Abstract
Growth factors regulated by specific macronutrients have been shown to promote aging and accelerate mortality in the great majority of the organisms studied. In particular, the enzymes activated by growth hormone (GH), insulin and insulin-like growth factor 1 (IGF-I) in mammals and their orthologs in simple model organisms represent perhaps the best-understood proteins involved in the aging process. Dietary restriction (DR), which reduces the level of IGF-I and of other growth factors, has been associated with protection from diabetes, cancer, and cardiovascular diseases and deficiencies in GH signaling and IGF-I are strongly associated with protection from cancer and diabetes in both mice and humans, but their role in cardiac function and cardiovascular diseases is controversial. Here we review the link between growth factors, cardiac function and heart disease with focus on the cardioprotective and sensitizing effect of growth factors in both model organisms and humans.
Keywords: IGF-I, Nutrition, Signaling, Stress Resistance
INTRODUCTION
Aging results in a progressive decline in multiple organs, and in particular, has profound effects on the heart and arterial system. Age-related cardiac and vascular changes include impaired endothelial function and intimal proliferation,1 increased arterial stiffness,2-7 left ventricular (LV) diastolic dysfunction,8-10 LV pathological hypertrophy,11 diminished LV systolic reverse capacity,9-10 decreased heart rate variability,12-14 and a reduction in maximal heart rate.15 Furthermore, as a consequence of aging, the interaction between the heart and arterial system is altered to preserve ventricle-arterial homeostasis. Therefore, the age-associated LV structural and functional deterioration due to the intrinsic effects of aging on the myocardium in conjunction with the compensatory reactive cardiac modifications in response to the progressive rise of systolic load imposed by increased arterial stiffness,4, 9 can have a significant detrimental effect on the senescent heart.
Dietary restriction and cardiovascular aging
Dietary restriction (DR), a 20-40% reduction in calorie intake, which reduces the levels of IGF-I and other growth factors, has been consistently shown to increase lifespan, and to prevent the development of age-associated cardiovascular functional and structural changes in several model organisms16-23. In particular, DR has been shown to improve arterial flow-mediated vasodilation 24-25 and to retard the development of atherosclerotic lesions in rodents.18 DR significantly ameliorates LV diastolic function of the aging heart and reduces arterial stiffness.17, 20-21, 24 Moreover, long-term DR has been shown to improve autonomic function, and in particular, increase the high-frequency component of the heart rate variability spectra, a marker for parasympathetic activity in rats.22 Finally, long-term DR has a powerful effect in preventing/delaying the age-related increase in the severity of cardiomiopathy in rodents as well as in monkeys23, 26.
There are a number of hypotheses regarding the mechanisms by which DR mediates its beneficial effects on aging in lower organisms that could have relevance to slowing cardiovascular aging in humans. These include a decrease in chronic inflammation, a reduction in the levels of various hormones and growth factors, an increased resistance to oxidative stress and a reduction in free radical-induced tissue damage that may be mediated by the decrease in oxygen consumption as well as the potentiation of antioxidant defense mechanisms (Figure 2).27 The protection from free radical-induced tissue damage is presumably conferred, at least in part, by the DR-mediated reduction in growth factors signalling. In the long-lived dwarf, GHR-KO, klotho transgenic and p66shc-/- mice the suppression of intracellular mitogenic signaling pathways increases the expression of reactive oxygen species (ROS) scavenging enzymes, such as catalase and superoxide dismutase, thereby facilitating removal of these toxic oxygen species.28 Also, human cells exposed to IGF-I-deficient human serum are more protected against oxidative DNA damage.29 In the heart of rats, these DR-dependent effects lead to a decrease in oxidative stress and an improved functional recovery following ischemia.30-32 These effects and the reduced heart hypertrophy in aging rats may be due to improvements in mitochondrial function.33 In fact, DR is well-known to retard the deterioration of mitochondrial respiratory function, a main source of ROS, by preserving enzymatic activities of the electron transport system and controlling proton leak.34-36
Figure 2.
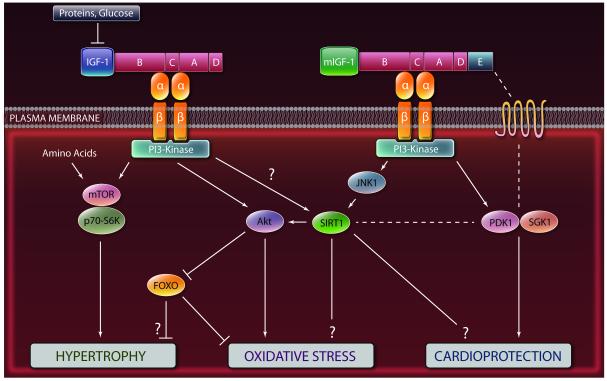
A simplified model representing the effect of nutrients and IGF-I on cellular protection and the differences between the intracellular signaling cascades triggered by the circulating IGF-I and the cardiomyocyte-produced mIGF-I isoform. Because studies on the effect and role of the mIGF-I isoform are relatively new, the right panel represents only a potential model for mIGF-I effects on cardioprotection. Additional studies are necessary to confirm this role. Question marks indicate mechanisms not fully clarified. Dashed lines indicate hypothetical interactions. See text for details and references. (Illustration credit: Cosmocyte/Ben Smith).
The protection against inflammation-mediated tissue damage and aging-associated deterioration in immune function may also play an important role in preventing/delaying cardiovascular aging, since it could lower the levels of inflammatory cytokines and oxidative stress involved in cardiovascular disease (CVD) progression.37 In fact, DR has been shown to lower the circulating levels of inflammatory cytokines (e.g. IL-6 and TNF-alpha), and to increase plasma adiponectin and cortisol concentration.38-43 In addition, DR simultaneously affects multiple processes that are involved in cardiovascular aging, including more rapid removal of damaged proteins and oxidized lipids and lipoproteins, decreases protein glycation, and decreased collagen cross-linking,44-46 effects which suggest the involvement of autophagy.
Long-term DR in human volunteers causes profound reductions in several cardiometabolic risk factors for coronary heart disease, including lowering of total cholesterol, LDL cholesterol, and triglycerides, and a large increase in HDL cholesterol concentrations, lower fasting glycemia and HOMA-IR index, and a remarkable lowering effect on systolic and diastolic blood pressure.27, 47-48 Long-term DR in humans has also a powerful anti-inflammatory effect reflected by almost undetectable/low circulating levels of C-reactive protein and TNF. This decrease in systemic inflammation and other cardiometabolic markers was accompanied by a significantly lower thickness of the carotid artery intima-media and by an improved LV diastolic function in the DR individuals than in the age- and sex-matched control group.47, 49
Recent studies have provided evidence for the role of inactivation of GH and IGF-I signaling pathways in the protective effects of DR on cellular resistance and aging. In agreement with this link, down-regulation of the insulin/IGF-I pathway slows aging and protects against several metabolic alterations that promote cardiovascular disease. However, the role of growth factors in modulating age-associated cardiovascular dysfunction has not been clearly defined.
Among the pathways whose inactivation is believed to mediate part of the protective effects of DR are the PI3K-AKT, Ras, and TOR-S6K pathways, all regulated by insulin and IGF-I.16 The adenylate cyclase/PKA pathway is also emerging as a sensitizing and aging-promoting pathway in yeast and mice, but its connection with growth factor signaling is still poorly understood.16, 50 In the next section we focus on the connection between IGF-I signaling pathways and cardiomyocyte protection.
GROWTH SIGNALING, STRESS RESISTANCE & HEART FUNCTION IN MODEL ORGANISMS
IGF-I signaling and function
IGF-I acts as an intermediate of several growth hormone (GH) responses and affects multiple signaling cascades, resulting in a potent proliferative signal that blocks apoptosis and stimulates growth in many different cells and organs.51 IGF-I actions are mediated through its receptor: a heterotetrameric transmembrane glycoprotein complex belonging to the receptor tyrosine kinase family (Figure 2). The IGF-I receptor is closely related to the insulin receptor although each has significantly different affinities for its cognate ligand.52 One branch of IGF-I’s mitogenic signaling involves the association of the receptor tyrosine kinase with Shc, Grb2, and Sos-1 to activate Ras and the MAP kinase cascades (Raf, Mek, Erk).53 One of the endpoints of the MAP kinase pathways is the modification of ELK transcription factors, serum response factor (SRF) and AP-1. Another important branch of IGF-I signaling involves the phosphorylation of IRS-1 and the activation of the PI3K/Akt signaling cascade, which is similarly activated by insulin, and which is known to sensitize cells in part by the phosphorylation and inactivation of FOXO transcription factors (Figure 1).53-54 These kinases and transcription factors have profound effects on cell survival, cell cycle and lipid & glucose metabolism by regulating downstream effectors such as NF-kB, BAD and many others and the expression of protective genes including superoxide dismutases and catalase.16, 55
Figure 1.
Mammalian IGF-I transcript processing. The IGF-I gene contains six exons that encode multiple isoforms, all of which include the core IGF-I protein body (dark red boxes). Exons 1 and 2 contain multiple transcription start sites and code for the N-terminal signal peptide of precursor IGF-I (class 1 and class 2). Exons 5 and 6 each encode distinct portions of the E-peptides. Class 1 IGF-IEa is also termed mIGF-I. (Illustration credit: Cosmocyte/Ben Smith).
The IGF-I core peptide is released by the liver into the bloodstream and it has a half-life of less than 10 minutes. It is usually stabilized by forming complexes with carrier proteins, the IGFBPs, which serve not only to transport IGF-I in circulation but also to prolong its half-life, modulate its tissue specificity, and strengthen or neutralize its biological actions.56 Studies on mice lacking the IGF-I gene have shown that normal IGF-I expression is critical for normal growth and tissue development: both pre- and postnatally, IGF-I contributes more to the total body weight then GH alone (35% versus 14%). Mice deficient for IGF-I have a birthweight of only 60% of that of their wild-type (wt) littermates and most of them die around birth due to respiratory failure. Depending on the genetic background only a small percentage survives until adulthood, and adult IGF-I KO mice are infertile and severely growth retarded, reaching only 30% of the normal adult body weight.57-58 When the IGF-IR is genetically removed, mice are even more profoundly affected: the birthweight of IGF-IR KO animals is only 45% of that of their wt littermates, and they all die immediately after birth because of respiratory failure. The essential role IGF-I plays in somatic growth is also illustrated by the finding that one single-nucleotide polymorphism in the IGF-I gene is responsible for the size difference between strains of small and large dogs.59 While these results firmly establish the importance of IGF-I in somatic growth, and its interplay with GH in this process, it remains unclear how the participation of endocrine, liver-derived IGF-I in the promotion of growth differs from the effects of locally produced IGF-I, which acts in an auto- and paracrine fashion in skeletal and heart muscle.
Because of these central effects on growth, survival, and metabolism, IGF-I and insulin signaling pathways are connected to the aging process in many organisms. 16, 60-62
IGF-II signaling and function
Whereas IGF-I is perhaps the most important GH-induced growth factor produced postnatally, IGF-II is considered the main IGF for the regulation of fetal and placental growth.63 IGF-II signals through the IGF-I receptor, triggering similar signaling cascades of IGF-I,64 and by binding to the IGF-II receptor, which is believed to signal through G-protein related mechanisms.65 Mice with disrupted IGF-II are growth-deficient but they do not have alterations in life span.66 Elevated levels of IGF-II in the postnatal period do not rescue the body and skeletal growth defects in the absence of IGF-I.67 There is evidence that IGF-II is induced by hypertrophic and apoptotic stressors in the heart68-69 and that it triggers cardiac hypertrophy in vitro.70 Given its role during development, it has been suggested that a finely tuned IGF-II signaling is crucial for a balanced heart growth.71 Polymorphisms in the human IGF-II gene have been associated with body mass index in adult males.72 Despite these observations, to date a role for IGF-II role in cardiovascular aging and the development remains unproven.
Growth signaling and stress resistance in simple organisms
The understanding of the basic mechanisms of cellular protection and the identification of the factors that regulate this protection are important to counteract cardiovascular aging and diseases. Yeast do not express insulin/IGF-I-like growth factors but respond to the presence of glucose, amino acids and other macromolecules by directly activating orthologs of genes that function in the mammalian IGF-I pathway including Tor/S6K, and Ras. Inactivation of the yeast Tor/Sch9 amino acid-response pathway or of the Ras/adenylate cyclase/PKA glucose-response pathway extends life span and also causes a major increase in the resistance to a variety of stresses including oxidative and heat stress.50, 73 These effects are mediated in large part by stress resistance transcription factors Gis1 and Msn2/Msn4, which promote extensive gene expression changes including increased expression of the mitochondrial superoxide scavenger SOD2.16, 50, 74-75 Similarly, the down-regulation of the PI3K age-1 gene or of the upstream daf-2 insulin/IGF-I-like receptor is associated with resistance to a variety of stresses in worms76 which, analogously to what is observed in yeast, is often mediated by the activation of forkhead transcription factor DAF-16, which regulates the expression of a number of protective genes including heat shock proteins and antioxidant enzymes.61
Fruit flies (Drosophila melanogaster) have been proposed as a valuable and simple model organism to study cardiac aging.77 In Drosophila, the insulin-like receptor (InR) and its downstream substrate chico regulate longevity and stress resistance as well. Loss of the activity of Chico, the insulin receptor substrate that regulates cell size and metabolism,78 increases lifespan and provides some resistance to paraquat.79-80 Interfering with InR signaling by overexpressing the phosphatase dPTEN or the forkhead transcription factor dFOXO, analogous to the worm DAF-16 transcription factor, prevents the age-dependent decline in cardiac performance.81 Drosophila has a simple “heart”/cardiovascular system composed by a cardiac tube or dorsal vessel, made of single layer of cardiomyocytes directly in contact with the internal and external environments.99 This constitutes the entire cardiovascular system of the organism that operates in an open circulation and that has been often regarded as a useful model of cardiac organogenesis environments.99 The fruit fly has also a simple and unique insulin/IGF-I signaling pathway without variant isoforms or complex gene regulation by alternative splicing. In this organism, a mutation in the InR, the only receptor homologous to mammalian insulin/IGF-I receptors, or Chico (the only homologous to the 3 insulin/IGF-I substrate IRS1-4) or other experimental interventions that dampen this pathway, reduce the progressive and detrimental changes in heart function observed when the fly ages (decrease in heart rate and heart failure).81 As introduced earlier, this evidence confirms that reduced IGF-I signaling can have both a positive and negative impact on cardiovascular function during stress and aging; a dichotomy which may be explained by the well-established role of IGF-I and of IGF-I signaling proteins in acting acutely to block apoptosis but to promote cellular sensitivity to stress in response to chronic exposure (see “Growth signaling and stress resistance in mammals” section).
Growth signaling and stress resistance in mammals
As anticipated by the conserved effect of pro-growth signaling on increasing sensitivity to stress in simple organisms, the down-regulation of nutrient signaling and cellular protection are also linked in mammals. Fibroblasts removed from different IGF-I deficient mouse models are resistant to a variety of toxins including H2O2, paraquat, UV, methylmethanesulfonate (MMS), heat, and cadmium.28, 82 Conversely, the levels of superoxide dismutases and catalase activity are reduced after exposure of hepatocytes to GH or IGF-I or overexpression of GH in transgenic mice.83-84 Also, IGF-I signaling sensitizes rat primary neurons to oxidative stress by a SirT1 and Ras/Erk-dependent mechanism85 and sensitizes glial cells against chemotherapy drugs.86 Furthermore, mice with a 70-80% deficiency in IGF-I (LID) mice, have been shown to be resistant to multiple toxins including doxorubicin, which is well known to promote cardiac damage.86 Thus, IGF-I signaling is likely to promote a chronic aging promoting effect on cardiomyocytes. These sensitizing effects of growth factor signaling genes in various mammalian cell types can be mediated by the inactivation of FOXO forkhead stress resistance transcription factors, which, analogously to Msn2/4 in yeast and DAF-16 in worms, regulate cellular protection in part by modulating the expression of antioxidant enzymes such as SOD2.54
Mitochondria play an important role during cardiac stress resistance and aging.11 Short-term (24 h) IGF-I subcutaneous administration in rats confers cardioprotection from ischemic stress by enhancing mitochondrial function, boosting anti-apoptotic mechanisms, inhibiting of Ca2+ induced mitochondrial swelling and cytochrome c release, and increasing in ATP synthesis.87 In mice with hypopituitary dwarfism (Ames dwarf), low plasma GH/IGF-I levels are associated with increased cardiac mitochondrial oxidative stress.88 Nonetheless, Ames dwarf mice have a significantly increased life span and are less sensitive to dobutamine-induced heart stress.88-89
Although IGF-I and adenylate cyclase/PKA are not closely associated in mammals, mice lacking adenylate cyclase 5 (AC), an ortholog of the pro-aging and stress sensitizing yeast adenylate cyclase, are resistant to oxidative stress and protected against cardiac damage including ventricular hypertrophy, apoptosis and fibrosis.90 Similar results were obtained for mice with deficiencies in PKA, downstream of AC, which are resistant to age-related changes in diastolic dysfunction, and myocardial performance.91 These results raise the possibility that some of the fundamental mechanisms of cellular sensitization by specific IGF-I signaling and AC/PKA signaling pathways may be conserved from non-dividing yeast to heart cells. However, the relationship between IGF-I/pro-growth signaling and stress resistance is not straightforward particularly in non-dividing cells. In fact, GHRD mice are more susceptible to the pro-oxidant paraquat, possibly because IGF-I can be a strong temporary protector of cardiomyocytes against acute damage such as that caused by oxidative and other forms of damage.92 This dual role of IGF-I-is not surprising considering the variety of enzymes that it activates and considering that some of them, such as AKT, are well known to activate blockers of apoptosis (Bcl-2 etc).
IGF-I and stress resistance in cardiomyocytes
Decades of studies pointed to IGF-I as a potential therapeutic agent. Paradoxically, as discussed earlier, decades of studies also pointed to reduced insulin and IGF-I signaling as a strategy to protect cells and organs against aging and diseases. The picture is complicated by recent evidence about the cardioprotective, cardiosensitizing, and regenerative effects exerted by different IGF-I isoforms, and by the newly characterized molecular cross-talk between IGF-I and other signaling pathways involved in aging-associated damage and diseases (Sirtuins), in animal models.
In some reports, over-expression of IGF-I in the heart protected and prevented myocardial cell death after infarction, limiting ventricular dilation, hypertrophy, and diabetic cardiomyopathy.93-96 In another study, over-expression of a different IGF-I-transgene in the heart produced hypertrophy and failure, despite an initial physiological hypertrophy.97 As for oxidative stress, the role of circulating IGF-I is also debated: cardiomyocyte-specific overexpression of IGF-I has been shown to protect from Ang II-mediated oxidative stress,94 but severe lack of IGF-I in hepatocyte-specific IGF-I knockout mice, antagonizes oxidative stress and cell death in cardiomyocytes induced by the potent oxidant agent paraquat,98 in agreement with a number of studies showing a prooxidation effect of IGF-I in a number of cells and tissue (see section Growth signaling and stress resistance in mammals).
Unlike Drosophila, mammals display a complex IGF-I signaling system with different isoforms that have distinct effects on cardiovascular function (Figure 1, Figure 2). The IGF-I gene spans more than 70 kb and has six exons, giving rise to multiple splice variants. These variants share a common core peptide, flanked by varying termini (Class 1 and 2 N-terminal peptides, and E peptides)(Figure 1). IGF-I is both a systemic growth factor produced mainly by the liver in response to GH and a local growth factor acting in an autocrine/paracrine manner in organs such as the heart.51 Post-transcriptionally, IGF-I isoforms are cleaved to give a mature 70 amino acid core hormone (identical for all isoforms) devoid of both the signal peptide and the extension peptide (Figure 1). Although there are little data available on the tissue-specific expression of the IGF-I splicing isoforms in mammals, several IGF-I variants have been discovered, resulting in complex speculative models to describe IGF-I function and regulation. The locally acting mIGF-I isoform comprises a Class 1 signal peptide and a C-terminal Ea extension peptide (Figure 1).51 mIGF-I is highly expressed in neonatal tissues and in the adult liver, but decreases during aging in the heart and skeletal muscle, where it is expressed only transiently in response to local damage.51, 100-101 Over the past decade mouse genetics have been used to show that enhancement of the mIGF-I signaling pathway is highly effective in countering tissue decline possibly by its regenerative properties and its promotion of cell survival and renewal demonstrated in senescent skeletal muscle.102-104 Continuous cardiac expression of this isoform throughout postnatal life does not perturb cardiac physiology, and does not evolve into a pathological phenotype.105 mIGF-I overexpression is able to recover heart functionality after HF-inducing injuries (infarct induced by ligation of the left coronary artery, or after cardiotoxin-induced injury),105 demonstrating a restoration of cardiac function in post-infarct and injury-challenged mIGF-I transgenic mice that is facilitated by modulation of the inflammatory response. Molecular analysis further revealed that mIGF-I enhances antioxidative cell defenses by up-regulating a subset of genes that display anti-oxidant, anti-apoptotic properties and are inversely correlated with cardiac adiposity [adiponectin, uncoupling protein 1 (UCP1) and methallothionein 2 (MT-2)].105-108 Moreover, mIGF-I ventricular tissue exhibits increased proliferative activity in the affected areas several weeks after injury. The canonical signaling pathway involving Akt, mTOR, and p70S6 kinase is not induced in mIGF-I hearts, which instead displays activated PDK1 and SGK1 signaling intermediates (Figure 2).105 This is in agreement with the conserved role of Akt, mTOR and p70S6 kinase in sensitizing different model organisms against stress and aging50, 109-110 and may explain part of the dichotomy underlined earlier. The robust response achieved with the mIGF-I isoform suggests a potential mechanistic basis for therapeutic strategies to improve the outcome of heart disease.
Against a cardioprotective role for mIGF-I, a recent ex vivo study showed that mIGF-I overexpression in cardiomyocytes diminishes heart functional recovery after acute ischemic challenge was applied.111 It is possible that the cardioprotective effects of mIGF-I overexpression occur only upon prolonged stress triggering an in vivo response of the immune system. In fact, mIGF-I may repair the heart from injury through production of specific cytokines, cross-talk with the bone marrow, and recruitment of endothelial-primed cells for de novo vascularization of the myocardium.112 These finely tuned immune responses are not taken into account during ex vivo analyses, possibly leading to confounding results. A main limitation of the in vivo data obtained with mIGF-I cardiac restricted transgenic mice is the lack of comparison with control core peptide IGF-I cardiac restricted transgenics, to study if the observed cardioprotection is due to the peculiar properties of mIGF-I isoform (Class 1 signal peptide and a C-terminal Ea extension peptide (Figure 1) in the same experimental settings. In the skeletal muscle tissue is well known that the mIGF-I isoform promotes cell survival and renewal by paracrine mechanisms: its local supplementation orchestrates efficient repair of injured skeletal muscle tissues without scar formation, prevents age-related muscle atrophy and enhances bone marrow stem cell recruitment to the damaged tissue. Importantly, neither fully processed IGF-I nor systemically administered IGF-I counteracts muscle loss.102-104
It would be also of great interest to explore the protective properties of cardiac-restricted mIGF-I versus IGF-I signaling against fat-induced cardiac adiposity and lipotoxicity, which have not been addressed to date. This is relevant to CVD, because when the storing capacity of cardiomyocytes is exceeded, lipotoxicity, which includes cellular dysfunction or cell death, gradually leads to HF.113
IGF-I, stem cells and myocardial regeneration
Great effort has been devoted to exploring the potential of adult stem cells using different approaches (cellular reprogramming, tissue engineering), to provide new therapeutic options for myocardial regeneration, although many challenges remain to be solved.114 Early work from Anversa, Nadal-Ginard and others provided evidence for the possibility to isolate and utilize a pool of resident cardiac stem cells (CSC) both in humans and in rodent models for research and therapeutic purposes.115-116 In humans, these cells can amplify and likely commit to the myocyte lineage in response to increased workload, thereby contributing to the generation of new fibers during stress-induced hypertrophy.116 Further, the existence of CSC was confirmed in the adult rat myocardium, and it was demonstrated that they are self-renewing, clonogenic, multipotent and can give rise to several different cardiogenic cell lines.115 CSC could regenerate myocardium upon infarct by functionally differentiating into adult cardiomyocytes.115 More recently, a study from Anversa’s group on human hearts collected from patients 19 to 104 years of age who died from causes other than cardiovascular diseases, showed that from 20 to 100 years of age, the cardiomyocyte compartment is replaced 15 times in women and 11 times in men, and this is regulated by a pool of resident CSC modulating cardiac homeostasis and aging.117
A mechanistic link between the IGF-I signaling system and CSC function in the heart was discovered: IGF-I signaling induces division of CSC by up-regulating telomerase activity and hindering replicative senescence, thereby preserving the pool of functionally competent CSC.118-121 In dogs, circulating IGF-I was injected after infarction to stimulate resident CPC: this intervention led to the formation of cardiomyocytes and coronary vessels within the infarct and resulted in a marked recovery of contractility of the infarcted heart.120 Similar results were obtained in mice.119 With chronological aging, CPC undergo telomere shortening which generate a differentiated progeny acquiring a senescent phenotype. CPC aging were mediated by attenuation of the IGF-I system in rats.118 Activation by IGF-I injection led CPC to migrate to the regions of damage, reversing partly the aging cardiomyopathy. Consequently, heart aging and failure were partially corrected, leading to an extension of the maximum lifespan.118 CPC can be activated locally and induced to proliferate by administration of IGF-I, but they could also be isolated from myocardial biopsies and, following their expansion in vitro, administered back to the myocardium to improve the response to the challenge.122 In this respect, the most impressive protective effects and reduction of tissue damage from myocardial infarct induced in rats were observed with a combination therapy, made of simultaneous injection of CPC and circulating IGF-I tethered to peptide nanofibers to prolong its release.123 Although this wealth of data needs to be confirmed independently, the prominent emerging notion is that stem cell based cell reprogramming and organismal lifespan share some common regulatory pathways.124-125 Because in some cases, such as for IGF-I, stem cell regeneration and aging may have opposite responses (IGF-I promotes regeneration but also aging) it will be essential to understand how the positive effects of regeneration can be combined with the anti-aging effects of reduced growth factor signaling. It will also be important to understand how IGF-I and most likely the locally-generated mIGF-I can have a protective and not sensitizing effect on the myocardium, particularly in the periods following ischemic injury.
IGF-I and SirT1
At least three reports indicate that IGF-I and SirT1 can influence the same signaling pathways: 1) SIRT1 modulates the IGF-I signaling critical for both growth regulation and mammary gland development in mice;126 2) in neurons, the inhibition of SIRT1 reduces IGF-I signaling through deacetylation of IRS-2 and thereby protects them;85 3) SIRT1 reduces IGF-I signaling in fibroblasts by inhibiting Akt activation and cardiomyocytes expressing SIRT1, but not those lacking it developed hypertrophy after treatment with IGF-I, indicating that SIRT1 is needed for IGF-I–mediated hypertrophy of cardiomyocytes.127
The Sirtuins family of enzymes plays a role in the regulation of organismal lifespan.128 In mammals, Sirtuins are composed of seven members of class III nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylases (SIRT1 to 7). SIRT1, the largest and best characterized among them, is the mammalian orthologue of yeast “longevity” gene Sir2.129 SIRT1 activation displays pleiotropic effects128 and, in fact, both protective and sensitizing effects for Sir2 and SirT1 have been described.130-131 Interventions capable of activating SIRT1 enzymatic activity have been shown to increase life span in some model organisms. These include chronic treatment with the polyphenol resveratrol132-133 as well as a DR regimen.134 In contrast, SIRT1 knock-out (KO) mice die prenatally or perinatally.135-136 In one report, moderate (about 3-8 fold) SIRT1 overexpression protected mice from paraquat-induced cardiac stress and delayed the onset of age-dependent heart fibrosis and apoptosis.137 In parallel, in vitro findings on cultured or primary cardiomyocytes models expanded our understanding of the cardio-protective effects of SIRT1, suggesting that pharmacological SIRT1 activation might be beneficial for the treatment of some cardiac diseases.128 Two other sirtuins, SIRT3 and SIRT7, may also play an important protective role against age-related-, genotoxic stress-, hypertrophic stress- and oxidative stress-induced cardiac pathology,138 indicating a diversified and non-redundant conserved role of the Sirtuin family members. Interestingly, recent evidence shows that mice lacking SIRT3 develop severe age-related pathologies in the heart, such as hypertrophy, due to mitochondrial swelling and dysfunction, possibly because SIRT3 can modulate directly the mitochondrial permeability transition pore (mPTP), a multi-protein complex involved in age-related mitochondrial diseases.139
SIRT1 has also been shown to have the opposite effect on the heart: sensitize cardiomyocytes to stress. For example, higher increases in SIRT1 levels (about 13-fold) induced oxidative stress and apoptosis, ultimately leading to cardiomyopathy.137 In agreement with these results, the lack of SirT1 has been shown to promote the acetylation and reduced activity of Akt, leading to protection against cardiac hypertrophy.127 These studies indicate that SIRT1 activation can be either beneficial or deleterious in the heart in agreement with the role of its yeast ortholog Sir2 in promoting either pro- or anti-aging effects,128, 131 but also in agreement with the dual role of IGF-I described earlier. The disparate effects of different doses of cardiac SIRT1 could represent an example of the concept of “hormesis”, according to which an exogenous perturbation or stress can be protective or induce a damage according to the dose and the length of administration.140
Other than for a few studies listed above, there is scarce information about the molecular mechanisms of crosstalk between IGF-I and SIRT1 in the heart and, in particular, about the impact of separate IGF-I isoforms, acting locally or systemically, on cardiac SIRT1, although it is evident that IGF-I and SIRT1 share molecular downstream targets in cardiomyocytes such as AKT and FOXOs, and this in turn may affect cardiovascular function.127, 141 In a recent study the role of the IGF-I core protein isoform and the locally acting mIGF-I isoform were tested to determine if they could display distinct effects in the protection from cardiac stress.142 Using Ang II and paraquat as hypertrophic/oxidative stressors, an important signaling pathway that protects cardiomyocytes and which relies on the activation of SIRT1 by the locally acting mIGF-I isoform was identified.142 Heart-specific transgenic mIGF-I mice displayed a two-fold increase in SIRT1 expression compared with wild type mice, and this correlated with a down-regulation in the acetylation of SIRT1 targets (H1, p53) in mouse hearts.142 In vitro, mIGF-I overexpression protected cardiomyocytes from cell damages induced by hypertrophic and oxidative stressors in a specific SIRT1-dependent fashion, again in agreement with the function of SIRT1 in IGF-I signaling described earlier.142 The beneficial activity of mIGF-I was mediated by SIRT1-dependent activation of the protective molecules UCP1, adiponectin and MT2, through their respective gene promoters. Interestingly, the circulating IGF-I isoform, either added exogenously or produced by plasmid overexpression in cardiomyocytes, did not regulate SIRT1 expression and activity and it was not beneficial during hypertrophy and oxidative stress conditions.142-143
Cardiac-specific mIGF-I transgenic mice in which SIRT1 was depleted from adult cardiomyocytes in a tamoxifen-inducible and conditional fashion were recently generated to evaluate the role of mIGF-I/SIRT1 signaling in vivo. Analysis of these mice confirmed that mIGF-I-induced SIRT1 activity is necessary to protect the heart from paraquat (PQ)-induced oxidative stress and lethality.143 In cultured cardiomyocytes, mIGF-I increases SIRT1 expression through a JNK1-dependent signaling mechanism, while circulating core peptide IGF-I activated preferentially JNK2.143 Thus, mIGF-I can protect the heart from oxidative stress via SIRT1/JNK1 enzymatic activities (Figure 2). Considering the studies listed above which indicate that SIRT1 facilitates IGF-I-dependent cardiomyocyte hypertrophy, it is possible that the mIGF-I isoform causes a set of signaling changes that turns SIRT1 from a promoter of cardiotoxicity to a protective deacetylase (Figure 2).
The field of study of the different signaling and crosstalk between the distinct IGFI isoforms in the heart is in its infancy. Intriguingly, exogenous recombinant IGF-I administration could be beneficial for heart diseases in patients but only during precise temporal windows and duration of IGF-I administration.144-145 Understanding the cellular signaling networks, the epigenetic mechanisms (such as SIRT1 activation and chromatin remodeling processes) and the temporal windows through which the distinct IGF-I isoforms signal into the cardiomyocytes using animal models, during growth and aging, could lead to the identification of therapeutic entry points to fight oxidative stress and cardiac hypertrophy. In addition, this will provide us with a framework to understand the mechanistic basis of the modulation of the immune system and of the proposed recruitment of cardiac stem cells by IGF-I signaling.
GROWTH FACTORS, AND CARDIOVASCULAR AGING IN HUMANS
IGF-I and Heart Disease: good or bad?
The relationship between IGF-I and cardiovascular diseases in humans is complex. In mammals ageing is negatively associated to serum concentrations of several growth factors and anabolic hormones. For example, the circulating levels of GH, IGF-I, sex hormones, and DHEA-s progressively decline between 20 and 80 years of age in humans, whereas serum concentrations of pro-inflammatory cytokines increase with age.146 Data from epidemiological data show an association between low serum IGF-I concentrations and increased cardiovascular mortality.147-148 In particular, a low serum IGF-I concentration is associated with coronary artery disease and diabetic vascular lesions.149-151 However, association is not equal to causation as indicated by recent data showing that several metabolic and hormonal factors play a crucial role in modulating the risk of developing chronic diseases and survival in mammals.16, 29
As discussed earlier, the down-regulation of IGF-I or similar signaling pathways by several dietary or genetic interventions has been shown to improve health and prolong lifespan in model organisms including mice.16 In agreement with these findings and in contrast to the epidemiological data described above, humans with mutations that cause constitutively very low levels of IGF-I do not display increased atherosclerosis but appear to be protected against diabetes and cancer (see following sections). Conversely, subjects with elevated GH/IGF-I due to acromegaly have a 2- to 3-fold increase in mortality due mostly to vascular disease but also to cancer and a variety of other diseases.152 The discrepancy between the above-mentioned studies on the effects of IGF-I on cardiovascular morbidity and mortality in humans may be due to confounding factors. Patients with coronary artery disease are exposed for many years to harmful cardiometabolic risk factors (i.e. type 2 diabetes, high blood pressure, dyslipidemia and most importantly chronic systemic inflammation).153 It has been shown that an antagonistic relationship exists between the elevated pro-inflammatory cytokines and serum IGF-I concentration during degenerative conditions.154-157 Therefore, it is likely that inflammation (or other metabolic alterations), rather than low IGF-I, is responsible for the increased cardiovascular mortality in patients affected by CHD and that low IGF-I is another consequence of inflammation. In fact, the dramatic decline in serum IGF-I concentration induced by infections, trauma, and critical illness is not reversed by treatment with GH.158-161. Further, growth hormone infusion increases serum IGF-I concentration and nitrogen balance in healthy control subjects, but septic patients treated with GH maintain low IGF-I levels and a negative nitrogen balance.160-162 A proof of our scarce and possibly misguided understanding of the implications of up-regulating the GH/IGF-I signaling pathway on health came from two prospective, multicenter, double-blind, randomized, placebo-controlled trials that were ended prematurely because of increased morbidity and mortality in critically ill patients treated with GH.163
Taken together, these data strongly suggest that the association of low circulating IGF-I levels with diseases is likely to represent a protective response to conditions that can promote heart disease and not a risk factor for the disease.Because of the rising trend of GH supplementation in older adults to increase muscle and bone mass, a stress sensitizing effect of circulating GH/IGF-I in cardiomyocytes would increase the risk for cardiac diseases in addition to diabetes and cancer.
GH/IGF-I deficiencies and CVDs
Obesity is a major risk factor for the metabolic syndrome, which in addition to playing a key role in diabetes is associated with diseases including coronary artery disease and stroke. Thus, GH and IGF-I deficiencies, which promote fat deposition in mice and humans, would be expected to also increase the incidence of cardiovascular diseases. However, the studies performed failed to provide evidence for either premature atherosclerosis or cardiovascular disease in subjects with deficiencies in growth hormones/factors. Salvatori and colleagues reported that whereas subjects from Brazil with homozygous mutations in the growth hormone releasing hormone receptor (GHRHR) gene were obese and had higher LDL and C-reactive protein levels, they did not develop premature atherosclerosis as evaluated by exercise echocardiography.162 Furthermore, a 6-month treatment with GH of these GH deficient subjects caused weight loss but also a progressive increase in the number of atherosclerotic plaques and in the intima-media thickness.163 GH replacement therapy instead resulted in an increase in both diastolic and systolic blood pressure.163 Results consistent with those from the GHRHRD subjects in Brazil were obtained for the growth hormone receptor deficient (GHRD) subjects living in Ecuador. Whereas 27% of the deaths of GHRDs were reported to be from cardiac disease and 3% from stroke, in the control population composed of first to fourth degree relative, in the GHRDs 21% of the deaths were reported to be from cardiac disease and 12% from stroke, providing evidence that CVD deaths are approximately the same for the GHRDs and normal relatives.29
Although the protection against both diabetes and cancer in GHRD subjects29 is in agreement with studies in mice, the normal longevity of GH and IGF-I deficient humans does not reflect the record mammalian life span extension observed in GH and IGF-I deficient rodents.164-165 One possible explanation for this disparity is the finding that many GHRD subjects die young of a variety of unusual causes.29, 166 For example, 17% of the deaths in GHRDs were reported to be caused by convulsive disorders whereas 13% were alcohol-related, 20% were from accidents, and 17% from unknown causes representing a combined total of 67% of the deaths caused by these non-age-related causes, versus 34% unknown causes of death and 2% accidental deaths in the relatives.29 Whereas neither GHR nor GHRHR deficient subject appear to be long-lived, mutations that reduce the activity of the IGF-IR protein were overrepresented among centenarians, suggesting that lower activity but not severe deficiency in GH/IGF-I signaling may be more beneficial for longevity extension.167
CONCLUSIONS AND FUTURE DIRECTIONS
In conclusion the studies in simple model organisms, mice and humans reviewed here point to a number of conclusions: 1) DR causes a decrease in growth factors and anabolic hormones including IGF-I, which may mediate many of its protective effect including those on heart disease; 2) Low IGF-I is associated with aging and cardiovascular disease in humans but the studies discussed here indicate that this may be a consequence and not a cause of aging and heart disease; 3) Different IGF-I isoforms can have either protective and sensitizing effects on cardiomyocytes. Also, IGF-I may have a temporary protective effect based on inhibition of apoptosis but a chronic cellular sensitizing and aging promoting effect. The IGF-I expressed by heart cells and not the circulating IGF-I appears to be the protective factor.
Additional studies are needed in both rodent and human studies to test the hypothesis that the severe IGF-I deficiency associated with reduced cancer and diabetes incidence rates does not contribute to CVD and to determine whether certain isoforms of IGF-I can in fact promote cardioprotection without activating the pro-aging signaling pathways. Because several drugs targeting the GHRH, GH, and GHR axis are FDA approved and widely used to treat acromegaly and other diseases, it will be important to monitor their effects on cardiovascular diseases in patients already being treated. More studies are also urgently needed to clarify the relationship between IGF-I signaling and cardiovascular health, particularly in older individuals eating high calorie Western diets that promote chronic inflammation, insulin resistance, and dyslipidemia.
Acknowledgements
We thank Dr. Min Wei for the careful reading of the manuscript and helpful comments.
Sources of Funding V.D.L. is funded by NIH/NIA grants AG20642, AG025135 and AG034906 and by the Bakewell Foundation. L.F. is supported by UL1 RR00036, P30DK056341, as well as grants from the Barnes Jewish Hospital Foundation, Istituto Superiore di Sanità/National Institutes of Health Collaboration Program, the Longer Life Foundation (an RGA/Washington University Partnership), the Bakewell Foundation and a donation from the Scott and Annie Appleby Charitable Trust.
Abbreviations
- Ang II
angiotensin II
- CRP
C-reactive protein
- CSC
cardiac stem cells
- CVD
cardiovascular diseases
- DR
dietary restriction
- GH
growth hormone
- GHR
growth hormone receptor
- GHRD
growth hormone receptor deficient
- GHRHR
growth hormone releasing hormone receptor
- GHRHRD
growth hormone releasing hormone receptor deficient
- HF
heart failure
- HOMA-IR
homeostatic model assessment – insulin resistance
- IGF-I
insulin growth factor
- 1 InR
insulin receptor
- mTOR
mammalian target of rapamycin
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- SIRT1
sirtuin 1
- S6K
p70 S6 ribosomal kinase
- TNF
tumor necrosis factor - alpha
Footnotes
Disclosures None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 2.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O’Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: Comparison between urban and rural communities in china. Circulation. 1985;71:202–210. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG. Cardiovascular aging research: The next horizons. J Am Geriatr Soc. 1999;47:613–625. doi: 10.1111/j.1532-5415.1999.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 4.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part ii: The aging heart in health: Links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 5.McGrath BP, Liang YL, Teede H, Shiel LM, Cameron JD, Dart A. Age-related deterioration in arterial structure and function in postmenopausal women: Impact of hormone replacement therapy. Arterioscler Thromb Vasc Biol. 1998;18:1149–1156. doi: 10.1161/01.atv.18.7.1149. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 7.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 8.Kitzman DW, Sheikh KH, Beere PA, Philips JL, Higginbotham MB. Age-related alterations of doppler left ventricular filling indexes in normal subjects are independent of left ventricular mass, heart rate, contractility and loading conditions. J Am Coll Cardiol. 1991;18:1243–1250. doi: 10.1016/0735-1097(91)90542-h. [DOI] [PubMed] [Google Scholar]
- 9.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part i: Aging arteries: A “Set up” For vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 10.Port S, Cobb FR, Coleman RE, Jones RH. Effect of age on the response of the left ventricular ejection fraction to exercise. N Engl J Med. 1980;303:1133–1137. doi: 10.1056/NEJM198011133032001. [DOI] [PubMed] [Google Scholar]
- 11.Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93:381–385. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 13.Fluckiger L, Boivin JM, Quilliot D, Jeandel C, Zannad F. Differential effects of aging on heart rate variability and blood pressure variability. J Gerontol A Biol Sci Med Sci. 1999;54:B219–224. doi: 10.1093/gerona/54.5.b219. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz JB, Gibb WJ, Tran T. Aging effects on heart rate variation. J Gerontol. 1991;46:M99–106. doi: 10.1093/geronj/46.3.m99. [DOI] [PubMed] [Google Scholar]
- 15.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 16.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castello L, Froio T, Cavallini G, Biasi F, Sapino A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto E. Calorie restriction protects against age-related rat aorta sclerosis. FASEB J. 2005;19:1863–1865. doi: 10.1096/fj.04-2864fje. [DOI] [PubMed] [Google Scholar]
- 18.Guo Z, Mitchell-Raymundo F, Yang H, Ikeno Y, Nelson J, Diaz V, Richardson A, Reddick R. Dietary restriction reduces atherosclerosis and oxidative stress in the aorta of apolipoprotein e-deficient mice. Mech Ageing Dev. 2002;123:1121–1131. doi: 10.1016/s0047-6374(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Haddad F, Bodell PW, McCue SA, Herrick RE, Baldwin KM. Food restriction-induced transformations in cardiac functional and biochemical properties in rats. J Appl Physiol. 1993;74:606–612. doi: 10.1152/jappl.1993.74.2.606. [DOI] [PubMed] [Google Scholar]
- 20.Seymour EM, Parikh RV, Singer AA, Bolling SF. Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the dahl-ss rat. J Mol Cell Cardiol. 2006;41:661–668. doi: 10.1016/j.yjmcc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Taffet GE, Pham TT, Hartley CJ. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol A Biol Sci Med Sci. 1997;52:B285–290. doi: 10.1093/gerona/52a.6.b285. [DOI] [PubMed] [Google Scholar]
- 22.Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- 23.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolinsky VW, Morton JS, Oka T, Robillard-Frayne I, Bagdan M, Lopaschuk GD, Des Rosiers C, Walsh K, Davidge ST, Dyck JR. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension. 2010;56:412–421. doi: 10.1161/HYPERTENSIONAHA.110.154732. [DOI] [PubMed] [Google Scholar]
- 25.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of fischer 344 rats: Ii. Pathology. J Gerontol. 1985;40:671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- 27.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- 28.Murakami S. Stress resistance in long-lived mouse models. Exp Gerontol. 2006;41:1014–1019. doi: 10.1016/j.exger.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 29.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial h2o2 production and oxidative damage. Cardiovasc Res. 2007;74:456–465. doi: 10.1016/j.cardiores.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Shinmura K, Tamaki K, Sano M, Nakashima-Kamimura N, Wolf AM, Amo T, Ohta S, Katsumata Y, Fukuda K, Ishiwata K, Suematsu M, Adachi T. Caloric restriction primes mitochondria for ischemic stress by deacetylating specific mitochondrial proteins of the electron transport chain. Circ Res. 2011;109:396–406. doi: 10.1161/CIRCRESAHA.111.243097. [DOI] [PubMed] [Google Scholar]
- 32.Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex i and lowers oxidative damage to mitochondrial DNA in the rat heart. Faseb J. 2001;15:1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- 33.Niemann B, Chen Y, Issa H, Silber RE, Rohrbach S. Caloric restriction delays cardiac ageing in rats: Role of mitochondria. Cardiovasc Res. 2010;88:267–276. doi: 10.1093/cvr/cvq273. [DOI] [PubMed] [Google Scholar]
- 34.Feuers RJ. The effects of dietary restriction on mitochondrial dysfunction in aging. Ann N Y Acad Sci. 1998;854:192–201. doi: 10.1111/j.1749-6632.1998.tb09902.x. [DOI] [PubMed] [Google Scholar]
- 35.Weindruch RH, Cheung MK, Verity MA, Walford RL. Modification of mitochondrial respiration by aging and dietary restriction. Mech Ageing Dev. 1980;12:375–392. doi: 10.1016/0047-6374(80)90070-6. [DOI] [PubMed] [Google Scholar]
- 36.Ash CE, Merry BJ. The molecular basis by which dietary restricted feeding reduces mitochondrial reactive oxygen species generation. Mech Ageing Dev. 2011;132:43–54. doi: 10.1016/j.mad.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Park Y, Zuidema MY, Hannink M, Zhang C. Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World J Cardiol. 2011;3:18–24. doi: 10.4330/wjc.v3.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ershler WB, Sun WH, Binkley N, Gravenstein S, Volk MJ, Kamoske G, Klopp RG, Roecker EB, Daynes RA, Weindruch R. Interleukin-6 and aging: Blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–230. [PubMed] [Google Scholar]
- 39.Han ES, Evans TR, Shu JH, Lee S, Nelson JF. Food restriction enhances endogenous and corticotropin-induced plasma elevations of free but not total corticosterone throughout life in rats. J Gerontol A Biol Sci Med Sci. 2001;56:B391–397. doi: 10.1093/gerona/56.9.b391. [DOI] [PubMed] [Google Scholar]
- 40.Jolly CA. Dietary restriction and immune function. J Nutr. 2004;134:1853–1856. doi: 10.1093/jn/134.8.1853. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki J, Kuwamura M, Yamaji R, Inui H, Nakano Y. Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice. J Nutr. 2001;131:2139–2144. doi: 10.1093/jn/131.8.2139. [DOI] [PubMed] [Google Scholar]
- 42.Sabatino F, Masoro EJ, McMahan CA, Kuhn RW. Assessment of the role of the glucocorticoid system in aging processes and in the action of food restriction. J Gerontol. 1991;46:B171–179. doi: 10.1093/geronj/46.5.b171. [DOI] [PubMed] [Google Scholar]
- 43.Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines tnf-alpha and il-6 in c3b10rf1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 44.Cefalu WT, Bell-Farrow AD, Wang ZQ, Sonntag WE, Fu MX, Baynes JW, Thorpe SR. Caloric restriction decreases age-dependent accumulation of the glycoxidation products, n epsilon-(carboxymethyl)lysine and pentosidine, in rat skin collagen. J Gerontol A Biol Sci Med Sci. 1995;50:B337–341. doi: 10.1093/gerona/50a.6.b337. [DOI] [PubMed] [Google Scholar]
- 45.Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys. 1997;346:74–80. doi: 10.1006/abbi.1997.0297. [DOI] [PubMed] [Google Scholar]
- 46.Sell DR, Lane MA, Obrenovich ME, Mattison JA, Handy A, Ingram DK, Cutler RG, Roth GS, Monnier VM. The effect of caloric restriction on glycation and glycoxidation in skin collagen of nonhuman primates. J Gerontol A Biol Sci Med Sci. 2003;58:508–516. doi: 10.1093/gerona/58.6.b508. [DOI] [PubMed] [Google Scholar]
- 47.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masoro EJ, Austad SN. Handbook of the biology of aging. Elsevier Academic Press; 2010. [Google Scholar]
- 49.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 50.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 51.Winn N, Paul A, Musaro A, Rosenthal N. Insulin-like growth factor isoforms in skeletal muscle aging, regeneration, and disease. Cold Spring Harb Symp Quant Biol. 2002;67:507–518. doi: 10.1101/sqb.2002.67.507. [DOI] [PubMed] [Google Scholar]
- 52.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 53.Foncea R, Andersson M, Ketterman A, Blakesley V, Sapag-Hagar M, Sugden PH, LeRoith D, Lavandero S. Insulin-like growth factor-i rapidly activates multiple signal transduction pathways in cultured rat cardiac myocytes. J Biol Chem. 1997;272:19115–19124. doi: 10.1074/jbc.272.31.19115. [DOI] [PubMed] [Google Scholar]
- 54.Salih DA, Brunet A. Foxo transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song G, Ouyang G, Bao S. The activation of akt/pkb signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fazio S, Palmieri EA, Biondi B, Cittadini A, Sacca L. The role of the gh-igf-i axis in the regulation of myocardial growth: From experimental models to human evidence. Eur J Endocrinol. 2000;142:211–216. doi: 10.1530/eje.0.1420211. [DOI] [PubMed] [Google Scholar]
- 57.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor i (igf-1) and type 1 igf receptor (igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 58.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. Igf-i is required for normal embryonic growth in mice. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 59.Sutter N, Bustamante CD, Chase K, Gray MM, Zhao K, Zhu L, Padhukasahasram B, Karlins E, Davis S, Jones PG, Quignon P, Johnson GS, Parker HG, Fretwell N, Mosher DS, Lawler DF, Satyaraj E, Nordborg M, Lark KG, Wayne RK, Ostrander EA. A single igf1 allele is a major determinant of small size in dogs. Science. 2007;316:112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Longo V. Linking sirtuins, igf-i signaling, and starvation. Exp Gerontol. 2009:1–2. doi: 10.1016/j.exger.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 62.Fadini GP, Ceolotto G, Pagnin E, de Kreutzenberg S, Avogaro A. At the crossroads of longevity and metabolism: The metabolic syndrome and lifespan determinant pathways. Aging Cell. 2011;10:10–17. doi: 10.1111/j.1474-9726.2010.00642.x. [DOI] [PubMed] [Google Scholar]
- 63.Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific igf-ii is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 64.Lamonerie T, Lavialle C, Haddada H, Brison O. Igf-2 autocrine stimulation in tumorigenic clones of a human colon-carcinoma cell line. Int J Cancer. 1995;61:587–592. doi: 10.1002/ijc.2910610425. [DOI] [PubMed] [Google Scholar]
- 65.Nishimoto I, Murayama Y, Katada T, Ui M, Ogata E. Possible direct linkage of insulin-like growth factor-ii receptor with guanine nucleotide-binding proteins. J Biol Chem. 1989;264:14029–14038. [PubMed] [Google Scholar]
- 66.DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor ii gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 67.Moerth C, Schneider MR, Renner-Mueller I, Blutke A, Elmlinger MW, Erben RG, Camacho-Hubner C, Hoeflich A, Wolf E. Postnatally elevated levels of insulin-like growth factor (igf)-ii fail to rescue the dwarfism of igf-i-deficient mice except kidney weight. Endocrinology. 2007;148:441–451. doi: 10.1210/en.2006-0385. [DOI] [PubMed] [Google Scholar]
- 68.Lee SD, Chu CH, Huang EJ, Lu MC, Liu JY, Liu CJ, Hsu HH, Lin JA, Kuo WW, Huang CY. Roles of insulin-like growth factor ii in cardiomyoblast apoptosis and in hypertensive rat heart with abdominal aorta ligation. Am J Physiol Endocrinol Metab. 2006;291:E306–314. doi: 10.1152/ajpendo.00127.2005. [DOI] [PubMed] [Google Scholar]
- 69.Vogt AM, Htun P, Arras M, Podzuweit T, Schaper W. Intramyocardial infusion of tool drugs for the study of molecular mechanisms in ischemic preconditioning. Basic Res Cardiol. 1996;91:389–400. doi: 10.1007/BF00788719. [DOI] [PubMed] [Google Scholar]
- 70.Chu CH, Tzang BS, Chen LM, Kuo CH, Cheng YC, Chen LY, Tsai FJ, Tsai CH, Kuo WW, Huang CY. Igf-ii/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/bnp expression via galphaq interaction and protein kinase c-alpha/camkii activation in h9c2 cardiomyoblast cells. J Endocrinol. 2008;197:381–390. doi: 10.1677/JOE-07-0619. [DOI] [PubMed] [Google Scholar]
- 71.Wang KC, Zhang L, McMillen IC, Botting KJ, Duffield JA, Zhang S, Suter CM, Brooks DA, Morrison JL. Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J Physiol. 2011;589:4709–4722. doi: 10.1113/jphysiol.2011.211185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaunt TR, Cooper JA, Miller GJ, Day IN, O’Dell SD. Positive associations between single nucleotide polymorphisms in the igf2 gene region and body mass index in adult males. Hum Mol Genet. 2001;10:1491–1501. doi: 10.1093/hmg/10.14.1491. [DOI] [PubMed] [Google Scholar]
- 73.Longo VD. The pro-senescence role of ras2 in the chronological life span of yeast. 1997 [Google Scholar]
- 74.Longo VD, Gralla EB, Valentine JS. Superoxide dismutase activity is essential for stationary phase survival in saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J Biol Chem. 1996;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 75.Fabrizio P, Liou LL, Moy VN, Diaspro A, Valentine JS, Gralla EB, Longo VD. Sod2 functions downstream of sch9 to extend longevity in yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson TE, Lithgow GJ, Murakami S. Hypothesis: Interventions that increase the response to stress offer the potential for effective life prolongation and increased health. J Gerontol A Biol Sci Med Sci. 1996;51:B392–395. doi: 10.1093/gerona/51a.6.b392. [DOI] [PubMed] [Google Scholar]
- 77.Nishimura M, Ocorr K, Bodmer R, Cartry J. Drosophila as a model to study cardiac aging. Exp Gerontol. 2011;46:326–330. doi: 10.1016/j.exger.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by chico, a drosophila homolog of vertebrate irs1-4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- 79.Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of chico, a drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 80.Giannakou ME, Partridge L. Role of insulin-like signalling in drosophila lifespan. Trends Biochem Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 82.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 83.Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol. 2000;35:199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 84.Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med (Maywood) 2002;227:94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- 85.Li Y, Xu W, McBurney MW, Longo VD. Sirt1 inhibition reduces igf-i/irs-2/ras/erk1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of igf-i mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamamura T, Otani H, Nakao Y, Hattori R, Osako M, Imamura H. Igf-i differentially regulates bcl-xl and bax and confers myocardial protection in the rat heart. Am J Physiol Heart Circ Physiol. 2001;280:H1191–1200. doi: 10.1152/ajpheart.2001.280.3.H1191. [DOI] [PubMed] [Google Scholar]
- 88.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived gh/igf-deficient ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64:819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 91.Enns LC, Morton JF, Mangalindan RS, McKnight GS, Schwartz MW, Kaeberlein MR, Kennedy BK, Rabinovitch PS, Ladiges WC. Attenuation of age-related metabolic dysfunction in mice with a targeted disruption of the cbeta subunit of protein kinase a. J Gerontol A Biol Sci Med Sci. 2009;64:1221–1231. doi: 10.1093/gerona/glp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hauck SJ, Aaron JM, Wright C, Kopchick JJ, Bartke A. Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm Metab Res. 2002;34:481–486. doi: 10.1055/s-2002-34787. [DOI] [PubMed] [Google Scholar]
- 93.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. Igf-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin ii-mediated oxidative stress. Diabetes. 2001;50:1414–1424. doi: 10.2337/diabetes.50.6.1414. [DOI] [PubMed] [Google Scholar]
- 95.Ren J, Duan J, Thomas DP, Yang X, Sreejayan N, Sowers JR, Leri A, Kajstura J, Gao F, Anversa P. Igf-i alleviates diabetes-induced rhoa activation, enos uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol. 2008;294:R793–802. doi: 10.1152/ajpregu.00713.2007. [DOI] [PubMed] [Google Scholar]
- 96.Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol. 2007;292:H1398–1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 97.Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ. Local insulin-like growth factor i expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. Faseb J. 1999;13:1923–1929. doi: 10.1096/fasebj.13.14.1923. [DOI] [PubMed] [Google Scholar]
- 98.Li Q, Yang X, Sreejayan N, Ren J. Insulin-like growth factor i deficiency prolongs survival and antagonizes paraquat-induced cardiomyocyte dysfunction: Role of oxidative stress. Rejuvenation Res. 2007;10:501–512. doi: 10.1089/rej.2007.0552. [DOI] [PubMed] [Google Scholar]
- 99.Medioni C, Senatore S, Salmand PA, Lalevee N, Perrin L, Semeriva M. The fabulous destiny of the drosophila heart. Curr Opin Genet Dev. 2009;19:518–525. doi: 10.1016/j.gde.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 100.Velloso CP, Harridge SD. Insulin-like growth factor-i e peptides: Implications for aging skeletal muscle. Scand J Med Sci Sports. 2010;20:20–27. doi: 10.1111/j.1600-0838.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- 101.Matheny RW, Jr., Nindl BC, Adamo ML. Minireview: Mechano-growth factor: A putative product of igf-i gene expression involved in tissue repair and regeneration. Endocrinology. 2010;151:865–875. doi: 10.1210/en.2009-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Musaro A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, Ottolenghi S, Cossu G, Bernardi G, Battistini L, Molinaro M, Rosenthal N. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci U S A. 2004;101:1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 104.Vinciguerra M, Musaro A, Rosenthal N. Regulation of muscle atrophy in aging and disease. Adv Exp Med Biol. 2010;694:211–233. doi: 10.1007/978-1-4419-7002-2_15. [DOI] [PubMed] [Google Scholar]
- 105.Santini MP, Tsao L, Monassier L, Theodoropoulos C, Carter J, Lara-Pezzi E, Slonimsky E, Salimova E, Delafontaine P, Song YH, Bergmann M, Freund C, Suzuki K, Rosenthal N. Enhancing repair of the mammalian heart. Circ Res. 2007;100:1732–1740. doi: 10.1161/CIRCRESAHA.107.148791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iozzo P, Lautamaki R, Borra R, Lehto HR, Bucci M, Viljanen A, Parkka J, Lepomaki V, Maggio R, Parkkola R, Knuuti J, Nuutila P. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab. 2009;94:4472–4482. doi: 10.1210/jc.2009-0436. [DOI] [PubMed] [Google Scholar]
- 107.Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, Cai L. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–554. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- 108.Villarroya F, Iglesias R, Giralt M. Ppars in the control of uncoupling proteins gene expression. PPAR Res. 2007;2007:74364. doi: 10.1155/2007/74364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in drosophila by modulation of genes in the tor signaling pathway. Current Biology. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prele CM, Reichelt ME, Mutsaers SE, Davies M, Delbridge LM, Headrick JP, Rosenthal N, Bogoyevitch MA, Grounds MD. Insulin-like growth factor-1 overexpression in cardiomyocytes diminishes ex vivo heart functional recovery after acute ischemia. Cardiovasc Pathol. 2011 doi: 10.1016/j.carpath.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 112.Santini MP, Lexow J, Borsellino G, Slonimski E, Zarrinpashneh E, Poggioli T, Rosenthal N. Igf-1ea induces vessel formation after injury and mediates bone marrow and heart cross-talk through the expression of specific cytokines. Biochem Biophys Res Commun. 2011;410:201–207. doi: 10.1016/j.bbrc.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 113.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 114.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 116.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogorek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D’Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 119.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 120.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 122.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, Urbanek K, Hosoda T, Rota M, Anversa P, Leri A, Lee RT, Kajstura J. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen T, Shen L, Yu J, Wan H, Guo A, Chen J, Long Y, Zhao J, Pei G. Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00722.x. [DOI] [PubMed] [Google Scholar]
- 125.Calvanese V, Fraga MF. Sirt1 brings stemness closer to cancer and aging. Aging (Albany NY) 2011;3:162–167. doi: 10.18632/aging.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li H, Rajendran GK, Liu N, Ware C, Rubin BP, Gu Y. Sirt1 modulates the estrogen-insulin-like growth factor-1 signaling for postnatal development of mammary gland in mice. Breast Cancer Res. 2007;9:R1. doi: 10.1186/bcr1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. The deacetylase sirt1 promotes membrane localization and activation of akt and pdk1 during tumorigenesis and cardiac hypertrophy. Sci Signal. 2011;4:ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 128.Guarente L, Franklin h. Epstein lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 129.Vinciguerra M, Fulco M, Ladurner A, Sartorelli V, Rosenthal N. Sirt1 in muscle physiology and disease: Lessons from mouse models. Dis Model Mech. 2010;3:298–303. doi: 10.1242/dmm.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 131.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 132.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 133.Howitz K, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 134.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the sirt1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 135.Cheng H, Mostoslavsky R, Saito S, Manis J, Gu Y, Patel P, Bronson R, Appella E, Alt F, Chua K. Developmental defects and p53 hyperacetylation in sir2 homolog (sirt1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McBurney M, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian sir2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 138.Schug TT, Li X. Surprising sirtuin crosstalk in the heart. Aging (Albany NY) 2010;2:129–132. doi: 10.18632/aging.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mptp by sirt3-mediated deacetylation of cypd at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 141.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. Foxo transcription factors activate akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vinciguerra M, Santini MP, Claycomb WC, Ladurner AG, Rosenthal N. Local igf-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via sirt1 activity. Aging (Albany NY) 2009;2:43–62. doi: 10.18632/aging.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vinciguerra M, Santini MP, Martinez C, Pazienza V, Claycomb WC, Giuliani A, Rosenthal N. Migf-1/jnk1/sirt1 signaling confers protection against oxidative stress in the heart. Aging Cell. 2011 doi: 10.1111/j.1474-9726.2011.00766.x. [DOI] [PubMed] [Google Scholar]
- 144.Abbas A, Grant PJ, Kearney MT. Role of igf-1 in glucose regulation and cardiovascular disease. Expert Rev Cardiovasc Ther. 2008;6:1135–1149. doi: 10.1586/14779072.6.8.1135. [DOI] [PubMed] [Google Scholar]
- 145.Conti E, Musumeci MB, Assenza GE, Quarta G, Autore C, Volpe M. Recombinant human insulin-like growth factor-1: A new cardiovascular disease treatment option? Cardiovasc Hematol Agents Med Chem. 2008;6:258–271. doi: 10.2174/187152508785909456. [DOI] [PubMed] [Google Scholar]
- 146.Leifke E, Gorenoi V, Wichers C, Von Zur Muhlen A, Von Buren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: Cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf) 2000;53:689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 147.Bulow B, Hagmar L, Eskilsson J, Erfurth EM. Hypopituitary females have a high incidence of cardiovascular morbidity and an increased prevalence of cardiovascular risk factors. J Clin Endocrinol Metab. 2000;85:574–584. doi: 10.1210/jcem.85.2.6346. [DOI] [PubMed] [Google Scholar]
- 148.Rosen T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285–288. doi: 10.1016/0140-6736(90)91812-o. [DOI] [PubMed] [Google Scholar]
- 149.Goodman-Gruen D, Barrett-Connor E, Rosen C. Igf-1 and ischemic heart disease in older people. J Am Geriatr Soc. 2000;48:860–861. doi: 10.1111/j.1532-5415.2000.tb04774.x. [DOI] [PubMed] [Google Scholar]
- 150.Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum total igf-i, free igf-i, and igfb-1 levels in an elderly population: Relation to cardiovascular risk factors and disease. Arterioscler Thromb Vasc Biol. 1998;18:277–282. doi: 10.1161/01.atv.18.2.277. [DOI] [PubMed] [Google Scholar]
- 151.Spallarossa P, Brunelli C, Minuto F, Caruso D, Battistini M, Caponnetto S, Cordera R. Insulin-like growth factor-i and angiographically documented coronary artery disease. Am J Cardiol. 1996;77:200–202. doi: 10.1016/s0002-9149(96)90600-1. [DOI] [PubMed] [Google Scholar]
- 152.Clayton RN. Cardiovascular function in acromegaly. Endocr Rev. 2003;24:272–277. doi: 10.1210/er.2003-0009. [DOI] [PubMed] [Google Scholar]
- 153.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 154.Fan J, Wojnar MM, Theodorakis M, Lang CH. Regulation of insulin-like growth factor (igf)-i mrna and peptide and igf-binding proteins by interleukin-1. Am J Physiol. 1996;270:R621–629. doi: 10.1152/ajpregu.1996.270.3.R621. [DOI] [PubMed] [Google Scholar]
- 155.Fan J, Char D, Bagby GJ, Gelato MC, Lang CH. Regulation of insulin-like growth factor-i (igf-i) and igf-binding proteins by tumor necrosis factor. Am J Physiol. 1995;269:R1204–1212. doi: 10.1152/ajpregu.1995.269.5.R1204. [DOI] [PubMed] [Google Scholar]
- 156.Wolf M, Bohm S, Brand M, Kreymann G. Proinflammatory cytokines interleukin 1 beta and tumor necrosis factor alpha inhibit growth hormone stimulation of insulin-like growth factor i synthesis and growth hormone receptor mrna levels in cultured rat liver cells. Eur J Endocrinol. 1996;135:729–737. doi: 10.1530/eje.0.1350729. [DOI] [PubMed] [Google Scholar]
- 157.De Benedetti F, Alonzi T, Moretta A, Lazzaro D, Costa P, Poli V, Martini A, Ciliberto G, Fattori E. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-i. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997;99:643–650. doi: 10.1172/JCI119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baxter RC. Changes in the igf-igfbp axis in critical illness. Best Pract Res Clin Endocrinol Metab. 2001;15:421–434. doi: 10.1053/beem.2001.0161. [DOI] [PubMed] [Google Scholar]
- 159.Dahn MS, Lange MP, Jacobs LA. Insulinlike growth factor 1 production is inhibited in human sepsis. Arch Surg. 1988;123:1409–1414. doi: 10.1001/archsurg.1988.01400350123019. [DOI] [PubMed] [Google Scholar]
- 160.Jenkins RC, Ross RJ. Acquired growth hormone resistance in catabolic states. Baillieres Clin Endocrinol Metab. 1996;10:411–419. doi: 10.1016/s0950-351x(96)80545-3. [DOI] [PubMed] [Google Scholar]
- 161.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 162.Alcantara MR, Salvatori R, Alcantara PR, Nobrega LM, Campos VS, Oliveira EC, Oliveira MH, Souza AH, Aguiar-Oliveira MH. Thyroid morphology and function in adults with untreated isolated growth hormone deficiency. J Clin Endocrinol Metab. 2006;91:860–864. doi: 10.1210/jc.2005-2555. [DOI] [PubMed] [Google Scholar]
- 163.Oliveira JL, Aguiar-Oliveira MH, D’Oliveira A, Jr., Pereira RM, Oliveira CR, Farias CT, Barreto-Filho JA, Anjos-Andrade FD, Marques-Santos C, Nascimento-Junior AC, Alves EO, Oliveira FT, Campos VC, Ximenes R, Blackford A, Parmigiani G, Salvatori R. Congenital growth hormone (gh) deficiency and atherosclerosis: Effects of gh replacement in gh-naive adults. J Clin Endocrinol Metab. 2007;92:4664–4670. doi: 10.1210/jc.2007-1636. [DOI] [PubMed] [Google Scholar]
- 164.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 165.Kopchick JJ, Andry JM. Growth hormone (gh), gh receptor, and signal transduction. Mol Genet Metab. 2000;71:293–314. doi: 10.1006/mgme.2000.3068. [DOI] [PubMed] [Google Scholar]
- 166.Oliveira CR, Salvatori R, Meneguz-Moreno RA, Aguiar-Oliveira MH, Pereira RM, Valenca EH, Araujo VP, Farias NT, Silveira DC, Vieira JG, Barreto-Filho JA. Adipokine profile and urinary albumin excretion in isolated growth hormone deficiency. J Clin Endocrinol Metab. 2010;95:693–698. doi: 10.1210/jc.2009-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor i receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]