Abstract
Objectives
To image cholesteatoma using optical coherence tomography (OCT) and correlate the results with clinical findings and conventional observations obtained using binocular microscopy and histology. OCT is a high-resolution optical imaging modality that generates cross-sectional images of turbid media, such as tissue with resolution approaching that of light microscopy. OCT relies on intrinsic differences in tissue optical properties for image contrast.
Study Design
In vivo prospective clinical study.
Setting
University Medical Center.
Patients
Patients with cholesteatoma undergoing otologic surgery.
Intervention
Using a commercial OCT imaging system, we obtained cross-sectional images (resolution, ~10 μm; depth penetration, ~1 mm) of cholesteatomas.
Main Outcome Measures
Images are obtained by raster scanning a single mode fiber across the interior of the probe. The imaging probe is sterilized and inserted into the middle ear or mastoid under microscopic guidance, and still images of the middle ear or mastoid mucosa and cholesteatoma when present were obtained.
Results
OCT images of cholesteatomas demonstrate differences in signal intensity, which are distinct from those of normal or inflamed middle ear/mastoid mucosa. Identification of keratin in cholesteatoma, even if very thin, distinguished it from inflamed mucosa.
Conclusion
This is the first study that systematically used OCT to image cholesteatoma during otologic surgery. Cholesteatomas can be distinguished from normal or inflamed adjacent mucosa.
Keywords: Cholesteatoma, Ear, Mastoid, Middle ear, Optical coherence tomography
Optical coherence tomography (OCT) is a noninvasive imaging modality that uses low-coherence interferometry to visualize tissue microstructure (1). Using discrepancies in phase and intensity of backscattered light, high-resolution images can be reconstructed. Currently, commercial OCT imaging systems have an axial resolution of approximately 10 μm and, in certain circumstances, image up to 2 mm in depth (2). Clinically, OCT has been used primarily to examine the eye (3) and the skin (4) because these organs do not require specialized hardware, endoscopes, scanner, or general anesthesia for imaging. For the past decade, several studies have used investigational OCT systems to image the upper aero-digestive tract, with an emphasis on laryngeal imaging (2,5–13). In the ear, animal studies have demonstrated the potential use for OCT to image the middle ear, cochlea (14,15), and the tympanic membrane (TM) (16).
In this study, OCT was used to image cholesteatoma and determine whether it can be distinguished from the surrounding middle ear mucosa. This is the first systematic study to image cholesteatomas using OCT intraoperatively.
MATERIALS AND METHODS
Patient Population
The research protocol was approved by the institutional review board at the University of California Irvine. OCT imaging was performed in 10 patients undergoing otologic surgery under general anesthesia, where consent was previously obtained. Five patients were male subjects, and 5 patients were female subjects. The average age of the patients was 43 years, with a range of 7 to 78 years.
OCT System
Each patient was examined using a commercially OCT imaging system (Niris; Imalux Corporation, Cleveland, OH, USA). This portable time-domain OCT system, which is described in detail elsewhere (17) uses a low coherence near-infrared light source with a central wavelength of 1300 nm, to acquire real-time images of 200 × 200 pixels at a frame rate of approximately 1 Hz. To acquire the images, it uses a reusable flexible probe of 2.7 mm in diameter, which contains a single-mode fiber raster scanned by a solenoid across the surface of a grin lens. This system has a lateral resolution of 25 μm, with a lateral scanning range of 1.5 to 2.5 mm. The axial (depth) resolution of the system is 10 to 20 μm, with a depth scanning range of 2.2 mm. In practice, because of the turbidity of living tissues, scanning depth is only approximately 1.5 mm. Images are acquired at a frame rate of 0.75 Hz.
Imaging
The images were obtained after surgical exposure. The sterilized flexible probe was inserted into the middle ear or mastoid under microscopic guidance and placed in light or near contact with the tissue of interest. Imaging of multiple areas required less than 5 minutes in each case. In the operating room, the OCT device is set up near the microscope, so the image would be easily seen by the surgeon.
RESULTS
Cholesteatomas were found to have characteristic findings on OCT imaging that differentiate them from normal mucosa (Fig. 1), which lacks a keratin layer, where no hyperintensity is observed. This also is illustrated in Figure 2, which shows both middle ear mucosa and cholesteatoma; the latter one has an overlying layer of keratin that creates the hyperintensity on the image. Cholesteatoma can be readily differentiated from the TM (figure not included); the normal TM is thin and lacks a thick keratin layer (hyperintensity) seen in the area of the cholesteatoma.
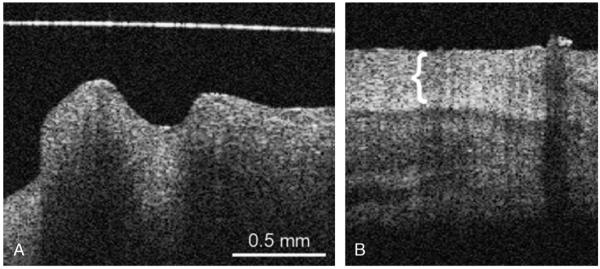
FIG. 1.
OCT image of the normal middle ear mucosa (A), where no distinct hyperintense layer is observed, in comparison with cholesteatoma (B) with the thick keratin layer (bracket). The horizontal line is an artifact (A).
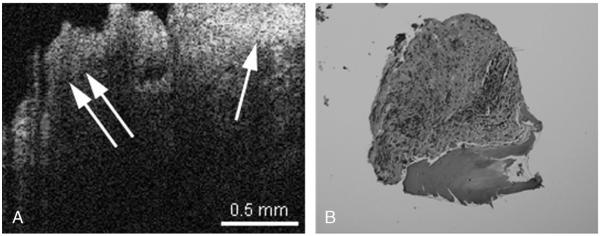
FIG. 2.
A, OCT image of cholesteatoma (single arrow) adjacent to inflamed middle ear mucosa (double arrow) can be observed. The cholesteatoma has an overlying layer of keratin, which creates the hyperintensity on the image (single arrow). B, Hematoxylin and eosin–stained histology of inflamed middle ear mucosa, with some underlying bone.
The cholesteatoma capsule cell layer can be distinguished from the keratin (Fig. 3). This image of the capsule is from the nonkeratinized side; cholesteatoma also can be clearly distinguished from the dura (figure not included). The keratin in the cholesteatoma shows the characteristic hyperintensity, whereas the underlying dura does not.
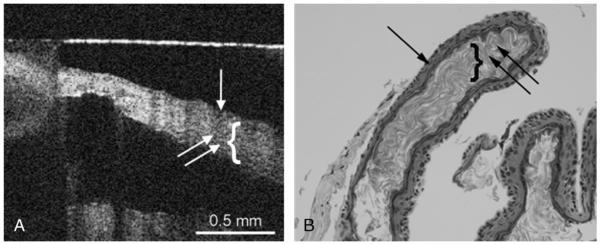
FIG. 3.
A, OCT image of cholesteatoma capsule. Capsule image from the nonkeratinized side, where the cholesteatoma matrix (single arrow) and the keratin (double arrow, bracket) can be observed. B, Histology image of cholesteatoma, where the cholesteatoma matrix (single arrow) and the keratin (double arrow, bracket) can be observed. The horizontal line is an artifact (A).
DISCUSSION
This is the first study evaluating the use of OCT imaging for intraoperative identification of cholesteatoma. We have shown that OCT imaging can be used to differentiate cholesteatoma from inflamed middle ear or mastoid mucosa. The characteristic imaging findings include greater signal intensity of the keratin compared with the lower gray scale values of the mucosa. When the cholesteatoma matrix was imaged from the outside (nonkeratinized side), a very thin layer of matrix could be identified on OCT imaging. Although this study is limited in the number of patients evaluated, it does suggest that there are frank differences between cholesteatoma and inflamed mucosa that can be resolved using OCT.
The diagnosis of cholesteatoma is primarily clinical, and it is unlikely that OCT will provide a quantitative method for diagnosis; any more than CT imaging can readily differentiate benign neoplasms from cancer in glands such as the thyroid. However, residual cholesteatoma plays a significant role in the high rate of recidivism after primary cholesteatoma surgery. If residual sub-clinical cholesteatoma can be identified intraoperatively, it can be removed promptly. The most challenging cases where residual cholesteatoma cannot be identified are in patients with significant inflammation in the middle ear or mastoid at the time of the primary surgery. OCT may be of benefit in these cases by identifying small collections of cholesteatoma in a bed of granulation tissue, given their distinct appearance during imaging.
There are currently several limitations to the broad adoption of OCT technology in otologic surgery. With this particular instrument, the size of the probe at 2.7 mm of diameter is somewhat large for examining certain areas of the middle ear. Specifically, areas in the posterior superior quadrant of the middle ear are difficult to image because the probe may injure the stapes because of its size. Thinner probes in the future may overcome this limitation. Second, because the current probe’s flexibility is limited, it cannot be used to image areas such as the sinus tympani through a transcanal approach. Although the sinus tympani can be visualized using angled optical rigid endoscopes, the current OCT device is unable to image tissue from several millimeters away and requires a near contact. It is plausible that, in the future, newer probes specific to otologic surgery can be developed for this purpose. Third, the resolution of the current commercial device (Niris system) is far below the capabilities of available research devices. Fourth, blood on the tip of the imaging probe will obscure the image, which will make interpretation of the images difficult. Because OCT relies on tissue optical properties to generate image contrast, optically dense structures (e.g., bone, tympano-sclerosis, or blood) will reflect or absorb incident light at the surface and limit signal penetration to deeper structures. During our procedures, good hemostasis was obtained with cotton balls soaked with 1:1000 epinephrine before imaging. Also, meticulous handling of the probe when used transcanal is important to not abut the edges of the tympanomeatal flap or the blood in the ear canal. Finally, the cost of the device and uncertainty over reimbursement matters are currently factors that limit its widespread use.
The quality of the OCT images also can be affected by blood because of its hemoglobin content. Hemoglobin is highly absorbent and forward-scattering to light, which creates an “optical shadow,” causing a loss or attenuation of the image beyond the point where the blood is. In contrast, clear fluid does not absorb nor scatter the light; thus, the images quality is not affected. The area to be imaged has to be dried of blood well using cotton ball and epinephrine before imaging.
CONCLUSION
In this study, we were able to successfully image cholesteatoma in vivo. Because OCT is a noninvasive image modality, it has great potential in imaging cholesteatoma in vivo, especially for differentiating between residual cholesteatoma and inflamed mucosa. As the OCT technology improves, the OCT imaging characteristics unique to cholesteatomas may permit imaging and identification of middle ear tissue in the office without the need for a tympanotomy if the TM is adequately thin. In addition, intraoperative differentiation of inflammatory tissue from cholesteatoma in granulation tissue can help guide the surgeon if further resection is necessary.
Acknowledgments
This work was supported by the National Institutes of Health (DC 006026, CA 91717, EB 00293, RR 01192, and RR00827), Flight Attendant Medical Research Institute (32456), State of California Tobacco Related Disease Research Program (12RT-0113), Air Force Office of Scientific Research (F49620_00_1_0371), and the Arnold and Mabel Beckman Foundation.
Footnotes
This work was presented at the 112th Annual Triological Society Meeting at Combined Otolaryngology Spring Meeting; May 29–31, 2009, Phoenix, Arizona.
REFERENCES
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong BJ, Jackson RP, Guo S, et al. In vivo optical coherence tomography of the human larynx: normative and benign pathology in 82 patients. Laryngoscope. 2005;115:1904–11. doi: 10.1097/01.MLG.0000181465.17744.BE. [DOI] [PubMed] [Google Scholar]
- 3.Krauss JM, Puliafito CA. Lasers in ophthalmology. Lasers Surg Med. 1995;17:102–59. doi: 10.1002/lsm.1900170203. [DOI] [PubMed] [Google Scholar]
- 4.Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1–9. doi: 10.1034/j.1600-0846.2001.007001001.x. [DOI] [PubMed] [Google Scholar]
- 5.Sepehr A, Armstrong WB, Guo S, et al. Optical coherence tomography of the larynx in the awake patient. Otolaryngol Head Neck Surg. 2008;138:425–9. doi: 10.1016/j.otohns.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridgway JM, Armstrong WB, Guo S, et al. In vivo optical coherence tomography of the human oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2006;132:1074–81. doi: 10.1001/archotol.132.10.1074. [DOI] [PubMed] [Google Scholar]
- 7.Mahmood U, Ridgway J, Jackson R, et al. In vivo optical coherence tomography of the nasal mucosa. Am J Rhinol. 2006;20:155–9. [PubMed] [Google Scholar]
- 8.Armstrong WB, Ridgway JM, Vokes DE, et al. Optical coherence tomography of laryngeal cancer. Laryngoscope. 2006;116:1107–13. doi: 10.1097/01.mlg.0000217539.27432.5a. [DOI] [PubMed] [Google Scholar]
- 9.Ridgway JM, Su J, Wright R, et al. Optical coherence tomography of the newborn airway. Ann Otol Rhinol Laryngol. 2008;117:327–34. [PMC free article] [PubMed] [Google Scholar]
- 10.Guo S, Hutchison R, Jackson RP, et al. Office-based optical coherence tomographic imaging of human vocal cords. J Biomed Opt. 2006;11:30501. doi: 10.1117/1.2200371. [DOI] [PubMed] [Google Scholar]
- 11.Ridgway JM, Ahuja G, Guo S, et al. Imaging of the pediatric airway using optical coherence tomography. Laryngoscope. 2007;117:2206–12. doi: 10.1097/MLG.0b013e318145b306. [DOI] [PubMed] [Google Scholar]
- 12.Klein AM, Pierce MC, Zeitels SM, et al. Imaging the human vocal folds in vivo with optical coherence tomography: a preliminary experience. Ann Otol Rhinol Laryngol. 2006;115:277–84. doi: 10.1177/000348940611500405. [DOI] [PubMed] [Google Scholar]
- 13.Kraft M, Glanz H, von Gerlach S, et al. Clinical value of optical coherence tomography in laryngology. Head Neck. 2008;30:1628–35. doi: 10.1002/hed.20914. [DOI] [PubMed] [Google Scholar]
- 14.Sepehr A, Djalilian HR, Chang JE, et al. Optical coherence tomography of the cochlea in the porcine model. Laryngoscope. 2008;118:1449–51. doi: 10.1097/MLG.0b013e318173dd6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong BJ, Zhao Y, Yamaguchi M, et al. Imaging the internal structure of the rat cochlea using optical coherence tomography at 0.827 microm and 1.3 microm. Otolaryngol Head Neck Surg. 2004;130:334–8. doi: 10.1016/j.otohns.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Djalilian HR, Ridgway J, Tam M, et al. Imaging the human tym-panic membrane using optical coherence tomography in vivo. Otol Neurotol. 2008;29:1091–4. doi: 10.1097/MAO.0b013e31818a08ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldchtein F, Bush J, Gelikonov G, et al. Cost-effective all-fiber autocorrelator-based 1300-nm OCT system. Proc SPIE. 2005;5690:349–55. [Google Scholar]