27% reduction in risk of oral cavity cancer was observed for vitamin E supplement use (OR=0.73, 95% CI=0.56-0.96).
Frequency, Duration and Cumulative Consumption of Vitamin or Mineral Supplement Intake
Table 3 shows the association between HNC risk and frequency of various vitamins and mineral supplements. High frequency (7-13 tablets/week) of Vitamin E intake was associated with a decreased HNC risk (OR=0.61, 95% CI=0.37-0.98), but the association was not significant after the adjustment of dietary vitamin E intake (OR=0.59, 95% CI=0.22-1.61). For other vitamin or mineral supplement intake, no significant association was observed between the frequency of vitamin or mineral supplements and HNC risk in this pooled analysis.
Table 3.
Frequency of vitamin or mineral supplement use and risk of head and neck cancer, INHANCE pooled analysis
| Cases / controls | ORa (95% CI) | Cases / controls | ORb (95% CI) | |
|---|---|---|---|---|
| Any vitamins | ||||
| None | 896/1263 | 1.00 | 639/1103 | 1.00 |
| 1-6 tablets/week | 84/281 | 0.71(0.45-1.12) | 60/266 | 0.64(0.33-1.24) |
| 7-13 tablets/week | 404/686 | 0.79(0.35-1.78) | 207/593 | 0.74(0.24-2.30) |
| >=14 tablets/week | 32/55 | 1.30(0.50-3.42) | 18/52 | 1.26(0.34-4.72) |
| Missing | 9/6 | 4/6 | ||
| P trend | 0.80 | 0.70 | ||
| P heterogeneity | <0.01 | <0.01 | ||
| Multiple vitamins | ||||
| None | 972/1594 | 1.00 | 694/1415 | 1.00 |
| 1-6 tablets/week | 52/95 | 1.29(0.68-2.45) | 34/88 | 1.14(0.31-4.14) |
| 7-13 tablets/week | 359/549 | 0.97(0.54-1.72) | 181/468 | 0.93(0.42-2.06) |
| >=14 tablets/week | 32/46 | 1.63(0.61-4.34) | 15/43 | 1.51(0.39-5.78) |
| Missing | 10/7 | 4/6 | ||
| P trend | 0.48 | 0.29 | ||
| P heterogeneity | <0.01 | <0.01 | ||
| Vitamin C | ||||
| None | 1251/1821 | 1.00 | 832/1594 | 1.00 |
| 1-6 tablets/week | 31/139 | 0.60(0.31-1.17) | 25/133 | 0.49(0.18-1.35) |
| 7-13 tablets/week | 113/279 | 0.57(0.27-1.12) | 51/243 | 0.58(0.30-1.12) |
| >=14 tablets/week | 21/45 | 1.22(0.45-3.30) | 16/44 | 1.16(0.34-3.95) |
| Missing | 9/7 | 4/6 | ||
| P trend | 0.85 | 0.88 | ||
| P heterogeneity | <0.01 | 0.02 | ||
| Vitamin E | ||||
| None | 1307/1850 | 1.00 | 853/1610 | 1.00 |
| 1-6 tablets/week | 25/145 | 0.59(0.29-1.21) | 22/144 | 0.52(0.18-1.50) |
| 7-13 tablets/week | 76/265 | 0.61(0.37-0.98) | 43/237 | 0.59(0.22-1.61) |
| >=14 tablets/week | 8/24 | 1.71(0.25-5.34) | 6/23 | 1.33(0.19-9.27) |
| Missing | 9/7 | 4/6 | ||
| P trend | 0.17 | 0.76 | ||
| P heterogeneity | <0.01 | 0.02 | ||
| Calcium | ||||
| None | 1369/2029 | 1.00 | 899/1773 | 1.00 |
| 1-6 tablets/week | 8/83 | 0.37(0.10-1.44) | 6/82 | 0.38(0.14-1.01) |
| 7-13 tablets/week | 32/124 | 0.57(0.28-1.17) | 14/113 | 0.54(0.19-1.53) |
| >=14 tablets/week | 7/49 | 0.66(0.13-3.38) | 5/46 | 0.82(0.10-7.13) |
| Missing | 9/6 | 4/6 | ||
| P trend | 0.62 | 0.50 | ||
| P heterogeneity | 0.02 | 0.20 |
: Odds ratio adjusted for age, sex, race/ethnicity, study center, educational level, pack-years of smoking (continuous), the frequency of alcohol drinking (continuous). All vitamins include New York, Seattle, North Carolina, Tampa and Puerto Rico.
: Odds ratio adjusted for age, sex, race/ethnicity, study center, educational level, pack-years of smoking (continuous), the frequency of alcohol drinking (continuous), vegetable and fruit intake (quartiles of center-specific controls). All vitamins include Seattle, North Carolina, Tampa and Puerto Rico.
We next examined whether there was a dose-response relationship between HNC risk and the years of supplement use (Table 4). Vitamin C supplementation for 10 or more years was associated with a reduced risk of HNC (OR=0.72, 95% CI 0.54-0.97). However, statistical evidence of a dose-response relationship was not observed (P-trend = 0.46) and the associations did not persist after adjustment for other vitamins. The durations of other individual vitamin or mineral supplements were not significantly associated with risk of HNC.
Table 4.
Duration of vitamin or mineral supplement use and risk of head and neck cancer, INHANCE pooled analysis
| Cases / controls |
ORa (95% CI) | Cases / controls |
ORb (95% CI) | |
|---|---|---|---|---|
| Any vitamins | ||||
| None | 1601/2015 | 1.00 | 1333/1832 | 1.00 |
| <1 year | 664/1136 | 0.84(0.67-1.07) | 617/1094 | 0.84(0.60-1.16) |
| 1-9 years | 374/684 | 0.89(0.58-1.37) | 367/676 | 1.01(0.72-1.43) |
| 10+ years | 354/893 | 0.75(0.54-1.05) | 353/892 | 0.84(0.57-1.26) |
| Missing | 13/15 | 13/15 | ||
| P trend | 0.02 | 0.93 | ||
| P heterogeneity | <0.01 | <0.01 | ||
| Multiple vitamins |
||||
| None | 1453/2347 | 1.00 | 1453/2347 | 1.00 |
| <1 year | 397/688 | 0.87(0.68-1.11) | 397/688 | 0.87(0.68-1.12) |
| 1-9 years | 379/641 | 1.00(0.82-1.22) | 379/641 | 1.06(0.87-1.30) |
| 10+ years | 441/818 | 1.03(0.86-1.24) | 441/818 | 1.11(0.91-1.35) |
| Missing | 13/15 | 13/15 | ||
| P trend | 0.74 | 0.33 | ||
| P heterogeneity | 0.12 | 0.21 | ||
| Vitamin C | ||||
| None | 2193/3393 | 1.00 | 2193/3393 | 1.00 |
| <1 year | 156/355 | 0.80(0.58-1.10) | 156/355 | 0.83(0.58-1.18) |
| 1-9 years | 154/292 | 0.88(0.61-1.27) | 154/292 | 0.97(0.67-1.14) |
| 10+ years | 167/454 | 0.72(0.54-0.97) | 167/454 | 0.80(0.57-1.14) |
| Missing | 13/15 | 13/15 | ||
| P trend | 0.46 | 0.52 | ||
| P heterogeneity | <0.01 | <0.01 | ||
| Vitamin E | ||||
| None | 2297/3477 | 1.00 | 2297/3477 | 1.00 |
| <1 year | 129/359 | 0.79(0.57-1.11) | 129/359 | 0.87(0.60-1.26) |
| 1-9 years | 131/312 | 0.74(0.38-1.41) | 131/312 | 0.80(0.39-1.65) |
| 10+ years | 113/346 | 0.77(0.41-1.42) | 113/346 | 0.89(0.52-1.50) |
| Missing | 13/15 | 13/15 | ||
| P trend | 0.77 | 0.75 | ||
| P heterogeneity | <0.01 | <0.01 | ||
| Calcium | ||||
| None | 1404/2658 | 1.00 | 1404/2658 | 1.00 |
| <1 year | 56/205 | 0.72(0.48-1.07) | 56/205 | 0.71(0.47-1.07) |
| 1-9 years | 61/194 | 0.69(0.46-1.02) | 61/194 | 0.71(0.47-1.07) |
| 10+ years | 38/172 | 0.71(0.45-1.10) | 38/172 | 0.68(0.43-1.08) |
| Missing | 10/12 | 10/12 | ||
| P trend | 0.79 | 0.76 | ||
| P heterogeneity | 0.06 | 0.05 |
Odds ratio adjusted for age, sex, race/ethnicity, study center, educational level, pack-years of smoking (continuous), the frequency of alcohol drinking (continuous), vegetable and fruit intake (quartiles of center-specific controls). Any vitamins include France, North Carolina, Tampa, Los Angeles, Puerto Rico, Boston and US multicenter studies; multiple vitamins, vitamin A, vitamin C and vitamin E include North Carolina, Tampa, Los Angeles, Puerto Rico, Boston and US multicenter studies; calcium includes North Carolina, Tampa, Los Angeles, Puerto Rico and Boston studies.
Odds ratio adjusted for age, sex, race/ethnicity, study center, educational level, pack-years of smoking (continuous), the frequency of alcohol drinking (continuous), vegetable and fruit intake (quartiles of center-specific controls), and other vitamins (Multiple vitamins, vitamin A, vitamin C and vitamin E). Any vitamins, multiple vitamins, vitamin A, vitamin C and vitamin E include North Carolina, Tampa, Los Angeles, Puerto Rico, Boston and US multicenter studies; calcium includes North Carolina, Tampa, Los Angeles, Puerto Rico and Boston studies.
We also explored dose-response relations for multiple vitamins, vitamin C, vitamin E and calcium, by cumulative consumption. Calcium supplement was associated inversely with the risk of HNC regardless of cumulative consumption level (OR for cumulative calcium <=365 tablets = 0.26, 95% CI=0.07-0.94; OR for cumulative calcium >365 tablets = 0.36, 95% CI=0.16-0.83), after adjustment for confounding factors, but no dose-response trend was apparent (P-trend = 0.75). There was no significant association between HNC risk and the cumulative consumption of other individual vitamin or mineral supplements (data not shown).
Stratification by smoking and drinking status
Finally, the analysis was further stratified on smoking and drinking status (Table 5). Among never alcohol users (212 cases and 866 controls), calcium intake was associated with reduced HNC risk (OR=0.39, 95% CI=0.16-0.94). Ever use of vitamin E supplement were reported by 25 cases (17.1%) and 133 controls (18.2%) among never users of tobacco and alcohol, but not associated with the risk of HNC. However, increased HNC risk was observed with the use of vitamin E supplement for one to nine years but the confidence interval was so wide (OR=5.31, 95% CI 1.43-19.76) because of the small number of subjects with vitamin E intake of one to nine years (11 cases and 37 controls). For the supplements of multiple vitamins and vitamin C, there was no significant association with HNC risk when stratified by smoking or drinking status.
Table 5.
Vitamin or mineral supplement use and the risk of HNC, stratified on smoking and alcohol status, INHANCE pooled analysis
| Ever tobacco users | Never tobacco users | Ever alcohol users | Never alcohol users | Ever tobacco/alcohol users | Never tobacco/alcohol users | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Cases / controls |
ORa (95% CI) | Cases / controls |
ORa (95% CI) | Cases / controls |
ORb (95% CI) | Cases / controls |
ORb (95% CI) | Cases / controls |
ORc (95% CI) | Cases / controls |
ORd (95% CI) | |
| Multiple vitamins* | ||||||||||||
| None | 1223/1404 | 1.00 | 230/939 | 1.00 | 1297/1714 | 1.00 | 1551/631 | 1.00 | 1158/1185 | 1.00 | 90/144 | 1.00 |
| Ever | 989/1291 | 0.96(0.81-1.14) | 228/855 | 1.07(0.80-1.44) | 1113/1755 | 1.07(0.92-1.24) | 103/391 | 0.81(0.19-3.47) | 938/1171 | 0.98(0.77-1.24) | 53/271 | 0.76(0.42-1.37) |
| <1 year | 334/425 | 0.86(0.64-1.15) | 63/263 | 0.78(0.47-1.32) | 353/563 | 0.85(0.65-1.11) | 44/124 | 0.81(0.42-1.56) | 312/374 | 0.85(0.62-1.16) | 22/74 | 0.89(0.35-2.30) |
| 1-9 years | 307/385 | 1.00(0.79-1.15) | 72/255 | 1.13(0.75-1.72) | 351/513 | 1.12(0.91-1.39) | 31/139 | 1.14(0.62-2.11) | 294/354 | 1.05(0.81-1.35) | 15/96 | 1.01(0.42-2.40) |
| 10+ years | 348/481 | 1.02(0.81-1.28) | 93/337 | 1.26(0.87-1.83) | 409/679 | 1.15(0.94-1.40) | 2/8 | 0.83(0.45-1.55) | 332/443 | 1.01(0.80-1.29) | 16/101 | 0.77(0.33-1.82) |
| Missing | 12/5 | 1/6 | 11/4 | 10/2 | 0/5 | |||||||
| P trend | 0.78 | 0.13 | 0.44 | 0.69 | 0.98 | 0.54 | ||||||
| P heterogeneity | 0.06 | 0.51 | 0.23 | 0.69 | 0.03 | 0.21 | ||||||
| Vitamin C* | ||||||||||||
| None | 1846/2021 | 1.00 | 347/1367 | 1.00 | 1975/2557 | 1.00 | 216/833 | 1.00 | 1746/1742 | 1.00 | 117/556 | 1.00 |
| Ever | 366/674 | 0.83(0.63-1.10) | 111/427 | 1.22(0.53-2.80) | 435/912 | 0.83(0.64-1.08) | 42/189 | 0.82(0.36-1.89) | 350/614 | 0.86(0.64-1.16) | 26/129 | 0.99(0.41-2.42) |
| <1 year | 121/210 | 0.86(0.58-1.26) | 35/145 | 1.01(0.60-1.67) | 144/291 | 0.87(0.61-1.25) | 11/64 | 0.68(0.26-1.76) | 116/187 | 0.93(0.62-1.41) | 7/41 | 1.26(0.33-4.80) |
| 1-9 years | 118/187 | 0.86(0.57-1.30) | 36/105 | 1.84(1.08-3.15) | 135/240 | 0.89(0.60-1.31) | 19/52 | 1.10(0.41-2.95) | 111/170 | 0.90(0.58-1.40) | 12/35 | 1.36(0.37-4.99) |
| 10+ years | 127/277 | 0.78(0.53-1.15) | 40/177 | 0.93(0.57-1.52) | 156/381 | 0.78(0.55-1.11) | 11/73 | 0.84(0.32-2.20) | 123/257 | 0.82(0.54-1.25) | 7/35 | 0.67(0.15-3.03) |
| Missing | 12/5 | 1/6 | 11/4 | 2/8 | 10/12 | 0/5 | ||||||
| P trend | 0.13 | 0.88 | 0.53 | 0.28 | 0.60 | 0.43 | ||||||
| P heterogeneity | <0.01 | 0.02 | <0.01 | 0.12 | <0.01 | 0.28 | ||||||
| Vitamin E* | ||||||||||||
| None | 1934/2070 | 1.00 | 363/1402 | 1.00 | 2083/2653 | 1.00 | 213/821 | 1.00 | 1839/1799 | 1.00 | 118/552 | 1.00 |
| Ever | 278/625 | 0.74(0.46-1.18) | 95/392 | 1.04(0.53-2.04) | 327/816 | 0.76(0.49-1.18) | 45/201 | 1.13(0.59-2.16) | 257/1557 | 0.73(0.48-1.12) | 25/133 | 1.63(0.68-3.95) |
| <1 year | 93/213 | 0.79(0.49-1.28) | 36/146 | 1.33(0.67-2.63) | 114/282 | 0.78(0.53-1.15) | 15/77 | 1.23(0.35-4.30) | 89/188 | 0.77(0.49-1.21) | 11/52 | 1.80(0.48-6.77) |
| 1-9 years | 100/192 | 0.71(0.41-1.23) | 31/120 | 1.24(0.26-6.01) | 111/251 | 0.72(0.37-1.40) | 20/61 | 2.43(0.71-8.35) | 91/168 | 0.71(0.42-1.22) | 11/37 | 5.31(1.43-19.76) |
| 10+ years | 85/220 | 0.77(0.49-1.21) | 28/126 | 0.96(0.31-2.98) | 102/283 | 0.82(0.50-1.36) | 10/63 | 1.08(0.15-7.78) | 77/201 | 0.71(0.44-1.14) | 3/44 | 1.69(0.23-12.54) |
| Missing | 12/5 | 1/6 | 11/4 | 2/8 | 10/2 | 0/5 | ||||||
| P trend | 0.16 | 0.89 | 0.77 | 0.55 | 0.44 | 0.73 | ||||||
| P heterogeneity | <0.01 | 0.04 | <0.01 | 0.06 | 0.01 | 0.28 | ||||||
| Calcium* | ||||||||||||
| None | 1145/1552 | 1.00 | 259/1101 | 1.00 | 1214/1946 | 1.00 | 188/709 | 1.00 | 1057/1315 | 1.00 | 101/474 | 1.00 |
| Ever | 111/321 | 0.67(0.43-1.04) | 44/250 | 0.73(0.37-1.44) | 133/422 | 0.75(0.49-1.14) | 22/149 | 0.39(0.16-0.94) | 102/267 | 0.75(0.45-1.24) | 13/95 | 0.44(0.14-1.40) |
| <1 year | 38/100 | 0.86(0.44-1.66) | 18/105 | 0.75(0.38-1.46) | 45/146 | 0.80(0.51-1.26) | 11/59 | 0.48(0.15-1.61) | 33/82 | 0.90(0.54-1.50) | 6/41 | 0.34(0.08-1.48) |
| 1-9 years | 46/117 | 0.74(0.32-1.75) | 15/77 | 0.87(0.42-1.81) | 54/137 | 0.86(0.56-1.33) | 7/57 | 0.33(0.05-2.05) | 44/95 | 0.79(0.49-1.28) | 5/35 | 0.50(0.05-4.60) |
| 10+ years | 27/104 | 0.63(0.30-1.32) | 11/68 | 0.72(0.33-1.57) | 34/139 | 0.75(0.46-1.22) | 4/33 | 1.11(0.20-6.05) | 25/90 | 0.70(0.40-1.22) | 2/19 | 2.93(0.19-44.76) |
| Missing | 10/5 | 0/6 | 8/4 | 2/8 | 8/2 | 0/5 | ||||||
| P trend | 0.13 | 0.48 | 0.65 | 0.47 | 0.79 | 0.28 | ||||||
| P heterogeneity | <0.01 | <0.01 | 0.02 | 0.28 | 0.01 | 0.32 | ||||||
: odds ratio adjusted for age, sex, race/ethnicity, study center, educational level, the frequency of alcohol drinking (continuous), vegetable and fruit intake (quartiles of center-specific controls), and other vitamins (Multiple vitamins, vitamin C and vitamin E).
: odds ratio adjusted for age, sex, race/ethnicity, study center, educational level, pack-years of smoking (continuous), the frequency of alcohol drinking (continuous), vegetable and fruit intake (quartiles of center-specific controls), and other vitamins (Multiple vitamins, vitamin C and vitamin E).
: odds ratio adjusted for age, sex, race/ethnicity, study center, educational level, pack-years of smoking (continuous), the frequency of alcohol drinking (continuous), vegetable and fruit intake (quartiles of center-specific controls), and other vitamins (Multiple vitamins, vitamin C and vitamin E).
: odds ratio adjusted for age, sex, race/ethnicity, study center, educational level, vegetable and fruit intake (quartiles of center-specific controls), and other vitamins (Multiple vitamins, vitamin C and vitamin E).
: multiple vitamins, vitamin C and vitamin E include North Carolina, Tampa, Los Angeles, Puerto Rico, Boston and US multicenter studies; calcium includes North Carolina, Tampa, Los Angeles, Puerto Rico and Boston studies.
Discussion
This study, based on a large pooled dataset, examined associations of vitamin or mineral supplements with HNC risk. Ever supplemental intake of vitamin C and calcium were associated with a reduced risk of HNC in this large pooled analysis of case-control study, but linear trends were not observed for the frequency or duration of any supplement intake. There did not appear to be any association between the HNC risk and ever use of any vitamins, multiple vitamins, vitamin A, beta-carotene, iron, selenium and zinc.
To date, only a handful of studies evaluated the association of supplemental vitamins and HNC. In a study focusing on pharyngeal cancer, Rossing et al reported increasing cancer risk with decreasing use of vitamin C supplement 12. Another case-control study by Barone et al, reported that vitamin C was not associated with the risk of oral cancer 13. Vitamin C is thought to play a role in cancer chemoprevention by stimulating immune function, inhibiting nitrosamine formation, blocking the metabolic activation of carcinogens, and preventing oxidative stress 39.
In our study, a 24% reduction in HNC risk was associated with ever use of vitamin C supplement (95% CI=0.59-0.96). The inverse association was also observed with the long term intake of supplemental vitamin C (more than 10 years). Our results are in agreement with earlier case-control studies. However, we note that dose response relations were not observed.
An inverse association between calcium supplement and the HNC risk was detected in our pooled analysis, even after adjustment for fruit and vegetable intake and other potential confounding factors. Cumulative calcium intake was also significantly associated with HNC risk. When stratified on smoking and drinking status, the significant association was stronger among never alcohol users. The finding may be explained by the fact that calcium is required for optimal activity of vitamin D, and has been found to participate in regulating apoptosis, cell proliferation, and differentiation 40. In animal and epidemiological studies, calcium intake has been suggested to have protective effects against many cancer types, including colorectal 41, breast 42, endometrial 43, prostate and ovarian 40,41. However in our study, there was no dose response relationship between calcium supplement and the risk of HNC. Vitamin E is a strong intracellular antioxidant 44, which has been shown to confer a cancer-inhibiting effect in animal studies 45. Topical application of this nutrient has been reported to inhibit the development of tumors in the hamster buccal pouch 46. Lower serum levels of alpha-tocopherol were related to a low oral cancer risk in some epidemiological studies 47,48. Two case-control studies also provide modest evidence for an inverse association between vitamin E supplements and oral and pharyngeal cancers 12,13. However, results from systematic review and meta-analysis do not provide support for vitamin E supplementation on the reduced risk of HNC 11,20. In addition, Bairati et al found vitamin E supplementation statistically significantly increased the risk of second primary cancers among HNC patients in a multicenter, double-blind, placebo-controlled, randomized chemoprevention trial 17,18. In our pooled analysis, the high frequency of vitamin E intake was associated with a reduced the risk of HNC, especially oral cancer. However, increased HNC risk was observed with the vitamin E supplement for one to nine years use, although the confidence interval was wide.
There did not appear to be any association with HNC risk and other vitamin or mineral supplements in our study, such as beta-carotene, selenium, iron and zinc. In a large randomized controlled trial, beta-carotene was observed to possibly be protective against early-stage laryngeal cancer 11. However, the results from the three prevention trials for HNC, beta-carotene supplement had no significant effect forsecond primary risk among head and neck cancer patients15-17. Systematic reviews have reported that beta-carotene might increase overall mortality as well as cardiovascular mortality 20-23. Selenium given singly or in combination with other supplements seemed to significantly decrease mortality 22,49. However, few studies have investigated the relation of mineral supplements and HNC. One possible explanation for the null associations in our study is the low prevalence of use of individual supplements.
In this analysis, there was significant heterogeneity between studies for some vitamins, such as vitamin C. Given the different characteristics of the various populations, variation in assessment in exposure, and the study design, a degree of heterogeneity across studies is to be expected. Meta-regression analysis showed that the geographic location of the studies might explain the source for the heterogeneity across studies. It is possible that the prevalence of susceptibility genes varies in different populations. For example, for vitamin C supplement, the 5 studies for which the point estimate was below 1 were from North America. Another explanation for the source of heterogeneity is that the case subtype distribution may differ by geographic location or hospital type/specialty. A few studies did not include cases of the larynx (Seattle, US multicenter, New York multicenter, Puerto Rico, and IARC studies) since all of the studies recruited eligible head and neck cancer patients sequentially. Moreover, the different types of questions and the different number of questions were across the studies.
There are several limitations in our pooled analysis. A potential limitation with regards to pooling data on ever use of vitamin or mineral supplements is the difference of definition of ever use. Accordingly, individuals with low vitamin or mineral supplement use might have been categorized as a “never user of vitamin or mineral supplements” in the analysis because of the wording on the questionnaires used to establish the “unexposed” group in the studies. The studies with the highest thresholds for classifying an individual as unexposed were the New York multicenter and Tampa studies (never users were defined as an individual who took any vitamins or minerals at least once a week for a year), and the US multicenter study (never users were defined as an individual who took any vitamins on a regular basis less than for six months). However, the odds ratios for these studies with higher thresholds were not necessarily towards the null, if inclusion of these minimal users had an impact on the association.
Recall bias is a potential limitation in retrospective studies because the subjects knew their disease status when they were interviewed. Supplemental vitamin use is not an established protective factor specifically for HNC, especially in the knowledge of the public at the time of the studies. Therefore, we would expect recall bias to be minimal during the interview for vitamin or mineral supplements assessment.
Another limitation is our inability to adjust for other potential confounding factors, such as micronutrients from dietary intake and HPV infection. We adjusted on fruit and vegetable intake as confounding factors in our study because most antioxidant vitamins are from fruit and vegetables. HPV would not be expected to result in major confounding because an association between vitamin or mineral supplements and HPV infection has not been established. It would be of interest to explore this area in the future when HPV data may be available with a standardized measure across the INHANCE studies.
Furthermore, although the pooled data provided large sample size for the investigation on vitamin or mineral supplements and HNC risk, the statistical power of analysis was limited for beta-carotene, iron, selenium and zinc because the prevalence of these supplements was low in the study population. It was difficult to analyze the duration and frequency of these supplements because of small sample size. The major strength of our pooled analyses was assembly of a very large series HNC patients and control subjects, which allowed us to examine HNC risks in detail and to explore differences in risks by cancer subsites, smoking and alcohol status. To our knowledge, the estimates we present are the most precise available for the relationship of vitamin supplement and HNC risk. In summary, though some associations were suggested for vitamin C and calcium supplement use, dose-response trends were not observed.
Supplementary Material
Acknowledgments
This International Head and Neck Cancer Epidemiology Consortium was supported by NIH, NCI grant R03CA113157 and NIDCR grant R03 DE016611. The work reported in this article was undertaken during the tenure of a Postdoctoral Fellowship awarded by the IARC (postdoctoral fellowship, Q. Li).
The individual studies were supported by:
France study: Swiss League against Cancer [KFS1069-09-2000], Fribourg League gainst Cancer [FOR381.88], Swiss Cancer Research [AKT 617] and Gustave-Roussy Institute [88D28].
New York study: National Institutes of Health (NIH) US [P01CA068384, K07CA104231].
Seattle study: National Institutes of Health (NIH) US [R01CA048896, R01DE012609].
North Carolina study: National Institutes of Health (NIH) US [R01CA61188], and in part by a grant from the National Institute of Environmental Health Sciences [P30ES010126].
Tampa study: National Institutes of Health (NIH) US [P01CA068384, K07CA104231, R01DE13158].
Los Angeles study: National Institute of Health (NIH) US [P50CA90388, R01DA11386, R03CA77954, T32CA09142, U01CA96134, R21ES011667] and the Alper Research Program for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center.
Puerto Rico study: jointly funded by National Institutes of Health (NCI) US and NIDCR intramural programs.
Latin America study: Fondo para la Investigacion Cientifica y Tecnologica (FONCYT) Argentina, IMIM (Barcelona), Fundação de Amparo a‘ Pesquisa no Estado de São Paulo (FAPESP) [No 01/01768-2], and European Commission [IC18-CT97-0222].
IARC Multicenter study: Fondo de Investigaciones Sanitarias (FIS) of the Spanish Government [FIS 97/0024, FIS 97/0662, BAE 01/5013], International Union Against Cancer (UICC), and Yamagiwa-Yoshida Memorial International Cancer Study Grant.
Boston study: National Institutes of Health (NIH) US [R01CA078609, R01CA100679].
US Multicenter study: The Intramural Program of the National Cancer Institute, National Institutes of Health, USA
MSKCC study: NIH [R01CA51845]
Abbreviations
- CI
confidence interval
- HNC
head and neck cancer
- HPV
human papillomavirus
- IARC
International Agency for Research on Cancer
- INHANCE
International Head and Neck Cancer Epidemiology
- MSKCC
Memorial Sloan-Kettering Cancer Center
- NOS
not otherwise specified
- OR
Odds ratios
Reference List
- 1.Ferlay J, SHin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 Cancer Incidence and Mortality Worldwide: IARC CancerBase No.10. International Agency for Research on Cancer; Lyon: 2010. [Google Scholar]
- 2.IARC Working Group . IARC monographs on the evaluation of carcinogenic risks to humans. Tobacco smoke and involuntary smoking. vol 83. International Agency for Research on Cancer; Lyon: 2004. [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Working Group . Alcohol drinking. vol 44. International Agency for Research on Cancer; Lyon: 1988. IARC monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund International . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. World Cancer Research Fund International; London: 2007. [Google Scholar]
- 5.Heck JE, Sapkota A, Vendhan G, Roychowdhury S, Dikshit RP, Jetly DH, Brennan P, Boffetta P, Hashibe M. Dietary risk factors for hypopharyngeal cancer in India. Cancer Causes Control. 2008;19:1329–37. doi: 10.1007/s10552-008-9204-z. [DOI] [PubMed] [Google Scholar]
- 6.Garavello W, Giordano L, Bosetti C, Talamini R, Negri E, Tavani A, Maisonneuve P, Franceschi S, La VC. Diet diversity and the risk of oral and pharyngeal cancer. Eur J Nutr. 2008;47:280–4. doi: 10.1007/s00394-008-0722-y. [DOI] [PubMed] [Google Scholar]
- 7.Diaz MN, Frei B, Vita JA, Keaney JF., Jr Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–16. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 8.Reddy BS. Micronutrients as chemopreventive agents. IARC Sci Publ. 1996:221–35. [PubMed] [Google Scholar]
- 9.Kelloff GJ, Boone CW, Steele VE, Fay JR, Lubet RA, Crowell JA, Sigman CC. Mechanistic considerations in chemopreventive drug development. J Cell Biochem Suppl. 1994;20:1–24. doi: 10.1002/jcb.240560903. [DOI] [PubMed] [Google Scholar]
- 10.Patterson RE, White E, Kristal AR, Neuhouser ML, Potter JD. Vitamin supplements and cancer risk: the epidemiologic evidence. Cancer Causes Control. 1997;8:786–802. doi: 10.1023/a:1018443724293. [DOI] [PubMed] [Google Scholar]
- 11.Wright ME, Virtamo J, Hartman AM, Pietinen P, Edwards BK, Taylor PR, Huttunen JK, Albanes D. Effects of alpha-tocopherol and beta-carotene supplementation on upper aerodigestive tract cancers in a large, randomized controlled trial. Cancer. 2007;109:891–8. doi: 10.1002/cncr.22482. [DOI] [PubMed] [Google Scholar]
- 12.Rossing MA, Vaughan TL, McKnight B. Diet and pharyngeal cancer. Int J Cancer. 1989;44:593–7. doi: 10.1002/ijc.2910440406. [DOI] [PubMed] [Google Scholar]
- 13.Barone J, Taioli E, Hebert JR, Wynder EL. Vitamin supplement use and risk for oral and esophageal cancer. Nutr Cancer. 1992;18:31–41. doi: 10.1080/01635589209514202. [DOI] [PubMed] [Google Scholar]
- 14.Gridley G, McLaughlin JK, Block G, Blot WJ, Gluch M, Fraumeni JF., Jr Vitamin supplement use and reduced risk of oral and pharyngeal cancer. Am J Epidemiol. 1992;135:1083–92. doi: 10.1093/oxfordjournals.aje.a116208. [DOI] [PubMed] [Google Scholar]
- 15.Toma S, Bonelli L, Sartoris A, Mira E, Antonelli A, Beatrice F, Giordano C, Benazzo M, Caroggio A, Cavalot AL, Gandolfo S, Garozzo A, et al. beta-carotene supplementation in patients radically treated for stage I-II head and neck cancer: results of a randomized trial. Oncol Rep. 2003;10:1895–901. [PubMed] [Google Scholar]
- 16.Mayne ST, Cartmel B, Baum M, Shor-Posner G, Fallon BG, Briskin K, Bean J, Zheng T, Cooper D, Friedman C, Goodwin WJ., Jr Randomized trial of supplemental beta-carotene to prevent second head and neck cancer. Cancer Res. 2001;61:1457–63. [PubMed] [Google Scholar]
- 17.Bairati I, Meyer F, Gelinas M, Fortin A, Nabid A, Brochet F, Mercier JP, Tetu B, Harel F, Masse B, Vigneault E, Vass S, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst. 2005;97:481–8. doi: 10.1093/jnci/dji095. [DOI] [PubMed] [Google Scholar]
- 18.Bairati I, Meyer F, Jobin E, Gelinas M, Fortin A, Nabid A, Brochet F, Tetu B. Antioxidant vitamins supplementation and mortality: a randomized trial in head and neck cancer patients. Int J Cancer. 2006;119:2221–4. doi: 10.1002/ijc.22042. [DOI] [PubMed] [Google Scholar]
- 19.Huang HY, Caballero B, Chang S, Alberg AJ, Semba RD, Schneyer CR, Wilson RF, Cheng TY, Vassy J, Prokopowicz G, Barnes GJ, Bass EB. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference. Ann Intern Med. 2006;145:372–85. doi: 10.7326/0003-4819-145-5-200609050-00135. [DOI] [PubMed] [Google Scholar]
- 20.Bardia A, Tleyjeh IM, Cerhan JR, Sood AK, Limburg PJ, Erwin PJ, Montori VM. Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis. Mayo Clin Proc. 2008;83:23–34. doi: 10.4065/83.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–28. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 22.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 23.Coulter ID, Hardy ML, Morton SC, Hilton LG, Tu W, Valentine D, Shekelle PG. Antioxidants vitamin C and vitamin e for the prevention and treatment of cancer. J Gen Intern Med. 2006;21:735–44. doi: 10.1111/j.1525-1497.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes RB, Bravo-Otero E, Kleinman DV, Brown LM, Fraumeni JF, Jr., Harty LC, Winn DM. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999;10:27–33. doi: 10.1023/a:1008876115797. [DOI] [PubMed] [Google Scholar]
- 25.Elahi A, Zheng Z, Park J, Eyring K, McCaffrey T, Lazarus P. The human OGG1 DNA repair enzyme and its association with orolaryngeal cancer risk. Carcinogenesis. 2002;23:1229–34. doi: 10.1093/carcin/23.7.1229. [DOI] [PubMed] [Google Scholar]
- 26.Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B, Pintos J, Fernandez L, Idris A, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 27.Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao J, Cao W, Cozen W, Mack TM, Zhang ZF. Polymorphism of Xeroderma Pigmentosum group G and the risk of lung cancer and squamous cell carcinomas of the oropharynx, larynx and esophagus. Int J Cancer. 2006;118:714–20. doi: 10.1002/ijc.21413. [DOI] [PubMed] [Google Scholar]
- 28.Olshan AF, Weissler MC, Watson MA, Bell DA. GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:185–91. [PubMed] [Google Scholar]
- 29.Benhamou S, Tuimala J, Bouchardy C, Dayer P, Sarasin A, Hirvonen A. DNA repair gene XRCC2 and XRCC3 polymorphisms and susceptibility to cancers of the upper aerodigestive tract. Int J Cancer. 2004;112:901–4. doi: 10.1002/ijc.20474. [DOI] [PubMed] [Google Scholar]
- 30.Rosenblatt KA, Daling JR, Chen C, Sherman KJ, Schwartz SM. Marijuana use and risk of oral squamous cell carcinoma. Cancer Res. 2004;64:4049–54. doi: 10.1158/0008-5472.CAN-03-3425. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Shi Q, Liu Z, Sturgis EM, Spitz MR, Wei Q. Polymorphisms of methionine synthase and methionine synthase reductase and risk of squamous cell carcinoma of the head and neck: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1188–93. doi: 10.1158/1055-9965.EPI-04-0501. [DOI] [PubMed] [Google Scholar]
- 32.Muscat JE, Richie JP, Jr., Thompson S, Wynder EL. Gender differences in smoking and risk for oral cancer. Cancer Res. 1996;56:5192–7. [PubMed] [Google Scholar]
- 33.Peters ES, McClean MD, Marsit CJ, Luckett B, Kelsey KT. Glutathione S-transferase polymorphisms and the synergy of alcohol and tobacco in oral, pharyngeal, and laryngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:2196–202. doi: 10.1158/1055-9965.EPI-06-0503. [DOI] [PubMed] [Google Scholar]
- 34.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Dal ML, Daudt AW, Fabianova E, Fernandez L, Wunsch-Filho V, Franceschi S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–89. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 35.Sun EC, Curtis R, Melbye M, Goedert JJ. Salivary gland cancer in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8:1095–100. [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–64. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 38.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley and Sons, Inc; New York: 1987. [Google Scholar]
- 39.Lee KW, Lee HJ, Surh YJ, Lee CY. Vitamin C and cancer chemoprevention: reappraisal. Am J Clin Nutr. 2003;78:1074–8. doi: 10.1093/ajcn/78.6.1074. [DOI] [PubMed] [Google Scholar]
- 40.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. 2009;29:3687–98. [PubMed] [Google Scholar]
- 41.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer. 2009;61:47–69. doi: 10.1080/01635580802395733. [DOI] [PubMed] [Google Scholar]
- 42.Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 43.McCullough ML, Bandera EV, Moore DF, Kushi LH. Vitamin D and calcium intake in relation to risk of endometrial cancer: a systematic review of the literature. Prev Med. 2008;46:298–302. doi: 10.1016/j.ypmed.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson R, Theron AJ. Physiological potential of ascorbate, beta-carotene and alpha-tocopherol individually and in combination in the prevention of tissue damage, carcinogenesis and immune dysfunction mediated by phagocyte-derived reactive oxidants. World Rev Nutr Diet. 1990;62:27–58. doi: 10.1159/000417534. [DOI] [PubMed] [Google Scholar]
- 45.Birt DF. Update on the effects of vitamins A, C, and E and selenium on carcinogenesis. Proc Soc Exp Biol Med. 1986;183:311–20. doi: 10.3181/00379727-183-42424. [DOI] [PubMed] [Google Scholar]
- 46.Odukoya O, Hawach F, Shklar G. Retardation of experimental oral cancer by topical vitamin E. Nutr Cancer. 1984;6:98–104. doi: 10.1080/01635588509513813. [DOI] [PubMed] [Google Scholar]
- 47.Zheng W, Blot WJ, Diamond EL, Norkus EP, Spate V, Morris JS, Comstock GW. Serum micronutrients and the subsequent risk of oral and pharyngeal cancer. Cancer Res. 1993;53:795–8. [PubMed] [Google Scholar]
- 48.Krishnamurthy S, Jaya S. Serum alpha-tocopherol, lipo-peroxides, and ceruloplasmin and red cell glutathione and antioxidant enzymes in patients of oral cancer. Indian J Cancer. 1986;23:36–42. [PubMed] [Google Scholar]
- 49.Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, Johnson LL, Gail MH, Dong ZW, Yu B, Mark SD, Taylor PR. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–18. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


