Abstract
INTRODUCTION
Blunt diaphragmatic rupture is a rare event that may occur after traumatic injury. Due to its rarity and difficulty in diagnosing, delayed detection of diaphragmatic injuries can occur. Management involves repair of the diaphragmatic defect via trans-thoracic and/or trans-abdominal approaches. Most small repairs may be repaired primarily, larger defects have been historically repaired with mesh.
PRESENTATION OF CASE
We report a case series of five patients with diaphragmatic injuries all repaired with biologic mesh via both trans-thoracic and trans-abdominal approaches.
DISCUSSION
Delayed presentation is the single most important contributor to increased morbidity and mortality in patients with blunt diaphragmatic rupture. Our case series corroborates other findings that patients with blunt diaphragmatic ruptures are at high risk for infection and thus may be repaired with use of biologic mesh versus the traditional use of synthetic mesh. This can be done without high rates of recurrence or complications from use of biologic mesh.
CONCLUSION
In our series, we successfully repaired 5 diaphragmatic defects with the use of biologic mesh. With follow-up as much as 4 years out, none of our patients have had an infectious complication with the use biologic mesh and there is no evidence of recurrence or eventration. The use of biologic mesh is an acceptable alternative to the traditional use of synthetic mesh in the repair of both acute and chronic diaphragmatic defects.
Keywords: Trauma, Diaphragm, Rupture, Biologic mesh, Delayed
1. Introduction
Blunt diaphragmatic rupture is a rare event that may occur after traumatic injury. Due to its rarity and difficulty in diagnosis, detection of diaphragmatic injury is often delayed. Similarly, missed congenital diaphragmatic hernia can present in a delayed fashion. Following blunt trauma, injury to the diaphragm involves both sides equally, as reported in autopsy and computed tomography (CT) studies.1 However, in clinical practice left sided injuries appear to be more frequent than right-sided injuries. Management involves repair of the diaphragmatic defect via trans-thoracic and/or trans-abdominal approaches.2 Most small repairs may be repaired primarily, historically larger defects have been repaired with mesh. This report presents a case series of five patients with diaphragmatic injuries all repaired with biologic mesh instead of synthetic mesh.
2. Case series
2.1. Case 1
21-year-old male who was involved in a motor vehicle collision (MVC) one year prior with several associated orthopedic injuries presented multiple times to the emergency department with complaint of worsening dyspnea on exertion. Chest x-ray (Fig. 1a) revealed evidence of left-sided diaphragmatic injury. CT scan of the chest and abdomen revealed large left sided defect with herniation of stomach and small intestine. Due to the patient's delayed presentation the repair occurred via a left thoracotomy, the diaphragmatic defect was closed primarily and reinforced with Human Acellular Dermal Matrix (HADM). One-year post repair chest x-ray with no evidence of recurrence (Fig. 1b).
Fig. 1.
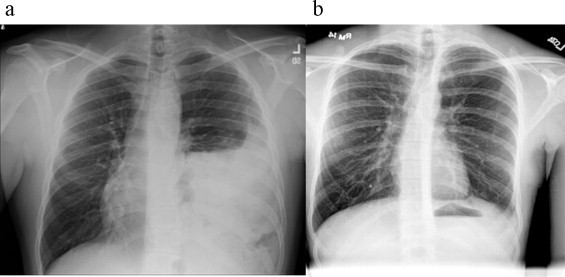
(a) Chest x-ray with elevation of left hemidiaphragm. (b) One-year post procedure chest x-ray with no evidence of recurrence and good expansion of left lung.
2.2. Case 2
42-year-old female presented to her primary care physician with complaints of chest pain and shortness of breath. Work-up included chest x-ray and CT scan (Fig. 2a) that revealed a large right anteriomedial diaphragmatic defect. This was repaired via right thoracotomy with primary repair of part of the defect and placement of HADM mesh to bridge the remainder of the defect. Chest x-ray two years post repair with no evidence of recurrence or eventration (Fig. 2b).
Fig. 2.
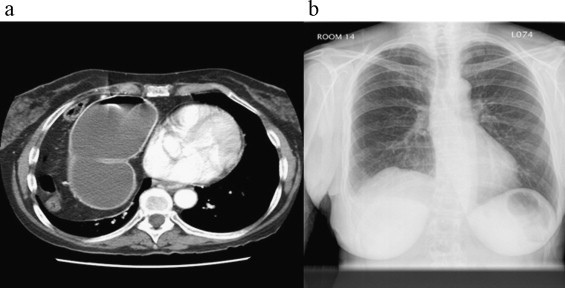
(a) CT scan of chest with evidence of herniated colon into the right chest. (b) Two-year post-operative chest x-ray with resolution of right hemidiaphragm and no evidence of recurrence of eventration.
2.3. Case 3
27-year-old male who was involved in a head on collision MVC 6-months prior. Patient presented to trauma clinic with worsening shortness of breath. Chest x-ray and CT-scan (Fig. 3a) revealed left-sided elevation of hemidiaphragm, large pneumothorax and herniation of colon into left chest. Patient underwent repair via left thoracotomy and laparotomy to assist in reduction of herniated viscera. There were 2 small enterotomies that occurred during the removal of the bowel from the hernia and thus wound classification changed to clean-contaminated. Due to the change in wound classification the diaphragmatic defect was closed primarily and reinforced with HADM mesh. One-year post repair chest x-ray with no evidence of recurrence (Fig. 3b).
Fig. 3.
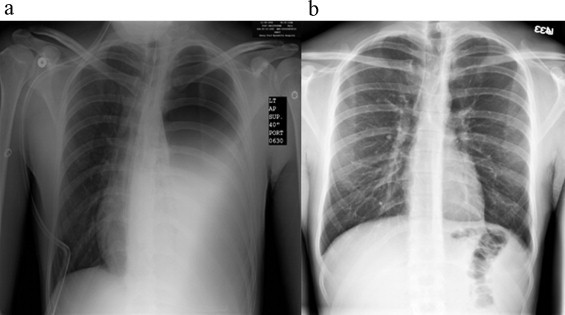
(a) Pre-operative chest x-ray with evidence of left hemidiaphragm and large pneumothorax. (b) One-year post-procedure chest x-ray with good expansion of left lung, no evidence of pneumothorax and no evidence of recurrent diaphragmatic hernia.
2.4. Case 4
20-year-old female unrestrained passenger in MVC collision found 6 h after injury. Patient with multiple orthopedic injuries that underwent repair. The patient's initial chest x-ray showed elevation of right hemidiaphragm and CT chest (Fig. 4a) with suspicion for possible diaphragmatic injury. A diagnosis of empyema was made and the patient was taken to the operating room for video-assisted thorascopic surgery, which was converted to a right thoracotomy with decortication and pleurodesis. It was discovered that the patient had a large diaphragmatic defect with herniation of liver. The defect was repaired via right thoracotomy and laparotomy with primary closure of the lateral aspect of defect. Remainder of defect was repaired with Porcine Acellular Dermal Matrix (PADM) mesh. Post-procedure chest x-ray shows no evidence of herniation (Fig. 4b).
Fig. 4.
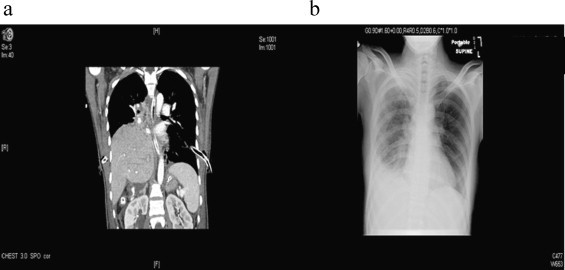
(a) CT scam of chest (coronal view) with suspicion for possible diaphragmatic injury and herniation of liver into right chest. (b) Post-operative chest x-ray with no evidence of recurrent pneumothorax and resolution of right hemidiaphragmatic elevation.
2.5. Case 5
68-year-old male with history of angiomyolipoma of the left kidney involving the diaphragm underwent en-bloc resection with repair of left diaphragm primarily. Patient presented several years later with shortness of breath, chest x-ray revealed recurrent diaphragmatic hernia (Fig. 5a). Patient underwent repair via laparoscopic converted to open laparotomy with closure of diaphragmatic defect with HADM. This patient has been followed for over 4 years and has no evidence of recurrence or eventration (Fig. 5b).
Fig. 5.
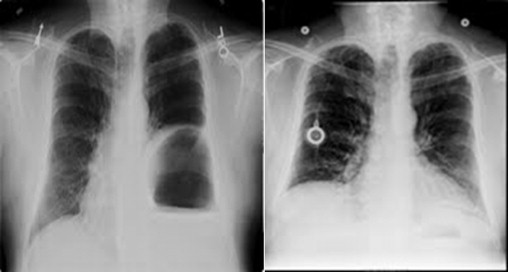
Pre-operative (left) chest x-ray with evidence of herniation of colon into left chest. Routine chest x-ray (right) 4 years later with no evidence of diaphragmatic hernia.
3. Discussion
The incidence of diaphragmatic ruptures secondary to trauma is 0.8–5%. Due to the difficulty in diagnosing traumatic diaphragmatic injuries, up to 30% present as late findings.3 Delayed presentation is the single most important contributor to increased morbidity and mortality. Mortality rates as high as 16–33% are seen, owning to the fact that many blunt diaphragmatic ruptures remain clinically silent until they present with life-threatening complications.4 Although post mortem studies report an equal incidence of right and left sided ruptures, antemortem reports suggest that 88–95% of diaphragmatic ruptures occur on the left and ruptures of the right side are associated with more severe injuries and therefore an increased morbidity and mortality.5 This disparity of increased incidence in left-sided rupture can be explained by multiple factors: under diagnosis of right-sided injuries, relative strength of the right hemidiaphragm compared to the left due to embryonic fusion points and hepatic protection of right-sided injuries.6
Delayed presentation and diagnosis makes the surgical repair of blunt diaphragmatic ruptures more difficult. Surgical treatment is performed either laparoscopically, thorascopically or through an open laparotomy or thoracotomy. Surgical treatment consists of hernia reduction, pleural drainage and repair of diaphragmatic defect.7 A recent review by Kishore et al.8 found that the vast majority (89%) of blunt diaphragmatic injuries were successfully repaired via open laparotomy. Similarly, Chughtai et al.9 in their review of 208 patients with diaphragmatic rupture found that 93% of diaphragmatic injuries can be repaired via open laparotomy. This is clearly the preferred approach in the acute setting of trauma as it allows evaluation for and repair of abdominal injuries. With that being said, patients who present with a delayed diagnosis of blunt diaphragmatic rupture (BDR) are often thought to benefit from initial attempted repair through the chest via thoracotomy or thorascopy. This allows for evaluation of the herniated viscera as well as the pleural cavity. Addition of a laparotomy may be necessary to assist in reduction of the herniated viscera.
Most diaphragmatic defects may be repaired primarily, especially in the acute setting due to the pliability of the diaphragm. However, for larger defects and patients that have a delayed presentation, the diaphragmatic defect may be too large to repair primarily or the edges have become to thin and weak to hold suture and needs to be closed with the use of mesh. The stress of the continued use of the diaphragm during breathing, coughing, valsava, and even during cardiac motion is reason enough for the use of a mesh repair for any large diaphragmatic defect or rupture.10 The gold standard repair for BDRs, that are not amenable to primary repair, is use of synthetic mesh. Synthetic mesh is a durable, cost-effective prosthesis that has been used for decades. However, an increasing amount of data is emerging regarding the complications of synthetic mesh repair, such as adhesion formation, erosion into surrounding structures, infections and need for subsequent explantation.11,12 This has led to an increase interest in the potential use of biologic mesh for this purpose.
The use of biologic mesh to repair traumatic or congenital diaphragmatic defects is limited to small case series and case reports. In other situations, namely abdominal ventral hernias, it has been well established that biologic mesh is more resistant to infection and may be placed in infected fields with minimal risk of infection compared to synthetic mesh.13,14 Biologic mesh will incorporate into the surrounding tissue and with inosculation will become part of the native diaphragm.15 Trauma patients that have blunt diaphragmatic rupture often have a concomitant bowel or lung injury with contamination. Biologic mesh can be placed in these hostile environments with less risk of infection when compared to synthetic mesh. The sequlae of infected mesh, especially in the polytrauma patient, can be cumbersome and require further operations for mesh removal and complex reconstruction of the diaphragm.
In case #1 HADM was used to reinforce the primary repair of a diaphragm injury. It was felt that the chronic diaphragmatic injury had substantially weakened the diaphragm and the edges of the defect were friable. Thus, a primary repair would not be a durable long-term repair given the constant movement of the diaphragm and would require a reinforced repair with biologic mesh. This is not unlike the concept of an underlay patch in treatment of large abdominal ventral hernias.16 In cases #2, #3, and #4 there was remaining diaphragmatic defect despite attempts at primary repair. In cases #3 and #4 there was a contaminated field with bowel perforation in case #3 and empyema and bile spillage in case #4. Anti-microbial management was attempted for a prolonged course in case #4, however, due to the empyema the patient required a decortication and repair of the diaphragmatic injury. It was felt that synthetic mesh would be at high risk for infection in this setting and primary repair in these cases was not technically feasible.
Biologic mesh has shown to be much less adhesiogenic and is less likely to adhere to the lung or abdominal viscera as is synthetic mesh.17 Additionally, some biologics have been shown to have vascular ingrowth and cell turnover. Specifically, in cases #2 and #4, both repairs were right-sided and adjacent to the pericardium. Biologic mesh would be less prone to from adhesions and is less likely to promote scarring to the pericardium. Using this type of material to reinforce a structure that continually moves like the diaphragm could be advantageous in that the diaphragm can repair itself via normal biologic pathways. Synthetic inert materials that do not have the ability to catalyze regeneration and incorporation into dynamic native tissues may be subject to fatigue stress of the material and suture due to the constant movement of the diaphragm.18
Several prospective animal models have been studied. Gonzalez et al.19 compared the characteristics of new diaphragmatic tissue between an absorbable biologic mesh and a nonabsorbable synthetic mesh for repairing diaphragmatic hernia in a growing animal model. The animals were randomly assigned to coverage of hernia defect and survived for 6 months. Patch disruption and herniation occurred in 3 animals in the synthetic mesh group and none in the biologic mesh group. The biologic mesh group had better integration into the chest wall, more muscle growth into the newly formed diaphragmatic tissue and less fibrotic tissue than the synthetic mesh group.
While the focus of this retrospective case series was primarily in traumatic BDR, certainly there are other circumstances in which consideration of biologic mesh use may be more pertinent. Specifically, treatment of mesothelioma or other malignancies requiring large resection of the diaphragmatic surfaces may warrant the use of biologic mesh either has overlay to reinforce a primary repair or in attempt to close the diaphragmatic defect.20
In our series, we successfully repaired 5 diaphragmatic defects with the use of biologic mesh. With follow-up as much as 4 years after repair, none of these patients have had an infectious complication and there is no evidence of recurrence or eventration. The use of biologic mesh can be used in the setting of BDR and is an acceptable alternative to the traditional use of synthetic mesh in the repair of both acute and chronic diaphragmatic defects. In addition, while there are cost differences between the two products, technically, they appear to be equivalent and from some theoretical perspectives, perhaps, more advantageous. Thus, the added expense of biologic mesh may be warranted in selected cases when there is fear of possible infection and subsequent mesh explanation.
Conflict of interest statement
None.
Funding
None.
Consent
“Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request”. No patient identifiers were used in the creation of this manuscript for publication.
Author contributions
Study design – OA, CT, BH; data collection – OA, CT, BH, BF; data analysis – OA, CT, BH, BF, TE; writing – OA, CT, BH, TE; editing – OA, CT, TE.
References
- 1.Meyers B.F., McCabe C.J. Traumatic diaphragmatic hernia. Occult marker of serious injury. Annals of Surgery. 1993;218:783–790. doi: 10.1097/00000658-199312000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwivedi S., Banode P., Gharde P., Bhatt M., Johrapurkar S. Treating traumatic injuries of the diaphragm. Journal of Emergencies, Trauma and Shock. 2010;3:173–176. doi: 10.4103/0974-2700.62122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crandall M., Popowich D., Shapiro M., West M. Posttraumatic hernias: historical overview and review of literature. American Surgeon. 2007;73:845–850. [PubMed] [Google Scholar]
- 4.Anderson D.W. Bilateral diaphragm rupture: a unique presentation. Journal of Trauma. 2002;52:560–561. doi: 10.1097/00005373-200203000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Rashid F., Chakrabarty M.M., Singh R., Iftikhar S.Y. A review on delayed presentation of diaphragmatic rupture. World Journal of Emergency Surgery. 2009;4 doi: 10.1186/1749-7922-4-32. (Article 32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah R., Sabanathan S., Mearns A.J., Choudhury A.K. Traumatic rupture of the diaphragm. Annals of Thoracic Surgery. 1995;60:1444–1449. doi: 10.1016/0003-4975(95)00629-Y. [DOI] [PubMed] [Google Scholar]
- 7.Hanna W.C., Ferri L.E. Acute traumatic diaphragm injury. Thoracic Surgery Clinics. 2009;19:485–489. doi: 10.1016/j.thorsurg.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Kishore G.S., Gupta V., Doley R.P., Kudari A., Kalra N., Yadav T.D. Traumatic diaphragmatic hernia: tertiary centre experience. Hernia. 2010;4:159–164. doi: 10.1007/s10029-009-0579-x. [DOI] [PubMed] [Google Scholar]
- 9.Chughtai T., Ali S., Sharkey P., Lins M., Rizoli S. Update on managing diaphragmatic rupture in blunt trauma: a review of 208 consecutive cases. Canadian Journal of Surgery. 2009;52:177–181. [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell I.C., Garcia N.M., Barber R., Ahmad N., Hicks B.A., Fisher A.C. Permacol: a potential biologic patch alternative in congenital diaphragmatic hernia repair. Journal of Pediatric Surgery. 2008;43:2161–2164. doi: 10.1016/j.jpedsurg.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 11.Frantzides C.T., Carlson M.A., Loizides S., Papafili A., Luu M., Roberts J. Hiatal hernia repair with mesh: a survey of SAGES members. Surgical Endoscopy. 2010;24(5):1017–1024. doi: 10.1007/s00464-009-0718-6. [DOI] [PubMed] [Google Scholar]
- 12.Hazebroek E.J., Leibman S., Smith G.S. Erosion of a composite PTFE/ePTFE mesh after hiatal hernia repair. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques. 2009;19(2):175–177. doi: 10.1097/SLE.0b013e3181a11926. [DOI] [PubMed] [Google Scholar]
- 13.Hyong K., Bruen K., Vargo D. Acellular dermal matrix in the management of high-risk abdominal wall defects. The American Journal of Surgery. 2006;192:705–709. doi: 10.1016/j.amjsurg.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Diaz J.J., Guy J., Berkes M., Guillamondegui O., Miller R. Acellular dermal allograft for ventral hernia repair in the compromised surgical field. The American Surgeon. 2006;72(12):1181–1188. [PubMed] [Google Scholar]
- 15.Hawn M.T., Synder C.W., Graham L.A., Gray S.H., Finan K.R., Vick C.C. Long-term follow-up of technical outcomes for incisional hernia repair. Journal of the American College of Surgeons. 2010;210(5):648–655. doi: 10.1016/j.jamcollsurg.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 16.Zardo P., Zhang R., Wiegmann B., Haverich A., Fischer S. Biological materials for diaphragmatic repair: initial experiences with the PeriGuard Repair Patch®. Thoracic and Cardiovascular Surgeon. 2011;59:40–44. doi: 10.1055/s-0030-1250499. [DOI] [PubMed] [Google Scholar]
- 17.Butler C.E., Prieto V.G. Reduction of adhesions with composite AlloDerm/polypropylene mesh implants for abdominal wall reconstruction. Plastic and Reconstructive Surgery. 2004;114(2):464–473. doi: 10.1097/01.prs.0000132670.81794.7e. [DOI] [PubMed] [Google Scholar]
- 18.Ventral Hernia Working Group, Breuing K., Butler C.E., Ferzoco S., Franz M., Hultman C.S. Incisional ventral hernia: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez R., Hill S.J., Mattar S.G., Lin E., Ramshaw B.J., Smith C.D. Absorbable versus nonabsorbable mesh repair of congenital diaphragmatic hernias in a growing animal model. Journal of Laparoendoscopic and Advanced Surgical Techniques Part A. 2011;21:449–454. doi: 10.1089/lap.2010.0409. [DOI] [PubMed] [Google Scholar]
- 20.Sugarbaker D.J., Mentzer S.J., Strauss G. Extrapleural pneumonectomy in the treatment of malignant pleural mesothelioma. Annals of Thoracic Surgery. 1992;54:941–946. doi: 10.1016/0003-4975(92)90654-m. [DOI] [PubMed] [Google Scholar]


