Abstract
INTRODUCTION
Gastric metastases from lung adenocarcinoma are rare and usually associated with disseminated disease. The great majority is asymptomatic and in few cases discovered during autopsy studies. Reports of single metachronous metastases during the lifetime are anecdotal. We describe a case of solitary gastric metastasis 5 years after lung surgery.
PRESENTATION OF CASE
A 68-year-old male submitted in 2006 to right lobectomy for lung adenocarcinoma was referred at Emergency Room department in 01/2011 because of chronic epigastric pain. Radiologic and endoscopic evaluation showed a bulky lesion inside the stomach, originating from the muscular layer, suspected for GIST. He underwent a subtotal gastrectomy and the pathologic examination revealed an undifferentiated adenocarcinoma, positive for Thyroid Transcriptional Factor-1, Cytokeratin 7, AE 1/3 and CEA, confirming the pulmonary origin.
DISCUSSION
At the time of diagnosis about 50% of lung cancer are metastatic, with survival rates of 1% at 5-year. Gastric metastasis is very rare; autopsy studies report an incidence of 0.2–0.5%. They develop in the submucosa, usually without any symptom and the diagnosis is incidental during the staging of primary cancer or the follow-up. There are no guidelines about surgical treatment; however few cases of long-term survival following the operation were reported. Pathologic diagnosis is difficult, but the immunohistochemical staining helps to recognize the primary origin.
CONCLUSION
Solitary metachronous gastric metastasis from pulmonary adenocarcinoma is an exceptional event, but it could happen during the follow-up. It seems that a radical resection, in absence of systemic implants, might provide survival benefits in selected patients.
Keywords: Lung cancer, Gastric metastasis, Outcome, Immunohistochemical analysis, Surgery
1. Introduction
Primary lung cancers rarely metastasize to the gastrointestinal tract. Gastric metastases are even rarer and usually associated with disseminated disease. Generally they are not detected during the lifetime of the patients, because most of them are asymptomatic.1 From autopsy studies was reported an incidence as 0.2–0.5%.2,3 Actually only sporadic cases were published in the literature, particularly considering metachronous metastases after several years from lung surgery. We report a case of isolated metachronous gastric metastasis 5 years after surgery for pulmonary adenocarcinoma, and review the literature on this unusual situation.
2. Presentation of case
In January 2011, a 68-year-old Caucasian male was referred at Emergency Room (ER) department because of chronic epigastric pain associated with nausea and anorexia. He reported the arising of the symptoms two months before, with weight loss of six kilograms during this period. Up to ten years before the patient was a smoker of about 50 packages of cigarettes per year. The past medical history was positive for coronary artery disease, treated with stenting in 2009, stable at the time of observation. No other comorbidities were reported.
The cancer-related anamnesis started in 2006, when a right upper lobectomy was performed for an undifferentiated lung adenocarcinoma (pT3N0M0). During the 2007, a solitary lung recurrence was diagnosed, and the patient underwent a left upper lobectomy; the subsequent pathologic analysis confirmed an undifferentiated lung adenocarcinoma with features similar to the 2006 histology.
After the surgical treatment, the patient continued the regular oncological follow-up, which was unremarkable for recurrent disease until the 2011. Particularly, he was submitted to CT and bone scans every 6 months for three years and annually thereafter, with no evidence of disease re-activation.
At admission to ER, laboratory tests showed an increasing of one the oncologic markers (CA125 = 1389.3 U/ml, n.v. 0–35; CA19.9 = 19.07 U/ml; CEA = 1.55 ng/ml) and of inflammatory indexes (CRP = 96.08 mg/l; Fibrinogen = 554.8 mg/dl). Moreover a mild anemia (Hb = 10.3 g/dl; Ht = 35.1%) and hypoalbuminemia (Albumin = 3.1 g/dl) were detected.
The patient underwent then a total body CT scan, which revealed a lesion of the stomach, involving most of the gastric antrum, measuring 7 cm × 5 cm; no other abnormalities were identified (Fig. 1). Subsequently an esophagogastroduodenoscopy (EGDS) was planned, with report of a bulky neoplasm, protruding into the gastric lumen, located in the posterior wall of the antrum. It was covered by normal mucosa, without any sign of ulceration or erosion, suggesting the presence of an extrinsic mass. Nevertheless the following endoscopic ultrasound (EUS) defined the lesion as an oval capsulated tumor, originating from the muscular layer of the stomach, measuring more than 4 cm in maximum diameter (Fig. 2).
Fig. 1.
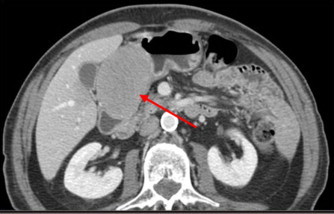
CT scan showing the gastric lesion involving most of the antrum (arrow).
Fig. 2.
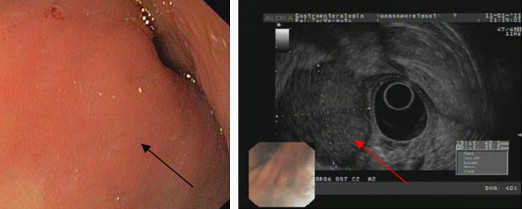
EGDS and EUS identifying a bulky oval capsulated neoplasm (arrows), located in the posterior wall of the antrum, protruding into the lumen. It originated from the muscular layer of the stomach, with a maximum diameter of more than 4 cm, and was covered by normal mucosa.
Based on the radiologic and endoscopic findings, suggesting a gastrointestinal stromal tumor (GIST), was planned the surgical resection.
At laparotomy a hard mass of about 7 cm was identified at the level of the posterior wall of the stomach, associated to enlarged locoregional lymph-nodes. No serosal invasion or other intraabdominal pathologies were evident. Consequently a subtotal gastrectomy with a Roux-en-Y reconstruction was performed. The postoperative course was uneventful and the patient was discharged on post-operative day (POD) 9 on normal diet.
Pathological examination of the surgical specimen revealed an undifferentiated adenocarcinoma, as shown in Fig. 3A and B. To settle if the tumor was a primary gastric cancer or a metastasis from the lung malignancy, the analysis was integrated with the immunohistochemical staining. The diagnosis of gastric metastasis from lung cancer was confirmed by positive staining for Thyroid Transcriptional Factor-1 (TTF-1), Cytokeratin AE 1/3, CEA and by the analysis of the pattern Cytokeratin 7 (CK7) positive/Cytokeratin 20 (CK20) negative (Fig. 3C and D).
Fig. 3.
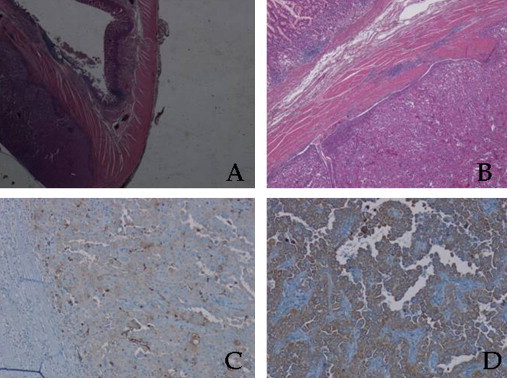
Pathological examination orienting toward an epithelial origin of the neoplasia, with specific characteristics of the lung. (A) Hematoxylin and eosin stain, 2×; (B) hematoxylin and eosin stain, 10×; (C) CK20 immunohistochemistry stain, clone name: SP33, Ventana Medical System (VMS), Tucson, AZ, USA, 20×; (D) CK7 immunohistochemistry stain, clone name: SP52, Ventana Medical System (VMS), Tucson, AZ, USA, 20×.
After the pathologic result, the staging of the disease was completed with a fluoro-deoxy-glucose positron emission tomography (FDG-PET)/CT scan, confirming the single gastric metastasis.
Subsequently to the lung and gastric surgery an adjuvant chemotherapy with cisplatin and vinorebine was offered, but the patient firmly did not consent and standard surveillance was hence started. At the time of writing, he is regularly continuing the follow-up at our center, without evidence of recurrence.
3. Discussion
Lung cancer accounted for approximately 27% of all new cancer cases of both men and women, and represented the leading cause of cancer death world-wide. At the time of diagnosis about 50% of the patients have metastatic disease, with reported survival rates to be 20% at 1-year and 1% at 5-year from diagnosis.4,5 The most common sites of extrapulmonary spread include regional lymph nodes (72–84%), liver (33–47%), bones (21–34%), brain (16–32%), adrenals (20–29%), and heart (12–29%).6,7
Gastrointestinal (GI) metastases from primary lung carcinoma rarely occur.1,3 In most cases, the diagnosis is possible only at autopsy; some necropsy studies report their incidence to be as high as 14% and usually as part of disseminated disease.8 The few clinical papers available indicate the incidence of symptomatic GI metastases to be about 2%, with the most common histotype being either squamous cells or adenocarcinoma. The small bowel is usually the most common metastatic site, while the duodenum and the stomach are the most rare.8,9
The pathogenesis is thought to be related to the tumor cell spread via the haematogenous and lymphatic routes, but there is not any specific data demonstrating a particular tropism for a segment of the GI tract.4
Symptoms and complications are secondary to a partial or complete invasion of the intestinal wall by the neoplasm. The most common consequences are bleeding or perforation; however, the latter was reported also due to the tumor necrosis during chemotherapy.10,11
Gastric metastases are very rare and in most cases asymptomatic. Autopsy reports show an incidence as 0.2–0.5%,2,3 but the actual value is difficult to assess. Symptomatic localizations to the stomach in living patients were described sporadically, as part of disseminated disease, or as synchronous lesions at the time of lung cancer diagnosis.12,13 The finding of a single metacronous metastasis, several years after the primary lung surgery as in our case, is exceptionally rare and incidental.
Metastases to the stomach usually develop in the submucosa, without any symptom; only after a considerable growth, with the involvement of other gastric layers, they could come up. Abdominal pain was reported to be the most frequent symptom, generally associated to chronic bleeding with haematemesis or melena, anaemia, nausea and vomiting.1 Moreover, gastric perforation and pyloric obstruction were described.2,14–17 The diagnosis is usually incidental during the staging procedures of primary lung cancer or during the follow-up, as part of concomitant distal recurrences. PET scan is the most common investigative and effective tool in detecting GI metastases, both symptomatic or not. However, if any symptom appears, upper GI endoscopy usually reveals the lesion as submucosal plaque, with or without mucosal ulceration.
In the present case, the symptoms were secondary to the growth of this bulky lesion at the antro-pyloric region, leading to epigastralgia and subtotal lumen obstruction, with consequently difficult feeding and weight loss. As the patient was referred at ER department, the first instrumental exam was the total body CT scan, which first revealed the gastric pathology. At the EGDS the gastric mucosa appeared normal, without erosions or ulcers; the EUS confirmed the presence of one single lesion developing at the submucosal/muscular interface, ruling out an extrinsic mass.
According to the radiographic findings, Menuck et al. described three distinct patterns of metastatic involvement of the stomach: (1) solitary polypoid submucosal mass, which may ulcerate; (2) multiple polypoid submucosal masses, which may ulcerate; (3) infiltrating constricting pattern similar to linitis plastica.15
In the literature was reported a high percentage of gastric metastases localized at the level of the cardias or fundus2; our case, instead, is included in the rarer group of lesions involving the distal portion of the organ.
From a pathologic point of view, usually is difficult to diagnose a metastasis from a primary lung cancer to the stomach when the histology is adenocarcinoma. However the integration with the immunohistochemical staining allows the recognition of the primary site, even in presence of an undifferentiated cancer. In our case the positivity of CEA and Cytokeratin AE 1/3 suggested the epithelial origin of the neoplasia; moreover the TTF-1 is positive in the great majority of lung adenocarcinomas, as in the present report, but negative in gastric adenocarcinomas. Eventually we reached the confirmation analyzing the CK7+/CK20− pattern; this is typical for lung in 90–100% of patients, conversely it is recognized only in 35–40% of gastric cancers.
Most extrathoracic recurrences after complete lung adenocarcinoma resection are multiple and disseminated, associated to a poor prognosis. Literature data on surgical treatment of single metastases is scant, and no definitive conclusions can be drawn from the small number of case reports. However some investigators showed long-term survival after operative treatments in selected patients; Hishida et al. reported a satisfactory outcome after resection of lung cancer recurrence in stage I patients.18 Aokage et al. observed prolonged survival after surgery in two patients with diagnosis of solitary gastric metastasis from pulmonary pleomorphic carcinoma.19 Moreover surgical treatment of such recurrences may be indicated if bleeding or obstruction is encountered.20
Table 1 shows a review of reported cases of gastric metastases from primary lung carcinoma, with time interval and characteristics of the cancer.
Table 1.
Reported cases of gastric metastases from primary lung carcinoma.
| Author | Age (yr)/gender | Symptom/sign | Location | Timing | Endoscopic features |
|---|---|---|---|---|---|
| Okazaki, 201012 | 68/male | Epigastric pain | Upper third and body | Synchronous | Linitis plastica-like tumor |
| Lee, 201021 | 77/male | Asymptomatic | Mid-antrum | Synchronous | Oval-shaped ulcer with raised margin |
| Ozdilekcan, 201022 | 46/male | Disphagia, epigastric pain | Lesser curvature of gastric body | Synchronous | Giant gastric ulcer |
| Kanthan, 200923 | 75/male | Epigastric-right upper quadrant pain | Not reported | Synchronous | Gastric polyps |
| Aokage, 200819 | 69/male | Severe anemia, fatigue | Anterior wall of mid-stomach | Metachronous | Mass with central depression (volcano-like) |
| Aokage, 200819 | 62/male | Asymptomatic | Posterior wall of the fornix | Synchronous | Submucosal gastric mass (CT scan) |
| Yang, 20064 | 71/male | Black colored stools | Not reported | Synchronous | Not reported |
| Suzaki, 200211 | 45/male | Epigastralgia, gastric perforation | Greater curvature | Metachronous | Not reported |
| Hamatake, 200124 | 65/male | Hematemesis | Posterior wall of mid-stomach | Synchronous | Gastric ulcer |
4. Conclusion
Solitary metachronous gastric metastasis from pulmonary adenocarcinoma several years after the primary treatment is an exceptional event. However during the follow up it is necessary to be aware of this rare situation. In presence of a single gastric localization, after ruled out any other systemic implants, surgical approach is justified, with the potentiality to significantly prolong the disease-free survival. To have the definitive diagnosis of a pulmonary origin is important to identify the expression of specific immunohistochemical patterns.
Conflict of interest
All the authors have no conflict of interest.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Pierpaolo Sileri, Giovanna Del Vecchio Blanco, Stefano D’Ugo: study design.
Stefano D’Ugo, Elisabetta Lolli, Vincenzo Formica, Lucia Anemona, Carmela De Luca: data collections.
Pierpaolo Sileri, Stefano D’Ugo, Luana Franceschilli: data analysis.
Pierpaolo Sileri, Stefano D’Ugo: writing.
Achille L. Gaspari: revising and final approval.
References
- 1.Kadakia S.C., Parker A., Canales L. Metastatic tumors to the upper gastrointestinal tract: endoscopic experience. American Journal of Gastroenterology. 1992;87:1418–1423. [PubMed] [Google Scholar]
- 2.Antler A.S., Ough Y., Pitchumoni C.S., Davidian M., Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer. 1982;49:170–172. doi: 10.1002/1097-0142(19820101)49:1<170::aid-cncr2820490134>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Green L.K. Hematogenous metastases to the stomach. A review of 67 cases. Cancer. 1990;65:1596–1600. doi: 10.1002/1097-0142(19900401)65:7<1596::aid-cncr2820650724>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Yang C.J., Hwang J.J., Kang W.Y., Chong I.W., Wang T.H., Sheu C.C. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer. 2006;54(3):319–323. doi: 10.1016/j.lungcan.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee R.T., Hill-Harmon M.B., Murray T., Thun M. Cancer Statistics, 2001. CA: A Cancer Journal for Clinicians. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Stenbygaard L.E., Sorensen J.B., Larsen H., Dombernowsky P. Metastatic pattern in non-resectable non-small cell lung cancer. Acta Oncologica. 1999;38:993–998. doi: 10.1080/028418699432248. [DOI] [PubMed] [Google Scholar]
- 7.Steinhart A.H., Cohen L.B., Hegele R., Saibil F.G. Upper gastrointestinal bleeding due to superior mesenteric artery to duodenum fistula: a rare complication of metastatic lung carcinoma. American Journal of Gastroenterology. 1991;86:771–774. [PubMed] [Google Scholar]
- 8.Garwood R.A., Sawyer M.D., Ledesma E.J., Foley E., Claridge J.A. A case and review of bowel perforation secondary to metastatic lung cancer. American Surgeon. 2005;71:110–116. [PubMed] [Google Scholar]
- 9.Yamamoto M., Matsuzaki K., Kusumoto H., Uchida H., Mine H., Kabashima A. Gastric metastasis from lung carcinoma. Case report. Hepato-Gastroenterology. 2002;49:363–365. [PubMed] [Google Scholar]
- 10.Yuen J.S., Chow P.K., Ahmed Q. Metastatic lung cancer causing bowel perforations: spontaneous or chemotherapy-related? ANZ Journal of Surgery. 2002;72:245–246. doi: 10.1046/j.1445-2197.2002.02236.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzaki N., Hiraki A., Ueoka H., Aoe M., Takigawa N., Kishino T. Gastric perforation due to metastasis from adenocarcinoma of the lung. Anticancer Research. 2002;22:1209–1212. [PubMed] [Google Scholar]
- 12.Okazaki R., Ohtani H., Takeda K., Sumikawa T., Yamasaki A., Matsumoto S. Gastric metastasis by primary lung adenocarcinoma. World Journal of Gastrointestinal Oncology. 2010;2(10):395–398. doi: 10.4251/wjgo.v2.i10.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casella G., Di Bella C., Cambareri A.R., Buda C.A., Corti G., Magri F. Gastric metastasis by lung small cell carcinoma. World Journal of Gastroenterology. 2006;12(25):4096–4097. doi: 10.3748/wjg.v12.i25.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins P.M. Pyloric obstruction due to metastatic deposit from carcinoma of bronchus. Cancer Journal of Surgery. 1962;5:538–541. [PubMed] [Google Scholar]
- 15.Menuck L.S., Amberg J.R. Metastatic disease involving the stomach. American Journal of Digestive Diseases. 1975;20:903–913. doi: 10.1007/BF01070875. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher M.S. Gastric perforation secondary to metastatic carcinoma of the lung: a case report. Cancer. 1980;46:1879–1882. doi: 10.1002/1097-0142(19801015)46:8<1879::aid-cncr2820460829>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Rubin S.A., Davis M. “Bull's eye” or “target” lesions of the stomach secondary to carcinoma of the lung. American Journal of Gastroenterology. 1985;80:67–69. [PubMed] [Google Scholar]
- 18.Hishida T., Nagai K., Yoshida J., Nishimura M., Ishii G., Iwasaki M. Is surgical resection indicated for a solitary non-small cell lung cancer recurrence? Journal of Thoracic and Cardiovascular Surgery. 2006;131:838–842. doi: 10.1016/j.jtcvs.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Aokage K., Yoshida J., Ishii G., Takahashi S., Sugito M., Nishimura M. Long-term survival in two cases of resected gastric metastasis of pulmonary pleomorphic carcinoma. Journal of Thoracic Oncology. 2008;3:796–799. doi: 10.1097/JTO.0b013e31817c925c. [DOI] [PubMed] [Google Scholar]
- 20.Rossi G., Marchioni A., Romagnani E., Bertolini F., Longo L., Cavazza A. Primary lung cancer presenting with gastrointestinal tract involvement: clinicopathologic and immunohistochemical features in a series of 18 consecutive cases. Journal of Thoracic Oncology. 2007;2(2):115–120. [PubMed] [Google Scholar]
- 21.Lee M.H., Kim S.R., Soh J.S., Chung M.J., Lee Y.C. A solitary gastric metastasis from pulmonary adenocarcinoma: a case report. Thorax. 2010;65(7):661–662. doi: 10.1136/thx.2009.122382. [DOI] [PubMed] [Google Scholar]
- 22.Ozdilekcan C., Songür N., Memiş L., Bozdoğan N., Köksal A.S., Ok U. Lung cancer associated with a single simultaneous solitary metastatic lesion in stomach: a case report with the review of literature. Tuberk Toraks. 2010;58(1):78–84. [PubMed] [Google Scholar]
- 23.Kanthan R., Sharanowski K., Senger J.L., Fesser J., Chibbar R., Kanthan S.C. Uncommon mucosal metastases to the stomach. World Journal of Surgical Oncology. 2009;7(August):62. doi: 10.1186/1477-7819-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamatake M., Ishida T., Yamazaki K., Baba H., Maehara Y., Sugio K. Lung cancer with p53 expression and a solitary metastasis to the stomach: a case report. Annals of Thoracic and Cardiovascular Surgery. 2001;7(3):162–165. [PubMed] [Google Scholar]


