Abstract
During pregnancy, it is evolutionary advantageous for inflammatory immune responses that might lead to fetal rejection to be reduced and anti-inflammatory responses that promote transfer of maternal antibodies to the fetus to be increased. Hormones modulate the immunological shift that occurs during pregnancy. Estrogens, including estradiol and estriol, progesterone, and glucocorticoids increase over the course of pregnancy and affect transcriptional signaling of inflammatory immune responses at the maternal-fetal interface and systemically. During pregnancy, the reduced activity of natural killer cells, inflammatory macrophages, and helper T cell type 1 (Th1) cells and production of inflammatory cytokines, combined with the higher activity of regulatory T cells and production of anti-inflammatory cytokines, affects disease pathogenesis. The severity of diseases caused by inflammatory responses (e.g., multiple sclerosis) is reduced and the severity of diseases that are mitigated by inflammatory responses (e.g., influenza and malaria) is increased during pregnancy. For some infectious diseases, elevated inflammatory responses that are necessary to control and clear a pathogen have a negative consequence on the outcome of pregnancy. The bidirectional interactions between hormones and the immune system contribute to both the outcome of pregnancy and female susceptibility to disease.
Keywords: autoimmunity, estrogen, IFN-γ, influenza, IL-10, malaria, multiple sclerosis, progesterone, Th1/Th2, regulatory T cell
Introduction
The state of pregnancy represents an extreme challenge for the immune system. From the perspective of the pregnant female's immune system, the fetus is an allograft that contains foreign antigens from the father. To support a successful pregnancy, it is evolutionarily advantageous for a pregnant female's immune responses to shift away from inflammatory responses that contribute to fetal rejection and toward anti-inflammatory immune responses that aid in passive transfer of antibodies to the developing fetus (Raghupathy, 1997). Hormones contribute significantly to the shift in immune function that occurs over the three trimesters of pregnancy (Figure 1). Importantly, pregnant females are not immunosuppressed, but rather their immune responses are biased toward an anti-inflammatory phenotype that influences not only the outcome of pregnancy but disease pathogenesis as well.
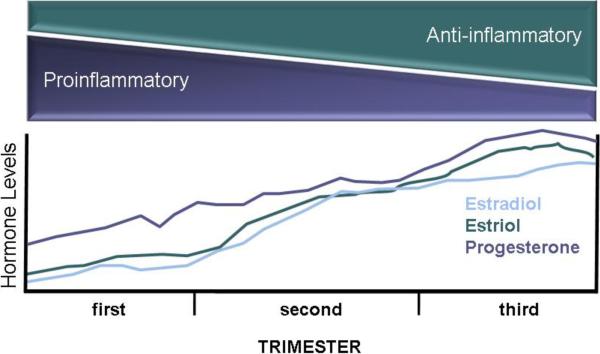
Figure 1.
During the three trimesters of pregnancy, there is a shift in the balance of proinflammatory and anti-inflammatory responses. By the third trimester, anti-inflammatory responses, including the activity of M2 macrophages, Th2 cells, and regulatory T cells, are elevated and inflammatory responses, including the activity of NK cells, M1 macrophages, and Th1 cells, are reduced. Changes in the concentrations of sex steroids, including estradiol, estriol, and progesterone, lead to the immunological shifts during pregnancy.
The hormonal and immunological changes that occur over the course of pregnancy are necessary to support a healthy pregnancy, but also dramatically affect female susceptibility to autoimmune and infectious diseases. While many studies of autoimmune and infectious disease pathogenesis report changes in immunological factors over the course of pregnancy, few studies consider the role that hormones play in orchestrating these immunological changes. The goals of this review are to: (1) evaluate the immunological shifts that occur during pregnancy; (2) determine the effects of pregnancy-associated hormones, in particular estrogens and progesterone (P4), on innate and adaptive immune responses; (3) provide relevant examples of pregnancy and pregnancy-associated hormones affecting the outcome of diseases caused by pathogens as well as recognition of self-antigens; and (4) identify general principles across diseases that might improve interpretation and treatment for immune-related diseases during pregnancy.
Inflammatory immune responses are skewed during pregnancy
Concentrations of steroid hormones, including estrogens and P4, are considerably higher during pregnancy than during other times in the female reproductive cycle and increase over the course of pregnancy, with highest levels achieved during the third trimester (Figure 1). Hormonal changes that occur during pregnancy underlie some of the distinct immunological changes associated with pregnancy. Elevated levels of P4 stimulate the synthesis of progesterone-induced binding factor (PIBF) by lymphocytes (Szekeres-Bartho and Polgar, 2010). In humans, PIBF increases over the course of pregnancy and drops significantly after birth, but in pathological pregnancies that result in preterm labor, abortion, or hypertension, concentrations of PIBF are low (Polgar et al., 2004). High concentrations of PIBF promote differentiation of CD4+ T cells into helper T cell type 2 (Th2) cells that secrete high concentrations of anti-inflammatory cytokines, including IL-4, IL-5, and IL-10 (Szekeres-Bartho et al., 1996). The Th2 bias that occurs during pregnancy corresponds with a reduction in inflammatory Th1 responses (e.g., production of IFN-γ), both at the maternal-fetal interface and systemically in humans and animal models (Krishnan et al., 1996a; Lin et al., 1993; Marzi et al., 1996; Ostensen, 1999; Sacks et al., 2001; Veenstra van Nieuwenhoven et al., 2002). In vitro, splenocytes from pregnant female mice produce less Th1 cytokines and more Th2 cytokines than do cells isolated from non-pregnant females following stimulation (Dudley et al., 1993; Krishnan et al., 1996a). Successful pregnancies in humans are associated with elevated IL-4 and IL-10 and reduced IL-2 and IFN-γ production by peripheral blood mononuclear cells (PBMCs), with differences in cytokine production being greatest during the third trimester of pregnancy (Marzi et al., 1996). Inflammatory cytokines, like IFN-γ and TNF-α, can damage the placenta and developing fetus either directly or by activating cytotoxic cells, including natural killer (NK) or T cells (Raghupathy, 1997).
In addition to T cells, the anti-inflammatory polarization of immune cells during pregnancy is observed for macrophages. In an inflammatory environment (i.e., caused by the presence of inflammatory cytokines or exposure to inflammatory stimuli), uterine decidual macrophages develop an inflammatory phenotype (often referred to as M1 macrophages) that is characterized by elevated secretion of the inflammatory cytokines, IL-12 and TNF-α (Nagamatsu and Schust, 2010). Macrophages that differentiate in an environment that is dominated by Th2-biased cytokines, such as IL-4, IL-10, or IL-13, or high glucocorticoid concentrations, develop an anti-inflammatory phenotype (referred to as M2 macrophages), which is characterized by arginase activity, scavenger receptor expression, and secretion of IL-1 receptor antagonist (Nagamatsu and Schust, 2010). In women with healthy, full-term pregnancies, there is increased M2 polarization of decidual macrophages as compared with women with preterm pregnancies (Nagamatsu and Schust, 2010).
The immunological shift away from inflammatory responses is necessary for a successful, full-term pregnancy. If the anti-inflammatory bias during pregnancy is altered, by infection for example, this can result in preterm labor or abortion in humans as well as mice (Hill et al., 1995; Krishnan et al., 1996b; Marzi et al., 1996). Elevated Th2 responses also correlate with increased antibody responses during pregnancy (Wegmann et al., 1993). Elevated concentrations of anti-inflammatory factors, such as IL-10, can prevent spontaneous abortions, at least in mice (Chaouat et al., 1995). Concurrent with the increase in Th2 responses during normal pregnancy is an increase in the activity of regulatory T cells at the maternal-fetal interface in mice (Kallikourdis and Betz, 2007). In women, migration of regulatory T cells to the pregnant uterus is mediated by human chorionic gonadotropin, which is a chemoattractant protein secreted by the blastocyst after fertilization (Schumacher et al., 2009). Regulatory T cells are hypothesized to orchestrate immune tolerance of the fetus during pregnancy in mammals.
Pregnancy-associated hormones alter immune function
Pregnancy is associated with changes in concentrations of several hormones, including estradiol (E2), estriol (E3), P4, corticosteroids, and prolactin. These hormonal changes contribute to the immunological shifts during pregnancy (Figure 1). Importantly, the effects of pregnancy-associated hormones on immune function extend beyond what has been examined in the context of pregnancy and may inform future studies. Altered activity of innate immune cells contributes to the differential induction of cell-mediated and humoral responses during pregnancy. Consideration of the diverse effects of sex steroids, in particular, on the functioning of the immune system may provide insight into why the pathogenesis of infectious and autoimmune diseases changes dramatically during pregnancy.
Estradiol
Estradiol (E2) occurs in high concentrations in non-pregnant as well as pregnant females and is responsible for a majority of the `classic' estrogenic effects in reproductive and non-reproductive tissues. Estrogen receptors (ERs) are expressed in various lymphoid tissue cells as well as in lymphocytes, macrophages, and dendritic cells (DCs) (Kovats et al., 2010). There are two subtypes of the receptor for estrogens, ERα and ERβ, that exhibit differential expression in subsets of immune cells, with ERα being highly expressed in T cells and ERβ being upregulated in B cells (Phiel et al., 2005). The differential effects of estrogens on parameters of immune function may reflect not only the concentration of estrogen (i.e., whether physiological or pregnancy doses are used), but the density, distribution, and type of ERs in immune cells.
Estradiol affects several aspects of innate immunity, including the functional activity of innate immune cells that influence downstream adaptive immune responses. Exposure to E2 in vitro enhances NK cytotoxicity and production of IFN-γ (Nakaya et al., 2006; Sorachi et al., 1993), but can also downregulate the expression of NK cell surface activation markers and secretion of granzyme B and FasL (Hao et al., 2007). Estradiol enhances the expression of pattern recognition receptors, like toll-like receptor (TLR) 4, on the surface of peritoneal macrophages as well as production of TNF-α (Rettew et al., 2009). Estradiol can have bipotential effects on monocytes and macrophages, with low doses enhancing proinflammatory cytokine production (e.g., IL-1, IL-6, and TNF-α) and high concentrations reducing production of these cytokines (Bouman et al., 2005). In vitro exposure to E2 facilitates differentiation of bone marrow precursor cells into functional CD11c+ DCs (Paharkova-Vatchkova et al., 2004) and increases the synthesis of chemokines, including CXCL8 and CCL2, by immature DCs (Bengtsson et al., 2004), but downregulates antiviral responses, including production of IFN-α and CXCL10, following viral infection (Escribese et al., 2008). Treatment of ovariectomized mice with physiological doses of E2 increases production of IFN-γ by CD11c+ DCs and synthesis of proinflammatory cytokines, including IL-1, IL-6, and TNF-α (Miller and Hunt, 1996; Siracusa et al., 2008). Estradiol acts primarily though ERα, not ERβ, to regulate differentiation of DCs (Carreras et al., 2008; Douin-Echinard et al., 2008; Paharkova-Vatchkova et al., 2004).
Estradiol can enhance both cell-mediated and humoral immune responses (Straub, 2007). Generally, low E2 concentrations promote Th1 responses and cell-mediated immunity and high concentrations of E2 augment Th2 responses and humoral immunity (Straub, 2007). The binding of E2 to the ER increases Ifnγ transcription by interacting with estrogen response elements in the promoter region of the Ifnγ gene (Fox et al., 1991). Low dose E2 also upregulates mitogen activated protein kinase (MAPK), T-bet, and select microRNAs to increase production of IFN-γ by T cells, which can be reversed by treatment of cells with the ER antagonist ICI 182,780 (Dai et al., 2008; Karpuzoglu et al., 2007; Suzuki et al., 2008). Estradiol regulates proinflammatory responses that are transcriptionally mediated by NF-κB (Dai et al., 2007).
Estradiol enhances the expansion of CD4+CD25+ T cells (regulatory T cells) in mice (Polanczyk et al., 2004). The number of regulatory T cells increases during proestrus and estrus in mice and during the follicular stage of the menstrual cycle in women (i.e., when E2 concentrations are highest) (Arruvito et al., 2007; Kallikourdis and Betz, 2007). Treatment of mice with high doses of E2 also decreases production of IL-17 by Th17 cells (Wang et al., 2009). Estradiol at physiological concentrations can stimulate antibody production by B cells (Lu et al., 2002). Levels of immunoglobulin (Ig) and numbers of Ig-secreting cells are highest prior to ovulation in females (Franklin and Kutteh, 1999; Lu et al., 2002).
Estriol
Estriol (E3) is produced in high concentrations by the fetoplacental unit during pregnancy and accounts for almost 90% of all estrogens produced during pregnancy (Soldan et al., 2003; Tulchinsky et al., 1972). Estriol is not present in non-pregnant females. The immunological effects of E3 have not been well characterized and it is assumed that the effects of E3 are broadly the same as E2 because both estrogens signal through the same ERs (Voskuhl, 2011). Much of the research on the immunological effects of E3 has been based on studies of multiple sclerosis (MS) in patients and animal models of MS, such as experimental autoimmune encephalomyelitis (EAE). Treatment of female MS patients or male EAE mice with doses of E3 that produce pregnancy levels in circulation significantly lowers proinflammatory cytokine production (e.g., TNF-α and IFN-γ), increases anti-inflammatory cytokines (e.g., IL-5), reduces numbers of CD4+ and CD8+ T cells, increases autoantibody responses, and increases proportions of CD19+ B cells in circulation (Kim et al., 1999; Liu et al., 2003; Palaszynski et al., 2004b; Sicotte et al., 2002; Soldan et al., 2003). In female mice, induction of EAE causes DCs from E3-treated mice to develop a tolerogenic phenotype, in which there is upregulation of activation and costimulatory surface markers, including inhibitory PD-L1, reduction of proinflammatory transcripts (i.e., IL-12 and IL-6 mRNA), and increased expression of anti-inflammatory transcripts (i.e., TGF-β and IL-10 mRNA) (Papenfuss et al., 2011). Adoptive transfer of DCs from E3-treated females prior to induction of EAE provides protection against development of disease by causing Th2-biased immune responses (Papenfuss et al., 2011). Stimulation of T cells from E3-treated EAE mice with myelin basic protein induces elevated production of the anti-inflammatory cytokine, IL-10 (Kim et al., 1999). The effects of E3 on T cell function are mediated by reduced degradation of IκB leading to inhibition of NF-κB activity and reduced concentrations of proinflammatory cytokines (Zang et al., 2002). Estriol treatment also reduces concentrations of matrix metalloproteinase 9 in EAE mice, which likely contributes to reduced infiltration of monocytes and inflammatory T cells into the central nervous system (CNS) (Gold et al., 2009). Estriol, like E2, stimulates antibody production against innocuous antigens (Ding and Zhu, 2008), which is likely one factor contributing to heightened humoral immunity during pregnancy.
Progesterone
Progesterone is produced by the corpus lutea in the ovaries in non-pregnant females and by the placenta during pregnancy, playing a critical role in reproduction and immune function. Progesterone is typically regarded as anti-inflammatory. Progesterone receptors (PRs) have been identified in epithelial cells as well as in mast cells, eosinophils, macrophages, DCs, and lymphocytes (Kovats et al., 2010). There are sex differences in PR expression. For example, the expression of PRs is higher in DCs from females, which may explain why P4 is better able to suppress the activity (e.g., secretion of TNF-α) of DCs from female than male rats (Butts et al., 2008). Progesterone can bind to glucocorticoid receptors (GRs), which are more abundant in the immune system than are PRs, and may represent an alternative mechanism for progesterone-induced changes in immune function (Jones et al., 2010). Progesterone inhibits TLR-induced cytokine production as well as surface receptor expression via PRs and GRs in DCs (Jones et al., 2010).
Progesterone suppresses innate immune responses, including macrophage and NK cell activity as well as NF-κB signal transduction (Baley and Schacter, 1985; Furukawa et al., 1984; McKay and Cidlowski, 1999; Miller and Hunt, 1996; Savita and Rai, 1998; Toder et al., 1984). Progesterone can inhibit nitrite and nitric oxide production as well as Tnfα mRNA expression by murine macrophages (Miller et al., 1996; Miller and Hunt, 1998; Savita and Rai, 1998). Elevated concentrations of progesterone during pregnancy inhibit the development of Th1 immune responses and promote production of Th2 immune responses, including IL-4 and IL-5 production (Piccinni et al., 1995; Piccinni et al., 2000). In humans, elevated concentrations of progesterone during the second trimester of pregnancy is correlated with reduced activity of regulatory T cells (Mjosberg et al., 2009). In contrast, in mice, the activity of regulatory T cells is increased at the maternal-fetal interface and in lymphoid tissues in pregnant females and in non-pregnant females exposed to P4 (Kallikourdis and Betz, 2007; Mao et al., 2010). Progesterone also suppresses antibody production (Lu et al., 2002).
Pregnancy and pregnancy-associated hormones affect disease pathogenesis
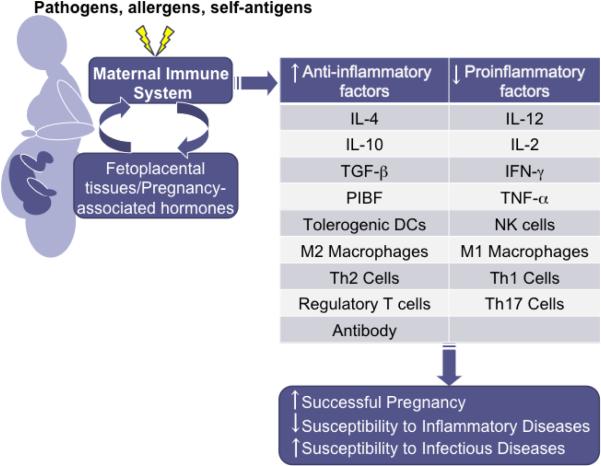
The hormonal and immunological changes that occur during pregnancy affect susceptibly to and the outcome of autoimmune and infectious diseases (Figure 2). Generally, the severity of diseases that are exacerbated by inflammatory immune responses, like MS, rheumatoid arthritis, and psoriasis, is reduced during pregnancy (Confavreux et al., 1998; Ostensen and Villiger, 2002; Raychaudhuri et al., 2003). In contrast, the severity of many infectious diseases, which require inflammatory responses for the initial control and clearance of pathogens, is increased during pregnancy (Jamieson et al., 2009; Krishnan et al., 1996a; Luft and Remington, 1982; Menendez, 1995). Hormones contribute significantly to the outcome of immune-related diseases during pregnancy by altering the functioning of immune cells. Hormones can have additional effects on the outcome of infection during pregnancy. For example, hormonal changes, including increased concentrations of P4, are hypothesized to alter not only local immune responses, but also genital tract mucosa, to increase the risk of HIV infection during pregnancy (Gray et al., 2005). For this review, the diseases selected are intended to provide examples of how pregnancy, pregnancy-associated hormones, or both affect the pathogenesis of disease, primarily by altering immune function.
Figure 2.
Hormonal changes and exposure to fetal antigens during pregnancy skew the maternal immune system toward higher anti-inflammatory responses and away from proinflammatory responses, especially during the third trimester. These immunological changes are necessary for successful pregnancy, but also affect the outcome of disease.
Multiple sclerosis
Multiple sclerosis is caused by inflammatory immune responses, including Th1 and Th17 responses, that target the myelin sheath of axons within the CNS to promote axon demyelination, axonal damage, and neurological dysfunction. Clinically, MS is characterized by two courses of disease including relapsing-remitting MS (RRMS), the more prevalent form of the disease defined by an acute phase of CNS inflammation and neurological dysfunction, and the chronic secondary progressive (SPMS) phase associated with more severe neurological damage (Tsui and Lee, 2011; Voskuhl, 2011). Although MS occurs more frequently in females than males, the frequency of MS relapses is reduced during pregnancy (Confavreux et al., 1998; Whitacre et al., 1999; Whitaker, 1998). A study of female MS patients monitored before, during, and post-pregnancy revealed that compared with pre-pregnancy relapse rates, pregnant MS patients suffer fewer relapses, an effect most pronounced during the third trimester, during which pregnancy-associated hormones reach their highest level (Confavreux et al., 1998). During the post-partum period, when pregnancy-associated hormones rapidly decline, relapse rates return to comparable levels observed prior to pregnancy (Confavreux et al., 1998).
There are several mechanisms by which pregnancy-associated hormones are hypothesized to alter MS disease pathogenesis, including modulation of the immune response. Elevated concentrations of E3 can reduce MS disease pathogenesis by significantly reducing CNS lesion size, circulating inflammatory cytokines (e.g., IFN-γ), and delayed type hypersensitivity responses (Sicotte et al., 2002). PBMCs isolated from female MS patients treated with pregnancy-level E3 show decreased numbers of CD4+ and CD8+ T cells and increased B cells in both RRMS and SPMS patients (Soldan et al., 2003). PBMC stimulation ex vivo with mitogens and autoantigens also display increased production of IL-5 from CD4+ and CD8+ T cells, IL-10 from CD64+ monocytes and macrophages, and decreased TNF-α production from CD8+ T cells (Soldan et al., 2003). One mechanism by which pregnancy-associated hormones modulate immune function is through inflammatory gene regulation (Soldan et al., 2003). Genome-wide microarray analysis of PBMC mRNA isolated from healthy age-matched females and MS patients before, during, and after pregnancy reveals a distinct pattern of several differentially expressed inflammation-related genes, including TNF-α induced protein 3 (Tnfaip3), suppressor of cytokine signaling 2 (Socs2), nuclear receptor subfamily-4 member 2 (Nr4a2), and CXC chemokine receptor-4 (Cxcr4) in MS patients prior to pregnancy; by the third trimester of pregnancy, however, these genes are expressed at levels that are similar to healthy females an effect that is lost post-partum (Gilli et al., 2010). Estrogens regulate several of the immune-related genes characterized in this study and may partially account for the distinct expression patterns observed during pregnancy and the rapid deregulation that occurs post-partum (Gilli et al., 2010).
In the EAE mouse model of MS, disease induced by autoantigen immunization during mid to late gestation is less severe than disease induced in virgin aged-matched females or following parturition (Langer-Gould et al., 2002; McClain et al., 2007). Despite reduced disease severity and decreased production of proinflammatory cytokines (e.g., TNF-α and IL-17) in pregnant EAE mice, cellular infiltrates in the CNS are not reduced in pregnant compared with non-pregnant females (Langer-Gould et al., 2002; McClain et al., 2007). Estriol or ERα agonist treatment prior to EAE induction in non-pregnant female mice reduces disease severity and TNF-α, IFN-γ, IL-2, and IL-6 production by splenocytes stimulated ex vivo with recall autoantigen (Palaszynski et al., 2004a; Tiwari-Woodruff et al., 2007; Tiwari-Woodruff and Voskuhl, 2009). Estriol treatment during the effector phase of EAE also mitigates the disease severity and increases circulating autoantigen-specific IgG1 and production of IL-10 from antigen-specific CD4+ T cells (Kim et al., 1999). Progesterone-treated EAE mice also have reduced disease severity and inflammatory cytokine concentrations (e.g., IL-2 and IL-17) and increased numbers of B cells and anti-inflammatory cytokine concentrations (e.g., IL-10) (Yates et al., 2010). Progesterone treatment also reduced the expression or several chemokines and related receptors, including chemokine (C-C motif) ligand 3 (CCL3), chemokine (C-C motif) receptor 2 (CCR2), and CCR7 in the CNS during EAE induction, which may influence cellular infiltration into the CNS (Yates et al., 2010). Additionally, P4 promotes axon remyelination and repair through oligodendrocyte progenitor cell activation, maturation, and recruitment to foci of damaged myelin sheaths (Garay et al., 2009; Hussain et al., 2011).
Influenza
Pregnancy is a risk factor for severe disease outcome during both seasonal epidemics and pandemics of influenza viruses, with pregnant women experiencing greater complications than either non-pregnant women of the same age or the general population (Harris, 1919; Jamieson et al., 2009; Klein et al., 2010b; Klein et al., 2010c; Van Kerkhove et al., 2011). During the 1918 H1N1 pandemic, for example, a study of 1350 cases of influenza virus infection in pregnant women revealed that half of the pregnant women developed severe complications, including pneumonia, and almost a third of the women died as a result (Harris, 1919). Hospitalization with severe disease and mortality rates for pregnant women are consistently higher when compared with the general population during the 1918 H1N1 pandemic (Harris, 1919; Rothberg and Haessler, 2010), the 1957 H2N2 pandemic (Lapinsky, 2010; Mosby et al., 2011), and the 2009 H1N1 pandemic (Ellington et al., 2011; Jamieson et al., 2009; Klein et al., 2010b; Lapinsky, 2010; Mosby et al., 2011). In addition to increased hospitalization and death rates during pregnancy, severe influenza can affect the outcome of pregnancy, including preterm delivery, low birth weight, and fetal loss (Creanga et al., 2011a; Creanga et al., 2011b; Harris, 1919; Schwandt et al., 2011).
The risk of severe influenza is greatest during the second and third trimester of pregnancy (Harris, 1919; Neuzil et al., 1998; Van Kerkhove et al., 2011). Women in their third trimester of pregnancy account for over half of the deaths from secondary pneumonia during the 1918 H1N1 pandemic (Harris, 1919). Seasonal influenza cases monitored between 1974 and 1993 revealed that pregnant women are 3–4 times more likely to die from influenza-related illness during the third trimester than are non-pregnant women (Neuzil et al., 1998). Pregnancy-associated changes in cardiovascular and pulmonary function are hypothesized to underlie severe influenza (Louie et al., 2009; Mosby et al., 2011). Although physical changes may contribute to influenza severity in some cases, there is no cluster of physiologic changes that is consistently correlated with increased risk of severe outcome from influenza in pregnant women (Dodds et al., 2007). Even in the absence of co-morbid medical conditions, including asthma, diabetes, and cardiovascular disease, pregnant women still have an increased risk of influenza-related hospitalization and death (Neuzil et al., 1998).
Dysregulated cytokine and chemokine production, excessive immune cell influx, and damage to the lungs are hypothesized underlie influenza pathology (Hennet et al., 1992; Kash et al., 2004; Kash et al., 2006; Kobasa et al., 2007; Perrone et al., 2008; Tumpey et al., 2005). There is a paucity of data on immune responses to influenza virus infection during pregnancy. During the 2009 H1N1 pandemic, compared to post-partum and non-pregnant women, pregnant women had lower circulating levels of IgG2 antibody, which at high levels is hypothesized to protect against secondary bacterial infections following influenza infection (Chan et al., 2011). Reduced IgG2 is associated with severe influenza outcome and dysregulated cytokine production (Chan et al., 2011; Gordon et al., 2010). Small animal models have been instrumental for understanding the pathogenesis of 2009 H1N1 in pregnant females. Pregnant mice exposed to pandemic 2009 H1N1 have greater mortality than non-pregnant age-matched females (Chan et al., 2011; Marcelin et al., 2011). There are some reports that pregnant females have greater virus replication in their lungs than non-pregnant females, but other reports suggest that virus replication is not affected by pregnancy (Chan et al., 2011; Marcelin et al., 2011). The increased mortality in pregnant female mice correlates with greater induction of proinflammatory cytokines and chemokines, including TNF-α, CCL2, CCL3, and CXCL1, in the lungs following infection (Chan et al., 2011; Marcelin et al., 2011). Pregnant female mice also have greater numbers of pulmonary macrophages and regulatory T cells, but similar numbers of CD8+ T cells and levels of neutralizing antibodies, compared with non-pregnant females (Marcelin et al., 2011). The underlying hormonal mechanisms that might contribute to differential immune responses to and outcome of influenza virus infection during pregnancy remain to be elucidated. Elevation of E2 in non-pregnant female mice reduces, rather than increases, the severity of influenza A virus infection (Robinson et al., 2011), suggesting that this is not the hormonal mechanism mediating increased severity of influenza during pregnancy. Whether other estrogens, P4, or even glucocorticoids alter immune responses to influenza virus infection to result in more severe disease in pregnant females requires consideration.
Toxoplasmosis
Infection with the parasite Toxoplasma gondii typically results in mild or asymptomatic disease in adults, but can become severe in pregnant females (Pfaff et al., 2007; Roberts et al., 2001). Congenital transmission is documented in humans and rodent models (Pfaff et al., 2007). There are, however, reports that chronic T. gondii infection, in either humans or mice, does not result in transmission of parasites to offspring (Roberts et al., 2001), even if re-infected during pregnancy (Roberts and Alexander, 1992), suggesting that maternal immunity, probably transmission of antibodies, is sufficient to protect offspring. If pregnant females are infected early during pregnancy, prior to the anti-inflammatory skewing of the immune response, then transmission of parasites to offspring is low. Infection during early pregnancy, however, can result in excessive IFN-γ production, apoptosis of placental cells, and fetal resorption (Senegas et al., 2009). If pregnant females are infected during later stages of pregnancy, when inflammatory responses are low, then congenital transmission is likely to occur (Pfaff et al., 2007; Roberts et al., 2001).
Pregnant female mice are more susceptible to infection with T. gondii and experience worse disease outcome than non-pregnant females (Luft and Remington, 1982; Shirahata et al., 1992). The activity of NK and T cells as well as production of IL-12, IFN-γ, and TNF-α during the early stages of infection are necessary for induction of adaptive immune responses and clearance of parasites (Roberts et al., 2001). Pregnant females produce significantly less IFN-γ than non-pregnant females during T. gondii infection (Luft and Remington, 1982; Shirahata et al., 1992). Administration of recombinant IFN-γ to pregnant female mice improves the outcome of T. gondii infection and can reduce congenital transmission of parasites (Abou-Bacar et al., 2004a; Abou-Bacar et al., 2004b; Shirahata et al., 1992) but can also directly harm the developing fetus (Pfaff et al., 2007). There is growing evidence that hormones underlie increased susceptibility of pregnant females to toxoplasmosis. In female mice, E2 exacerbates, whereas gonadectomy reduces, parasite burden and disease pathogenesis (Kittas and Henry, 1979, 1980). High concentrations of P4 also increase susceptibility to T. gondii during pregnancy by suppressing production of IL-12 and IFN-γ (Jones et al., 2008).
Malaria
In malaria endemic areas, susceptibility to malarial infections is increased during pregnancy and is higher among primiparous (i.e., females that are in their first pregnancy) than multiparous (i.e., females that have had multiple pregnancies) females. Females are more susceptible to infection during the first pregnancy because they are immunologically naïve to the parasite adhesion proteins (i.e., they do not possess anti-adhesion antibodies) that are expressed in the placenta during pregnancy (Fried et al., 1998; Hviid et al., 2010; Rogerson et al., 2007). Increased susceptibility to infection among pregnant females is attributed to both increased parasites sequestered in the placenta and pregnancy-associated suppression of inflammatory responses caused by hormonal changes during pregnancy (Rogerson et al., 2007). In primiparous women infected with Plasmodium falciparum during pregnancy, elevated proinflammatory responses (e.g., IFN-γ and TNF-α) combined with low anti-inflammatory responses (e.g., IL-10) in the placenta are related to low birth weights (Fried et al., 1998). Following stimulation with parasite antigen, NK cells from infected primiparous women produce more IFN-γ that those from infected multiparous women suggesting that these innate immune cells play a role in the immunological profile of infected females during pregnancy (Bouyou-Akotet and Mavoungou, 2009).
Maternal infection is often associated with negative fetal outcomes. For example, offspring of women infected with P. falciparum, nonhuman primates infected with P. coatneyi, and rodents inoculated with P. berghei during pregnancy have lower birth weights and slower growth rates than offspring from uninfected females (Akingbade, 1992; Davison et al., 1998; Menendez, 1995). Although Plasmodium parasites sequester in the placenta of humans and rodents, infected females do not transmit parasites to offspring in utero (Rogerson et al., 2007). In rodent malaria models, negative pregnancy outcome, including an inability to maintain a viable pregnancy, is associated with elevated inflammatory responses, including IL-1β and IFN-γ production, both systemically and in the placenta (Poovassery and Moore, 2009; Poovassery et al., 2009).
Pregnancy-associated changes in cell-mediated immune responses and increased susceptibility to Plasmodium infections have been attributed to hormonal changes that occur during pregnancy (Rogerson et al., 2007). Studies of women in malaria endemic regions, as well as mouse models of P. berghei, reveal that concentrations of glucocorticoids (i.e., cortisol in human and nonhuman primates and corticosterone in rodents) are higher in pregnant females infected with malaria parasites than in uninfected pregnant females (Bayoumi et al., 2009; Van Zon et al., 1983; Van Zon et al., 1986; Vleugels et al., 1989; Vleugels et al., 1987). Elevated glucocorticoids increase, while adrenalectomy decreases, parasitemia in pregnant female mice which may be caused by glucocorticoid-induced suppression of inflammatory responses (van Zon et al., 1982). Prolactin concentrations are either reported to not change with malaria infection during pregnancy (Bouyou-Akotet et al., 2005) or to be lower in P. falciparum infected than uninfected pregnant females (Bayoumi et al., 2009). Malaria infection also reduces E2 concentrations in late pregnancy (Watkinson et al., 1985). The bi-directional interactions between hormones and malaria infection contribute both to female susceptible to infection as well as the outcome of pregnancy.
Conclusions and future directions
The immunological shifts that occur during pregnancy are necessary for reproductive success and, thus, are favored by natural selection. Hormones are the driving factors behind changes in immune function and disease susceptibility during pregnancy. This provides insight into how to therapeutically manipulate the hormonal milieu in non-pregnant individuals to reduce the pathogenesis of infectious and autoimmune diseases (Klein et al., 2010b; Voskuhl, 2011). The observation that the impact of malaria infection is less detrimental among multiparous than primiparous females should be expanded to other infectious as well as autoimmune diseases as this might provide novel insights into disease pathogenesis among pregnant females. Is the severity of disease, in general, reduced with an increased number of pregnancies? Whether the memory responses mounted are an evolved host-mediated response to infectious diseases during pregnancy requires consideration.
Congenital transmission of pathogens from an infected female to her offspring, although not extensively discussed in this review, is of significant consideration during pregnancy. Several pathogens, ranging from T. gondii, P. falciparum, and L. major to HIV and influenza can be transmitted from mother to offspring in utero (Andiman, 2002; Pfaff et al., 2007; Rogerson et al., 2007; Yawn et al., 1971). The mechanisms mediating why some pathogens (e.g., T. gondii and P. falciparum) but not others (e.g., seasonal influenza viruses) harm the fetus when vertically transmitted are diverse (Henriquez et al., 2010; Klein et al., 2010c). The factors that appear to be most significant determinants of the impact of infection on pregnancy outcome include the timing of infection (i.e., whether exposure occurs during early or late pregnancy), the magnitude of the host inflammatory responses mounted, and whether the pathogen is sequestered in the placenta and causes physical damage (Henriquez et al., 2010). Future studies should systematically evaluate the role of endocrine-immune interactions in the context of congenital transmission of pathogens.
Mechanistically, several proteins and pathways emerge as being most consistently altered during pregnancy. The activity of sex steroids signaling through intracellular steroid receptors generally suppresses transcriptional regulation of inflammatory cytokines. Hormonal-suppression of IFN-γ, which has critical anti-viral and anti-parasitic properties, contributes significantly to the worse outcome from infectious diseases and improved outcome of inflammatory autoimmune diseases during pregnancy. Studies of mice in which recombinant IFN-γ is administered to pregnant females and improves outcome of infection (Abou-Bacar et al., 2004a; Abou-Bacar et al., 2004b; Shirahata et al., 1992) should be explored further. Because global increases in IFN-γ can compromise the outcome of pregnancy, greater attention should be paid to designing prophylaxis and therapeutic treatments that boost pathogen-specific immunity or select arms of the immune system. The concurrent upregulation of regulatory T cell activity and anti-inflammatory cytokines also consistently contributes to altered disease outcome among pregnant females and should be explored further.
Host immunity is typically not therapeutically manipulated in pregnant females and this population is often not enrolled in drug or vaccine efficacy trials. Use of animal models and primary cell cultures (e.g., placental cell or umbilical vein cultures) will continue to elucidate the direct effects of hormones on immune cell function and disease pathogenesis and may provide insight into the aspects of immunity that could be safely manipulated in pregnant females. From a public health perspective, knowledge that protective immunity against pathogens is reduced during pregnancy provides grounds for evaluating interventions, like vaccinations, that could improve protection of pregnant females and their fetuses. Because the hormonal and immunological environment of a pregnant females is vastly different from that of non-pregnant females, these females must be considered separately when analyzing the efficacy of treatments for disease (Klein et al., 2010a).
Highlights
Inflammatory responses are lower and anti-inflammatory responses are higher during pregnancy
The immunological shift during pregnancy promotes healthy fetal development
the severity of diseases that are caused by inflammation is reduced during pregnancy
the severity of diseases mitigated by inflammatory responses is increased during pregnancy
sex steroids mediate the immunological shift and altered disease pathogenesis during pregnancy
Acknowledgements
NIH AI079342 and AI090344 and a Medtronic SWHR award provided support for this work.
Abbreviations
- DC
dendritic cell
- E2
estradiol
- E3
estriol
- IFN
interferon
- IL
interleukin
- NK
natural killer
- PBMCs
peripheral blood mononuclear cells
- P4
progesterone
- PIBF
progesterone-induced binding factor
- Th1
helper T cell type 1
- Th2
helper T cell type 2
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Bacar A, Pfaff AW, Georges S, Letscher-Bru V, Filisetti D, Villard O, Antoni E, Klein JP, Candolfi E. Role of NK cells and gamma interferon in transplacental passage of Toxoplasma gondii in a mouse model of primary infection. Infect Immun. 2004a;72:1397–1401. doi: 10.1128/IAI.72.3.1397-1401.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Bacar A, Pfaff AW, Letscher-Bru V, Filisetti D, Rajapakse R, Antoni E, Villard O, Klein JP, Candolfi E. Role of gamma interferon and T cells in congenital Toxoplasma transmission. Parasite Immunol. 2004b;26:315–318. doi: 10.1111/j.0141-9838.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Akingbade OA. Embryotoxic and growth-retarding effects of malaria on pregnant mice. J Reprod Med. 1992;37:273–276. [PubMed] [Google Scholar]
- Andiman WA. Transmission of HIV-1 from mother to infant. Curr Opin Pediatr. 2002;14:78–85. doi: 10.1097/00008480-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- Baley JE, Schacter BZ. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J Immunol. 1985;134:3042–3048. [PubMed] [Google Scholar]
- Bayoumi NK, Elhassan EM, Elbashir MI, Adam I. Cortisol, prolactin, cytokines and the susceptibility of pregnant Sudanese women to Plasmodium falciparum malaria. Ann Trop Med Parasitol. 2009;103:111–117. doi: 10.1179/136485909X385045. [DOI] [PubMed] [Google Scholar]
- Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104:1404–1410. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- Bouyou-Akotet MK, Adegnika AA, Agnandji ST, Ngou-Milama E, Kombila M, Kremsner PG, Mavoungou E. Cortisol and susceptibility to malaria during pregnancy. Microbes Infect. 2005;7:1217–1223. doi: 10.1016/j.micinf.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Bouyou-Akotet MK, Mavoungou E. Natural killer cell IFN-gamma-activity is associated with Plasmodium falciparum infection during pregnancy. Exp Parasitol. 2009;123:265–268. doi: 10.1016/j.exppara.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Butts CL, Bowers E, Horn JC, Shukair SA, Belyavskaya E, Tonelli L, Sternberg EM. Inhibitory effects of progesterone differ in dendritic cells from female and male rodents. Gend Med. 2008;5:434–447. doi: 10.1016/j.genm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, Kovats S. Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GMCSF or Flt3 ligand-mediated dendritic cell differentiation. J Immunol. 2008;180:727–738. doi: 10.4049/jimmunol.180.2.727. [DOI] [PubMed] [Google Scholar]
- Chan JF, To KK, Tse H, Lau CC, Li IW, Hung IF, Chan KH, Cheng VC, Lai TS, Woo PC, Chan EY, Yuen KY. The lower serum immunoglobulin G2 level in severe cases than in mild cases of pandemic H1N1 2009 influenza is associated with cytokine dysregulation. Clin Vaccine Immunol. 2011;18:305–310. doi: 10.1128/CVI.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Mosmann T, Wegmann TG. IL-10 prevents naturally occurring fetal loss in the CBA × DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol. 1995;154:4261–4268. [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Creanga AA, Kamimoto L, Newsome K, D'Mello T, Jamieson DJ, Zotti ME, Arnold KE, Baumbach J, Bennett NM, Farley MM, Gershman K, Kirschke D, Lynfield R, Meek J, Morin C, Reingold A, Ryan P, Schaffner W, Thomas A, Zansky S, Finelli L, Honein MA. Seasonal and 2009 pandemic influenza A (H1N1) virus infection during pregnancy: a population-based study of hospitalized cases. Am J Obstet Gynecol. 2011a;204:S38–45. 45. doi: 10.1016/j.ajog.2011.02.037. [DOI] [PubMed] [Google Scholar]
- Creanga AA, Shapiro-Mendoza CK, Bish CL, Zane S, Berg CJ, Callaghan WM. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011b;117:837–843. doi: 10.1097/AOG.0b013e3182113c10. [DOI] [PubMed] [Google Scholar]
- Dai R, Phillips RA, Ahmed SA. Despite inhibition of nuclear localization of NF-kappa B p65, c-Rel, and RelB, 17-beta estradiol up-regulates NF-kappa B signaling in mouse splenocytes: the potential role of Bcl-3. J Immunol. 2007;179:1776–1783. doi: 10.4049/jimmunol.179.3.1776. [DOI] [PubMed] [Google Scholar]
- Dai R, Phillips RA, Zhang Y, Khan D, Crasta O, Ahmed SA. Suppression of LPS-induced Interferon-gamma and nitric oxide in splenic lymphocytes by select estrogen-regulated microRNAs: a novel mechanism of immune modulation. Blood. 2008;112:4591–4597. doi: 10.1182/blood-2008-04-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison BB, Cogswell FB, Baskin GB, Falkenstein KP, Henson EW, Tarantal AF, Krogstad DJ. Plasmodium coatneyi in the rhesus monkey (Macaca mulatta) as a model of malaria in pregnancy. Am J Trop Med Hyg. 1998;59:189–201. doi: 10.4269/ajtmh.1998.59.189. [DOI] [PubMed] [Google Scholar]
- Ding J, Zhu BT. Unique effect of the pregnancy hormone estriol on antigen-induced production of specific antibodies in female BALB/c mice. Steroids. 2008;73:289–298. doi: 10.1016/j.steroids.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Douin-Echinard V, Laffont S, Seillet C, Delpy L, Krust A, Chambon P, Gourdy P, Arnal JF, Guery JC. Estrogen receptor alpha, but not beta, is required for optimal dendritic cell differentiation and [corrected] CD40-induced cytokine production. J Immunol. 2008;180:3661–3669. doi: 10.4049/jimmunol.180.6.3661. [DOI] [PubMed] [Google Scholar]
- Dudley DJ, Chen CL, Mitchell MD, Daynes RA, Araneo BA. Adaptive immune responses during murine pregnancy: pregnancy-induced regulation of lymphokine production by activated T lymphocytes. Am J Obstet Gynecol. 1993;168:1155–1163. doi: 10.1016/0002-9378(93)90361-l. [DOI] [PubMed] [Google Scholar]
- Ellington SR, Hartman LK, Acosta M, Martinez-Romo M, Rubinson L, Jamieson DJ, Louie J. Pandemic 2009 influenza A (H1N1) in 71 critically ill pregnant women in California. Am J Obstet Gynecol. 2011;204:S21–30. doi: 10.1016/j.ajog.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Escribese MM, Kraus T, Rhee E, Fernandez-Sesma A, Lopez CB, Moran TM. Estrogen inhibits dendritic cell maturation to RNA viruses. Blood. 2008;112:4574–4584. doi: 10.1182/blood-2008-04-148692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- Franklin RD, Kutteh WH. Characterization of immunoglobulins and cytokines in human cervical mucus: influence of exogenous and endogenous hormones. J Reprod Immunol. 1999;42:93–106. doi: 10.1016/s0165-0378(98)00086-2. [DOI] [PubMed] [Google Scholar]
- Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–2530. [PubMed] [Google Scholar]
- Furukawa K, Itoh K, Okamura K, Kumagai K, Suzuki M. Changes in NK cell activity during the estrous cycle and pregnancy in mice. J Reprod Immunol. 1984;6:353–363. doi: 10.1016/0165-0378(84)90045-7. [DOI] [PubMed] [Google Scholar]
- Garay L, Deniselle MC, Meyer M, Costa JJ, Lima A, Roig P, De nicola AF. Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res. 2009;1283:177–185. doi: 10.1016/j.brainres.2009.04.057. [DOI] [PubMed] [Google Scholar]
- Gilli F, Lindberg RL, Valentino P, Marnetto F, Malucchi S, Sala A, Capobianco M, di Sapio A, Sperli F, Kappos L, Calogero RA, Bertolotto A. Learning from nature: pregnancy changes the expression of inflammation-related genes in patients with multiple sclerosis. PLoS ONE. 2010;5:e8962. doi: 10.1371/journal.pone.0008962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SM, Sasidhar MV, Morales LB, Du S, Sicotte NL, Tiwari-Woodruff SK, Voskuhl RR. Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor alpha (ERalpha) Lab Invest. 2009;89:1076–1083. doi: 10.1038/labinvest.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, Nalugoda F, Kiddugavu M, Sewankambo N, Quinn TC, Reynolds SJ, Wawer MJ. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- Hao S, Zhao J, Zhou J, Zhao S, Hu Y, Hou Y. Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int Immunopharmacol. 2007;7:1765–1775. doi: 10.1016/j.intimp.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Harris JW. Influenza occurring in pregnant women: A Statistical Study of Thirteen Hundred and Fifty Cases. JAMA. 1919;72:978–980. [Google Scholar]
- Hennet T, Ziltener HJ, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149:932–939. [PubMed] [Google Scholar]
- Henriquez FL, Menzies FM, Roberts CW. Pregnancy and Susceptibility to Parasites. In: Klein S.L.a.R., C. W., editors. Sex Hormones and Immunity to Infection. Springer-Verlag; Berlin: 2010. pp. 227–256. [Google Scholar]
- Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273:1933–1936. [PubMed] [Google Scholar]
- Hussain R, El-Etr M, Gaci O, Rakotomamonjy J, Macklin WB, Kumar N, Sitruk-Ware R, Schumacher M, Ghoumari AM. Progesterone and Nestorone facilitate axon remyelination: a role for progesterone receptors. Endocrinology. 2011;152:3820–3831. doi: 10.1210/en.2011-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid L, Marinho CR, Staalsoe T, Penha-Goncalves C. Of mice and women: rodent models of placental malaria. Trends Parasitol. 2010;26:412–419. doi: 10.1016/j.pt.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- Jones LA, Anthony JP, Henriquez FL, Lyons RE, Nickdel MB, Carter KC, Alexander J, Roberts CW. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology. 2008;125:59–69. doi: 10.1111/j.1365-2567.2008.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA, Kreem S, Shweash M, Paul A, Alexander J, Roberts CW. Differential Modulation of TLR3- and TLR4-Mediated Dendritic Cell Maturation and Function by Progesterone. J Immunol. 2010;185:4525–4534. doi: 10.4049/jimmunol.0901155. [DOI] [PubMed] [Google Scholar]
- Kallikourdis M, Betz AG. Periodic accumulation of regulatory T cells in the uterus: preparation for the implantation of a semi-allogeneic fetus? PLoS ONE. 2007;2:e382. doi: 10.1371/journal.pone.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuzoglu E, Phillips RA, Gogal RM, Jr., Ansar Ahmed S. IFN-gamma-inducing transcription factor, T-bet is upregulated by estrogen in murine splenocytes: role of IL-27 but not IL-12. Mol Immunol. 2007;44:1808–1814. doi: 10.1016/j.molimm.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash JC, Basler CF, Garcia-Sastre A, Carter V, Billharz R, Swayne DE, Przygodzki RM, Taubenberger JK, Katze MG, Tumpey TM. Global host immune response: pathogenesis and transcriptional profiling of type A influenza viruses expressing the hemagglutinin and neuraminidase genes from the 1918 pandemic virus. J Virol. 2004;78:9499–9511. doi: 10.1128/JVI.78.17.9499-9511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, Swayne DE, Katze MG. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443:578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52:1230–1238. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- Kittas C, Henry L. Effect of gonadectomy and oestrogen administration on the response of lymph-node post-capillary venules to infection with Toxoplasma gondii. J Pathol. 1979;127:129–136. doi: 10.1002/path.1711270305. [DOI] [PubMed] [Google Scholar]
- Kittas C, Henry L. Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. Br J Exp Pathol. 1980;61:590–600. [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010a;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Passaretti C, Anker M, Olukoya P, Pekosz A. The impact of sex, gender and pregnancy on 2009 H1N1 disease. Biol Sex Differ. 2010b;1:5. doi: 10.1186/2042-6410-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Pekosz A, Passaretti C, Anker M, Olukoya P. Sex, gender and influenza. World Health Organization; Geneva: 2010c. pp. 1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Kovats S, Carreras E, Agrawal H. Sex steroid receptors in immune cells. In: Klein SL, Roberts CW, editors. Sex hormones and immunity to infection. Springer-Verlag; Berlin: 2010. pp. 53–92. [Google Scholar]
- Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996a;156:644–652. [PubMed] [Google Scholar]
- Krishnan L, Guilbert LJ, Wegmann TG, Belosevic M, Mosmann TR. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-gamma and TNF and reduced IL-10 production by placental cells. J Immunol. 1996b;156:653–662. [PubMed] [Google Scholar]
- Langer-Gould A, Garren H, Slansky A, Ruiz PJ, Steinman L. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: evidence for a suppressive pregnancy-related serum factor. J Immunol. 2002;169:1084–1091. doi: 10.4049/jimmunol.169.2.1084. [DOI] [PubMed] [Google Scholar]
- Lapinsky SE. Critical illness as a result of influenza A/H1N1 infection in pregnancy. BMJ. 2010;340:c1235. doi: 10.1136/bmj.c1235. [DOI] [PubMed] [Google Scholar]
- Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- Liu HB, Loo KK, Palaszynski K, Ashouri J, Lubahn DB, Voskuhl RR. Estrogen receptor alpha mediates estrogen's immune protection in autoimmune disease. J Immunol. 2003;171:6936–6940. doi: 10.4049/jimmunol.171.12.6936. [DOI] [PubMed] [Google Scholar]
- Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. N Engl J Med. 2009;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- Lu FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, McChesney M, Miller CJ. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 2002;128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft BJ, Remington JS. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect Immun. 1982;38:1164–1171. doi: 10.1128/iai.38.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Wang J, Kang Y, Tai P, Wen J, Zou Q, Li G, Ouyang H, Xia G, Wang B. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology. 2010;151:5477–5488. doi: 10.1210/en.2010-0426. [DOI] [PubMed] [Google Scholar]
- Marcelin G, Aldridge JR, Duan S, Ghoneim HE, Rehg J, Marjuki H, Boon AC, McCullers JA, Webby RJ. Fatal Outcome of Pandemic H1N1 2009 Influenza Virus Infection Is Associated with Immunopathology and Impaired Lung Repair, Not Enhanced Viral Burden, in Pregnant Mice. J Virol. 2011;85:11208–11219. doi: 10.1128/JVI.00654-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain MA, Gatson NN, Powell ND, Papenfuss TL, Gienapp IE, Song F, Shawler TM, Kithcart A, Whitacre CC. Pregnancy suppresses experimental autoimmune encephalomyelitis through immunoregulatory cytokine production. J Immunol. 2007;179:8146–8152. doi: 10.4049/jimmunol.179.12.8146. [DOI] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995;11:178–183. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- Miller L, Alley EW, Murphy WJ, Russell SW, Hunt JS. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukoc Biol. 1996;59:442–450. doi: 10.1002/jlb.59.3.442. [DOI] [PubMed] [Google Scholar]
- Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life Sci. 1996;59:1–14. doi: 10.1016/0024-3205(96)00122-1. [DOI] [PubMed] [Google Scholar]
- Miller L, Hunt JS. Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J Immunol. 1998;160:5098–5104. [PubMed] [Google Scholar]
- Mjosberg J, Svensson J, Johansson E, Hellstrom L, Casas R, Jenmalm MC, Boij R, Matthiesen L, Jonsson JI, Berg G, Ernerudh J. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17{beta}-estradiol. J Immunol. 2009;183:759–769. doi: 10.4049/jimmunol.0803654. [DOI] [PubMed] [Google Scholar]
- Mosby LG, Ellington SR, Forhan SE, Yeung LF, Perez M, Shah MM, MacFarlane K, Laird SK, House LD, Jamieson DJ. The Centers for Disease Control and Prevention's maternal health response to 2009 H1N1 influenza. Am J Obstet Gynecol. 2011;204:S7–12. doi: 10.1016/j.ajog.2011.02.057. [DOI] [PubMed] [Google Scholar]
- Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Tachibana H, Yamada K. Effect of estrogens on the interferon-gamma producing cell population of mouse splenocytes. Biosci Biotechnol Biochem. 2006;70:47–53. doi: 10.1271/bbb.70.47. [DOI] [PubMed] [Google Scholar]
- Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–1102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- Ostensen M. Sex hormones and pregnancy in rheumatoid arthritis and systemic lupus erythematosus. Ann N Y Acad Sci. 1999;876:131–143. doi: 10.1111/j.1749-6632.1999.tb07630.x. [DOI] [PubMed] [Google Scholar]
- Ostensen M, Villiger PM. Immunology of pregnancy-pregnancy as a remission inducing agent in rheumatoid arthritis. Transpl Immunol. 2002;9:155–160. doi: 10.1016/s0966-3274(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172:1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- Palaszynski KM, Liu H, Loo KK, Voskuhl RR. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004a;149:84–89. doi: 10.1016/j.jneuroim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004b;146:144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Papenfuss TL, Powell ND, McClain MA, Bedarf A, Singh A, Gienapp IE, Shawler T, Whitacre CC. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J Immunol. 2011;186:3346–3355. doi: 10.4049/jimmunol.1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff AW, Abou-Bacar A, Letscher-Bru V, Villard O, Senegas A, Mousli M, Candolfi E. Cellular and molecular physiopathology of congenital toxoplasmosis: the dual role of IFN-gamma. Parasitology. 2007;134:1895–1902. doi: 10.1017/S0031182007000200. [DOI] [PubMed] [Google Scholar]
- Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- Piccinni MP, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1-and Th2-type cytokines in successful pregnancy. J Neuroimmunol. 2000;109:30–33. doi: 10.1016/s0165-5728(00)00299-x. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- Polgar B, Nagy E, Miko E, Varga P, Szekeres-Bartho J. Urinary progesterone-induced blocking factor concentration is related to pregnancy outcome. Biol Reprod. 2004;71:1699–1705. doi: 10.1095/biolreprod.104.030437. [DOI] [PubMed] [Google Scholar]
- Poovassery J, Moore JM. Association of malaria-induced murine pregnancy failure with robust peripheral and placental cytokine responses. Infect Immun. 2009;77:4998–5006. doi: 10.1128/IAI.00617-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovassery JS, Sarr D, Smith G, Nagy T, Moore JM. Malaria-induced murine pregnancy failure: distinct roles for IFN-gamma and TNF. J Immunol. 2009;183:5342–5349. doi: 10.4049/jimmunol.0901669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Navare T, Gross J, Raychaudhuri SK. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518–520. doi: 10.1046/j.1365-4362.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- Rettew JA, Huet YM, Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150:3877–3884. doi: 10.1210/en.2009-0098. [DOI] [PubMed] [Google Scholar]
- Roberts CW, Alexander J. Studies on a murine model of congenital toxoplasmosis: vertical disease transmission only occurs in BALB/c mice infected for the first time during pregnancy. Parasitology. 1992;104(Pt 1):19–23. doi: 10.1017/s0031182000060753. [DOI] [PubMed] [Google Scholar]
- Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev. 2001;14:476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza a virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7:e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med. 2010;38:e91–97. doi: 10.1097/CCM.0b013e3181c92eeb. [DOI] [PubMed] [Google Scholar]
- Sacks GP, Clover LM, Bainbridge DR, Redman CW, Sargent IL. Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta. 2001;22:550–559. doi: 10.1053/plac.2001.0686. [DOI] [PubMed] [Google Scholar]
- Savita, Rai U. Sex steroid hormones modulate the activation of murine peritoneal macrophages: receptor mediated modulation. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119:199–204. doi: 10.1016/s0742-8413(97)00207-7. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M, Alexander T, Taran A, Malfertheiner SF, Costa SD, Zimmermann G, Nitschke C, Volk HD, Alexander H, Gunzer M, Zenclussen AC. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182:5488–5497. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- Schwandt HM, Creanga AA, Danso KA, Adanu RM, Agbenyega T, Hindin MJ. A comparison of women with induced abortion, spontaneous abortion and ectopic pregnancy in Ghana. Contraception. 2011;84:87–93. doi: 10.1016/j.contraception.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Senegas A, Villard O, Neuville A, Marcellin L, Pfaff AW, Steinmetz T, Mousli M, Klein JP, Candolfi E. Toxoplasma gondii-induced foetal resorption in mice involves interferon-gamma-induced apoptosis and spiral artery dilation at the maternofoetal interface. Int J Parasitol. 2009;39:481–487. doi: 10.1016/j.ijpara.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Shirahata T, Muroya N, Ohta C, Goto H, Nakane A. Correlation between increased susceptibility to primary Toxoplasma gondii infection and depressed production of gamma interferon in pregnant mice. Microbiol Immunol. 1992;36:81–91. doi: 10.1111/j.1348-0421.1992.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Sicotte NL, Liva SM, Klutch R, Pfeiffer P, Bouvier S, Odesa S, Wu TC, Voskuhl RR. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002;52:421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- Siracusa MC, Overstreet MG, Housseau F, Scott AL, Klein SL. 17{beta}-Estradiol Alters the Activity of Conventional and IFN-Producing Killer Dendritic Cells. J Immunol. 2008;180:1423–1431. doi: 10.4049/jimmunol.180.3.1423. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol. 2003;171:6267–6274. doi: 10.4049/jimmunol.171.11.6267. [DOI] [PubMed] [Google Scholar]
- Sorachi K, Kumagai S, Sugita M, Yodoi J, Imura H. Enhancing effect of 17 beta-estradiol on human NK cell activity. Immunol Lett. 1993;36:31–35. doi: 10.1016/0165-2478(93)90065-a. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Mitogen activated protein kinase (MAPK) mediates non-genomic pathway of estrogen on T cell cytokine production following trauma-hemorrhage. Cytokine. 2008;42:32–38. doi: 10.1016/j.cyto.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Faust Z, Varga P, Szereday L, Kelemen K. The immunological pregnancy protective effect of progesterone is manifested via controlling cytokine production. Am J Reprod Immunol. 1996;35:348–351. doi: 10.1111/j.1600-0897.1996.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Polgar B. PIBF: the double edged sword. Pregnancy and tumor. Am J Reprod Immunol. 2010;64:77–86. doi: 10.1111/j.1600-0897.2010.00833.x. [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Voskuhl RR. Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice. J Neurol Sci. 2009;286:81–85. doi: 10.1016/j.jns.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toder V, Nebel L, Elrad H, Blank M, Durdana A, Gleicher N. Studies of natural killer cells in pregnancy. II. The immunoregulatory effect of pregnancy substances. J Clin Lab Immunol. 1984;14:129–133. [PubMed] [Google Scholar]
- Tsui A, Lee MA. Multiple sclerosis and pregnancy. Curr Opin Obstet Gynecol. 2011;23:435–439. doi: 10.1097/GCO.0b013e32834cef8f. [DOI] [PubMed] [Google Scholar]
- Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–1100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhove MD, Vandemaele KAH, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, Carlino LO, Owen R, Paterson B, Pelletier L, Vachon J, Gonzalez C, Hongjie Y, Zijian F, Chuang SK, Au A, Buda S, Krause G, Haas W, Bonmarin I, Taniguichi K, Nakajima K, Shobayashi T, Takayama Y, Sunagawa T, Heraud JM, Orelle A, Palacios E, van der Sande MAB, Wielders CCHL, Hunt D, Cutter J, Lee VJ, Thomas J, Santa-Olalla P, Sierra-Moros MJ, Hanshaoworakul W, Ungchusak K, Pebody R, Jain S, Mounts AW, on behalf of the, W.H.O.W.G.f.R.F.f.S.H.N.p.I. Risk Factors for Severe Outcomes following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis. PLoS Med. 2011;8:e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zon AA, Eling WM, Hermsen CC, Koekkoek AA. Corticosterone regulation of the effector function of malarial immunity during pregnancy. Infect Immun. 1982;36:484–491. doi: 10.1128/iai.36.2.484-491.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zon AA, Eling WM, Hermsen CC, Van de Wiel TJ, Duives ME. Malarial immunity in pregnant mice, in relation to total and unbound plasma corticosterone. Bull Soc Pathol Exot Filiales. 1983;76:493–502. [PubMed] [Google Scholar]
- Van Zon AA, Termaat RM, Schetters TP, Eling WM. Plasmodium berghei: reduction of the mouse's specific lymphoproliferative response in relation to corticosterone and pregnancy. Exp Parasitol. 1986;62:71–78. doi: 10.1016/0014-4894(86)90009-3. [DOI] [PubMed] [Google Scholar]
- Veenstra van Nieuwenhoven AL, Bouman A, Moes H, Heineman MJ, de Leij LF, Santema J, Faas MM. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil Steril. 2002;77:1032–1037. doi: 10.1016/s0015-0282(02)02976-x. [DOI] [PubMed] [Google Scholar]
- Vleugels MP, Brabin B, Eling WM, de Graaf R. Cortisol and Plasmodium falciparum infection in pregnant women in Kenya. Trans R Soc Trop Med Hyg. 1989;83:173–177. doi: 10.1016/0035-9203(89)90632-9. [DOI] [PubMed] [Google Scholar]
- Vleugels MP, Eling WM, Rolland R, de Graaf R. Cortisol and loss of malaria immunity in human pregnancy. Br J Obstet Gynaecol. 1987;94:758–764. doi: 10.1111/j.1471-0528.1987.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Voskuhl R. Sex differences in autoimmune diseases. Biol Sex Differ. 2011;2:1. doi: 10.1186/2042-6410-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Li Y, Kaler LJ, Vandenbark AA, Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009;126:329–335. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson M, Rushton DI, Lunn PG. Placental malaria and foetoplacental function: low plasma oestradiols associated with malarial pigmentation of the placenta. Trans R Soc Trop Med Hyg. 1985;79:448–450. doi: 10.1016/0035-9203(85)90059-8. [DOI] [PubMed] [Google Scholar]
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Whitaker JN. Effects of pregnancy and delivery on disease activity in multiple sclerosis. N Engl J Med. 1998;339:339–340. doi: 10.1056/NEJM199807303390509. [DOI] [PubMed] [Google Scholar]
- Yates MA, Li Y, Chlebeck P, Proctor T, Vandenbark AA, Offner H. Progesterone treatment reduces disease severity and increases IL-10 in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;220:136–139. doi: 10.1016/j.jneuroim.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn DH, Pyeatte JC, Joseph JM, Eichler SL, Garcia-Bunuel R. Transplacental transfer of influenza virus. Jama. 1971;216:1022–1023. [PubMed] [Google Scholar]
- Zang YC, Halder JB, Hong J, Rivera VM, Zhang JZ. Regulatory effects of estriol on T cell migration and cytokine profile: inhibition of transcription factor NF-kappa B. J Neuroimmunol. 2002;124:106–114. doi: 10.1016/s0165-5728(02)00016-4. [DOI] [PubMed] [Google Scholar]