Abstract
Capillary morphogenesis protein-2 (CMG2) functions as an anthrax toxin receptor that plays an essential role in anthrax pathogenesis. Although mutations in CMG2 have been identified to cause two human autosomal recessive disorders, Juvenile Hyaline Fibromatosis and Infantile Systemic Hyalinosis, both characterized by excess hyaline material deposition in connective tissues, the physiologic function of CMG2 remains elusive. To study the roles of CMG2 in normal physiology, here we performed detailed histological analyses of the CMG2-null mice we generated previously. While no morphological or histological defects were observed in CMG2−/− male mice, CMG2−/− female mice were unable to produce any offspring due to a defect in parturition. We found that deletion of CMG2 resulted in a diffuse deposition of collagen within the myometrium of CMG2−/− females, causing remarkable morphological changes to their uteri. This collagen accumulation also led to loss of smooth muscle cells in the myometrium of CMG2−/− mice, apparently disabling uterine contractile function during parturition. As a consequence, even though pregnant CMG2−/− mice were able to carry the gestation to full term, they were unable to deliver pups. However, the fully-developed fetuses could be successfully delivered by Cesarean section and survived to adulthood when fostered. Our results demonstrate that CMG2 is not required for normal mouse embryonic development but is indispensable for murine parturition. In parallel to its role in anthrax toxin binding and internalization, herein we provide evidence that CMG2 may function as a collagen receptor which is essential for maintaining collagen homeostasis in the uterus.
Keywords: Anthrax, anthrax toxin receptor, collagen, capillary morphogenesis protein-2, parturition
Introduction
Capillary morphogenesis protein-2 (CMG2), also known as anthrax toxin receptor 2, is a ubiquitously-expressed type I cell surface protein. CMG2 was first described as one of the proteins up-regulated during capillary morphogenesis in embryonic development [1]. Following the discovery that a highly homologous protein, tumor endothelium marker-8 (TEM8, also termed anthrax toxin receptor 1) was an anthrax toxin receptor [3], CMG2 was also found to function as a toxin receptor [14]. Mutations in CMG2 have also been demonstrated to cause two human autosomal recessive conditions, namely, Juvenile Hyaline Fibromatosis (JHF) and the more severe form Infantile Systemic Hyalinosis (ISH), both of which are characterized by excess deposition of hyaline material in many connective tissues, including gingiva, skin, and gastrointestinal tract [4;5;7]. Despite the relevance of CMG2 to human disease, however, the physiologic function of CMG2 remains unclear.
To study the roles of CMG2 in anthrax pathogenesis and normal physiology, we previously generated CMG2-null (CMG2−/−) mice by deleting the transmembrane domain of CMG2. This modification makes the receptor unable to anchor to the cell surface and in the case of anthrax toxin, prevents the CMG2 receptor from mediating anthrax toxin internalization [9]. When the CMG2−/− mice were challenged with anthrax toxin, it was determined that CMG2 was the major anthrax toxin receptor in vivo whereas TEM8 played only a minor role [9;10]. Surprisingly, the CMG2−/− mice did not exhibit any of the characteristic lesions observed in human JHF and ISH patients [5;7;9]. The only observed phenotype in the CMG2-null mice was the inability of CMG2−/− females to produce offspring [9]. In this study, we found that CMG2 is not required for normal mouse embryonic development but is indispensable for uterine collagen homeostasis and parturition.
Materials and Methods
CMG2-null mice
Generation of CMG2 gene-targeted mice with the CMG2 transmembrane domain-coding exon 12 flanked by loxP sites (a “floxed” allele), namely, the CMG2flox/flox mice, and their further use to make CMG2-null (CMG2−/−) mice and myeloid cell lineage specific CMG2−/− mice have been described previously [9;10]. To generate fibroblast-specific CMG2−/− mice, the CMG2flox/flox mice were first mated with S100a4-Cre transgenic mice [2] (Jackson Laboratories, Bar Harbor, ME). Fibroblast-specific CMG2−/− mice (CMG2flox/flox/S100a4-Cre) were obtained by the subsequent intercrossing of the resulting CMG2+/flox/ S100a4-Cre mice. Genotyping was performed by PCR using mouse ear DNA. All animal studies were carried out in accordance with protocols approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
Body weight tracking and Cesarean section delivery of pups
CMG2−/− females and CMG2+/+ males that were 8–10 weeks old were set up as breeding pairs. When a vaginal plug was found, the female mouse was separated from the male and the day was counted as day 0.5 post coitum. Body weight was monitored daily and rapid body weight increase was used to verify pregnancy. When gestation reached full term, the CMG2−/− females were euthanized by CO2 inhalation followed in quick succession by Cesarean section to deliver the pups. The pups were raised to weaning age by foster mice.
Histopathological analyses
Uteri from nulliparous 6–8 month-old CMG2−/− mice and littermate controls were collected, fixed in Z-Fix (Anantech Ltd., Battle Creek, MI) for 24 hours, dehydrated, embedded in paraffin and sectioned. Staining with H&E (hematoxylin and eosin), Masson’s trichrome, and Alcian Blue/Periodic acid-Schiff were performed by Histoserv, Inc. (Germantown, MD). Immunohistochemistry to identify lymphatic vasculature was performed according to standard protocols using a polyclonal goat anti-mouse LYVE-1 (R&D Systems, Minneapolis, MN). Images were captured using an Aperio T3 Scanscope and were quantified using Aperio Imagescope Software (both Aperio Technologies, Vista, CA). Myometrial thickness for each uterus was determined by averaging ten individual measurements spaced throughout both uterine horns. LYVE-1 positive vessel density was determined by counting all positive vessels within the myometrium of each uterus and dividing the count by the total myometrial length. LYVE-1 positive vessel area was also determined for all positive staining vessels within the myometrium of each uterus. Statistical significance of differences for uterine horn length, myometrial thickness, LYVE-1 positive vessel density and LYVE-1 positive vessel lumen area were determined by two-tailed Student's t-tests.
Results
CMG2 is required for mouse parturition
We previously observed that CMG2−/− female mice were unable to produce any offspring during breeding with CMG2+/+ or CMG2−/− males [9]. To further explore this “infertility” of CMG2−/− female mice, we performed staged embryo dissections at embryonic days 12 (E12) and 17 (E17) on litters collected from CMG2−/− and CMG2+/+ females. We found that the E12 and E17 embryos and placentas harvested from the CMG2−/− females were macroscopically and histologically normal (data not shown), suggesting that the inability of CMG2−/− female mice to bear pups was not due to a developmental insufficiency but was more likely due to a defect in the delivery process. To examine this, we carefully tracked the body weight changes of the CMG2−/− female mice during pregnancy. As expected, both CMG2−/− and CMG2+/+ pregnant mice gained body weight rapidly until full term embryonic development at E20–21 days (Figure 1A). While the CMG2+/+ mice delivered pups on the day of full term of gestation, indicated by a sharp drop in body weight, remarkably, all of the CMG2−/− females failed to give birth even after 2–3 weeks overdue. During this overdue period, theCMG2−/− females gradually lost body weight, indicating that fetal reabsorption was taking place (Figure 1A). When 3-week overdue CMG2−/− females were dissected, it was possible to identify reabsorbed fetal remains with distinguishable skulls and limbs (the insert in Figure 1A). To verify that the fetuses from CMG2−/− females developed normally to full term, we performed Cesarean section to surgically deliver the pups from the CMG2−/− mice at one-day overdue (Figure 1B). All of the pups from two litters (7 and 6 pups respectively) were found alive in utero and survived to weaning age under the care of foster mothers. All 13 rescued pups had the expected CMG2 heterozygous genotype. The above results demonstrate that CMG2 is not required for normal embryonic development but that it is essential for normal mouse parturition.
Figure 1.
CMG2 is not required for mouse embryonic development but is essential for parturition. (A) Body weight changes of pregnant CMG2+/+ and CMG2−/− female mice at days post coitum (DPC). Upon delivering pups at DPC 20, the CMG2+/+ female showed a dramatic decrease in body weight. The CMG2−/− females failed to deliver pups atfull term gestation, and instead exhibited slower decreases in body weight accompanied by fetal reabsorption. (B) Successful delivery of pups carried by CMG2−/− females by Cesarean sections at DPC 22. All pups were raised by foster mothers and survived to adulthood.
CMG2 deletion leads to severe myometrial collagen accumulation and smooth muscle cell depletion in the uterus
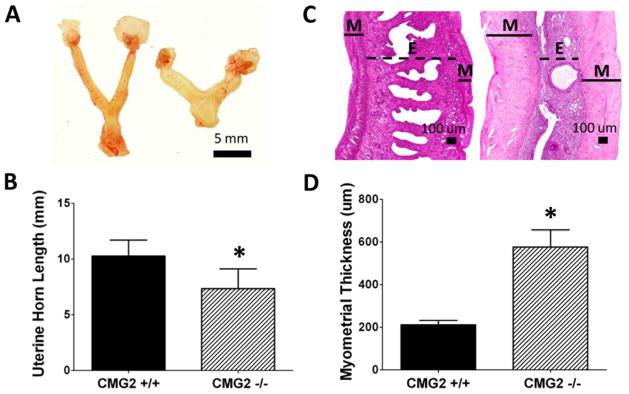
To further investigate how CMG2 deletion affects the female reproductive system, we performed a detailed histological analysis of uteri collected from nulliparous CMG2−/− female mice and their littermate CMG2+/+ mice at 6–8 months of age. Remarkably, all CMG2−/− uteri displayed morphological changes apparent on gross examination; the uteri were pale in color and were increased in stiffness while the uterine horns were significantly shortened (Figure 2, A and B). Histological analysis identified diffuse deposition of a hyaline material throughout the entire myometrial layer of the CMG2−/− uteri (Figure 2, C and D). This excess hyaline material deposition was confined to the myometrium, where it greatly increased myometrial thickness and also decreased myometrial cellularity (Figure 2, C and D and Figure 3, A and B). Masson’s Trichrome and Alcian Blue/PAS staining identified the deposited hyaline material as collagen in nature (Figure 3, D–G). Thus, nulliparous adult CMG2−/− females exhibit severe uterine fibrosis selectively affecting the myometrium, while having minimal to no effect on other regions of the reproductive tract including the cervix, vagina and ovaries (data not shown). This uterine fibrosis was observed to be progressive in nature, with excess myometrial collagen identified in CMG2−/− females as young as 7-weeks old and with the severity of fibrosis increasing with age (data not shown).
Figure 2.
Loss of CMG2 leads to alterations in uterine size. (A) Gross images of representative uteri from CMG2+/+ (Left, n=4) and CMG2−/− (Right, n=5) nulliparous littermates. (B) Uterine horn length is significantly shorter in CMG2−/− females. (Mean ± S.D.; *, p ≤ 0.01) (C) H&E stained sections of uterine horns from CMG2+/+ (Left) and CMG2−/− (Right) mice. M = myometrium, E = Endometrium. (D) Thickness of the myometrial layer is increased 2.7-fold in CMG2−/− females. (Mean ± S.D.; *, p ≤ 0.01)
Figure 3.
Lymphangiectasia and myometrial collagen accumulation are histopathological features of uteri from nulliparous, adult CMG2−/− females. M: myometrium, E: endometrium. Representative images are shown. (A) H&E stained section of a CMG2−/− uterine horn. Dilated lymphatic vessels are present throughout the myometrium, examples noted with arrowheads. There is diffuse accumulation of hyaline material and reduced myometrial cellularity in contrast with (B) H&E stained uterine horn from a CMG2+/+ littermate. (C) Higher magnification inset displaying prominent dilation of lymphatic vessels in a CMG2−/− uterus. (D, E, F) Masson’s trichrome staining of (D) wild-type and (E, F) CMG2−/− uteri highlighting the accumulation of collagen (Blue) within the myometrial layer of the CMG2−/− uterus. (G) Alcian-blue/PAS staining of a CMG2−/− uterus depicting collagen (Pink) within the myometrium.
The myometrium is the muscular layer of the uterine wall, and it is predominantly composed of uterine smooth muscle cells which play a major role in generating uterine contractions during parturition. In all CMG2−/− uteri examined, the fibrosis of the myometrium was always accompanied with the loss of uterine smooth muscle cells normally present. The increased rigidity of the uterine horn coupled with a depletion of uterine smooth muscle cells likely led to a decrease in uterine contractile function, providing an explanation as to why CMG2−/− females were able to carry fetuses to full term but were unable to deliver their pups.
In addition to myometrial fibrosis, another characteristic change in all CMG2−/− uteri was that the lymphatic vessels present within the myometrium were extremely congested/dilated (lymphangiectasia) (Figure 4), suggesting the presence of diffuse lymphedema. Atrophy of the endometrium was also observed in all CMG2−/− uteri. This observed atrophy is likely a secondary change directly related to the fibrosis of the myometrium (Pressure Atrophy). Detailed histological analyses did not identify abnormalities in other organs and tissues including, lung, heart, liver, intestines, kidney and adrenal glands, stomach, pancreas, spleen, thyroid, bladder, esophagus, skeletal muscle, thymus, gingiva, and lymph nodes in either female or male CMG2−/− mice (data not shown).
Figure 4.
Lymphatic vascularization of the myometrium is altered in the absence of CMG2. (A,B) LYVE-1 staining of myometrial lymphatic vessels in wild-type (n=4) (A) and CMG2−/− (n=5) (B) littermates. Representative images are shown. (C) Lymphatic vessel density is reduced in CMG2−/− (Mean ± S.D.; *, p ≤ 0.01). (D) Lymphatic vessels are significantly larger in size in CMG2−/− (Mean ± S.D.; *, p ≤ 0.01).
Specific deletion of CMG2 in fibroblasts or myeloid cells is dispensable for uterine fibrosis
The diffuse, progressive, deposition of collagen in virgin CMG2−/− uteri suggests that CMG2 expression on certain cell types is required for collagen homeostasis in the uterus. We hypothesized that CMG2 may function, in parallel to its role in anthrax toxin internalization, as a cellular receptor for collagen internalization and cellular degradation. Since fibroblasts have been reported to be a cell type involved in intracellular collagen degradation [6;8;12], we next examined whether specific deletion of CMG2 on fibroblasts could replicate the phenotype observed in CMG2−/− mice. We generated fibroblast-specific CMG2−/− (CMG2flox/flox/S100a4-Cre) mice by breeding CMG2flox/flox mice with S100a4-Cre transgenic mice and intercrossing the resulting CMG2+/flox/S100a4-Cre mice. The fibroblast-specific CMG2−/− female mice did not exhibit any abnormalities in parturition and the uteri dissected from these mice did not show any gross morphological changes (Data not shown). Similarly, myeloid-specific CMG2−/− female mice were also able to deliver litters normally. These results demonstrate that deletion of the CMG2 receptor on either fibroblasts or myeloid cells, such as macrophages, is not responsible for the parturition defect observed in CMG2−/− female mice.
Discussion
Interstitial collagen is the main component of connective tissue and is the most abundant protein in mammals, comprising 25% to 35% of the whole-body protein content. Like other matrix components, collagen is undergoing continuous synthesis and degradation to maintain its steady-state. Although collagen turnover rates are generally low in homeostatic tissues, the rates increase greatly in numerous physiological and pathological conditions including tissue remodeling, wound healing, and cancer invasion. In this study, we found that CMG2 is essential for maintaining collagen homeostasis in the mouse uterus. Loss of expression of CMG2 on the cell surface results in a progressive, diffuse, deposition of collagen throughout the myometrium of CMG2−/− mice which in turn causes remarkable morphological changes of the uteri including increased uterine rigidity and decreased uterine horn length. This collagen accumulation also causes loss of smooth muscle cells in the myometrium of CMG2−/− mice, presumably leading to functional changes in the contractile ability of the uterus during parturition. As a consequence, even though CMG2−/− females are able to become pregnant and to carry the gestation to full term, they are unable to deliver pups. However, the fully developed fetuses can be successfully delivered by Cesarean section and can survive to adulthood when fostered, demonstrating that CMG2 is not required for normal embryonic development. Another striking characteristic change in all CMG2−/− uteri is myometrial lymphangiectasia, suggesting the presence of diffuse lymphedema. Whether this is a primary or a secondary change remains unknown.
Tissue collagen homeostasis is maintained by multiple pathways, including intracellular collagen degradation; a process by which large, soluble collagen fragments in the extracellular milieu are internalized and degraded [17]. Previous studies have demonstrated that several cell surface proteins including the urokinase plasminogen activator receptor-associated protein (uPARAP)/Endo180, β1-integrins, and the mannose receptor play important roles in intracellular collagen degradation[6;8;15]. Because CMG2 is a cell surface protein which functions as a receptor for anthrax toxin and is required for binding and internalization of the anthrax toxin complex, it is tempting to speculate that CMG2 may have a parallel function as a physiologic receptor for collagen, where it may contribute to uterine collagen homeostasis by binding, internalizing and degrading collagen. In fact, the CMG2 homologous protein TEM8 has been reported to interact with collagen α3 (VI) [13].
Type I collagen is the most abundant collagen component of extracellular matrix in many tissues including the uterus. Interestingly, a previously described type I collagen-cleavage resistant mouse appears similar to the CMG2−/− mouse described here, with both exhibiting uterine fibrosis as a primary phenotype [11]. This type I collagen mutant mouse has three point mutations (Gln774Pro/Ile776Met/Ala777Pro) in the collagenase cleavage site of type I collagen, causing it to be resistant to cleavage by many collagenases. However, in contrast to our CMG2−/− mice, the uterine fibrosis observed in the type I collagen mutant mice only occurred post-partum, where it affected both the myometrium and the endometrium. Additionally, the type I collagen mutant mice were able to become pregnant and deliver pups multiple times and myometrial lymphangiectasia was not documented. Unlike the CMG2−/− mice, the type I collagen mutant mice also developed dermal fibrosis, affecting both male and female mice. The phenotypic differences between the CMG2−/− mice and the type I collagen mutant mice suggest that other type(s) of collagen might contribute to the uterine fibrosis observed in the CMG2−/− mice. It is also possible that the natural ligand of CMG2 might be a protein other than collagen, and that the observed accumulation of collagen in the uterine myometrium is secondary to dysregulation of this unknown ligand in CMG2−/− mice. In support of this hypothesis, a case study has been reported in the literature where electron microscopic analysis was performed on affected skin samples from an ISH patient [16]. This study found that while the patient did have characteristic abnormal collagen deposition in the skin, that this collagen accumulation appeared to be secondary to the deposition of an amorphous, “mucinoid” material with unknown nature [16]. Identification of the natural ligand(s) for CMG2, which is a subject of further research in our laboratory, would provide insightful information towards understanding underlying mechanisms of ISH and JHF diseases.
It remains unknown why the CMG2−/− mice do not exhibit skin lesions like those observed in human ISH and JHF patients. It is unlikely that the related protein TEM8 is compensating for CMG2 in tissues outside of the than uterus, as TEM8 and CMG2 double null mice have been generated and these mice, just like CMG2−/− mice, exhibit fibrosis solely in the uterus (Liu, et al unpublished data). As observed in JHF patients that the onset of disease symptoms needs years to develop, CMG2−/− mice may only be able to develop collagen accumulation in tissues with high collagen turnover rate like uterus in their 2~3 years lifetime.
Highlights.
CMG2 is not required for normal mouse embryonic development.
CMG2-null females are unable to produce any offspring due to a defect in parturition.
Deletion of CMG2 results in deposition of collagen and loss of smooth muscle cells within myometrium.
CMG2 may function as a collagen receptor for maintaining collagen homeostasis in uterus.
Acknowledgments
This research was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases and the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Abbreviations
- CMG2
Capillary Morphogenesis Protein-2
- TEM8
Tumor Endothelium Marker-8
- ISH
Infantile Systemic Hyalinosis
- JHF
Juvenile Hyaline Fibromatosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 2.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 3.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 4.Deuquet J, Lausch E, Superti-Furga A, van der Goot FG. The dark sides of capillary morphogenesis gene 2. EMBO J. 2012;31:3–13. doi: 10.1038/emboj.2011.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowling O, Difeo A, Ramirez MC, Tukel T, Narla G, Bonafe L, Kayserili H, Yuksel-Apak M, Paller AS, Norton K, Teebi AS, Grum-Tokars V, Martin GS, Davis GE, Glucksman MJ, Martignetti JA. Mutations in capillary morphogenesis gene-2 result in the allelic disorders juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:957–966. doi: 10.1086/378781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelholm LH, List K, Netzel-Arnett S, Cukierman E, Mitola DJ, Aaronson H, Kjoller L, Larsen JK, Yamada KM, Strickland DK, Holmbeck K, Dano K, Birkedal-Hansen H, Behrendt N, Bugge TH. uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J Cell Biol. 2003;160:1009–1015. doi: 10.1083/jcb.200211091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanks S, Adams S, Douglas J, Arbour L, Atherton DJ, Balci S, Bode H, Campbell ME, Feingold M, Keser G, Kleijer W, Mancini G, McGrath JA, Muntoni F, Nanda A, Teare MD, Warman M, Pope FM, Superti-Furga A, Futreal PA, Rahman N. Mutations in the gene encoding capillary morphogenesis protein 2 cause juvenile hyaline fibromatosis and infantile systemic hyalinosis. Am J Hum Genet. 2003;73:791–800. doi: 10.1086/378418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee W, Sodek J, McCulloch CA. Role of integrins in regulation of collagen phagocytosis by human fibroblasts. J Cell Physiol. 1996;168:695–704. doi: 10.1002/(SICI)1097-4652(199609)168:3<695::AID-JCP22>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Crown D, Miller-Randolph S, Moayeri M, Wang H, Hu H, Morley T, Leppla SH. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci U S A. 2009;106:12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Miller-Randolph S, Crown D, Moayeri M, Sastalla I, Okugawa S, Leppla SH. Anthrax toxin targeting of myeloid cells through the CMG2 receptor Is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe. 2010;8:455–462. doi: 10.1016/j.chom.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Wu H, Byrne M, Jeffrey J, Krane S, Jaenisch R. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J Cell Biol. 1995;130:227–237. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjoller L, Gardsvoll H, Hoyer-Hansen G, Holmbeck K, Bugge TH, Behrendt N. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem. 2007;282:27037–27045. doi: 10.1074/jbc.M701088200. [DOI] [PubMed] [Google Scholar]
- 13.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer Res. 2004;64:817–820. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- 14.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segal G, Lee W, Arora PD, McKee M, Downey G, McCulloch CA. Involvement of actin filaments and integrins in the binding step in collagen phagocytosis by human fibroblasts. J Cell Sci. 2001;114:119–129. doi: 10.1242/jcs.114.1.119. [DOI] [PubMed] [Google Scholar]
- 16.Stucki U, Spycher MA, Eich G, Rossi A, Sacher P, Steinmann B, Superti-Furga A. Infantile systemic hyalinosis in siblings: clinical report, biochemical and ultrastructural findings, and review of the literature. Am J Med Genet. 2001;100:122–129. doi: 10.1002/1096-8628(20010422)100:2<122::aid-ajmg1236>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Wagenaar-Miller RA, Engelholm LH, Gavard J, Yamada SS, Gutkind JS, Behrendt N, Bugge TH, Holmbeck K. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol. 2007;27:6309–6322. doi: 10.1128/MCB.00291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]